Abstract
Objectives
This study sought to determine if metoprolol succinate ER (MET), and nebivolol (NEB), a β1-AR with increased bioavailability of nitric oxide (NO), would have differing effects on plasma asymmetric dimethylarginine concentration in hypertensives.
Background
It was hypothesized that NEB, a β1-AR antagonist and β3-AR agonist with NO- releasing properties and MET, only a β1-AR antagonist, would have different effects on plasma ADMA concentration.
Methods
Forty-one hypertensive subjects randomly received either 50 mg of MET (n = 19) or 5 mg of NEB (n = 22) for 4 weeks followed by 100 mg MET and 10 mg NEB for 4 weeks. ADMA and IGF-1 were measured by ELISA kit; endothelial progenitor cells were estimated using fluorescein-labeled monoclonal antibody to KDR and CD133 receptors; arterial augmentation index was measured by radial tonometry.
Results
Baseline systolic/diastolic blood pressure was 155.1 ± 18.7/85.3 ± 12.5 mm Hg for MET subjects and 157.6 ± 20.7/87.1 ± 14.0 mm Hg for NEB subjects. Baseline ADMA was 0.32 ± 0.123 μmol/L in the MET group and 0.4035 ± 0.1378 in the NEB group. ADMA increased 44.78% and 72% in the MET group at weeks 4 and 8 (p < 0.05 for both), respectively, without increase in the NEB group. At week 8 augmentation index was increased in the MET group (p<0.05). IGF-1 and EPC were unchanged by treatment.
Conclusions
Plasma ADMA and augmentation index are increased in a dose-dependent fashion by MET but not with NEB.
INTRODUCTION
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase (NOS) and a reflection of oxidative stress.1 ADMA is formed by methylation of arginine residues by the enzyme protein arginine methyltransferase-1 (PRMT-1) and is metabolized by dimethylarginine dimethylaminohydrolase 1,2 (DDAH1,2).2,3 Plasma ADMA is widely recognized as a biomarker for cardiovascular disease risk and adverse cardiovascular and renal outcomes.4
Nebivolol (NEB) is a β1 adrenoceptor (AR) antagonist and a β3-AR agonist.5 Stimulation of the β3-AR results in an increase in nitric oxide (NO) formation. NEB has been shown to decrease ADMA concentration in cultured human umbilical vein cells (HUVEC)6,7 whereas this decrease in ADMA was not seen with metoprolol or carvedilol.7 The decrease may be accomplished by the stimulation of DDAH by the l-enantiomer of nebivolol.8 The l-enantiomer of NEB is responsible for the β3-AR agonism of NEB while the d-enantiomer is responsible for the β1-AR antagonism.
Growth hormone, through insulin-like growth factor-1 (IGF-1), improves markers of systemic NO availability and reduces ADMA plasma concentration.9 IGF-1 has been shown to increase eNOS-dependent NO production by increasing phosphorylation of the enzyme at Ser117710 Endothelial progenitor cells (EPCs) are impaired in hypertension and increased by shear-induced NO.11 Finally, systemic arterial augmentation index (AI), a measure of arterial stiffness, is increased in association with increased plasma ADMA.12,13
Based on the differences in pharmacology, we hypothesized that nebivolol and metoprolol succinate would have differing effects on plasma concentration of ADMA, arterial augmentation index, plasma IGF-1 and number of circulating EPCs. To test this hypothesis a prospective, randomized, parallel-group trial comparing NEB (5 to 10 mg daily) to MET (50 to 100 mg daily) on these parameters in 41 subjects with primary hypertension was performed.
METHODS
The trial consisted of a one to two week placebo period, followed by an eight week treatment period. The protocol was approved by Quorum Review, Inc, Seattle, WA. The primary endpoint of the trial was plasma ADMA concentration. Secondary endpoints included systolic and diastolic blood pressure, arterial augmentation index, plasma IGF-1, and circulating EPCs.
Inclusion criteria were men and women over the age of 21 years and a systolic blood pressure >140 mm Hg (>130 mm Hg in type 2 diabetes). Subjects were excluded if they had a secondary form of hypertension, type 1 diabetes mellitus, macroproteinuria, estimated glomerular filtration rate <60 ml/min, heart failure or significant hepatic disease, atrioventricular conduction disturbance greater than 1st degree AV block, and women of child-bearing potential not using birth-control measures.
After subjects gave informed consent to participate in the study, those who met the inclusion and exclusion criteria had screening labs and measurement of blood pressure. All subjects were placed on placebo and previous antihypertensive therapy withdrawn.
Subjects were seen every week during the placebo period for monitoring of blood pressure. At each visit sitting and standing blood pressure were measured. At the end of the placebo period subjects were randomized (if the last two systolic BP recordings varied by <10% and were >140 mm Hg (or 130 mm Hg in diabetics)) to receive NEB 5 mg daily or MET 100 mg daily. On the day of randomization the following procedures were done: recording of blood pressure and augmentation index, blood drawn for measurement of ADMA, IGF-1 and EPC number.
Subjects were seen at two-week intervals. If systolic BP was still elevated over 140 mm Hg (or 130 mm Hg in subjects with type 2 diabetes mellitus) up-titration to 10 mg of nebivolol or 200 mg of metoprolol succinate was performed. At each visit, blood pressure and AI were measured and blood drawn for measurement of ADMA, IGF-1 and EPC number.
PROCEDURES
Blood Pressure
Blood pressure and AI were measured by an Omron device with subjects seated and both feet on the floor. The arm was at heart level and recordings were taken after 5 min rest (no talking). Three measurements were recorded and the average of the last two measurements analyzed.
Measurement of ADMA, IGF-1 and EPCs
Blood specimens were obtained from a brachial vein 2–4 hours post-dosing after overnight fasting. Blood (10 ml) was extracted in two EDTA-containing tubes. One tube was used for measurement of circulating endothelial progenitor cell (EPC) number and the other one centrifuged at 1500 × g for 10 min. Plasma was separated and stored at −80 °C until assays for ADMA and IGF-1 concentrations were performed. Laboratory personnel were blinded as to the case-control status of the blood samples. Samples pertaining to matched study subjects were analyzed together in the same batch (that is, on the same day and utilizing the same immunoassay kit).
Plasma concentration of ADMA was measured in 3 replicas for each patient using direct ELISA kit (ALPCO Diagnostics). ADMA was not normalized to L-arginine concentrations. However, treatment of patients with hypertension with NEB for 4 weeks did not result in any change in L-arginine plasma concentrations.14
IGF-1 assay included an acid-ethanol extraction step (to release IGF-1 from its binding proteins) and free IGF-1 purification step (SEP-PAK C18 columns, Waters Inc.). Plasma IGF-1 levels were assayed using Human IGF-I Quantikine ELISA Kit (R&D systems).
EPC number were assessed as follows: peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Histopaque-1077 (1.077 g/ml, Sigma) (400 ×g for 40 min at 4 °C). Approximately 2 × 105 PBMCs were incubated with a biotinylated monoclonal antibody against human kinase-insert domain receptor (KDR, Sigma-Aldrich, St Louis, MO), followed by staining with Streptavidin-Phycoerythrin conjugate (BD Biosciences, San Jose, CA). Samples were then further incubated with fluorescein isothiocyanate (FITC) - conjugated anti-CD34 (BD Biosciences, San Jose, CA) and Allophycocyanin-conjugated anti-CD133 (Miltenyi Biotec, Auburn, CA) antibodies. Negative controls for each sample were co-incubated with appropriate isotype controls. Samples were analyzed in a FC 500 System (Beckman Coulter). At least 10,000 events in the lymphomonocytic gate were acquired and analyzed using CXP software (Beckman Coulter).
Safety and Tolerability
Subjects were monitored for possible adverse events associated with the study drugs.
Randomization Process
Study subjects were randomly allocated into the NEB and MET treatment groups at the beginning of the trial using a random number generated by SAS. We did not stratify the participants by race and sex in the randomization process, which led to an unbalanced proportion of males and females. At the beginning of the study, 25 subjects were recruited in each group. Due to withdrawal of consent, lost to follow-up and missing values of some outcome variables, the final sample size was 22 in the NEB group and 19 in the MET group.
Sample Size Calculation
Sample size need for comparing mean changes during follow-up between two groups with equal size was calculated using the method described by Rosner.15 We use ADMA as a primary outcome and expected a decrease of 25% in ADMA in NEB treatment group compared with MET. Based on the parameters (mean and SD) in a previous study,16 the sample size was calculated as n=20 for each group, with a power >=80% at a significance level α=0.05.
Statistical Analysis
Data analysis was performed using SAS 9.1. A general linear model (GLM) was performed to test the significance of differences in changes of outcome variables during follow-up between groups (NEB vs MET). Relevant confounder variables were included in the model for adjustment. The effects of NEB v MET on the relationship between outcome variables were examined by comparing correlation coefficients (Pearson or Spearman) between ADMA, IGF-1 and EPC between the two groups; the difference in the correlation coefficients between the two groups were tested using Fisher’s Z-test. The significance level was set at 0.05 for all analyses. The normality of the outcome variables was tested using the Shapiro-Wilk statistic. Log-transformation was applied where necessary to improve the distribution; however, their means and SDs are presented in the original scale in tables and text.
Results
Forty-four subjects were suitable for randomization and 41 subjects completed the study. There were no serious adverse side effects during the study. The baseline demographics are shown in Table 1.
TABLE 1.
| BASELINE DEMOGRAPHICS | ||
|---|---|---|
| NEB (n = 23) | MET (n = 18) | |
| Age(yrs) | 59.9 ± 8.6 | 57.5 ± 12.2 |
| Male | 10 | 13 |
| Female | 13 | 5 |
| White | 3 | 4 |
| Black | 20 | 14 |
| Wt (lbs) | 193 ± 43 | 199 ± 54 |
| Waist (in.) | 43.1 ± 6.15 | 42.9 ± 7.52 |
| Diabetes | 2 | 5 |
Mean ± SD are given for continuous variables. MET = metoprolol succinate ER; NEB = nebivolol; Wt = body weight; waist = waist circumference.
Blood Pressure
Baseline systolic/diastolic blood pressure was 155.1 ± 18.7/85.3 ± 12.5 mm Hg for MET subjects and 157.6 ± 20.7/87.1 ± 14.0 mm Hg for NEB subjects and lowered comparably in both groups. Systolic/diastolic blood pressure for NEB was 135 ± 26/75±13 mm Hg at week 4 and 137±17/75± 10 mm Hg at week 8 and for MET 142 ± 25/78 ± 16 mm Hg at week 4 and 149 ± 28/83 ± 17 mm Hg at week 8. There was no statistical difference in blood pressure lowering between the groups.
Asymmetric Dimethylarginine (ADMA)
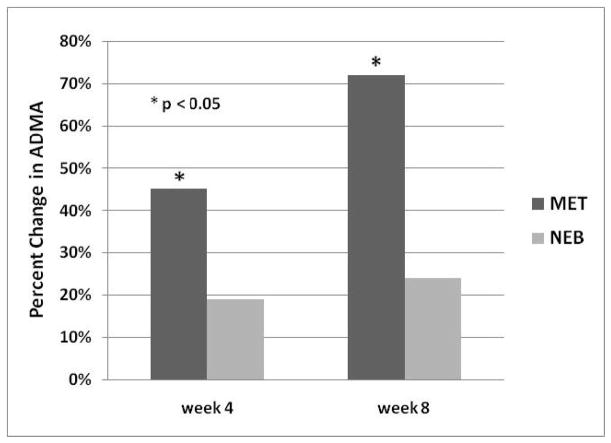
Baseline plasma ADMA was 0.4035 ± 0.1378 μmol/L in the nebivolol group and 0.3248 ± 0.1228 μmol/L in the metoprolol group (NS). NEB treatment was not associated with a change in ADMA at week 4 (0.4363 ± 0.9739 μmol/L) or week 8 (0.4353 ± 0.640 μmol/L) while treatment with MET resulted in a dose-dependent increase of 44.78% (0,3946 ± 0.0633) at week four (50 mg/day) and 71.72% (0.4628 ± 0.1052 μmol/L) at week eight (100 mg/day) compared to baseline (p<0.05) (Figure 1).
Figure 1.
Plasma concentration of ADMA is increased in a dose-dependant manner by MET, whereas there is no effect of treatment with NEB. MET dosage was 100 mg daily for the first 4 weeks and 200 mg daily for the last 4 weeks of treatment while NEB dosage was 5 mg daily for the first 4 weeks and 10 mg daily for the last 4 weeks of treatment.
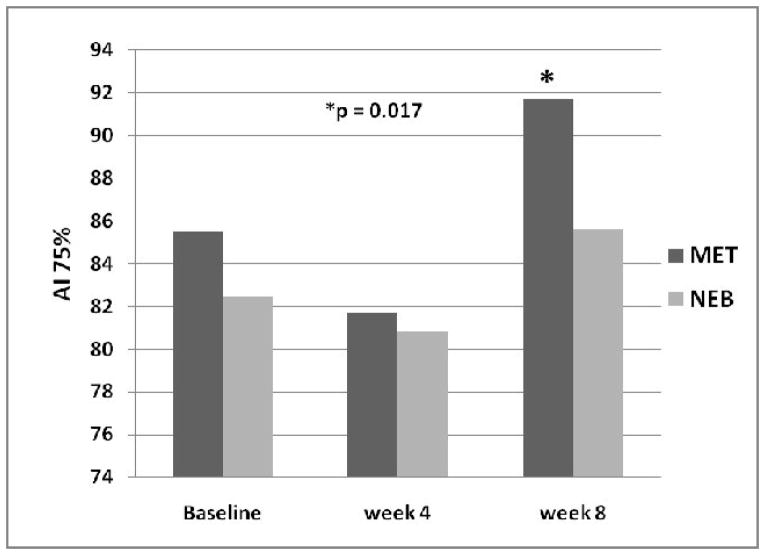
Augmentation Index
Augmentation index adjusted for heart rate {AI (75%)} was 82.5 ± 12.3 at baseline in the NEB group and 85.5 ± 12.0 in the MET group (NS). After 8 weeks of treatment AI(75%) was 85.6 ± 11.9 in the NEB group compared to 91.7 ± 17.1 in the MET group (P = 0.017) (Figure 2).
Figure 2.
Augmentation index corrected for heart rate (AI 75%) is increased after 8 weeks of treatment with MET, but was not affected by treatment with NEB.
Insulin-like Growth Factor-1
IGF-1 was 127.2 ± 46.4 μmol/L in the NEB group and 136.0 ± 44.1 μmol/L in the MET group (p = NS). With NEB treatment IGF-1 was 116.1 ± 36.1 and 118.9 ± 40.1 μmo/L at weeks 4 and 8 respectively while with MET treatment IGF-1 was 109.8 ± 36.7 and 114.2 ± 29.8 μmol/L at weeks 4 and 8 respectively. There was no correlation of IGF-1 with any of the other measured parameters.
Endothelial Progenitor Cells
Baseline CD34/KDR cells were 71.9 ± 55.9 in the NEB group and 108.5 ± 93.9 in the MET group (NS) while CD34/KKDR/CD133 cells were 32 ± 21.1 in the NEB group and 45.6 ± 54.7 in the MET group (NS). EPCs did not correlate with any other measured parameter at baseline and were unchanged by treatment with NEB or MET.
Discussion
NEB, a β1-AR antagonist and a β3-AR agonist, did not produce a change in plasma ADMA concentration in subjects with hypertension while MET, primarily a β1-AR antagonist only, increased ADMA in a dose-dependant fashion (Figure 1). Similar findings were reported but without the dose-response data and identification of MET as succinate or tartrate.13 The increase in ADMA with the β1-AR antagonist, MET, might result from increased production, decreased metabolism or both. However, a clue to the mechanism may come from the lack of effect of the β1-AR antagonist and β3-AR agonist NEB. Stimulation of the β3-AR results in an increase in activity of DDAH-2 with increased metabolism of ADMA thus neutralizing the effect of β1-AR antagonism alone. One may speculate that β1-AR stimulation would result in a decrease in ADMA to accommodate the increased blood flow resulting from increased heart rate and myocardial contractility.
Thus, the net effect on ADMA by nebivolol is the result of β1-AR antagonism and β3-AR agonism. The presence of two pharmacological mechanisms of action that influence the concentration of ADMA may account for the finding that in some studies, nebivolol decreases ADMA where as in ours, and other studies, ADMA concentration is not changed.
Interestingly, the l-enantiomer of nebivolol is an agonist at the β3 receptor and also has been shown to increase the activity of DDAH1,2, enzymes responsible for the metabolism of ADMA. Interestingly, mutation of the DDAH gene is associated with increased cardiovascular risk and hypertension.17
Interplay between the β1- and β3-ARs has been found in rat neonatal cardiomyocytes.18 Chronic β1- or β3-AR stimulation leads to the modulation of β1- and β3-ARs by a cross-regulation involving PKC, PI3K, p38MAPK and MEK/ERK1/2 pathway, and through protein kinase A when β1-ARs are chronically activated. Up-regulation of β3-ARs is seen in human myocardium from subjects with heart failure.19 In myocardium, stimulation of β3-ARs produces a negative inotropic effect that is mediated by NO. In the periphery, β3-AR stimulation produces vasodilation following NO release. Thus, the β3 adrenoceptor may assist in compensating for excessive catecholamine stimulation.
IGF-1 and ADMA have an interesting relationship in that increasing IGF-1 decreases ADMA while increasing the production of NO. Treatment of growth hormone deficient subjects results in a reduction of ADMA concentration and increased NO production. The failure of nebivolol to change the plasma concentration of IGF-1 suggests that NEB modulates ADMA due to a different mechanism.
The highest dose of MET (100 mg/day) resulted in an increase in AI (75%). This is consistent with previous studies showing that an increase in ADMA is associated with increased arterial stiffness.20 The lack of a negative effect of NEB may be related not only to the lack of effect on ADMA, but to the increased production of NO as well.
The number of EPCs observed in blood from subjects in this study was small and may reflect the degree of hypertension and co-morbidity, e.g. diabetes mellitus and smoking.21 The lack of effect of treatment may reflect the short duration of the study as well as a lack of effect of the specific drugs.
Conclusion
Beta-blockers are a heterogenous class of cardiovascular drugs that produce effects that are the net result of their actions on various receptors. The increase in ADMA with MET suggests that may β1-AR antagonism alone may not produce optimal benefit for the treatment of some diseases, e.g. hypertension as demonstrated in some meta-analyses. It remains to be seen if drugs such as nebivolol are associated with better outcomes, particularly on stroke.
Acknowledgments
This Investigator Initiated Trial was supported by an unrestricted grant from Forest Labs.
Footnotes
Dr. Giles is a consultant and speaker for Forest Labs.
Dr. Sander is a speaker for Forest Labs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vallance P, Leone A, Calver A, Colier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 2.MacAllister RJ, Fickling SA, Whitley GS, Vallance P. Metabolism of methylarginines by human vasculature: implications for the regulation of nitric oxide synthesis. Br J Pharmacol. 1994;112:43–8. doi: 10.1111/j.1476-5381.1994.tb13026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth J, Racz A, Kaminski PM, Wolin MS, Bagi Z, Koller A. Asymmetrical dimethylarginine inhibits shear stress-induced nitric oxide release and dilation and elicits superoxide-mediated increase in arteriolar tone. Hypertension. 2007;49:563–8. doi: 10.1161/01.HYP.0000256764.86208.3d. [DOI] [PubMed] [Google Scholar]
- 4.Böger BH, Sullivan LM, Schwedheim E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ignarro LJ. Different pharmacological properties of two enantiomers in a unique β-blocker, nebivolol. Cardiovascular Therapeutics. 2008;26:115–134. doi: 10.1111/j.1527-3466.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 6.Evangelista S, Garbin U, Prasini AF, Stranieri C, Boccioletti V, Cominacini L. Effect of DL-nebivolol, its enantiomers and metabolites on the intracellar production of superoxide and nitric oxide in human endothelial cells. Pharmacological Research. 2007;55:303–309. doi: 10.1016/j.phrs.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Bank A, Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Hypertens. 2007;20:777–783. doi: 10.1016/j.amjhyper.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Garbin U, Pasini AF, Stranieri C, Manfro S, Boccioletti V, Cominacini L. Nebivolol reduces asymmetric dimethylarginine in endothelial cells by increasing dimethylarginine dimethylaminohydrolase 2 (DDAH2) expression and activity. Pharmacol Res. 2007;56:515–21. doi: 10.1016/j.phrs.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Thum T, Fleissner F, Klink I, Tsikas D, Jakob M, Bauersachs J, Stichtenoth DO. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-1. J Clin Endocrinol Metab. 2007;92:4172–4179. doi: 10.1210/jc.2007-0922. [DOI] [PubMed] [Google Scholar]
- 10.Michell BJ, Griffiths JE, Mitchehill KL, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–80. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 11.Shantsila E, Watson T, Lip GYH. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:7412–52. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Böger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–2029. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 13.Oğuz A, Uzunlulu M, Yoruimaz E, Yalçin Y, Helim N, Fici F. Effect of nebivolol and metoprolol treatments on serum asymmetric dimethylarginine levels in hypertensive patients with type 2 diabetes mellitus. Anadolu Kardiyol Derg. 2007;7:383–7. [PubMed] [Google Scholar]
- 14.Pasini AF, Garbin U, Stranier C, Boccioletti V, Mozzini C, Manfro S, Pasini A, Cominacin M, Cominacin L. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257. doi: 10.1038/ajh.2008.260. [DOI] [PubMed] [Google Scholar]
- 15.Rosner B. Fundamentals of Biostatistics. 5. Boston, MA: Duxbury Press; 2000. pp. 309–312. [Google Scholar]
- 16.Bank A, Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Hypertens. 2007;20:777–783. doi: 10.1016/j.amjhyper.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Valkonen VP, Tuomainen TP, Laaksonen R. DDAH gene and cardiovascular risk. Vasc Med. 2005;(Suppl 1):S45–6. doi: 10.1191/1358863x05vm600oa. [DOI] [PubMed] [Google Scholar]
- 18.Ufer C, Germack R. Cross-regulation between β1- and β3-adrenoceptors following chronic β-adrenergic stimulation in neonatal rat cardiomyocytes. Brit J Pharmacol. 2009;158:300–313. doi: 10.1111/j.1476-5381.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozec B, Erfanian M, Laurent K, Trochu J-N, Gautheir C. Nebivolol, a vasodilating selective β1-blocker, is a β3-adrenoceptor agonist in the nonfailing transplanted human heart. J Am Coll Cardiol. 2009;53:1532–1538. doi: 10.1016/j.jacc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, Kario K. Role of the augmentation index in hypertension. Therapeutic Advances in Cardiovasc Dis. 2008;2:25–35. doi: 10.1177/1753944707086935. [DOI] [PubMed] [Google Scholar]
- 21.Delva P, Degan M, Vallerio P, et al. Endothelial progenitor cells in patients with essential hypertension. J Hypertens. 2007;25:2093–9. doi: 10.1097/HJH.0b013e3280109271. [DOI] [PubMed] [Google Scholar]