Abstract
Cancer is a disease characterized by dysregulation of multiple genes and is associated with symptoms such as cachexia, anorexia, fatigue, depression, neuropathic pain, anxiety, cognitive impairment, sleep disorders and delirium (acute confusion state) in medically ill patients. These symptoms are caused by either the cancer itself or the cancer treatment. During the past decade, increasing evidence has shown that the dysregulation of inflammatory pathways contributes to the expression of these symptoms. Cancer patients have been found to have higher levels of proinflammatory cytokines such as interleukin-6. The nuclear factor (NF)- κB is a major mediator of inflammatory pathways. Therefore, anti-inflammatory agents that can modulate the NF-κB activation and inflammatory pathways may have potential in improving cancer-related symptoms in patients. Because of their multitargeting properties, low cost, low toxicity and immediate availability, natural agents have gained considerable attention for prevention and treatment of cancer-related symptoms. How NF-κB and inflammatory pathways contribute to cancer-related symptoms is the focus of this review. We will also discuss how nutritional agents such as curcumin, genistein, resveratrol, epigallocatechin gallate and lycopene can modulate inflammatory pathways and thereby reduce cancer-related symptoms in patients.
Keywords: cancer-related symptoms, cytokines, inflammation, NF-κB, nutraceuticals
Introduction
As the disease of cancer progresses, patients develop multiple symptoms that can impair their function and quality of life. The symptoms common in cancer patients include cachexia, anorexia, fatigue, depression, neuropathic pain, anxiety, cognitive impairment, sleep disorders and delirium (acute confusion state). These symptoms can result from the disease itself or can be produced by the treatment of disease, including surgery, chemotherapy and radiation (Figure 1).1,2 For example, patients undergoing chemotherapy or radio-therapy report significant fatigue during the course of treatment.3– 5 In one study, the prevalence of cancer-related fatigue among untreated patients was 75%, with even higher prevalences in patients treated with chemotherapy or radiotherapy.6 Cancer survivors can continue to experience symptoms even when their disease is in remission or after treatment has ended. For example, cancer survivors of bone marrow transplantation reported cognitive impairment and emotional distress many years after the transplant.7 – 9
Figure 1.
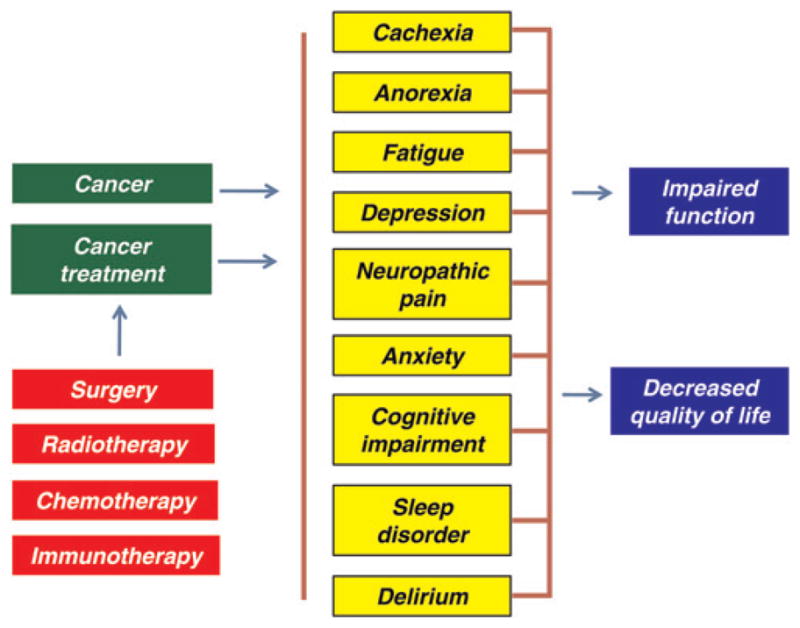
Effect of cancer and cancer treatment on the patient’s life. Cancer and its treatment can cause various symptoms, which may impair the function and decrease the quality of life (A color version of this figure is available in the online journal)
How cancer patients develop such symptoms is not properly known; however, different contributing physiological and psychosocial factors have been proposed.10 Mounting evidence has indicated that inflammatory cytokines such as interleukin (IL)-6, IL-1β and IL-10 and tumor necrosis factor (TNF)-α produced by the disease or the treatment contribute to these symptoms (Table 1).1,2,11–14 These inflammatory cytokines are regulated by the proinflammatory transcription factor, nuclear factor (NF)- κB (Figure 2). A recent study of lung cancer patients showed that polymorphisms in the cytokine genes IL1A and IL1B were important risk factors for the disease.15 Another study of the same patients showed that cytokine genes were also important in the development of the risk for pain severity.16 The role of TNF-α in producing symptoms in cancer patients was evident from one study. Combinations of recombinant TNF-α (rTNF-α) and recombinant interferon (rIFN)-γ was given by intramuscular injections for five consecutive days every two weeks for a total of four weeks to 25 patients with metastatic cancer. Administration of cytokines was associated with fever, chills and fatigue in patients, and severity of symptoms corresponded to increasing cytokines dose levels.17 Similarly, chemotherapeutic agents have also been shown to induce proinflammatory cytokines and pain. For example, co-administration of vincristine in combination with granulocyte–macrophage colony-stimulating factor markedly increased the severity and magnitude of treatment-induced pain and other neurological impairments.18,19 The cytokines IFN α/ γ, TNF-α, IL-1 and IL-6 are increased in vitro by cisplatin,20– 22 paclitaxel,23,24 and both ionizing and ultraviolet irradiation.25– 27 These chemotherapy drugs have also been shown to activate the NF-κB signaling pathway.28
Table 1.
Role of nuclear factor-κB-regulated inflammatory molecules in cancer-related symptoms
| Molecule | Cancer type | Finding |
|---|---|---|
| Cachexia | ||
| TNF-α | Hepatoma | Rats bearing AH-130 hepatoma cells showed enhanced protein degradation in gastrocnemius muscle, heart and liver that was diminished by administration of anti-TNF-α74 |
| Lung | Wild-type mice implanted with LLC had enhanced loss of fat and muscle concomitant with activation of the ubiquitin–proteasome pathway that was absent in TNF-α receptor type I deficient mice75 | |
| Colon | Mice bearing colon-26 adenocarcinoma exhibited cachexia in association with a significant increase in TNF-α76 | |
| Random | Skeletal muscle of cancer cachectic patients exhibited increased expression and activity of the TNF-α signaling pathway77 | |
| Lung | Increased level of serum TNF-α in cachexia patients compared with those without cachexia78 | |
| IL-6 | Colon | Mice bearing colon-26 adenocarcinoma exhibited cachexia in association with a significant increase in IL-676 |
| Pancreas | Serum from cachectic patients exhibited an elevated IL-679 | |
| IL-1β | Squamous | Wild-type mice had less lean body mass and fat mass and increased IL-1β compared with the TLR-non-functional mice80 |
| Anorexia | ||
| IL-1α | Sarcoma | Tumor-bearing rats exhibited negative correlation between CSF IL-1α and food intake85 |
| Sarcoma | Intrahypothalamic microinjections of IL-1ra were associated with an improvement in food intake in tumor-bearing rats86 | |
| IL-1β | Prostrate | Rats bearing prostrate tumor cells developed anorexia that was associated with enhanced IL-1β in cerebellum, cortex and hypothalamus regions of the brain87 |
| Fatigue | ||
| IL-6 | Random | A significant correlation between IL-6 and fatigue in cancer patients92 |
| Colorectal | Significant correlation between IL-6 and fatigue in patients32,94 | |
| Breast | Exhibited significant increase in IL-6 following stimulation with LPS95 | |
| Random | A significant correlation between IL-6 and fatigue in cancer patients96 | |
| Lymphoma | Treatment of patients with humanized monoclonal antibodies against IL-6 caused a relief from fatigue97 | |
| NSCLC | Worsened the symptoms in the patients undergoing CXCRT98 | |
| Mouse model | Exercise-induced IL-6 decreased the TNF-α levels in skeletal muscle of TNF-α transgenic mice and added to the beneficial effect of exercise against cancer treatment-related fatigue102,103 | |
| TNF-α | Breast | A significant increase in TNF-α following stimulation with LPS95 |
| Depression | ||
| IL-6 | Random | Significant correlation between plasma IL-6 and depression in cancer patients106 |
| Ovarian | Close association between plasma IL-6 and facets of depression107 | |
| Leukemia | Significant correlation between IL-6 gene expression and depression108 | |
| Pancreatic, esophageal, breast | Exhibited higher concentrations of IL-6 compared with normal subjects and cancer patients without depression109 | |
| Neuropathic pain | ||
| IL-1β | Rat with tumors | Increase in IL-1β was correlated with spontaneous pain in female rats131 |
| Prostrate | Rats injected with prostrate cancer cells exhibited an increase in IL-1β and bone pain132 | |
| TNF-α | Rat with tumors | Increase in TNF-α was correlated with spontaneous pain in female rats131 |
| Anxiety | ||
| IL-2 | Renal carcinoma, melanoma | Patients treated with a combination of IL-2 and INFα-2b had enhanced anxiety135 |
| Cognitive impairment | ||
| IL-6 | Leukemia, myeloma | Patients exhibited higher level of IL-6 in association with poorer executive function144 |
| Sleep disorder | ||
| IL-6 | NSCLC | Patients undergoing CXCRT exhibited a strong correlation between serum IL-6 and severity of sleep disorder98 |
| VEGF | Random | Poor sleep in cancer patients was associated with elevated VEGF153 |
CSF, cerebrospinal fluid; CXCRT, concurrent chemoradiation therapy; IL, interleukin; IL-1ra, IL-1-receptor antagonist; INFα-2b, interferon α-2b; LLC, Lewis lung carcinoma; LPS, lipopolysaccharide; NSCLC, non-small-cell lung cancer; TLR, toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Figure 2.
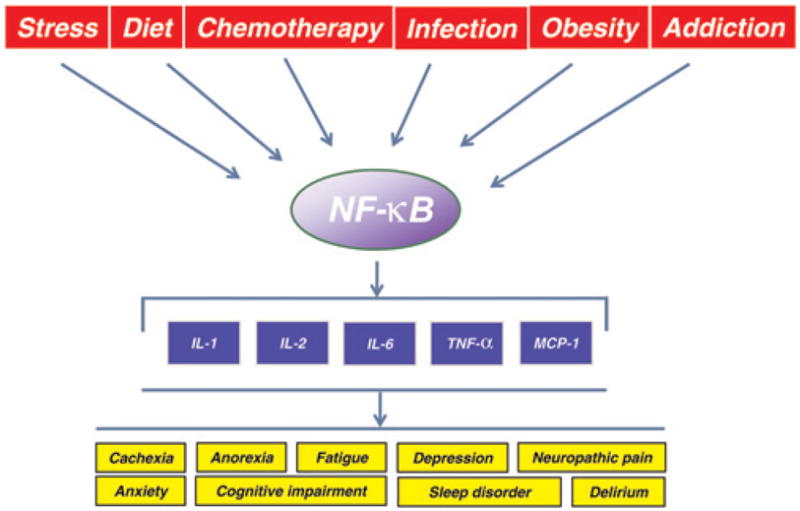
Regulation of inflammatory cytokines through activation of nuclear factor κB (NF-κB). NF-κB is activated in response to various stimuli, including stress, diet, chemotherapeutic agents, infection and also by disease conditions such as obesity and addiction. The inflammatory molecules may induce symptoms in cancer patients. IL, interleukin; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemotactic protein-1 (A color version of this figure is available in the online journal)
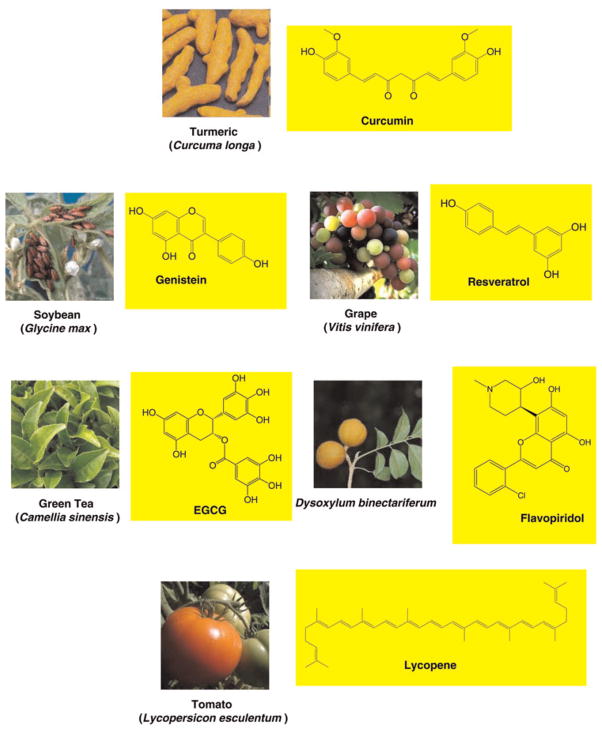
There is now ample evidence that multiple symptoms can occur simultaneously and can have an additive effect on cancer patients.1,29– 33 Thus it is likely that these symptoms are multifactorial in nature and caused by dysregulation of multiple genes. However, most currently available therapies are based on the modulation of a single target. Therefore, treating only one target might be potentially unsuccessful.34 In addition, these drugs are unsafe and expensive. Therefore, the current paradigm for treatment is either to combine several monotargeted drugs or to design drugs that modulate multiple targets. Recent research has indicated that nutraceuticals derived from nutritional sources are naturally multitargeting, and are less expensive, safer and immediately available.35,36 In fact, some nutraceuticals such as curcumin, genistein, resveratrol, epigallocatechin gallate (EGCG) and lycopene have been shown to reduce symptoms through modulation of inflammatory molecules. These nutraceuticals are chemically diverse (Figure 3) and affect production of various inflammatory cytokines (Table 2).
Figure 3.
Chemical structure of nutraceuticals having the potential to modulate cancer-related symptoms. Sources of these nutraceuticals are also shown. EGCG, epigallocatechin gallate (A color version of this figure is available in the online journal)
Table 2.
Effect of common nutritional agents on cancer-related symptoms
Curcumin
|
Genistein
|
| Resveratrol |
| EGCG |
| Lycopene |
EGCG, epigallocatechin gallate; i.c.v., intracerebroventicular; IL, interleukin; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B; STZ, streptozotocin; TNF, tumor necrosis factor
In this review, we describe how NF-κB-mediated inflammatory molecules contribute to cancer-related symptoms. We also discuss how nutritional agents can affect cancer-related symptoms by modulating inflammatory cytokines.
NF-κB and inflammatory pathways
Inflammation is a biological process that represents the response of an organism to infection or injury.37 In most cases, the inflammatory response is resolved by the release of endogenous anti-inflammatory cytokines and by the accumulation of intracellular negative regulatory factors. However, when the inflammatory conditions are persistent, or when resolution mechanisms fail, a state of chronic inflammation ensues that can lead to loss of normal physiological functions. Extensive research in recent years has indicated that chronic inflammation leads to various chronic disorders associated with cancer.38– 42 A central role in the induction of chronic inflammation is played by a set of genes encoding proinflammatory cytokines such as IL-1, IL-2, IL-6, TNF-α and monocyte chemotactic protein 1. What is common to all these molecules is that they are regulated by the transcription factor NF-κB (Figure 2).43 –45 This makes NF-κB an appealing target for therapeutic intervention. A number of compounds have been identified that can suppress NF-κB activation, including antioxidants, pro-tease inhibitors, proteasome inhibitors, salicylates, immuno-suppressants and anti-inflammatory agents.46 Some NF-κB-regulated cytokines, such as TNF- α and IL-1β, in turn regulate NF-κB itself.47
Numerous lines of evidence from preclinical and clinical studies have shown that NF-κB and NF-κB-mediated proinflammatory cytokines play a central role in the progression of cancer-related symptoms.1,11,48 –50 For instance, administration of selective inhibitors of the NF-κB pathway was shown to abrogate the expression of cytokines such as IL-1β and TNF-α in animal models of chronic inflam-mation.51,52 In patients with myelodysplastic syndrome and acute leukemia, a correlation was observed between the severity of symptoms and increased levels of cytokines (TNF-α, IL-6, IL-8).53 Similarly, patients without cancer have also been shown to display many of the symptoms of cancer patients after receiving cytokine therapy. For example, patients with hepatitis C virus infection and those with acquired immunodeficiency syndrome (AIDS) who received IFN-α therapy had symptoms of pain, fatigue, cognitive impairment and depression.54– 56 In another study, IL-6 was found to induce fatigue, inactivity and poor concentration when administered to normal subjects.57 These studies unequivocally indicate that NF-κB-regulated cytokines play a significant role in the development of cancer-related symptoms.
NF-κB is a dimeric transcription factor formed by the homodimerization or heterodimerization of Rel family proteins, including c-Rel, RelA (p65), RelB, NF-κB1 (p50 and its precursor p105) and NF-κB2 (p52 and its precursor p100). It was first discovered in 1986 by Sen and Baltimore58 in the nucleus, bound to an enhancer element of the immunoglobulin kappa light chain gene in B-cells. NF-κB is now known to be ubiquitous in nature, present in all cell types in an inactive form in the cytoplasm and evolutionarily conserved.59,60 NF-κB is maintained under such an inactive cytoplasmic form by virtue of its association with the inhibitory molecule IκB. NF-κB is activated in response to diverse stimuli such as stress, diet, chemotherapeutic agents and microbial infection.60– 63 NF-κB is also found active under some disease conditions such as obesity and addiction (Figure 2).64,65 The NF-κB inducers act through distinct signaling pathways that converge on the activation of an IκB kinase (IKK). IKK activation initiates IκB phosphorylation at specific amino-terminal serine residues (Ser32, Ser36),66 followed by ubiquitination at lysine residues (Lys21, Lys22) and subsequent degradation by 26S proteasome.67 The active NF-κB dimer is then translocated to the nucleus where it binds to the enhancer elements of target genes.
Role of inflammatory molecules in cancer-related symptoms
Role of inflammatory molecules in cachexia
Cachexia is a frequently observed symptom among cancer patients. It is characterized by profound loss of both adipose tissue and skeletal muscle.68 Suppression of protein synthesis and induction of protein degradation through the induction of unfolded protein response have been proposed as mechanisms of cachexia.69,70 Evidence for the role of proinflammatory molecules in the induction of cachexia comes from animal studies, with supporting evidence from work with humans.71,72 In particular, TNF-α, IL-1β and IL-6 have been proposed as mediators of cachexia.73
The role of TNF-α in the development of cancer cachexia is evident from a number of animal studies. In one study, rats bearing Yoshida AH-130 hepatoma cells showed enhanced protein degradation in gastrocnemius muscle, heart and liver. When these animals were administered anti-TNF-α, a decrease in protein degradation was observed, suggesting the involvement of TNF-α in the protein degradation process.74 The role of TNF-α in cancer cachexia was evident from another study in which cachexia-inducing Lewis lung carcinoma was implanted in wild-type mice and mice deficient for TNF-α receptor type I protein. Whereas the wild-type mice had a loss of both fat and muscle, in the gene-knockout mice, muscle wastage was not present to the same extent. In both groups, however, tumor burden resulted in significant increases in circulating TNF-α. Muscle wastage in wild-type mice was accompanied by an increase in the rate of protein degradation and a decrease in protein accumulation. The increase in protein degradation in the tumor-bearing wild-type mice was accompanied by an enhanced activation of the ubiquitin–proteasome pathway. Tumor-bearing gene-deficient mice did not show any increase in gene expression. It was concluded that TNF-α is responsible for the activation of protein breakdown in skeletal muscle of tumor-bearing mice.75 The role of TNF-α in inducing cachexia was evident from another mouse model bearing colon-26 adenocarcinoma. These mice developed cachexia in nine days that was associated with a significant increase in TNF-α.76
The correlation between TNF-α and cancer cachexia is evident from clinical studies as well. For example, in a recent study of patients with muscle wasting due to cancer, skeletal muscle of cachectic patients exhibited increased expression and activity of the TNF-α signaling, including TNF-α mRNA, activation of TNF receptor (TNFR) 1 and TNF-α-associated to TNFR1.77 Similarly, in a group of lung cancer patients, a significant increase in serum TNF-α was observed in the cachectic patients compared with patients without cachexia.78
In a few models of cancer, cachexia has been correlated with significant increases in IL-6. For example, mice implanted with colon-26 adenocarcinoma developed cachexia concomitant with a significant increase in IL-6.76 The mechanism behind aggressive development of cachexia in patients suffering from pancreatic cancer was investigated in one study that used pancreatic specimens obtained from non-cachectic and cachectic patients diagnosed with pancreatic ductal adenocarcinoma.79 Among numerous analyzed factors, IL-6 was significantly overexpressed in pancreatic specimens and elevated in the serum of cachectic patients. These results suggest that IL-6 is a prominent cachexia-associated factor in pancreatic cancer.79
Whether mice unable to mount an intact inflammatory response because of a defect in the Toll-like receptor (TLR) pathway will develop less cancer cachexia was investigated recently.80 Both wild-type and TLR-non-functional mice were inoculated with squamous carcinoma cells and compared. The wild-type mice weighed less on average than the TLR-non-functional mice. The wild-type mice had less lean body mass and fat mass and increased IL-1β compared with the TLR-non-functional mice. These results indicated that the impaired ability to secrete IL-1β may protect TLR-deficient mice from developing severe cancer cachexia.80 One study of gastric cancer patients from China examined the predictive value of IL-1β gene polymorphism for cachexia. Of the four alleles (IL-1β-31 T/C, −511 C/T, +3954 C/T, IL-1RN), IL-1β +3954 C/T was found to be a major risk for cachexia in gastric cancer patients.81
Role of inflammatory molecules in anorexia
Like cachexia, anorexia is a frequent symptom among cancer patients. Anorexia is defined as the loss of the desire to eat, which frequently leads to reduced food intake. The pathogenesis of cancer anorexia is complex and multifactorial, implying perturbations of the physiological regulation of eating behavior at the hypothalamic level.82 In healthy individuals, peripheral signals are integrated by the hypothalamus to modulate energy intake. In cancer patients, however, increased cytokine expression in the brain prevents the hypothalamus from responding appropriately to peripheral signals, by persistently activating anorexigenic systems and inhibiting prophagic pathways.83 Hypothalamic monoaminergic neurotransmission has been reported to contribute to these effects.84
Opara et al.85 found a negative correlation between cerebrospinal fluid IL-1α and food intake in a tumor-bearing rat model. Consistent with these data, intrahypothalamic microinjections of IL-1-receptor antagonist (IL-1ra) were associated with improvement in food intake in sarcoma-bearing rats.86 The mRNA profiles of IL-1β in the cerebellum, cortex and hypothalamus were investigated in a rat model bearing prostate tumor cells. These rats developed anorexia in association with an upregulation of IL-1β mRNA in their brain regions.87 In a few cases, cancer chemotherapeutic drugs have been shown to induce proinflammatory cytokines and anorexia. For example, the cancer chemotherapy drug etoposide (VP-16) activated p38 mitogen-activated protein kinase (p38MAPK) and induced IL-6 production in murine macrophages in a p38 MAPK-dependent manner. Etoposide administration rapidly increased serum levels of IL-6 in healthy mice and induced decreases in food intake, body weight, hemoglobin level and voluntary wheel-running activity.88
Although the role of cytokines in cancer anorexia is supported by a number of experimental models, a few studies failed to show a direct correlation and suggested the involvement of the nitric oxide pathways and the systemic or local production of eicosanoids.89
Role of inflammatory molecules in fatigue
Fatigue is one of the most commonly reported symptoms in patients with cancer.90,91 Such fatigue may be caused both by the disease itself and by cancer treatments. In addition, sleep disturbances, environmental conditions, activity level and nutritional status may also contribute to fatigue.
There is growing evidence that inflammatory molecules play a major role in the pathogenesis of fatigue.13 IL-6 is one of the most widely studied cytokines with the potential to induce cancer fatigue. For example, one study examined the association between fatigue and plasma IL-6 in 46 cancer patients; the IL-6 level was significantly higher in patients with clinical fatigue than in those without fatigue.92 In patients with metastatic colorectal cancer, high concentrations of IL-6 were associated with fatigue and appetite loss.93,94 Similarly, fatigued breast cancer survivors could be distinguished from non-fatigued survivors by their significant increases in monocyte production of IL-6 after lipopolysaccharide (LPS) stimulation.95 An elegant study conducted by Schubert and colleagues found a significant positive correlation between fatigue and circulating levels of inflammatory cytokines in cancer patients. Further analysis of individual inflammatory markers revealed significant positive correlations between fatigue and IL-6 and between fatigue and IL-1ra.96
A correlation between IL-6 and fatigue was also found in a study of patients with Castleman disease. Castleman disease is a lymphoproliferative disorder characterized by constitutional inflammatory symptoms and dysregulated IL-6 overproduction. Treatment of these patients with humanized monoclonal antibodies against IL-6 was associated with relief from fatigue immediately after the first administration of anti-IL-6.97
Some studies have shown that enhanced production of cytokines after cancer treatment is correlated with fatigue. For example, serum concentrations of IL-6 and IL-10 were found to be significantly correlated in patients undergoing concurrent chemoradiation therapy for locally advanced non-small-cell lung cancer. Further analysis indicated that the increase in IL-6 was associated with the severity of the fatigue.98 Another cytokine with a role in cancer-related fatigue includes TNF-α.95
In a few cancer types, NF-κB activity has been found to be directly correlated with fatigue. For example, a study using genome-wide microarray analysis found a significant correlation between NF-κB activity and cancer-related fatigue in breast cancer survivors.99 In a few studies, inflammatory molecules were not found to be correlated with cancer-related fatigue. For example, in one study, serum levels of inflammatory molecules from cancer individuals with early-stage breast and prostate cancers were assessed before, during and after a course of radiation therapy. A significant increase in fatigue during radiation treatment was observed in these individuals. Although changes in C-reactive protein (CRP) and IL-1ra were positively associated with increases in fatigue symptoms, IL-1β and IL-6 were not associated with fatigue.100 Another study of Norwegian long-term survivors of testicular cancer investigated the circulating levels of various inflammatory markers in relation to chronic fatigue. Higher levels of IL-1ra and CRP were found in patients compared with controls, but no difference was observed for IL-6.101 Schubert et al.96 also found no correlation between fatigue and IL-1β or TNF-α in cancer patients.
Some studies have shown beneficial effects of inflammatory molecules against cancer-related fatigue. For example, although IL-6 is considered proinflammatory, there is substantial evidence for anti-inflammatory properties of IL-6, in that it decreases the production or activity of IL-1β and TNF-α. Exercise-induced IL-6 production was shown to decrease the TNF-α levels in skeletal muscle of TNF-α transgenic mice.102 Furthermore, muscle-derived IL-6 has been proposed as a mechanism for the beneficial effects of exercise against cancer treatment-related fatigue.103
Role of inflammatory molecules in depression
Depression, one of the well-defined symptoms in cancer patients, is characterized not only by depressed mood but also by alterations in appetite, sleep, activity levels and cognitive functions. Prevalence rates for depression in patients with cancer have been reported to range from 1.5% to 50%.104 Depression has also been shown to increase as disease severity intensifies.105
Inflammatory molecules, especially IL-6, may play an important role in the pathophysiology of depression in cancer patients. For example, one study examined the efficacy of IL-6 as an adjunct to the diagnosis of depression in cancer patients. Depression was associated with increased plasma concentrations of IL-6.106 In another study, a close association was observed between plasma IL-6 and facets of depression in epithelial ovarian cancer patients.107
The association between IL-6, TNF-α, depression and stressful life events in patients with acute leukemia was investigated. Significant elevations in IL-6 gene expression, fatigue and perceived stress were observed among depressed patients compared with the non-depressed group. Although TNF-α was not associated with depression, it was associated with leukemia.108 Musselman et al. investigated whether cancer patients with pancreatic, esophageal or breast cancer with and without major depression exhibit immune system abnormalities similar to those reported in medically healthy (without cancer) subjects with depression. Cancer patients with depression had markedly higher plasma concentrations of IL-6 than did healthy comparison subjects or cancer patients without depression.109
In a few cases, cancer treatment has been shown to increase depression and cytokine expression. For example, an increased serum concentration of IL-6 was correlated with depressive symptoms in patients undergoing abdominal surgery.110 Similar observations have been reported in breast cancer patients111,112
Although a number of studies have shown a close association between cytokines and depression, a few reports were unable to find a correlation. For example, circulating levels of IL-6, soluble IL-6 receptor (sIL-6R), TNF-RII and soluble intercellular adhesion molecule were found to be almost the same among cancer patients with and without depression.113
Role of inflammatory molecules in neuropathic pain
Neuropathic pain is defined as pain ‘initiated or caused by a primary lesion or dysfunction in the nervous system’ or ‘arising as a direct consequence of a lesion or disease affecting the somatosensory system’.114 Cytokines have key roles in the pathogenesis of several preclinical models of neuropathic and inflammatory pain. For example, LPS-induced hyperalgesia (increased sensitivity to pain) has been shown to be blocked by antagonists to IL-1.115– 117 Similarly, glial fibrillary acidic protein-positive activated astrocytes118,119 that are the source for TNF, IL-1β, IL-15 and IL-6120– 123 are increased in the spinal cord segments to which the nerves affected by neuropathic pain project. Cytokines produce neuropathic pain by directly producing discharges of nociceptors124,125 and by altering the trafficking of growth factors along nerve fibers. This results in phenotypic changes in sensory endings,126 by inducing an alteration of glial-cell-mediated support of neural activities127 or by inducing the degeneration of neurons and the retraction of cell processes.128
Neuropathic pain is recognized as a common consequence of cancer, the major causes being cancer treatment and direct infiltration of nerves by cancer cells.13,129,130 The role of inflammatory molecules in inducing neuropathic pain in cancer patients has been shown by few studies. Liu et al. recently demonstrated that tactile allodynia and spontaneous pain of female rats with tibia tumors were correlated with the increase of both phosphorylated-p38MAPK and IL-1β and TNF-α in the spinal cord of rats. These changes were specific to bone cancer pain because rats without tibia tumors failed to show an increase. The authors further demonstrated that administration of an inhibitor of p38MAPK suppressed tactile allodynia and spontaneous pain of the bone cancer pain in rats and also decreased the phosphorylation of p38 as well as the expression of IL-1β and TNF-α. To characterize the cellular events upstream of p38MAPK, these authors examined the role of the TLR4. They observed that prolonged knockdown of TLR4 could attenuate hyperalgesia and also phosphorylation of p38 and the increases in IL-1β and TNF-α. On the basis of these observations, these authors concluded that TLR4-dependent phosphorylation of p38MAPK in spinal cord of rats might contribute to the development and maintenance of bone cancer pain, and p38MAPK and TLR4 would possibly be the potential targets for pain therapy in cancer patients.131 In another study, rats injected with prostrate cancer cells exhibited an increase in IL-1β and bone pain.132
Role of inflammatory molecules in anxiety
Anxiety can be described as vague, uneasy and unpleasant feelings of potential harm or distress. The symptoms of anxiety can be a reaction to the illness or can be related to the direct physiological effects of the disease or to drug therapies. Recent findings indicate that cancer patients facing death may often be plagued with recurrent unpleasant thoughts including fears of pain, death or dependency on others.133 Research has indicated that anxiety increases with the diagnosis of cancer, peaks before surgical interventions and frequently remains high thereafter, declining gradually during the first postoperative years.134
Although anxiety is common in cancer patients, we could find only one study showing a role for inflammatory molecules in cancer-related anxiety. That study evaluated the anxiety symptoms induced by IL-2 and/or INFα-2b in cancer patients. A total of 48 patients with renal cell carcinoma or melanoma were treated with subcutaneous IL-2 or INFα-2b, either alone or in combination. Although IL-2 alone had no effect, an enhancement in the anxiety scores was observed in the patients treated with IL-2 in combination with INFα-2b.135
Role of inflammatory molecules in cognitive impairment
Cognitive dysfunction in patients with cancer can include memory loss, distraction, difficulty with multitasking and mood disturbance.29 Although the majority of reports have been focused on women with breast cancer, patients with lung cancer and malignant glioma also exhibit cognitive impairment.136 As with other cancer-related symptoms, cognitive impairment in cancer patients may be due to cancer itself or its treatment.137– 143
Although the underlying mechanism remains poorly understood, there is some evidence that increased proinflammatory cytokine production plays a role in the development of cognitive impairment in cancer patients. One clinical study showed an association between increased circulating levels of cytokines and cognitive impairment in patients with acute myeloid leukemia or myelodysplastic syndrome. These patients had higher circulating levels of IL-6 at diagnosis and poorer executive function. The higher level of IL-8 in these patients, however, was associated with better memory.144
Role of inflammatory molecules in sleep disorders
Sleep disorders include an array of problems that are characterized by difficulty in falling asleep, problems maintaining sleep, poor sleep efficiency, early awakening and excessive daytime sleepiness. Recent research has indicated that patients with cancer experience sleep disturbances.145–147 However, despite data correlating inflammatory molecules with sleep disorders,148– 152 very few studies have shown a clear relationship between inflammatory markers and sleep disorder in cancer. In one study, 62 patients undergoing chemoradiation therapy for locally advanced non-small-cell lung cancer were examined. The severity of sleep disorder and serum IL-6 were examined on a weekly basis for 15 weeks using the MD Anderson Symptom Inventory. An increase in serum IL-6 was significantly correlated with increased mean severity of the disturbed sleep in these patients.98 Another study proposed that elevated vascular endothelial growth factor plays a role in the development of poor sleep in cancer patients.153
Role of inflammatory molecules in delirium
Delirium (acute confusion state) is a common psychiatric complication of patients with cancer.154 The incidence of the symptoms increases with the advancement of the disease.155 In one study, 85% of terminally ill cancer patients suffered from delirium.156 The causes for delirium in cancer patients are multifactorial and include metabolic abnormalities, infections, vascular complications, metastatic brain disease and treatment side-effects.154
Studies showing the role of inflammatory cytokines in the development of delirium in cancer patients are lacking. However, IL-1, IL-6, IL-8 and TNF have been reported to induce delirium in general.157– 160 One study examined the expression of proinflammatory cytokines in elderly patients with and without delirium. In patients with delirium, significantly more IL-6 and IL-8 levels were observed than in patients without delirium.157 van Munster et al. investigated serum levels of TNF-α, IL-1β, IL-6, IL-8 and IL-10 in patients with and without delirium. Ninety-six percent of these patients had TNF-α, IL-1β and IL-10 levels below the detection level. Differences between patients with and without delirium were observed in IL-6 and IL-8 levels. Further comparison indicated that patients with the hyperactive or mixed subtype of delirium had higher IL-6 levels than patients with hypoactive delirium. Based on these observations, the authors concluded that IL-6 and IL-8 may contribute to the pathogenesis of delirium and that IL-6 may play a role in the hyperactive behavior of delirium.161
Regulation of cancer-related symptoms by nutritional agents
From the above discussion, it is clear that cancer-related symptoms are multifactorial, with NF-κB-mediated inflammatory molecules playing a major role. Therefore, approaches aiming to downmodulate production of these cytokines could be beneficial in reducing symptoms in cancer patients. One such anti-inflammatory approach could be the use of nutritional agents that have multitargeting properties, are cost-effective, have low toxicity and are immediately available. In this section, we will describe how nutritional agents such as curcumin, genistein, resveratrol, EGCG and lycopene can modulate inflammatory molecules. Although none of these agents have demonstrated potential to reduce symptoms in cancer patients, their ability to downmodulate production of inflammatory cytokines and the role of these cytokines in the causation of symptoms strongly suggest further investigation of the use of nutraceuticals for reducing symptom burden and improving quality of life.
Curcumin
Curcumin [1,7-bis(4-hydroxy 3-methoxy phenyl)-1,6-heptadiene-3,5-dione] is a polyphenol derived from Curcuma longa (turmeric). Curcumin has been used for centuries throughout Asia as a food additive, cosmetic and traditional herbal medicine. It has been shown to reduce fatigue, neuropathic pain and cognitive function through modulation of inflammatory molecules in various models.
The ability of curcumin to reduce fatigue was investigated in a mouse model. Fatigue in the mice was induced immunologically by administration of LPS and Brucella abortus.162 The assessment of chronic fatigue syndrome was based on the chronic water-immersion stress test for 10 min daily for 19 days, and the immobility time was taken as the marker of fatigue. Mice challenged with LPS or B. abortus for 19 days showed significant increases in the immobility time and hyperalgesia on day 19, as well as a marked increase in serum TNF-α levels. Concurrent treatment with curcumin resulted in significant decreases in the immobility time, hyperalgesia and TNF-α levels. These results indicated that curcumin can be a valuable option in the treatment of chronic fatigue syndrome.162 In another mouse model, cur-cumin was found to reduce fatigue in association with decreases in IL-1β, IL-6 and TNF-α in soleus muscle.163 The role of curcumin in reducing neuropathic pain in mice with streptozotocin (STZ)-induced diabetes was investigated. The treatment of mice with insulin in combination with curcumin significantly reduced diabetic neuropathic pain that was associated with a reduction in TNF-α level.164,165 Curcumin has also been shown to improve cognitive function in animal models. One study investigated the effect of curcumin on cognitive functions and inflammation in diabetic rats. These rats exhibited cognitive deficits in association with an enhancement in serum TNF-α level that was significantly attenuated after chronic treatment with curcumin (60 mg/kg).166 The role of curcumin in improving body weight was evident from a recent study of patients with colorectal cancer. Curcumin administration (360 mg/day) for 10–30 days in these patients significantly improved body weight that was associated with a significant decrease in serum TNF-α level.167
Genistein
Genistein, a natural isoflavonoid found in soybean products, has demonstrated potential to inhibit NF-κB activation and modulate inflammatory pathways.168–170 It has a weak estrogenic effect and well-known non-specific tyro-sine kinase inhibitory activity at pharmacological doses.171 Genistein has been shown to reduce neuropathic pain and anorexia through modulation of inflammatory molecules.
In a mouse model of STZ-induced diabetes, induction of neuropathic pain was associated with elevations in IL-1β, IL-6 and TNF-α. When genistein (3 and 6 mg/kg) was administered to these mice from the second week until the fifth week after disease induction, neuropathic pain and cytokine production were significantly alleviated.170 Valsecchi et al. investigated the effects of genistein on neuropathic pain induced by mouse sciatic nerve chronic constriction injury. The authors investigated a number of molecular mechanisms possibly involved in the genistein-induced suppression of neuropathic hypersensitivity and found that suppression in neuropathic pain by genistein was associated with a reduction in NF-κB activation and IL-1β and IL-6 levels.172
In one study that investigated the role of genistein in reducing anorexia, LPS was injected into the lateral ventricle of rats to induce anorexia.173 Anorexia in these rats was inhibited by intracerebroventicular injections of IL-1β antibody and genistein. Furthermore, the decrease in body weight induced by injections of IL-1β (50 ng) was inhibited by genistein. These findings suggested that genistein mediates its effect on anorexia through modulation of IL-1β.
Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) has broad-spectrum beneficial health effects, including anti-infective, antioxidant and cardioprotective functions.174,175 Although initially isolated in 1940 as an ingredient of the roots of white hellebore (Veratrum grandiflorum O. Loes), resveratrol has now been identified in extracts from more than 70 other plant species.175,176 The anticancer activities of resveratrol are mediated through modulation of several cell signaling molecules involved in cell cycle progression, inflammation, proliferation, apoptosis, invasion, metastasis and angiogenesis of tumor cells.176–178
Resveratrol has been shown to ameliorate neuropathic pain and cancer cachexia through modulation of inflammatory molecules. For example, the antinociceptive effect of resveratrol alone or in combination with insulin on diabetic neuropathic pain and on serum TNF-α level was investigated in a mouse model of STZ-induced diabetes.165,179 Diabetic mice exhibited significant hyperalgesia along with increased plasma glucose and decreased body weights compared with control mice. Daily treatment with resveratrol (5, 10 and 20 mg/kg) for four weeks starting from the fourth week of STZ injection was associated with a significant attenuation of thermal hyperalgesia. Resveratrol also decreased TNF-α levels in the serum in a dose-dependent manner in these diabetic mice.165,179 To assess the potential therapeutic benefit of NF-κB inhibitors on muscle wasting in cancer cachexia, resveratrol (30 mmol/L) was used in a murine model. Resveratrol completely attenuated proteolysis-inducing factor-induced protein degradation in murine myotubes, as well as the increase in activity and expression of the molecules of the ubiquitin–proteasome pathway. In addition, resveratrol significantly attenuated the weight loss and protein degradation observed in the skeletal muscle of mice bearing the cachexia-inducing MAC16 tumor and produced a significant reduction in NF-κB DNA-binding activity. These observations suggested that agents that inhibit NF-κB activation may prove useful for the treatment of muscle wasting in cancer cachexia.180
Epigallocatechin gallate
EGCG is chiefly present in green tea. It has potent antioxidative, chemopreventive and antitumor activity.181 –187 Recent studies using mouse and rat models have indicated that EGCG can reduce fatigue and cognitive deficit through mediation of inflammatory molecules.
Sachdeva et al. produced chronic fatigue in mice by subjecting them to a forced swim inside a rectangular jar of specific dimensions for six minutes daily for 15 d. EGCG (25, 50 and 100 mg/kg) was administered daily 30 min before the forced swim session and immobility period, and post-swim fatigue was assessed on alternate days during the study. The authors observed a significant increase in TNF-α levels in the brain of mice subjected to water-immersion stress compared with the naive group. When the mice were treated with EGCG, behavioral and biochemical alterations were restored in a dose-dependent manner. The study suggested that EGCG could be of therapeutic potential in the treatment of chronic fatigue.188
Consumption of alcohol during pregnancy has been shown to induce mental retardation and long-term cognitive and behavioral deficits in developing offspring. In one study, EGCG was found to ameliorate ethanol-induced cognitive deficit in rat pups in association with a decrease in TNF-α, IL-1β and NF-κB levels.189
Lycopene
Lycopene is a bright red carotene found in tomatoes and other red fruits and vegetables, such as red carrots, watermelons and papayas. Studies have claimed that lycopene decreases the risk for some chronic diseases, including cardiovascular and inflammatory diseases such as atherosclerosis and rheumatoid arthritis.190,191 Lycopene has been shown to inhibit NF-κB activity and to modulate inflammatory pathways.192– 194 Two recent studies using mouse and rat models indicated that lycopene can improve neuropathic pain and cognitive deficit through mediation of inflammatory molecules.195,196
The effect of lycopene in ameliorating neuropathic pain was investigated in a mouse model of STZ-induced diabetes. Four weeks after a single intraperitoneal injection of STZ (200 mg/kg), mice were tested using the tail immersion and hot-plate assays. These mice exhibited significant hyperalgesia along with increased plasma glucose and decreased body weights compared with control mice. Lycopene (1, 2 and 4 mg/kg) treatment, from the fourth to eighth week after STZ injection, significantly attenuated thermal hyperalgesia and the hot-plate latencies. In addition, lycopene also inhibited the TNF-α release in a dose-dependent manner. These results indicated an antinociceptive activity of lycopene that could possibly be mediated through its inhibitory action on TNF-α release.196 Subsequently, this group examined the ability of lycopene to improve cognitive deficit in rats with STZ-induced diabetes. A deficit in the cognitive function in association with an increase in serum TNF-α level was observed in these diabetic rats. Chronic treatment of these rats with lycopene (1, 2 and 4 mg/kg) dose-dependently attenuated cognitive deficit and serum TNF-α level. The study emphasized the utility of lycopene in improving cognitive deficit in the diabetic rats.195
Flavopiridol
Flavopiridol is a flavone derived from a medicinal plant (Dysoxylum binectariferum) that is native to India. It is a potent cyclin-dependent kinase inhibitor, and its use is presently under investigation for a variety of solid tumors as well as hematological cancers.197–199 Although the nutraceuticals discussed so far have been shown to reduce symptoms, administration of flavopiridol was shown to induce symptoms in cancer patients. In a phase I clinical trial, infusion of flavopiridol for 72 h to patients with refractory malignancies was associated with fever, fatigue and pain,200 which were later shown to be associated with an increase in the serum IL-6 level.201
Conclusions and future perspective
Cancer and its treatment produce multiple symptoms that collectively cause a symptom burden for patients and impair function and rehabilitation. Inflammatory cytokines are involved in the development and progression of cancer and cancer-related symptoms. These cytokines are pleiotropic in nature and in turn are regulated by NF-κB. Therefore, strategies to downmodulate NF-κB and cytokines seem highly promising for suppressing cancer-related symptoms. However, most of the studies conducted to date have found only correlation between the severity of the symptoms and inflammatory cytokines. Correlation does not necessarily indicate the cause, and how these cytokines induce symptoms is largely unknown. Furthermore, none of the studies has examined whether inhibiting NF-κB to downregulate a network of cytokines would be helpful for symptom reduction in cancer patients. Additionally, some cytokines such as IL-6 have also shown beneficial effects against cancer-related fatigue. Because of their multitargeting properties, low cost, low toxicity and immediate availability, the use of nutritional agents in reducing symptoms seems attractive. Although nutraceuticals have shown promising results against symptoms in non-cancer models, their potential against cancer is unknown. Observations that some nutritional agents, such as flavopiridol, worsen the symptoms provide further obstacles in reaching a conclusion. Future studies should therefore be directed towards better defining the mechanism involved in the induction of symptoms by inflammatory molecules and towards determining the potential of nutritional agents, using animal models relevant to human cancer.
Acknowledgments
We thank Michael Worley and the Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr Aggarwal is the Ransom Horne, Jr, Professor of Cancer Research. This work was supported by a core grant from the National Institutes of Health (CA-16 672), a program project grant from the National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
Footnotes
Author contributions: All authors contributed to the writing and proofreading of the manuscript.
References
- 1.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 2.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 3.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367–78. [PubMed] [Google Scholar]
- 4.Smets EM, Garssen B, Cull A, de Haes JC. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. 1996;73:241–5. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 (Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 7.Andrykowski MA, Greiner CB, Altmaier EM, Burish TG, Antin JH, Gingrich R, McGarigle C, Henslee-Downey PJ. Quality of life following bone marrow transplantation: findings from a multicentre study. Br J Cancer. 1995;71:1322–9. doi: 10.1038/bjc.1995.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuellon RP, Craven B, Russell GB, Hoffman S, Cruz JM, Perry JJ, Hurd DD. Quality of life in breast cancer patients before and after autologous bone marrow transplantation. Bone Marrow Transplant. 1996;18:579–84. [PubMed] [Google Scholar]
- 9.Prieto JM, Saez R, Carreras E, Atala J, Sierra J, Rovira M, Batlle M, Blanch J, Escobar R, Vieta E, Gomez E, Rozman C, Cirera E. Physical and psychosocial functioning of 117 survivors of bone marrow transplantation. Bone Marrow Transplant. 1996;17:1133–42. [PubMed] [Google Scholar]
- 10.Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–50. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 11.Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, Shete S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–85. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penna F, Minero VG, Costamagna D, Bonelli G, Baccino FM, Costelli P. Anti-cytokine strategies for the treatment of cancer-related anorexia and cachexia. Expert Opin Biol Ther. 2010;10:1241–50. doi: 10.1517/14712598.2010.503773. [DOI] [PubMed] [Google Scholar]
- 13.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 14.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–42. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Wu X, Gu J, Dong Q, Liu J, Spitz MR. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67:6520–7. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Gibby CC, Spitz M, Wu X, Merriman K, Etzel C, Bruera E, Kurzrock R, Shete S. Cytokine genes and pain severity in lung cancer: exploring the influence of TNF-alpha-308 G/A IL6-174G/C and IL8-251T/A. Cancer Epidemiol Biomarkers Prev. 2007;16:2745–51. doi: 10.1158/1055-9965.EPI-07-0651. [DOI] [PubMed] [Google Scholar]
- 17.Kurzrock R, Feinberg B, Talpaz M, Saks S, Gutterman JU. Phase I study of a combination of recombinant tumor necrosis factor-alpha and recombinant interferon-gamma in cancer patients. J Interferon Res. 1989;9:435–44. doi: 10.1089/jir.1989.9.435. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub M, Adde MA, Venzon DJ, Shad AT, Horak ID, Neely JE, Seibel NL, Gootenberg J, Arndt C, Nieder ML, Magrath IT. Severe atypical neuropathy associated with administration of hematopoietic colony-stimulating factors and vincristine. J Clin Oncol. 1996;14:935–40. doi: 10.1200/JCO.1996.14.3.935. [DOI] [PubMed] [Google Scholar]
- 19.Rowinsky EK, Chaudhry V, Forastiere AA, Sartorius SE, Ettinger DS, Grochow LB, Lubejko BG, Cornblath DR, Donehower RC. Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J Clin Oncol. 1993;11:2010–20. doi: 10.1200/JCO.1993.11.10.2010. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Sodhi A. Increased release of interleukin-1 and tumour necrosis factor by interleukin-2-induced lymphokine-activated killer cells in the presence of cisplatin and FK-565. Immunol Cell Biol. 1992;70 (Part 1):15–24. doi: 10.1038/icb.1992.3. [DOI] [PubMed] [Google Scholar]
- 21.Gan XH, Jewett A, Bonavida B. Activation of human peripheral-blood-derived monocytes by cis-diamminedichloroplatinum: enhanced tumoricidal activity and secretion of tumor necrosis factor-alpha. Nat Immun. 1992;11:144–55. [PubMed] [Google Scholar]
- 22.Pai K, Sodhi A. Effect of cisplatin, rIFN-Y, LPS and MDP on release of H2O2, O2- and lysozyme from human monocytes in vitro. Indian J Exp Biol. 1991;29:910–5. [PubMed] [Google Scholar]
- 23.Zaks-Zilberman M, Zaks TZ, Vogel SN. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15:156–65. doi: 10.1006/cyto.2001.0935. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien JM, Jr, Wewers MD, Moore SA, Allen JN. Taxol and colchicine increase LPS-induced pro-IL-1 beta production, but do not increase IL-1 beta secretion. A role for microtubules in the regulation of IL-1 beta production. J Immunol. 1995;154:4113–22. [PubMed] [Google Scholar]
- 25.Galdiero M, Cipollaro de l’Ero G, Folgore A, Cappello M, Giobbe A, Sasso FS. Effects of irradiation doses on alterations in cytokine release by monocytes and lymphocytes. J Med. 1994;25:23–40. [PubMed] [Google Scholar]
- 26.Ibuki Y, Goto R. Contribution of inflammatory cytokine release to activation of resident peritoneal macrophages after in vivo low-dose gamma-irradiation. J Radiat Res. 1999;40:253–62. doi: 10.1269/jrr.40.253. [DOI] [PubMed] [Google Scholar]
- 27.Wasserman J, Petrini B, Wolk G, Vedin I, Glas U, Blomgren H, Ekre HP, Strannegard O. Cytokine release from mononuclear cells in patients irradiated for breast cancer. Anticancer Res. 1991;11:461–4. [PubMed] [Google Scholar]
- 28.Das KC, White CW. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem. 1997;272:14914–20. doi: 10.1074/jbc.272.23.14914. [DOI] [PubMed] [Google Scholar]
- 29.Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Cleeland CS. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–92. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 30.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L, Scher H. The memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–36. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 31.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–70. [PubMed] [Google Scholar]
- 32.Kurzrock R. Cytokine deregulation in cancer. Biomed Pharmacother. 2001;55:543–7. doi: 10.1016/s0753-3322(01)00140-8. [DOI] [PubMed] [Google Scholar]
- 33.Valentine AD, Meyers CA. Cognitive and mood disturbance as causes and symptoms of fatigue in cancer patients. Cancer. 2001;92:1694–8. doi: 10.1002/1097-0142(20010915)92:6+<1694::aid-cncr1499>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Mencher SK, Wang LG. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue) BMC Clin Pharmacol. 2005;5:3. doi: 10.1186/1472-6904-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an ‘old-age’ disease with an ‘age-old’ solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 38.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 39.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:36–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 43.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 46.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 1799:775–87. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr Opin Cell Biol. 1999;11:226–32. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 48.Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: could increased activity of pro-inflammatory cytokines be the common denominator? Eur J Cancer. 2008;44:175–81. doi: 10.1016/j.ejca.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802–7. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- 50.Cleeland CS. Cancer-related symptoms. Semin Radiat Oncol. 2000;10:175–90. doi: 10.1053/srao.2000.6590. [DOI] [PubMed] [Google Scholar]
- 51.Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–64. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomita T, Takeuchi E, Tomita N, Morishita R, Kaneko M, Yamamoto K, Nakase T, Seki H, Kato K, Kaneda Y, Ochi T. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappaB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999;42:2532–42. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Meyers CA, Seabrooke LF, Albitar M, Estey EH. Association of cancer-related symptoms with physiological parameters. J Pain Symptom Manage. 2002;24:359–61. doi: 10.1016/s0885-3924(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 54.Bridge TP. Neuropsychiatrically active lymphokines and AIDS. Clin Neuropharmacol. 1986;9 (Suppl 4):473–5. [PubMed] [Google Scholar]
- 55.Brenard R. Practical management of patients treated with alpha interferon. Acta Gastroenterol Belg. 1997;60:211–3. [PubMed] [Google Scholar]
- 56.Nozaki O, Takagi C, Takaoka K, Takata T, Yoshida M. Psychiatric manifestations accompanying interferon therapy for patients with chronic hepatitis C: an overview of cases in Japan. Psychiatry Clin Neurosci. 1997;51:175–80. doi: 10.1111/j.1440-1819.1997.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 57.Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–9. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 58.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 61.Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–94. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Nomura Y. NF-kappaB activation and IkappaB alpha dynamism involved in iNOS and chemokine induction in astroglial cells. Life Sci. 2001;68:1695–701. doi: 10.1016/s0024-3205(01)00967-5. [DOI] [PubMed] [Google Scholar]
- 63.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 64.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kapparcub;B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–92. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennis PA, Van Waes C, Gutkind JS, Kellar KJ, Vinson C, Mukhin AG, Spitz MR, Bailey-Wilson JE, Yeh GC, Anderson LM, Wiest JS. The biology of tobacco and nicotine: bench to bedside. Cancer Epidemiol Biomarkers Prev. 2005;14:764–7. doi: 10.1158/1055-9965.EPI-04-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–3. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin–proteasome pathway. Genes Dev. 1995;9:1586–97. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 68.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 69.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 70.Attaix D, Combaret L, Bechet D, Taillandier D. Role of the ubiquitin-proteasome pathway in muscle atrophy in cachexia. Curr Opin Supportive and Palliative Care. 2008;2:262–6. doi: 10.1097/spc.0b013e3283196ac2. [DOI] [PubMed] [Google Scholar]
- 71.Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia–cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427–34. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 72.Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37:2036–46. doi: 10.1016/j.biocel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 73.Lelbach A, Muzes G, Feher J. Current perspectives of catabolic mediators of cancer cachexia. Med Sci Monit. 2007;13:RA168–73. [PubMed] [Google Scholar]
- 74.Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argiles JM, Baccino FM. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–9. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llovera M, Garcia-Martinez C, Lopez-Soriano J, Carbo N, Agell N, Lopez-Soriano FJ, Argiles JM. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol. 1998;142:183–9. doi: 10.1016/s0303-7207(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 76.Chen SZ, Qiu ZG. Combined treatment with growth hormone, insulin and indomethacin alleviates cancer cachexia in a mouse model. J Endocrinol. 2011;208:131–6. doi: 10.1677/JOE-10-0341. [DOI] [PubMed] [Google Scholar]
- 77.Ramamoorthy S, Donohue M, Buck M. Decreased Jun-D and myogenin expression in muscle wasting of human cachexia. Am J Physiol Endocrinol Metab. 2009;297:E392–401. doi: 10.1152/ajpendo.90529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fortunati N, Manti R, Birocco N, Pugliese M, Brignardello E, Ciuffreda L, Catalano MG, Aragno M, Boccuzzi G. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep. 2007;18:1521–7. [PubMed] [Google Scholar]
- 79.Martignoni ME, Kunze P, Hildebrandt W, Kunzli B, Berberat P, Giese T, Kloters O, Hammer J, Buchler MW, Giese NA, Friess H. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005;11:5802–8. doi: 10.1158/1078-0432.CCR-05-0185. [DOI] [PubMed] [Google Scholar]
- 80.Cannon TY, Guttridge D, Dahlman J, George JR, Lai V, Shores C, Buzkova P, Couch ME. The effect of altered Toll-like receptor 4 signaling on cancer cachexia. Arch Otolaryngol Head Neck Surg. 2007;133:1263–9. doi: 10.1001/archotol.133.12.1263. [DOI] [PubMed] [Google Scholar]
- 81.Zhang D, Zheng H, Zhou Y, Tang X, Yu B, Li J. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer. 2007;7:45. doi: 10.1186/1471-2407-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 83.Plata-Salaman CR. Anorexia during acute and chronic disease. Nutrition. 1996;12:69–78. doi: 10.1016/s0899-9007(96)90702-9. [DOI] [PubMed] [Google Scholar]
- 84.Laviano A, Meguid MM, Rossi-Fanelli F. Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003;4:686–94. doi: 10.1016/s1470-2045(03)01247-6. [DOI] [PubMed] [Google Scholar]
- 85.Opara EI, Laviano A, Meguid MM, Yang ZJ. Correlation between food intake and CSF IL-1 alpha in anorectic tumor bearing rats. Neuroreport. 1995;6:750–2. doi: 10.1097/00001756-199503270-00011. [DOI] [PubMed] [Google Scholar]
- 86.Laviano A, Gleason JR, Meguid MM, Yang ZJ, Cangiano C, Rossi Fanelli F. Effects of intra-VMN mianserin and IL-1ra on meal number in anorectic tumor-bearing rats. J Invest Med. 2000;48:40–8. [PubMed] [Google Scholar]
- 87.Plata-Salaman CR, Ilyin SE, Gayle D. Brain cytokine mRNAs in anorectic rats bearing prostate adenocarcinoma tumor cells. Am J Physiol. 1998;275:R566–73. doi: 10.1152/ajpregu.1998.275.2.R566. [DOI] [PubMed] [Google Scholar]
- 88.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs. 2006;8:157–69. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 89.Wang W, Lonnroth C, Svanberg E, Lundholm K. Cytokine and cyclooxygenase-2 protein in brain areas of tumor-bearing mice with prostanoid-related anorexia. Cancer Res. 2001;61:4707–15. [PubMed] [Google Scholar]
- 90.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer. 1996;4:82–96. doi: 10.1007/BF01845757. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 92.Inagaki M, Isono M, Okuyama T, Sugawara Y, Akechi T, Akizuki N, Fujimori M, Mizuno M, Shima Y, Kinoshita H, Uchitomi Y. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage. 2008;35:153–61. doi: 10.1016/j.jpainsymman.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 93.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92:1684–8. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 94.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–64. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 95.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–66. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 96.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–27. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K, Hara M, Taguchi H, Kimura Y, Kato Y, Asaoku H, Kumagai S, Kodama F, Nakahara H, Hagihara K, Yoshizaki K, Kishimoto T. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 98.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–74. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25:147–50. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23:868–74. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Keller C, Keller P, Giralt M, Hidalgo J, Pedersen BK. Exercise normalises overexpression of TNF-alpha in knockout mice. Biochem Biophys Res Commun. 2004;321:179–82. doi: 10.1016/j.bbrc.2004.06.129. [DOI] [PubMed] [Google Scholar]
- 103.Wood LJ, Nail LM, Winters KA. Does muscle-derived interleukin-6 mediate some of the beneficial effects of exercise on cancer treatment-related fatigue? Oncol Nurs Forum. 2009;36:519–24. doi: 10.1188/09.ONF.519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB. Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry. 1995;52:89–99. doi: 10.1001/archpsyc.1995.03950140007002. [DOI] [PubMed] [Google Scholar]
- 105.Ciaramella A, Poli P. Assessment of depression among cancer patients: the role of pain, cancer type and treatment. Psychooncology. 2001;10:156–65. doi: 10.1002/pon.505. [DOI] [PubMed] [Google Scholar]
- 106.Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–9. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- 107.Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, III, Cole S, Sood AK, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–7. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.El-Gohary GM, Azzam HM, Ahmed OI, El-Shokry MH. Pro-inflammatory cytokines and depression in patients with acute leukemia. Egypt J Immunol. 2008;15:13–24. [PubMed] [Google Scholar]
- 109.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 110.Kudoh A, Katagai H, Takazawa T. Plasma inflammatory cytokine response to surgical trauma in chronic depressed patients. Cytokine. 2001;13:104–8. doi: 10.1006/cyto.2000.0802. [DOI] [PubMed] [Google Scholar]
- 111.Omne-Ponten M, Holmberg L, Burns T, Adami HO, Bergstrom R. Determinants of the psycho-social outcome after operation for breast cancer. Results of a prospective comparative interview study following mastectomy and breast conservation. Eur J Cancer. 1992;28A:1062–7. doi: 10.1016/0959-8049(92)90457-d. [DOI] [PubMed] [Google Scholar]
- 112.Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, ‘depression due to a general medical condition,’ and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 113.O’Connor MF, Irwin MR, Seldon J, Kwan L, Ganz PA. Pro-inflammatory cytokines and depression in a familial cancer registry. Psychooncology. 2007;16:499–501. doi: 10.1002/pon.1108. [DOI] [PubMed] [Google Scholar]
- 114.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–5. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 115.Wiertelak EP, Smith KP, Furness L, Mooney-Heiberger K, Mayr T, Maier SF, Watkins LR. Acute and conditioned hyperalgesic responses to illness. Pain. 1994;56:227–34. doi: 10.1016/0304-3959(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 116.Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–99. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 117.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 118.Bao L, Zhu Y, Elhassan AM, Wu Q, Xiao B, Zhu J, Lindgren JU. Adjuvant-induced arthritis: IL-1 beta, IL-6 and TNF-alpha are up-regulated in the spinal cord. Neuroreport. 2001;12:3905–8. doi: 10.1097/00001756-200112210-00010. [DOI] [PubMed] [Google Scholar]
- 119.Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp Neurol. 1994;129:237–43. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- 120.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625–9. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 121.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 122.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 123.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–95. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 124.Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 125.Leem JG, Bove GM. Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain. 2002;3:45–9. doi: 10.1054/jpai.2002.27138. [DOI] [PubMed] [Google Scholar]
- 126.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–4. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 127.Honore P, Menning PM, Rogers SD, Nichols ML, Mantyh PW. Neurochemical plasticity in persistent inflammatory pain. Progress Brain Res. 2000;129:357–63. doi: 10.1016/S0079-6123(00)29027-4. [DOI] [PubMed] [Google Scholar]
- 128.Allan SM. The role of pro- and antiinflammatory cytokines in neurodegeneration. Ann N Y Acad Sci. 2000;917:84–93. doi: 10.1111/j.1749-6632.2000.tb05373.x. [DOI] [PubMed] [Google Scholar]
- 129.Chang VT, Janjan N, Jain S, Chau C. Update in cancer pain syndromes. J Palliat Med. 2006;9:1414–34. doi: 10.1089/jpm.2006.9.1414. [DOI] [PubMed] [Google Scholar]
- 130.Lema MJ, Foley KM, Hausheer FH. Types and epidemiology of cancer-related neuropathic pain: the intersection of cancer pain and neuropathic pain. Oncologist. 2010;15(Suppl 2):3–8. doi: 10.1634/theoncologist.2009-S505. [DOI] [PubMed] [Google Scholar]
- 131.Liu S, Yang J, Wang L, Jiang M, Qiu Q, Ma Z, Liu L, Li C, Ren C, Zhou J, Li W. Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res. 1346:213–223. doi: 10.1016/j.brainres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 132.Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008;154:1533–8. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roth AJ, Massie MJ. Anxiety and its management in advanced cancer. Curr Opin Supportive Palliative Care. 2007;1:50–6. doi: 10.1097/SPC.0b013e32813aeb23. [DOI] [PubMed] [Google Scholar]
- 134.Miller K, Massie MJ. Depression and anxiety. Cancer J. 2006;12:388–97. doi: 10.1097/00130404-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 135.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–51. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 136.Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90:1691–6. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–68. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 138.Correa DD. Cognitive functions in brain tumor patients. Hematol Oncol Clin North Am. 2006;20:1363–76. doi: 10.1016/j.hoc.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 139.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 140.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–9. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 143.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63:183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–93. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 145.Clark J, Cunningham M, McMillan S, Vena C, Parker K. Sleep–wake disturbances in people with cancer part II: evaluating the evidence for clinical decision making. Oncol Nurs Forum. 2004;31:747–71. doi: 10.1188/04.ONF.747-771. [DOI] [PubMed] [Google Scholar]
- 146.Vena C, Parker K, Cunningham M, Clark J, McMillan S. Sleep–wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncol Nurs Forum. 2004;31:735–46. doi: 10.1188/04.ONF.735-746. [DOI] [PubMed] [Google Scholar]
- 147.Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Med Rev. 2004;8:199–212. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 148.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 149.Hatipoglu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration. 2003;70:665–71. doi: 10.1159/000075218. [DOI] [PubMed] [Google Scholar]
- 150.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 151.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 152.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 153.Guess J, Burch JB, Ogoussan K, Armstead CA, Zhang H, Wagner S, Hebert JR, Wood P, Youngstedt SD, Hofseth LJ, Singh UP, Xie D, Hrushesky WJ. Circadian disruption, Per3, and human cytokine secretion. Integr Cancer Ther. 2009;8:329–36. doi: 10.1177/1534735409352029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stiefel F, Fainsinger R, Bruera E. Acute confusional states in patients with advanced cancer. J Pain Symptom Manage. 1992;7:94–8. doi: 10.1016/0885-3924(92)90120-7. [DOI] [PubMed] [Google Scholar]
- 155.Stiefel F, Razavi D. Common psychiatric disorders in cancer patients. II. Anxiety and acute confusional states. Support Care Cancer. 1994;2:233–7. doi: 10.1007/BF00365727. [DOI] [PubMed] [Google Scholar]
- 156.Massie MJ, Holland J, Glass E. Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048–50. doi: 10.1176/ajp.140.8.1048. [DOI] [PubMed] [Google Scholar]
- 157.de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–5. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 158.Adamis D, Lunn M, Martin FC, Treloar A, Gregson N, Hamilton G, Macdonald AJ. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing. 2009;38:326–32. doi: 10.1093/ageing/afp014. discussion 251. [DOI] [PubMed] [Google Scholar]
- 159.Dunlop RJ, Campbell CW. Cytokines and advanced cancer. J Pain Symptom Manage. 2000;20:214–32. doi: 10.1016/s0885-3924(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 160.Bush SH, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;14:1039–49. doi: 10.1634/theoncologist.2009-0122. [DOI] [PubMed] [Google Scholar]
- 161.van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–9. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 162.Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. 2009;214:33–9. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 163.Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2168–73. doi: 10.1152/ajpregu.00858.2006. [DOI] [PubMed] [Google Scholar]
- 164.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–61. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 165.Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278–83. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- 166.Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol. 2007;576:34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 167.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–13. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 168.Gadgeel SM, Ali S, Philip PA, Wozniak A, Sarkar FH. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009;115:2165–76. doi: 10.1002/cncr.24250. [DOI] [PubMed] [Google Scholar]
- 169.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650:694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 171.McClain RM, Wolz E, Davidovich A, Edwards J, Bausch J. Reproductive safety studies with genistein in rats. Food Chem Toxicol. 2007;45:1319–32. doi: 10.1016/j.fct.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 172.Valsecchi AE, Franchi S, Panerai AE, Sacerdote P, Trovato AE, Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008;107:230–40. doi: 10.1111/j.1471-4159.2008.05614.x. [DOI] [PubMed] [Google Scholar]
- 173.Tsushima H, Mori M. Involvement of protein kinase C and tyrosine kinase in lipopolysaccharide-induced anorexia. Pharmacol Biochem Behav. 2001;69:17–22. doi: 10.1016/s0091-3057(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 174.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann NY Acad Sci. 2011;1215:150–60. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 176.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 177.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 178.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 179.Sharma S, Kulkarni SK, Chopra K. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam Clin Pharmacol. 2007;21:89–94. doi: 10.1111/j.1472-8206.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 180.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–50. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–44. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 182.Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436:69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 183.Mukhtar H, Ahmad N. Mechanism of cancer chemopreventive activity of green Tea. Proc Soc Exp Biol Med. 1999;220:234–8. doi: 10.1046/j.1525-1373.1999.d01-40.x. [DOI] [PubMed] [Google Scholar]
- 184.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–80. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 185.Tran PL, Kim SA, Choi HS, Yoon JH, Ahn SG. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer. 2010;10:276. doi: 10.1186/1471-2407-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (2)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–9. [PubMed] [Google Scholar]
- 187.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389:134–5. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 188.Sachdeva AK, Kuhad A, Tiwari V, Arora V, Chopra K. Protective effect of epigallocatechin gallate in murine water-immersion stress model of chronic fatigue syndrome. Basic Clin Pharmacol Toxicol. 2010;106:490–6. doi: 10.1111/j.1742-7843.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- 189.Tiwari V, Kuhad A, Chopra K. Epigallocatechin-3-gallate ameliorates alcohol-induced cognitive dysfunctions and apoptotic neurodegeneration in the developing rat brain. Int J Neuropsychopharmacol. 2010;13:1053–66. doi: 10.1017/S146114571000060X. [DOI] [PubMed] [Google Scholar]
- 190.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 191.Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65:2979–99. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim GY, Kim JH, Ahn SC, Lee HJ, Moon DO, Lee CM, Park YM. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Immunology. 2004;113:203–11. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2010.04.016. (In Press) [DOI] [PubMed] [Google Scholar]
- 194.Feng D, Ling WH, Duan RD. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm Res. 2010;59:115–21. doi: 10.1007/s00011-009-0077-8. [DOI] [PubMed] [Google Scholar]
- 195.Kuhad A, Sethi R, Chopra K. Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci. 2008;83:128–34. doi: 10.1016/j.lfs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 196.Kuhad A, Sharma S, Chopra K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pain. 2008;12:624–32. doi: 10.1016/j.ejpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 197.Park DS, Farinelli SE, Greene LA. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J Biol Chem. 1996;271:8161–9. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- 198.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–8. [PubMed] [Google Scholar]
- 199.Smith ME, Cimica V, Chinni S, Jana S, Koba W, Yang Z, Fine E, Zagzag D, Montagna C, Kalpana GV. Therapeutically targeting cyclin D1 in primary tumors arising from loss of Ini1. Proc Natl Acad Sci USA. 2011;108:319–24. doi: 10.1073/pnas.0913297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, Hill K, Steinberg SM, Figg WD, Tompkins A, Arbuck SG, Sausville EA. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol. 1998;16:2986–99. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 201.Messmann RA, Ullmann CD, Lahusen T, Kalehua A, Wasfy J, Melillo G, Ding I, Headlee D, Figg WD, Sausville EA, Senderowicz AM. Flavopiridol-related proinflammatory syndrome is associated with induction of interleukin-6. Clin Cancer Res. 2003;9:562–70. [PubMed] [Google Scholar]