Abstract
The development of cardiovascular fibrosis is associated with chronic inflammation, where activation of NF-κB signaling may play a critical role. NF-κB activation is tightly regulated by the cellular inhibitory proteins IκBα and IκBβ. IκBα and IκBβ display different regulation kinetics in response to inflammatory stimulation. The present study tested the hypothesis that IκBα and IκBβ may have different roles in modulating cardiovascular inflammation and fibrosis, using a model of angiotensin II-infusion-induced hypertension in wild type (WT) mice and IκBβ knockin mice, in which the IκBα gene is replaced by IκBβ cDNA (AKBI). In WT mice, subcutaneous angiotensin II infusion for 7 days induced increased perivascular and interstitial collagen deposition and fibrotic lesions, associated with myocardial interstitial hemosiderin accumulation and extensive macrophage infiltration. These effects of angiotensin II were dramatically limited in AKBI mice. Replacement of IκBα with IκBβ significantly attenuated angiotensin II infusion-induced expression of interleukin-1β, interleukin-6, monocyte chemotactic protein (MCP)-1, collagen I and III, fibronectin, and tissue inhibitor of metalloproteinase (TIMP)-1 in the hearts. Furthermore, using cultured vascular smooth muscle cells, we demonstrated that interleukin-1β-induced NF-κB activation and MCP-1, VCAM-1, and TIMP-1 expression were suppressed in the AKBI cells due to the replacement of IκBα with IκBβ. These results indicate that NF-κB has an essential role in mediating the cardiovascular inflammatory response to angiotensin II and suggest that targeting the balance of IκBα and IκBβ expression might be a novel therapeutic modality in preventing fibrosis in hypertensive cardiovascular disease.
Keywords: fibrosis, inflammation, iron, NF-kappa B, tissue inhibitor of metalloproteinase
Cardiovascular fibrosis is the most common consequence of hypertensive disease, and contributes to the development of cardiovascular dysfunction.1, 2 Fibrosis is thought to develop as a result of a tissue repair process associated with excessive chronic inflammation.3, 4 Activation of nuclear factor-κB (NF-κB) is essential for expression of numerous inflammatory mediators including adhesion molecules, cytokines/chemokines, growth factors, and extracellular matrix (ECM) metabolic regulators that participate in the tissue repair and remodeling process.5–8 Modulation of NF-κB signaling may, therefore, influence the inflammatory and fibrotic response in hypertensive disease.
NF-κB activation is tightly regulated by the cellular inhibitory proteins such as IκBα and IκBβ.5, 7, 9, 10 Various stimuli such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and lipopolysaccharide (LPS) can induce activation of IκB kinases (IKKs) that phosphorylate IκB, leading to subsequent IκB ubiquitination and proteasomal degradation. NF-κB released from the IκB/NF-κB complex then translocates to the nucleus, where it initiates transcription of NF-κB-regulated genes.9, 10 Although IκBα and IκBβ have overlapping functions,11 there are unique and non-redundant functional roles of IκBα and IκBβ in regulating immune and inflammatory response. IκBα-deficiency results in mouse neonatal lethality with extensive dermatitis and granulopoiesis,12 while IκBβ-deficiency does not;13 An IκBβ knockin mouse model, in which the IκBα gene is replaced by IκBβ cDNA (named AKBI),11 develops normally but shows attenuated neutrophil recruitment and liver dysfunction following liver ischemia/reperfusion injury,14 suggesting that replacement of IκBα by IκBβ may not only prevent IκBα-deficiency-induced mouse neonatal lethality, but also protect tissue from damage in certain conditions. However, the different functional roles of IκBα and IκBβ remain largely unknown, and whether IκBα and IκBβ could have different roles in modulating cardiovascular inflammation and fibrosis has not been previously explored.
Angiotensin (Ang) II is one of the best-characterized vasoconstrictors that, at abnormal high levels, can cause hypertension, cardiovascular inflammation and fibrosis. In vivo experiments using rodent models suggest that Ang II may contribute to fibrosis by inducing iron deposition, macrophage infiltration, and up-regulation of transforming growth factor (TGF)-β1.15–19 In the present study, we used Ang II infusion-induced hypertensive models in wild type (WT) and AKBI mice to test the hypothesis that IκBα and IκBβ may play different roles in modulating cardiovascular inflammation, iron deposition, and fibrosis. We also used cultured vascular smooth muscle cells (VSMCs) isolated from WT and AKBI mouse aortas to further uncover the different roles of IκBα and IκBβ in regulating NF-κB activation and gene expression.
Methods
Animal Models
AKBI transgenic mice were kindly made available by Dr. Cordula Stamme (Leibniz-Center for Medicine and Biosciences, Borstel, Germany). The mice were maintained on CD-1 genetic background. AKBI mice and WT CD-1 controls (both females, at 20 weeks of age) were randomly divided into 2 groups, and infused with either saline or Ang II (3.2 mg/kg/day) via subcutaneously implanted osmotic pumps (Alzet, model 1007D) following a procedure as described previously.20 Systolic blood pressure (SBP) was measured by the tail cuff plethysmography20 before starting treatment and again on day 6 after Ang II infusion. Mice were euthanized on day 7 of the treatment. Blood was drawn from abdominal vein and serum was isolated. Hearts and aortas were harvested. For histology and immunohistochemistry, the tissues were fixed with 10% buffered formalin acetate, processed, and embedded in paraffin. For isolation of RNA, cardiac ventricles were directly added to 1 mL of Trizol reagent (Invitrogen) and total RNA was extracted following manufacturer’s protocol. All animal experiments were carried out with the approval of the Institutional Animal Care and Use Committee of Boston University Medical Center and Harvard Medical School.
Histology and Immunohistochemistry
To assess morphological changes, Masson’s trichrome staining was performed in 5-μm thick cross sections of heart and descending aorta. Collagen was stained using Picrosirius Red. Collagen deposition (red color) and whole section area were analyzed using NIH ImageJ. Iron (hemosiderin) accumulation was detected by Prussian blue staining. Tissue macrophage infiltration was detected by immunohistochemistry with an antibody against Mac3 antigen (Online supplement, please see http://hyper.ahajournals.org). To measure aortic medial thickness and adventitial fibrotic area, microscopic images of descending aorta cross sections stained with Masson’s trichrome were analyzed using NIH ImageJ.
Cell Culture
Vascular smooth muscle cells were isolated from the medial layers of thoracic aorta of AKBI and WT littermates using the explant method described previously.21 Cells were cultured in DMEM/F12 (Invitrogen) with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were used between passages 4 and 7. The cells at confluence were washed and maintained in DMEM/F12 with 0.1% FBS for 24 hours. The cells were then treated with or without IL-1β for designated time periods. For determination of NF-κB activation, confluent cells cultured in 24-well plates were infected with NF-κB-luciferase reporter adenovirus (Ad-NFκBLuc)22 for 24 h, and then treated with IL-1β for either 4 or 16 hours. Cells were lysed and 5 μg proteins of each cell lysate were used for luciferase activity assay using the assay kit from Promega. Relative light units (RLU) were measured using the SpectraMax M5 Microplate Reader (Molecular Devices).
Determination of Serum Cytokines
Serum cytokines were determined by ELISA using the MILLIPLEX Mouse Cytokine/Chemokine Assay kit (MPXMCYTO-70K, Millipore) following manufacturer’s protocol.
Real Time PCR and Western Blot Analysis
Total RNA was extracted from tissues or cells using Trizol reagent (Invitrogen). cDNA was synthesized from 1 μg total RNA using AMV reverse transcriptase XL and random 9 mers (Takara). RNA expression levels were determined by real time PCR using SYBR Green premix reagents (SABiosciences) and target gene-specific primers (Online supplement, Table S1, please see http://hyper.ahajournals.org). Western blot analyses were performed using the method as described previously.5
Materials
(Online supplement, please see http://hyper.ahajournals.org).
Statistical Analysis
Data are expressed as mean ± SD or SEM as indicated in the results. 1-way ANOVA were performed for statistical analysis. P<0.05 was considered significant.
Results
AKBI Mice Resist Developing Cardiovascular Fibrosis in Response to Ang II Infusion
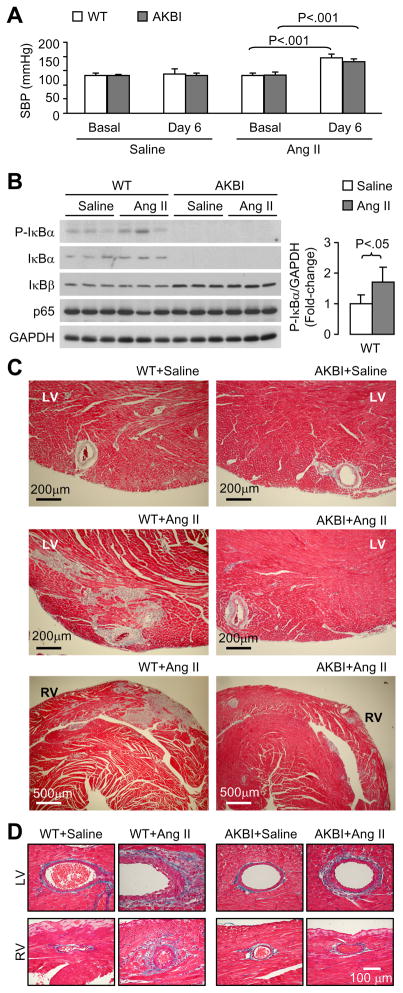
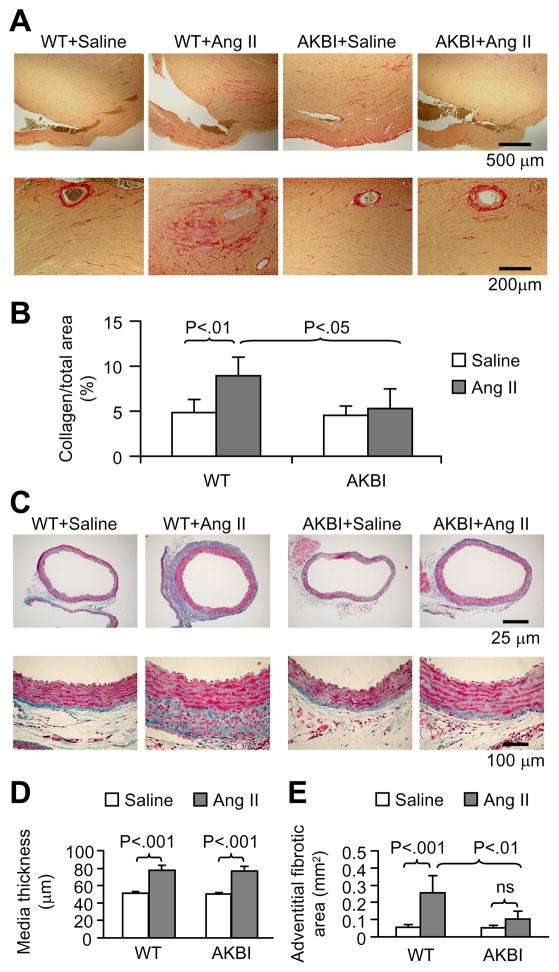
Ang II infusion increased systolic blood pressure similarly in WT and AKBI mice (Fig 1A). As shown in Fig 1B, AKBI mice did not express IκBα, but showed an increased IκBβ expression in the heart tissues. There was a basal level of phosphorylated IκBα (P-IκBα) in WT mice, which was enhanced by Ang II infusion. Endogenous phosphorylated IκBβ was not detectable (data not shown). There was no difference in NF-κB p65 expression between WT and AKBI mice. Ang II infusion caused perivascular and interstitial fibrotic lesions in both left ventricles (LV) and right ventricles (RV) (Fig 1C and D), suggesting that Ang II-induced cardiac fibrosis is at least partially, independent of increased systolic blood pressure. Ang II-induced fibrotic lesions were much less obvious in AKBI mice than in WT mice. Collagen staining showed that Ang II infusion enhanced ventricular tissue collagen deposition, which was attenuated in AKBI mice (Fig 2A and B). Furthermore, Ang II infusion increased aortic medial thickness in both WT and AKBI mice (Fig 2C and D). Interestingly, Ang II infusion increased aortic adventitial area and ECM deposition in WT mice, which was attenuated in AKBI mice (Fig 2C and E). These results demonstrate that replacement of IκBα with IκBβ attenuates Ang II infusion-induced fibrosis and this effect is independent of systolic blood pressure.
Figure 1.
AKBI mice resist developing cardiovascular fibrosis in response to Ang II infusion. A, Ang II infusion increases systolic blood pressure (SBP) in wild type (WT) and AKBI mice. Means ± SD, n = 5 in each group. B, Western blot analysis of IκBα, IκBβ, and NFκB p65 expression in heart tissues. C and D, Masson’s trichrome staining of heart sections. Ang II infusion causes perivascular and interstitial fibrosis in WT mice. These effects of Ang II were much less obvious in AKBI mice. LV, left ventricle; RV, right ventricle.
Figure 2.
AKBI mice have less myocardial collagen deposition and adventitial fibrosis after Ang II infusion. A, Picrosirius red staining on heart sections showing collagen deposition. B, Quantitative analysis of collagen area, expressed as percentage of total section area. Means ± SD, n = 5 in each group. C, Masson’s trichrome staining of descending aorta cross sections. Note that Ang II infusion-caused adventitial fibrotic area and matrix deposition were obviously less in AKBI mice than in wild type mice. D and E, Quantitative analysis of aortic media thickness (D) and adventitial fibrotic area (E). Means ± SD, n = 5 in each group. ns, no significance.
AKBI Prevents Cardiac Hemosiderin Deposition and Reduces Macrophage Accumulation
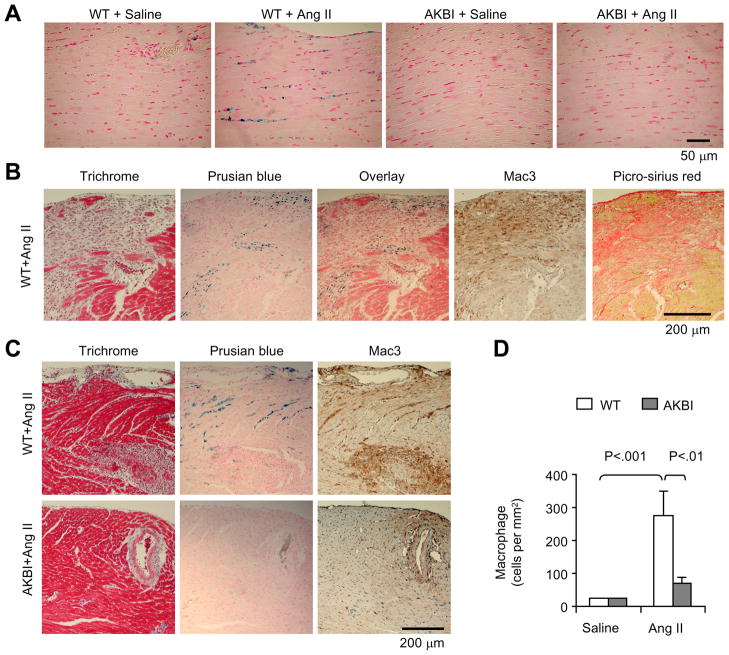
Abnormal hemosiderin deposition was observed in the ventricular interstitium of all Ang II-infused WT mice, but was nearly absent in those of Ang II-infused AKBI mice and saline-infused control mice (Fig 3A). Staining of serial sections revealed that hemosiderin deposition was mainly located in either the border regions of fibrotic lesions (Fig 3B) or in the regions lacking obvious fibrotic changes (Fig 3C), suggesting that Ang II-induced hemosiderin deposition may constitute an early sign of tissue damage that precedes fibrogenesis. Macrophage infiltration, as shown by positive Mac3 staining, was extensively seen in both fibrotic regions and regions with hemosiderin deposition in Ang II-infused WT mice (Fig 3B and C). In contrast, Ang II infusion-induced macrophage infiltration was significantly reduced in AKBI mice (Fig 3C and D).
Figure 3.
Effect of Ang II infusion on myocardial hemosiderin deposition and macrophage infiltration. A, Prusian blue staining for Hemosiderin on heart sections. B and C, Staining of serial sections showing the relation of fibrotic lesions, hemosiderin depositon, and macrophage infiltration. D, Quantitative analysis of the number of macrophage infiltration. Means + SD, n = 5 in each group.
AKBI Attenuates Pro-inflammatory and Pro-fibrotic Gene Expression in Response to Ang II Infusion
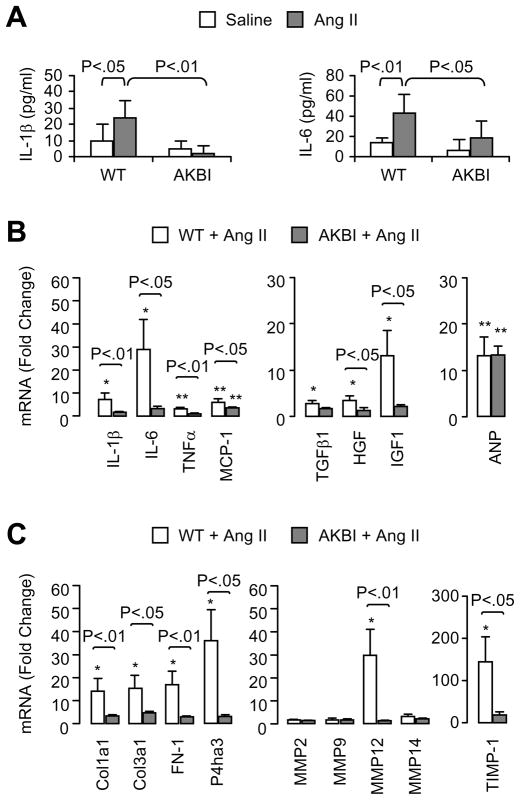
To examine how AKBI mice attenuate Ang II infusion-induced inflammation and fibrosis, we analyzed serum cytokine levels and ventricular tissue expression of selected genes related to inflammation and ECM metabolism. Serum IL-1β and IL-6 levels significantly increased in Ang II-infused WT mice, but showed no significant change in AKBI mice (Fig 4A). Serum TNF-α, monocyte chemotactic protein (MCP)-1, and TGF-β1 levels did not change significantly in response to Ang II infusion (data not shown). Ang II infusion enhanced the cardiac mRNA levels of IL-1β, IL-6, TNFα, MCP-1, TGFβ1, hepatocyte growth factor (HGF), and insulin-like growth factor (IGF)-1 in WT mice (Fig 4B). In contrast, Ang II infusion had little or no effect on the expression of these genes in AKBI mice. Interestingly, atrial natriuretic peptide (ANP) mRNA was enhanced to a similar levels by Ang II infusion in WT and AKBI mice, suggesting that ANP expression is not affected by the different IκB-mediated NF-κB regulatory mechanism.
Figure 4.
Serum cytokine levels and ventricular tissue gene expression in wild-type and AKBI mice infused with saline or Ang II for 7 days. A, Serum IL-1β and IL-6 levels, determined by ELISA. Means ± SD, n = 5. B and C, Ventricular tissue mRNA expression of selected genes related to inflammation and extracellular matrix metabolism. The mRNA levels were determined by real-time PCR and normalized to GAPDH mRNA levels. Data shown are fold-change with saline-infused wild-type mice as 1-fold. Means ± SEM, n = 5. * P<0.05, ** P<0.01, vs. saline-infused wild-type control.
Ang II infusion significantly enhanced cardiac mRNA levels of collagen I (Col1a1), collagen III (Col3a1), fibronectin (FN-1), P4ha3 (a component of prolyl 4-hydroxylase, the key enzyme in collagen synthesis), matrix metalloprotease (MMP)-12 and the tissue inhibitor of MMP (TIMP)-1 in WT mice, as compared to the levels in saline-infused control mice (Fig 4C). In contrast, the expression of these genes in AKBI mice did not increase significantly in response to Ang II infusion (Fig 4C), suggesting that abnormal degradation and deposition of ECM induced by Ang II infusion is inhibited by the replacement of IκBα with IκBβ.
Differential NF-κB Activation and Gene Expression in WT and AKBI VSMCs
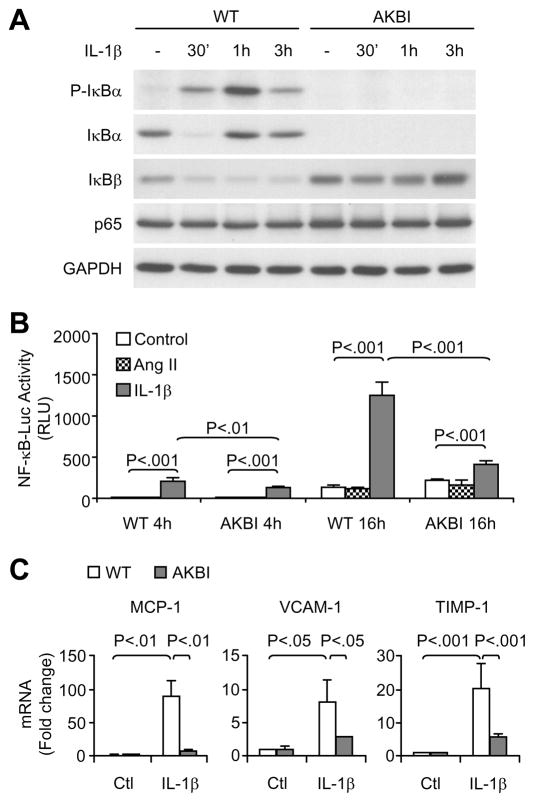
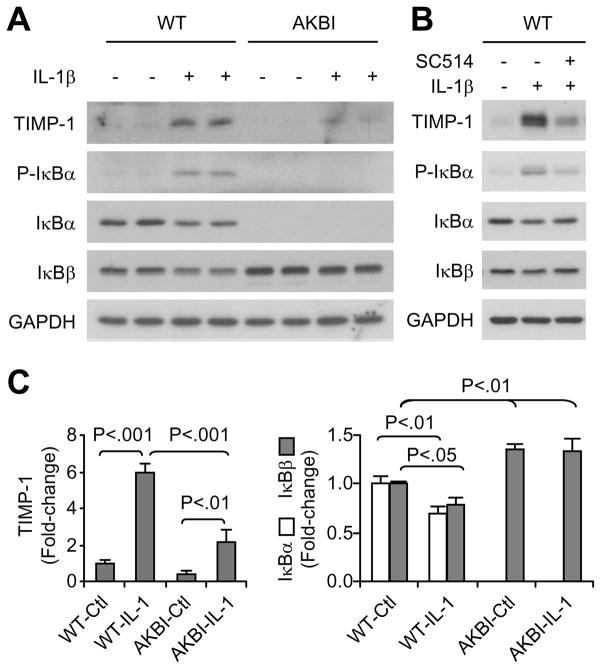
We further determined the effects of replacement of IκBα with IκBβ on VSMC response to IL-1β, a major cytokine known to activate NF-κB signaling and shown to be up-regulated in Ang II-infused WT mice. As shown in Fig 5A, treatment of WT cells with IL-1β resulted in IκBα phosphorylation and degradation, followed by rapid resynthesis, as evidenced by increased IκBα levels at 1 hour and 3 hours after the treatment. IκBβ was decreased during the 3-hour IL-1β treatment in WT cells. In AKBI cells, IL-1β induced a transient decrease in IκBβ levels, after which IκBβ gradually increased and restored to basal levels at 3 hours. IL-1β treatment did not change NF-κB p65 expression in WT and AKBI cells. Furthermore, IL-1β induced NF-κB activation, as shown by NF-κB-dependent luciferase activity assay, and the activation was significantly lower in AKBI cells than WT cells (Fig 5B). Treatment of the cells with Ang II alone did not activate NF-κB (Fig 5B). IL-1β treatment upregulated the mRNA levels of MCP-1, vascular cell adhesion molecule (VCAM)-1, and TIMP-1, however, the increase was significantly lower in AKBI cells than in WT cells (Fig 5C). The lower expression of TIMP-1 in AKBI than in WT cells was further demonstrated at protein levels (Fig 6A and C). Inhibition of IKKβ activity by SC-514 attenuated IL-1β-induced IκBα phosphorylation, IκBα and IκBβ degradation, and TIMP-1 expression in the WT cells (Fig 6B).
Figure 5.
IL-1β-induced NF-κB activation and gene expression in cultured vascular smooth muscle cells isolated from wild type (WT) and AKBI mouse aorta. A, Cells were untreated or treated with IL-1β (3 ng/mL) for designated times, and cell lysates (10 μg proteins per lane) were used for Western blot analysis. GAPDH was shown as an equal loading reference. B, Cells infected with Ad-NFκBLuc were untreated or treated with IL-1β (3 ng/mL) or Ang II (10−7 mol/L) for 4 h or 16 h. Cell lysates (5 μg proteins) were used for luciferase activity assay. Results are expressed as relative light units (RLU). Data are means ± SD, n = 3. C, Real time PCR analysis of MCP-1, VCAM-1, and TIMP-1 mRNA expression in cells untreated or treated with IL-1β (3 ng/mL) for 20 h. Data are means ± SD, n = 3.
Figure 6.
IL-1β-induced TIMP-1 expression in cultured vascular smooth muscle cells isolated from wild type (WT) and AKBI mouse aorta. A and B, Western blot analysis of TIMP-1, p-IκBα, IκBα, and IκBβ expression in cells untreated or treated with IL-1β (3 ng/mL) for 24 h. In lane 3 of C, a selective IKKβ inhibitor SC-514 (25 μmol/L) was add 1 h prior to IL-1β. Each Western blot image is a representative of 2 separate experiments. C, Densitometric analysis for A. Data are means ± SD, n = 3.
Discussion
In the present study, we demonstrated that the replacement of IκBα gene by IκBβ cDNA in mice results in resistance to development of cardiovascular fibrosis in response to Ang II infusion, which is evidenced by significantly reduced formation of fibrotic lesions and decreased collagen deposition in cardiac ventricular tissues, and reduced aortic adventitial fibrotic area, when compared with WT mice. Because NF-κB is specifically targeted in the AKBI mice these data also demonstrate the principle role of NF-κB signaling in Ang II-mediated cardiovascular fibrosis.
Ang II may not directly induce NF-κB activation, at least in cultured VSMCs, which is consistent with our previous observation.6 The in vivo tissue inflammatory response to Ang II infusion is probably a consequence of Ang II-induced hypertrophic remodeling, which may cause ECM destruction and cell death, and generate “danger signals” triggering IL-1β production and activation of NF-κB signaling cascade.23 Studies by Saito et al suggested that iron and iron-mediated generation of free radicals contribute to Ang II-induced upregulation of profibrotic and inflammatory genes.15, 16 Hemosiderin deposition may constitute a sign of the Ang II-induced tissue damage and trigger excessive tissue inflammatory response and fibrogenesis, because hemosiderin deposition is associated with increased macrophage infiltration as observed in our mouse model and reported by others in rat models.15, 16, 19 The reduction of hemosiderin deposition and macrophage infiltration in the interstitial regions of AKBI mouse hearts suggest that replacement of IκBα with IκBβ may protect tissue from Ang II-induced damage. Like hemosiderin, macrophage infiltration, as a marker of inflammation, has been implicated in stimulating fibrogenesis in various hypertensive animal models including spontaneously hypertensive rats, hypertensive mice or rats induced by infusion of Ang II, and rats with aortic constriction.4, 16, 24 In addition to less macrophage infiltration, the attenuated inflammatory response in AKBI mice is further supported by the decreased expression of numerous inflammatory genes including IL-1β, IL-6, TNFα, MCP-1, and TGFβ1. The lower expression of MCP-1, a key mediator of macrophage recruitment,4 may contribute to the reduction of macrophage infiltration observed in AKBI mouse heart tissues.
Excessive and chronic inflammation may result in maladaptive ECM remodeling and play a central role in fibrogenesis. Ang II infusion enhanced both ECM degradation and formation, as indicated by enhanced mRNA levels of MMP-12, TIMP-1, collagen I, collagen III, fibronectin, and P4ha3, which was markedly attenuated in AKBI mice. These gene expression data are consistent with the histological findings showing increased collagen deposition in WT mice but less in AKBI mice. A dramatically increased TIMP-1 expression may greatly contribute to the increased collagen deposition and fibrosis in Ang II-infused WT mice. Increased cardiac expression of TIMP-1 has been found to correlate with cardiac fibrosis in chronically pressure-overloaded human hearts.25 Overexpression of TIMP-1 promotes the development of liver fibrosis, and may also inhibit the spontaneous resolution of liver fibrosis.26, 27 In addition to its regulation of MMP-mediated ECM degradation, TIMP-1 has additional biological functions including regulation of cell growth, apoptosis, and angiogenesis28 that could also be related to its role in tissue remodeling. Importantly, IL-1β can up-regulate TIMP-1 expression through activation of NF-κB, as shown in our study and reported by others.29 Our data from Ang II-infused WT and AKBI mice and from IL-1β-treated WT and AKBI cells suggest that the differential regulation of NF-κB signaling by IκBα and IκBβ may differentially regulate the TIMP-1 expression and subsequently influence the ECM deposition and fibrogenesis.
Our data support the hypothesis that IκBα and IκBβ have different roles in modulating cardiovascular inflammation and fibrosis induced by Ang II infusion. It is known that IκBα and IκBβ display different kinetics of degradation and resynthesis in response to inflammatory stimuli. Upon IL-1β stimulation, in WT mouse VSMCs, IκBα is rapidly degraded and then resynthesized due to the presence of NF-κB-binding motifs in the IκBα gene promoter, whereas IκBβ is degraded and sustained at a low level because its synthesis is not up-regulated by NF-κB. This is consistent with previous observations in rat VSMCs and other cell types.5, 30, 31 Although IκBα returned to basal levels within 1 hour after stimulation, NF-κB remained active and was not suppressed by the increased IκBα, as revealed by the increased NF-κB-driven luciferase activity at later time-point (16 hours). This may result from continuous phosphorylation and degradation of IκBα and constantly low level of IκBβ. In AKBI mouse VSMCs that do not express IκBα, IκBβ was degraded upon IL-1β stimulation and then returned to basal levels within 3 hours, probably due to that the knockin IκBβ cDNA that replaces the IκBα gene is controlled under IκBα promoter and is NF-κB inducible.11, 14 However, an important difference between IκBα and IκBβ is that the increased IκBβ expression significantly attenuates NF-κB activation and reduces the expression of inflammatory genes, which could be ascribed to the lower sensitivity of IκBβ than IκBα to IKKs-induced phosphorylation and degradation.32 Our results, together with previous findings, indicate that IκBβ does not act the same as IκBα in regulating NF-κB activation and inflammatory gene expression principally due to its lower sensitivity to IKKs, and support the notion that a sustained reduction of IκBβ contributes to prolonged NF-κB activation.5, 31, 33–35 Taken together, the deletion of IκBα and overexpression of IκBβ in mice attenuate NF-κB-responsive inflammatory and profibrotic gene expression, hemosiderin deposition, and macrophage infiltration in response to Ang II infusion, which slows the development of cardiovascular fibrosis in the Ang II infusion-induced hypertension.
Supplementary Material
Perspective.
Our data suggest that up-regulating IκBβ expression or blocking IκBβ degradation may prevent excessive inflammatory response and could serve as a therapeutic target in preventing fibrosis in hypertensive disease.
Acknowledgments
Sources of Funding
This work was supported by American Heart Association award 09GRNT2110017 (BJ), and National Institute of Health grants HL083358 (BJ) and R37 HL104017 (RAC).
Footnotes
Disclosures
None
References
- 1.Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res. 1992;26:671–677. doi: 10.1093/cvr/26.7.671. [DOI] [PubMed] [Google Scholar]
- 2.Diez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens (Greenwich) 2007;9:546–550. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 5.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–20368. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 8.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S, Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 10.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JD, Ryseck RP, Attar RM, Dambach D, Bravo R. Functional redundancy of the nuclear factor kappa B inhibitors I kappa B alpha and I kappa B beta. J Exp Med. 1998;188:1055–1062. doi: 10.1084/jem.188.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 13.Mizgerd JP, Scott ML, Spieker MR, Doerschuk CM. Functions of IkappaB proteins in inflammatory responses to Escherichia coli LPS in mouse lungs. Am J Respir Cell Mol Biol. 2002;27:575–582. doi: 10.1165/rcmb.2002-0015OC. [DOI] [PubMed] [Google Scholar]
- 14.Fan C, Li Q, Zhang Y, Liu X, Luo M, Abbott D, Zhou W, Engelhardt JF. IkappaBalpha and IkappaBbeta possess injury context-specific functions that uniquely influence hepatic NF-kappaB induction and inflammation. J Clin Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizaka N, Saito K, Noiri E, Sata M, Ikeda H, Ohno A, Ando J, Mori I, Ohno M, Nagai R. Administration of ANG II induces iron deposition and upregulation of TGF-beta1 mRNA in the rat liver. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1063–1070. doi: 10.1152/ajpregu.00281.2004. [DOI] [PubMed] [Google Scholar]
- 16.Saito K, Ishizaka N, Aizawa T, Sata M, Iso-o N, Noiri E, Mori I, Ohno M, Nagai R. Iron chelation and a free radical scavenger suppress angiotensin II-induced upregulation of TGF-beta1 in the heart. Am J Physiol Heart Circ Physiol. 2005;288:H1836–1843. doi: 10.1152/ajpheart.00679.2004. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda K, Kai H, Kuwahara F, Yasukawa H, Tahara N, Kudo H, Takemiya K, Koga M, Yamamoto T, Imaizumi T. Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension. 2004;43:499–503. doi: 10.1161/01.HYP.0000111831.50834.93. [DOI] [PubMed] [Google Scholar]
- 18.Weber KT, Brilla CG, Campbell SE, Guarda E, Zhou G, Sriram K. Myocardial fibrosis: role of angiotensin II and aldosterone. Basic Res Cardiol. 1993;88 (Suppl 1):107–124. doi: 10.1007/978-3-642-72497-8_8. [DOI] [PubMed] [Google Scholar]
- 19.Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol. 2005;25:2282–2288. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
- 20.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 21.Jiang B, Haverty M, Brecher P. N-acetyl-L-cysteine enhances interleukin-1beta-induced nitric oxide synthase expression. Hypertension. 1999;34:574–579. doi: 10.1161/01.hyp.34.4.574. [DOI] [PubMed] [Google Scholar]
- 22.Bayat H, Xu S, Pimentel D, Cohen RA, Jiang B. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arterioscler Thromb Vasc Biol. 2008;28:127–134. doi: 10.1161/ATVBAHA.107.150250. [DOI] [PubMed] [Google Scholar]
- 23.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariety J, Michel JB. Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest. 1994;70:286–294. [PubMed] [Google Scholar]
- 25.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 26.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–860. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 27.Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Thorgeirsson SS, Fukui H. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248–1254. doi: 10.1053/jhep.2000.20521. [DOI] [PubMed] [Google Scholar]
- 28.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilczynska KM, Gopalan SM, Bugno M, Kasza A, Konik BS, Bryan L, Wright S, Griswold-Prenner I, Kordula T. A novel mechanism of tissue inhibitor of metalloproteinases-1 activation by interleukin-1 in primary human astrocytes. J Biol Chem. 2006;281:34955–34964. doi: 10.1074/jbc.M604616200. [DOI] [PubMed] [Google Scholar]
- 30.Le Bail O, Schmidt-Ullrich R, Israel A. Promoter analysis of the gene encoding the I kappa B-alpha/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J. 1993;12:5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Ghosh S. Differential phosphorylation of the signal-responsive domain of I kappa B alpha and I kappa B beta by I kappa B kinases. J Biol Chem. 2003;278:31980–31987. doi: 10.1074/jbc.M304278200. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DR, Douglas I, Jahnke A, Ghosh S, Pober JS. A sustained reduction in IkappaB-beta may contribute to persistent NF-kappaB activation in human endothelial cells. J Biol Chem. 1996;271:16317–16322. doi: 10.1074/jbc.271.27.16317. [DOI] [PubMed] [Google Scholar]
- 34.Bourke E, Kennedy EJ, Moynagh PN. Loss of Ikappa B-beta is associated with prolonged NF-kappa B activity in human glial cells. J Biol Chem. 2000;275:39996–40002. doi: 10.1074/jbc.M007693200. [DOI] [PubMed] [Google Scholar]
- 35.Jiang B, Brecher P, Cohen RA. Persistent activation of nuclear factor-kappaB by interleukin-1beta and subsequent inducible NO synthase expression requires extracellular signal-regulated kinase. Arterioscler Thromb Vasc Biol. 2001;21:1915–1920. doi: 10.1161/hq1201.099424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.