Abstract
Despite the fact cancer is primarily a preventable disease, recent statistics indicate cancer will become the number one killer worldwide in 2010. Since certain cancers are more prevalent in the people of some countries than others, suggests the role of lifestyle. For instance cancer incidence among people from the Indian subcontinent, where most spices are consumed, is much lower than that in the Western World. Spices have been consumed for centuries for a variety of purposes—as flavoring agents, colorants, and preservatives. However, there is increasing evidence for the importance of plant-based foods in regular diet to lowering the risk of most chronic diseases, so spices are now emerging as more than just flavor aids, but as agents that can not only prevent but may even treat disease. In this article, we discuss the role of 41 common dietary spices with over 182 spice-derived nutraceuticals for their effects against different stages of tumorigenesis. Besides suppressing inflammatory pathways, spice-derived nutraceuticals can suppress survival, proliferation, invasion, and angiogenesis of tumor cells. We discuss how spice-derived nutraceuticals mediate such diverse effects and what their molecular targets are. Overall our review suggests “adding spice to your life” may serve as a healthy and delicious way to ward off cancer and other chronic diseases.
Keywords: nutraceuticals, spices, inflammation, cancer, apoptosis, invasion, angiogenesis
Introduction
Spices have shaped a large part of the world’s history; for example, they led such legendary explorers as Christopher Columbus and Vasco de Gama to search the world for its most precious commodities—spices. Throughout the ancient and medieval world, spices carried a high value and were considered so special they often came with a very high price tag. Spices have been used for centuries, serving a variety of purposes in a wide variety of cultures. They have been used as flavor agents, as colorants to add special taste to dishes, and also as a preservative to prevent the growth of bacteria. But today, the importance of spices has become even more evident than they were throughout history. Due to their high antioxidant and anti-inflammatory properties, the common spices in today’s diet have been demonstrated to also have medicinal value, and much of this potential has only been realized over the last 50 years.
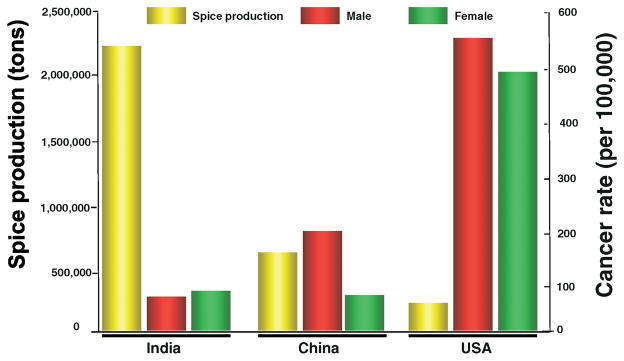
The major focus of this review is on the role that spices play in the various phases of tumorigenesis, serving as agents both for prevention and treatment. Examinations of dietary patterns of people around the world have demonstrated that populations that consume more spices have been shown to have a lower incidence of cancer. For example, in a comparison of the incidence of the various types of cancer between the United States and India, the US was found to have much higher rates of colorectal cancer. In 2000, the US had 356 colon cancer cases reported and 139 deaths per 1 million people. In contrast, India only had 40 reported cases of colon cancer and 26 deaths per 1 million people. Why cancer incidence is so much lower in India than in most Western countries, is not fully understood, but the high spice consumption could be one of the contributing factor (Fig. 1) (1).
Figure 1.
Relationship between production of spices and cancer incidence. Data is modified from 2000 faostat.fao.org (http://www.foodmarketexchange.com/datacenter/product/herb/herb/detail/dc_pi_hs_herb0406.htm) and cancer data from the World Health Organization GLOBOCAN 2002. A color version of the figure is available in the online journal.
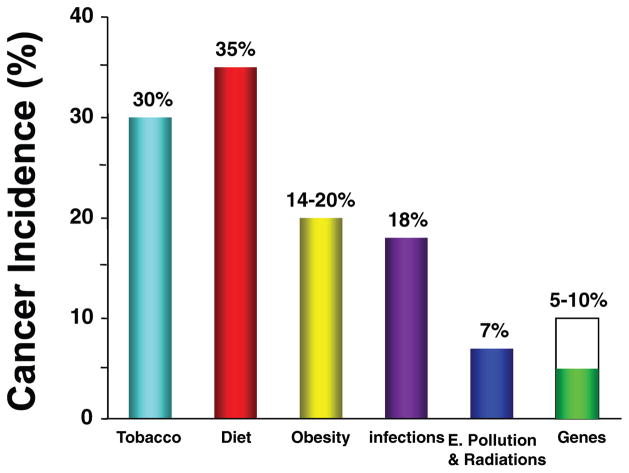
Despite continued cancer research and positive breakthroughs, the overall rates of cancer over the past 50 years have not changed substantially. The percentage of people who develop cancer and the percentage of people who die from cancer have shown virtually no improvement over the past few decades. Among the chronic diseases, cancer is expected to become the number one killer in 2010, accounting for more deaths than even heart attack. Yet it is now believed that 90–95% of all cancers are attributable to lifestyle, while the remaining 5–10% can be attributed to faulty genes (2) (see Fig. 2). The implications of the above statements indicate that as of today, the best and only true “cure” for cancer is through prevention.
Figure 2.
Cancer is a preventable disease that requires major changes in lifestyle. A color version of the figure is available in the online journal.
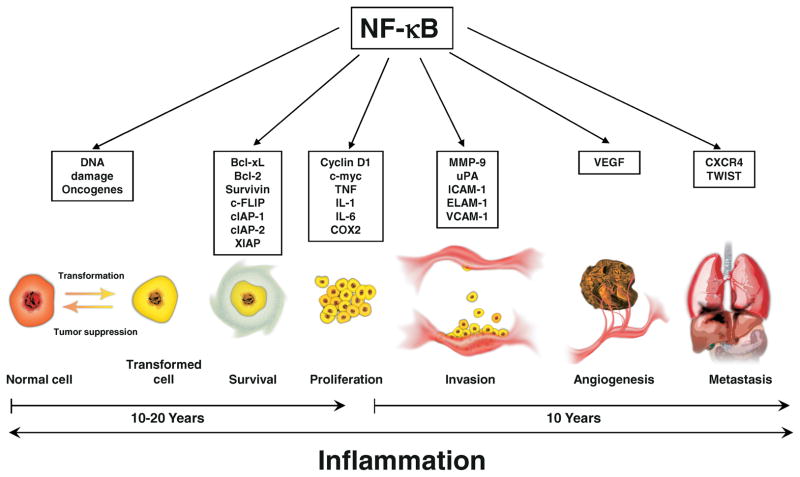
Cancer is a complicated disease that develops over a number of years (see Fig. 3). The basic theory of tumorigenesis starts with a normal cell that is transformed through the activation of proto-oncogenes and the suppression of tumor suppressor genes such as p53. After the cell has been transformed, it no longer behaves like a normal cell, but begins to exhibit the properties of a cancer cell. These transformed cells acquire the capability to proliferate uncontrollably through self-sufficiency in growth signals and insensitivity to anti-growth signals. In addition, these cells are able to evade apoptosis, resulting in tumor growth. The transformation of a normal cell to a cancer cell along with uncontrolled proliferation and evasion of apoptosis is a process that can take between ten to twenty years. As the tumor continues to develop, this growth is aided through the development of new blood vessels that provide nutrients to the tumor, allowing it to sustain itself and even begin to invade other tissues, resulting in metastasis that is ultimately lethal.
Figure 3.
Roles of NF-κB-mediated inflammatory pathway in cellular transformation, cancer survival, proliferation, invasion, angiogenesis and metastasis. A color version of the figure is available in the online journal.
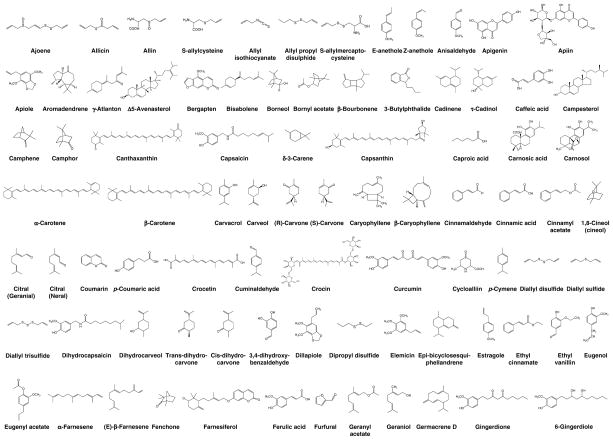
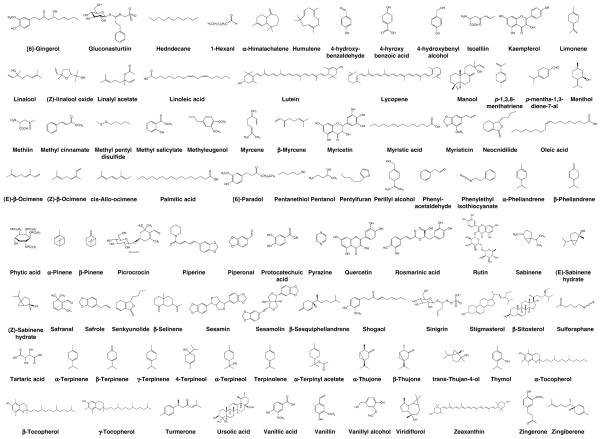
Nutraceutical is a term first coined by Stephen DeFelice in 1989 from “nutrition” and “pharmaceutical”. According to DeFelice, nutraceutical can be defined as, “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease” (3, 4). This review will focus on the specific nutraceuticals derived from 41 common herbs and spices (see Fig. 4) (Table 1) (5–50) that target one or more of the various phases of tumorigenesis. The five focuses are on targets for inflammation, proliferation, apoptosis, invasion, and angiogenesis. Over 182 spice-derived nutraceuticals have been identified and the major well-studied compounds are listed in Figure 5. A large portion of these nutraceuticals do indeed show great potential for targeting cancer at various phases, showing down-regulation of transcription factor (e.g. nuclear factor-κB), anti-apoptotic proteins (e.g. Bcl-2 and Bcl-xL), promoters of cell proliferation (e.g. cyclooxygenase-2, cyclin D1 and c-Myc), and invasive and metastatic genes (e.g. matrix metalloproteinases, intracellular adhesion molecule-1, and angiogenic protein vascular endothelial growth factor) (Table 2) (51–130). This article shows that “adding spice to your life” has the potential to not only improve the flavor of your favorite dishes, but to help fight the various stages of tumorigenesis.
Figure 4.
Common dietary spices. A color version of the figure is available in the online journal.
Table 1.
A List of Common Spices and Nutraceuticals from Spices
| Spice | Nutraceuticals |
|---|---|
| Allspice | Eugenol, methyl eugenol, myrcene, 1,8-cineol, α-phellandrene, quercetin, myricetin (5) |
| Anise | Anethole, bergapten, estragole, anisaldehyde, α-himalchalene (6) |
| Asafoetida | α-Pinene, phellandrenes, Farnesiferoles, hendecylsulphonyl acetic acid (7) |
| Basil | Ursolic acid, eugenol, caffeic acid, β-sitosterol, limonene, estragole, methyl eugenol, geraniol, 1,8-cineol, linalool, citral, methyl cinnamate (8) |
| Bay leaves | Linalool, α-terpinol, α-terpinyl acetate, thymol, caryophyllene, aromadendrene, β-selinene, farnesene, cadinene, methyl eugenol, myrcene, eugenol (9) |
| Black pepper | Piperine, β-caryophyllene, limonene, δ-3-carene, α-pinene, β-pinene, α-phellandrene, myrcene, terpinolene (10) |
| Caraway | S-Carvone, germacrene D, limonene, dihydrocarveol, α-pinene, β-pinene, sabinene, perillyl alcohol, carveol (11, 12) |
| Cardamom | α-Terpinyl acetate, 1,8-cineol, limonene, linalool, linalyl acetate, terpinolene, myrcene (13) |
| Celery seed | Apigenin, limonene, β-selinene, humulene, 3-butylphthalide, senkyunolide, α-pinene, β-pinene, myrcene, (Z)-β-ocimene, γ-terpinene, cis-allo-ocimene, (E)-β-farnesene, apiole, senkyunolide, neocnidilide (14) |
| Chervil | Estragole, apiin, hendecane (undecane) (15, 16) |
| Chives | Dipropyl disulfide, methyl pentyl disulfide, pentyl hydrodisulfidea, cis/trans 3,5-diethyl-1,2,4-trithiolanea, pentanethiol, diallyl sulfide (17) |
| Cinnamon | Cinnamaldehyde, cinnamyl acetate, cineol, eugenol, coumarin, ethyl cinnamate, linalool, humulene, β-caryophyllene, τ-cadinol (18) |
| Cloves | Carvacrol, thymol, eugenol, cinnamaldehyde, eugenyl acetate (19) |
| Coriander | Linalool, geraniol, geranyl acetate, camphor (20) |
| Cumin | Cuminaldehyde, γ-terpinene, β-pinene, p-cymene, p-mentha-1,3-diene-7-al, p-mentha-1,4-dien-7-ala (11, 21) |
| Dill | Carvones, limonene, dillapiole, trans-dihydrocarvone, cis-dihydrocarvone, myristicin (22) |
| Fennel | (E)-Anethole, limonene, fenchone, estragole, anisaldehyde, bergapten, β-sitosterol (23, 24) |
| Garlic | Ajoene, allicin, alliin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, S-allylcysteine, methiin, isoalliin, cycloalliin, S-allylmercaptocysteine (25) |
| Ginger | [6]-Gingerol, [6]-paradol, shogoal, 6-gingerdiol, gingerdione, zingiberene, citral (neral and geranial), bisabolene, α-farnesene, β-phellandrene, cineol, zingerone (26, 27) |
| Horseradish | Sinigrin, allyl isothiocyanate, gluconasturtiin, phenylethyl isothiocyanate, quercetin, kaempferol (28) |
| Marjoram | 4-Terpinenol, (E)-sabinene hydrate, γ-terpinene, sabinene, β-pinene, limonene, β-phellandrene, (Z)-sabinene hydrate, terpinolene (12) |
| Mint (spearmint) | Menthol, R-carvone, limonene, β-pinene, β-myrcene, trans-thujan-4-ol, dihydrocarvone, β-bourbonene, β-caryophyllene, epi-bicyclosesquiphellandrene (29) |
| Mustard | Allyl isothiocyanate, sulforaphane (30) |
| Nutmeg | Eugenol, myristicin, elemicin, sabinene, safrole, methyl eugenol, α-pinene, β-pinene, myristic acid, 4-terpineol (31, 32) |
| Onion | Quercetin, allicepina, allyl propyl disulphide, protocatechuic acid, quercetin dimera, quercetin trimera (33, 34) |
| Oregano | Carvacrol, cis-sabinene hydrate, thymol, linalyl acetate, β-caryophyllene, 4-terpineol, α-terpineol, caffeic acid (35) |
| Paprika | β-Carotene, α-, β-, and γ-tocopherols, canthaxanthin, capsaicin, dihydrocapsaicin (36) |
| Parsley | p-1,3,8-Menthatriene, β-phellandrene, apiole, myrcene, myristicin, rutin, apigenin (37) |
| Poppy seed | 1-Pentanol, 1-hexanal, pentylfuran, caproic acid, linoleic acid, oleic acid, palmitic acid (38) |
| Red pepper | Capsaicin, β-carotene, zeaxanthin, lutein, caffeic acid, capsanthin (39) |
| Rosemary | Carnosol, rosmarinic acid, carnosic acid, α-pinene, camphor, limonene, camphene, borneol, cineole, (Z)-linalool oxide, bornyl acetate (40) |
| Saffron | Crocin, safranal, picrocrocin, crocetin, α- and β-carotene, lycopene, zeaxanthin (41) |
| Sage | 1,8-Cineol, camphor, α-thujone, β-thujone, borneol, viridiflorol, manool, humulene, β-caryophyllene (11, 42) |
| Savory | Carvacrol, α-pinene, γ-terpinene, 4-terpineol, α-terpineol, cadinene, τ-cadinol, caryophyllene (43) |
| Sesame seed | Sesamin, sesamolin, phytic acid, linoleic acid, oleic acid, β-sitosterol, campesterol, stigmasterol, γ-tocopherol, Δ5-avenasterol, palmitic acid (44) |
| Tamarind | Tartaric acid, limonene, geraniol, safrole, cinnamic acid, ethyl cinnamate, methyl salicylate, pyrazine, phenylacetaldehyde, 2-furfural, palmitic acid (45) |
| Tarragon | (Z)-Anethole, (Z)-β-ocimene, (E)-β-ocimene, limonene, methyl eugenol, camphor, cineol (46) |
| Thyme | Thymol, carvacrol, p-cymene, γ-terpinene, linalool, borneol, β-caryophyllene, caffeic acid, β-pinene, thymodihydroquinone (12, 47) |
| Turmeric | Curcumin, zingiberene, turmerone, γ-atlantone, β-sesquiphellandrene, turmerol, bisabolone (48) |
| Vanilla | Vanillin, ethyl vanillin, 4-hydroxybenzyl alcohol, vanillyl alcohol, piperonal, ferulic acid, vanillic acid, 3,4-dihydroxybenzaldehyde, 4-hydroxybenzoic acid, 4-hydroxybenzaldehyde, p-coumaric acid (49) |
| White pepper | Piperine, β-caryophyllene, limonene, δ-3-carene, α-pinene, β-pinene, α-phellandrene, myrcene, terpinolene (50) |
The structure is not available.
Figure 5.
Chemical structures of nutraceuticals derived from different spices.
Table 2.
Molecular Targets of Nutraceuticals Derived from Spices
| Pathwaysa | Inhibitors |
|---|---|
| Inflammation | |
| NF-κB | Anethole, carnosol, caryophyllene, cinnamaldehyde, curcumin, humulene, perillyl alcohol, quercetin, sulforaphane, ursolic acid (51–59) |
| TNF | Ajoene, allicin, allyl isothiocyanate, apigenin, curcumin, diallyl sulfide, eugenol, gingerol, humulene, kaempferol, paradol, piperine, zingerone (53, 60–68) |
| IL-1β | Allicin, apigenin, diallyl sulfide, eugenol, gingerol, humulene, kaempferol, phytic acid, piperine (53, 61, 63–66, 68, 69) |
| IL-8 | Allicin, phytic acid (61, 69) |
| IL-6 | Diallyl sulfide, phytic acid, piperine (64, 68, 69) |
| STAT3 | Capsaicin (70) |
| Survival | |
| Bcl-2 | Ajoene, allyl isothiocyanate, β-carotene, β-sitosterol, capsaicin, carnosol, cinnamaldehyde, curcumin, diallyl sulfide, gingerol, limonene, lutein, rosmarinic acid, S-allylcysteine, sul foraphane, ursolic acid (70–85) |
| Bcl-xL | Allyl isothiocyanate, β-carotene, capsaicin, sulforaphane (70, 72, 73, 84) |
| Bax | Cinnamaldehyde, curcumin, diallyl sulfide, limonene, lutein (76–78, 80) |
| Survivin | Capsaicin, curcumin (70, 77) |
| Caspases | Allicin, β-sitosterol, cinnamaldehyde, citral, diallyl disulfide, diallyl trisulfide, kaempferol, limonene, myristicin, paradol, shogaol, sulforaphane, terpinen-4-ol, ursolic acid (74, 76, 84–95) |
| p53 | Diallyl sulfide, limonene, lutein, shogaol (78, 80, 95, 96) |
| Proliferation | |
| c-Myc | Apigenin, perillyl alcohol (97, 98) |
| Cyclin D1 | Apigenin, capsaicin, curcumin, lutein, sesamin, sulforaphane, ursolic acid (59, 70, 97, 99–102) |
| COX-2 | Eugenol, curcumin (55, 97) |
| Other cyclins | Bergapten, β-carotene, carnosol, diallyl trisulfide, geraniol, perillyl alcohol, rosmarinic acid (73, 82, 89, 103–105) |
| p53 | Phytic acid, quercetin (106, 107) |
| Invasion | |
| MMP-9 | Allyl isothiocyanate, caffeic acid, carnosol, curcumin, diallyl disulfide, quercetin, ursolic acid, vanillin (64, 108–113) |
| MMP-2 | Allyl isothiocyanate, curcumin, diallyl disulfide, myricetin, quercetin (64, 108, 111, 114–116) |
| MMPs (non-specific) | Crocetin, piperine, sulforaphane (68, 117, 118) |
| ICAM-1 | Allicin, apigenin, crocetin, kaempferol (117, 119, 120) |
| Angiogenesis | |
| VEGF | Alliin, caffeic acid, capsaicin, curcumin, diallyl disulfide, diallyl sulfide, gingerol, perillyl alcohol, phytic acid, rosmarinic acid, sulforaphane (58, 64, 121–130) |
COX-2, cyclooxygenase-2; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin-1β; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; STAT3, signal transducers and activator of transcription 3; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Molecular Targets of Nutraceuticals
As we now know, cancer is not a simple disease, but a complex interaction between multiple genes, within the cell itself, and with its neighboring tissues. In order for a tumor to develop, somewhere between 300–500 genes have gone wrong. These “ bad genes” can lead to up-regulation of undesired products such as anti-apoptotic proteins or down-regulation of tumor suppressor proteins such as p53. This section will focus on some of the most commonly known targets that are shown to cause undesired effects in tumor development.
Effect of Nutraceuticals on Inflammation
Inflammation comes from the Latin word inflammatio, which means, “to set on fire.” The four classic hallmarks of inflammation are redness, heat, swelling, and pain. Inflammation is the body’s natural response to harmful stimuli and is achieved by the movement of plasma and leukocytes from the blood into injured tissues. This particular type of immune response is important for the body to ward off harmful pathogens and is classified as acute inflammation. Chronic inflammation, on the other hand, occurs when there is a progressive change in the type of cells that are present at the sight of inflammation where the body is attempting to heal, but tissue damage is occurring at the same time. Recently, more and more attention has been focused on chronic inflammation and its link to cancer. Research has shown that inflammation is a critical component of tumor progression and that many cancers arise from sites of infection, chronic irritation, and inflammation. The micro-environment provided by inflammatory cells is an indispensable participant in the neoplastic process, fostering proliferation, survival and migration (131). The inflammatory microenvironment containing various inflammatory cells and a network of signaling molecules is necessary for the malignant progression of transformed cells, which is attributed to the mutagenic predisposition of persistent infection-fighting agents at sites of chronic inflammation. The inflammatory cells and regulators may facilitate angiogenesis and promote the growth, invasion, and metastasis of tumor cells (132).
One of the most important links between inflammation and cancer is the transcription factor nuclear factor-κB (NF-κB). This transcription factor can be activated in response to various types of stress, such as pro-inflammatory cytokines (TNF), viruses, γ-radiation, bacterial cell wall components (lipopolysaccharide, LPS), or chemotherapeutic agents (133). This DNA binding of NF-κB signals transcription of various cancer-promoting genes such as anti-apoptotic genes, pro-angiogenic genes, and pro-invasion genes (134). It is believed that NF-κB DNA binding leads to the activation of over 400 genes, many of which lead to a variety of diseases besides cancer such as Alzheimer’s disease and arthritis (135).
Other factors such as tumor necrosis factor (TNF), interleukins (IL-1β and IL-6), and chemokines (IL-8 or CXCL8) play an important role in the link between inflammation and cancer as well. TNF is part of a family of cytokines, mainly secreted from macrophages, that is responsible for the regulation of immune cells. However, its disregulation and overproduction can lead to diseases, including cancer, and is known to be responsible for the activation of NF-κB. It does this by binding to a TNF-receptor on the cell’s surface that triggers a pathway that leads to the activation of IκBα kinase (IKK) (136). Interleukins are a group of cytokines that are released from a wide variety of bodily cells. However, the two interleukins this article discusses, IL-1β and IL-6, are mainly released from macrophages. IL-1β is an important part of the inflammatory response against infection that increases the expression of adhesion factors on endothelial cells to allow movement of leukocytes to the sight of infection. IL-6 is a pro-inflammatory cytokine that is released in response to trauma or tissue damage. IL-8, also known as CXCL8, is a member of the CXC chemokine family. It has been shown to contribute to human cancer progression through its potential functions as a mitogenic, angiogenic, and mutagenic factor (137).
Many spice-derived nutraceuticals have been found to play a role in reducing inflammation. These include ajoene, allicin, allyl isothiocyanate, anethole, apigenin, capsaicin, carnosol, caryophyllene, cinnamaldehyde, curcumin, diallyl sulfide, eugenol, [6]-gingerol, humulene, kaempferol, limonene, myrcene, [6]-paradol, perillyl alcohol, phytic acid, piperine, quercetin, sulforaphane, ursolic acid, and zingerone (Tables 2 and 3) (51–70, 138, 139). Many of these nutraceuticals target the transcription factor NF-κB, leading to its down-regulation.
Table 3.
Inhibition of Inflammation by Spice-Derived Nutraceuticals
| Nutraceutical | Responsesa |
|---|---|
| Ajoene | Inhibits tumor-endothelial cell adhesion, as well as the in vivo TNF-α response to LPS in mouse melanoma cells (60). |
| Allicin | Inhibits the spontaneous and TNF-α-induced secretion of IL-1β, IL-8, IP-10 and MIG in a dose-dependent manner from intestinal epithelial cells in vitro, suppresses the expression of IL-8 and IL-1β mRNA levels and the degradation of IκB (61). |
| Allyl isothiocyanate | Significantly inhibits the cellular production of proinflammatory mediators such as TNF-α and NO and inhibits the release of MCP-1 from 3T3-L1 adipocytes in vitro (62). |
| Anethole | Inhibits NF-κB activation, IκBα phosphorylation and degradation, and NF-κB-reporter gene expression by induced by TNF, TRAF2, and NIK in vitro, suppresses TNF-induced activation of the transcription factor AP-1, JNK and MAPK in vitro (51). |
| Apigenin | Inhibits TNF-α in LPS stimulated macrophages resulting in diminished MCP-1 and inhibition of IL-1β in vitro (63). |
| Capsaicin | Blocks the STAT3 activation pathway in multiple myeloma cells in vitro leading to down-regulation of cyclin D1, Bcl-2, Bcl-xL, survivin, and VEGF (70). |
| Carnosol | Decreases LPS-induced iNOS mRNA and protein expression, reduces NF-κB subunits translocation and NF-κB DNA binding activity in activated macrophages due to inhibition of IKK, inhibits iNOS and NF-κB promoter activity (52). |
| Caryophyllene | Inhibits the LPS-induced NF-κB activation and neutrophil migration in rat paw edema in vivo (53). |
| Cinnamaldehyde | Inhibits age-related NF-κB activation and targets inflammatory iNOS and COX-2, inhibits the activation of NF-κB via three signal transduction pathways, NIK/IKK, ERK, and p38 MAPK (54). |
| Curcumin | Down-regulates the constitutive activity of NF-κB, decreases levels of phospho-IκBα, decreases expression of NF-κB-target genes COX-2 and cyclin D1, and induces apoptosis in mouse melanoma cells in vitro (55). Significantly inhibits the cellular production of proinflammatory mediators such as TNF-α and NO and inhibits the release of MCP-1 from 3T3-L1 adipocytes (62). |
| Diallyl sulfide | Significantly reduces the production of and serum levels of IL-1β, IL-6, TNF-α and GM-CSF in mice with melanoma (64). |
| Eugenol | Blocks the release of IL-1β, TNF-α, and prostaglandin E2 and suppresses the mRNA expression of IL-1β, TNF-α, and COX-2 in LPS-stimulated human macrophages in vitro (65). |
| [6]-Gingerol | Inhibits the production of TNF-α, IL-1β, and IL-12 in murine peritoneal macrophages exposed to several doses of 6-gingerol in the presence of LPS stimulation (66). |
| Humulene | Inhibits the LPS-induced NF-κB activation and neutrophil migration in rat paw edema, prevents the production of TNF-α and IL-1β and the in vivo up-regulation of kinin B(1) receptors (53). |
| Kaempferol | Inhibits TNF-α in LPS stimulated macrophages resulting in diminished MCP-1 and inhibition of IL-1β in vitro (63), inhibits IL-4-induced STAT6 activation by specifically targeting JAK3 in hemopoietic cells from human and mouse origin in vitro (138). |
| Limonene | Inhibits the LPS-induced inflammation including cell migration and production of NO along with significant inhibition of γ-interferon and IL-4 production in mouse model of pleurisy (139). |
| Myrcene | Inhibits the LPS-induced inflammation including cell migration and production of NO along with significant inhibition of γ-interferon and IL-4 production in mouse model of pleurisy (139). |
| Paradol | Significantly inhibits the tumor-promoter-stimulated inflammation, TNF-α production, and activation of epidermal ornithine decarboxylase in mice (67). |
| Perillyl alcohol | Reduces NF-κB DNA-binding activity and targets gene induction, which is associated with an increase in apoptosis in B-lymphoma cells and in estrogen receptor-negative breast cancer cells (56). |
| Phytic acid | Modulates IL-8 and IL-6 release from colonic epithelial cells stimulated with LPS and IL-1β, suppresses IL-8 basal release, and it dose-dependently reduces IL-8 secretion by colonocytes and down-regulates IL-6 (69). |
| Piperine | Significantly reduces the expression of IL-1β, IL-6, TNF-α, GM-CSF and IL-12p40 genes in melanoma cells (68). |
| Quercetin | Attenuates PMACI-induced activation of NF-κB and p38 MAPK in human mast cell line HMC-1 (57). |
| Sulforaphane | Inhibits NF-κB transcriptional activity, nuclear translocation of p65, and gene expression of NF-κB-regulated VEGF, cyclin D1, and Bcl-xL in human prostate cancer cell (58). |
| Ursolic acid | Inhibits IKK and p65 phosphorylation leading to the suppression of NF-κB activation induced by various carcinogens; this correlates with the down-regulation of COX-2, MMP-9, and cyclin D1 in vitro (59). |
| Zingerone | Significantly inhibits the cellular production of proinflammatory mediators such as TNF-α and NO and inhibits the release of MCP-1 from 3T3-L1 adipocytes (62). |
AP-1, activator protein-1; COX-2, cyclooxygenase-2; ERK, extracellular signal regulated kinase; GM-CSF, granulocyte-macrophage colony-stimulating factor; IKK, IκB kinase; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; IP-10, inducible protein-10; Jak 3; janus kinase 3; LPS, lipopolysaccharide; MAPK, mitogen activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MIG, monokine induced by interferon gamma; NF-κB, nuclear factor-κB; NIK, NF-κB-inducing kinase; NO, nitric oxide; PMACI, phorbol-12-myristate 13-acetate plus calcium ionophore A23187; TRAF2, TNF receptor associated factor-2; VEGF, vascular endothelial growth factor.
The compound 2,8-Biapigenin blocks the action of NF-κB by preventing nuclear translocation of p65; this blocks the transactivation of inducible nitric oxide synthase (iNOS) and COX-2 genes, thus playing an anti-inflammatory role and causing cell cycle arrest (140). Anethole inhibits NF-κB activation, IκBα phosphorylation and degradation, and NF-κB-reporter gene expression induced by TNF, TNF receptor-associated factor 2 (TRAF2), and NF-κB-inducing kinase (NIK) in vitro (51). Capsaicin has an antioxidant potential and helps decrease oxidative stress induced cancer during exposure to benzopyrene in the lungs of mice (141). Carnosol limits the translocation of NF-κB subunits to the nucleus and reduces NF-κB DNA binding activity in activated macrophages due to the inactivation of IKK (52). Caryophyllene and humulene inhibit LPS-induced NF-κB activation and neutrophil migration in rat paw edema in vivo (53). Cinnamaldehyde inhibits age-related NF-κB activation and targets inflammatory iNOS and COX-2 (54). In addition, cinnamaldehyde can inhibit the activation of NF-κB through three different signal transduction pathways—NIK/IKK, extracellular signal-regulated kinase (ERK), and p38 mitogen activated protein kinase (MAPK) (54). Perillyl alcohol reduces NF-κB DNA-binding activity and target gene induction in estrogen receptor-negative breast cancer cells in vitro (56). Quercetin attenuates the expression of phorbol-12-acetate-13-myristate (PMA)-induced activation of NF-κB and p38 MAPK in human mast cells in vitro (57). Sulforaphane inhibits NF-κB transcriptional activity, nuclear translocation of p65, and gene expression of NF-κB-regulated gene products such as VEGF, cyclin D1, and Bcl-xL in human prostate cancer cells in vitro (58). Ursolic acid inhibits IKK and p65 phosphorylation leading to the suppression of NF-κB activation induced by various carcinogens in vitro. This NF-κB suppression leads to the down-regulation of its regulated gene products COX-2, MMP-9, and cyclin D1 (59).
Many of the spice-derived nutraceuticals target TNF-α, either by blocking its production or its function. Ajoene inhibits TNF-α production in response to LPS stimulation in mouse melanoma cells in vivo (59). Allyl isothiocyanate (AITC), curcumin, and zingerone significantly inhibit the cellular production of TNF-α and nitric oxide and inhibit the release of monocyte chemoattractant protein-1 (MCP-1) from adipocytes in vitro (62). [6]-Paradol inhibits TNF-α production in mouse skin papillomagenesis initiated by 7,12-dimethylbenz[a]anthracene in female ICR mice (67). Many of the spice-derived nutraceuticals target both TNF-α and IL-1β. Apigenin and kaempferol inhibit TNF-α in LPS stimulated macrophages resulting in diminished MCP-1 and inhibition of IL-1β in vitro (63). Apigenin inhibits the production of nitric oxide, prostaglandin E(2), p38 MAPK and c-Jun N-terminal kinase (JNK) phosphorylation. This apigenin also has an inhibitory effect on the expression of TNF-α and IL-1β genes in macrophages, thus playing a protective role against inflammation. Apigenin also plays an important role in decreasing oxidative stress causing a decrease in macromolecular damage to hepatocellular cells, thus preventing hepatocellular carcinogenesis (142). Diallyl sulfide significantly reduces the production of and serum levels of TNF-α and IL-1β in mice with melanoma (64). Eugenol is the essential active substance in clove; it possesses antimicrobial, antioxidant, anti-inflammatory and cytotoxic properties (19). Eugenol blocks the release of TNF-α and IL-1β and suppresses their mRNA expression in LPS-stimulated human macrophages in vitro (143). [6]-Gingerol inhibits the production of TNF-α and IL-1β in LPS-stimulated murine peritoneal macrophages in vitro (66). Humulene prevents the production of pro-inflamma-tory cytokines TNF-α and IL-1β in rat paw edema in vivo (53). Piperine significantly reduces the expression of TNF-α and IL-1β in human melanoma cells in vitro (68).
AITC also down-regulates proinflammatory cytokines such as IL-1β, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Eugenol causes initiation of membrane oxidative damage and intracellular reactive oxygen species (ROS) generation in combination with gamma-radiation, which plays an important role in radio-sensitization of various tumors (144). Also it increases sensitivity of cisplatin-induced cytotoxicity by decreasing the expression of multidrug resistance protein 2 (MRP2) (145). Eugenol decreases the incidence of radiation-induced thymic lymphoma in mice (146). Eugenol contained in clocimum oil was found to have modulating effects on murine skin papillomagenesis; it was found to exert its effect by increasing levels of glutathione S-transferase (GST) and cytochrome B5 (147). It also inhibited CCl4-induced hepatotoxicity in rat (148). In vivo treatment of rats with eugenol resulted in decrease in mutagenicity of B[a]P in Salmonella typhimurium mutagenicity assay in hepatoma cell line HepG2 (149). Eugenol inhibited tobacco induced mutagenicity and nitrosation of methylurea in a dose-dependent manner (150). Jeng et al. found that frequent exposure of oral mucosa to low concentrations of eugenol at a concentration less than 1 mmol/L might protect cells from the genetic attack of ROS via inhibition of xanthine oxidase activity and lipid peroxidation, thus preventing oral submucosal fibrosis; however, higher concentrations may predispose to carcinogenesis (151). In rat liver cells, dietary administration of eugenol was found to enhance UDP-glucuronyltransferase, UDP-glucose dehydrogenase, and GST activities, which would reduce intermediates of various carcinogens, and active intermediates of drugs (152).
The remaining nutraceuticals target IL-6 and IL-8. Allicin inhibits the spontaneous and TNF-α induced secretion of IL-1β and IL-8 from intestinal epithelial cells in vitro. It also suppresses the mRNA levels of IL-1β and IL-8 and the degradation of IκBα (61). Diallyl sulfide significantly reduces the production and serum levels of IL-6 in mice with melanoma (64). Phytic acid modulates IL-6 and IL-8 release from colonic epithelial cells stimulated with LPS and IL-1β. It suppresses IL-8 basal release and reduces the secretion of IL-8 by colonocytes, as well as down-regulates IL-6 (69). Piperine reduces the expression of IL-6 in human melanoma cells in vitro (68).
Effect of Nutraceuticals in Tumor Cell Survival and Apoptosis
Apoptosis is a process of programmed cell death that in Greek literally means “falling away.” Apoptosis is a natural, organized process that cells go through in the human body on a daily basis. The human body is made up of 6 trillion cells, with approximately 3 billion cells replaced every minute. This equates to 1% of the human body being turned over every day, and a “new body” being generated every three months. Cells are also triggered to go through apoptosis when intracellular sensors detect abnormalities such as DNA damage, oncogene activation, nutrient deficiency, or hypoxia. However, cancer cells are able to acquire the capability to evade apoptosis, allowing tumors to grow rapidly and uncontrollably. One of the most common ways this can occur is through mutation of the p53 tumor suppressor gene, resulting in functional inactivation of the p53 protein. Without the p53 protein, the cell lacks important DNA damage sensor capability that would normally induce the apoptotic cascade. For this reason, many pharmaceutical companies are trying to develop new drugs that target steps in the apoptotic pathway. Many are still in clinical trials, and it is unclear at this time which targeted therapies will be most effective. Current cancer treatments, such as radiation and chemotherapy, are believed to shrink tumors through triggering apoptosis, but this is still uncertain, and cells often become resistant to such therapies.
There are two different types of apoptosis: mitochondria dependent and mitochondria independent. In mitochondria dependent form, apoptotic signals cause the release of cytochrome C from the mitochondria, a potent catalyst of apoptosis that activates caspases. Caspases are proteases that cleave other proteins and therefore are essential for apoptotic cell death. Apoptotic signals may come in the form of members of the Bcl-2 family of proteins, such as pro-apoptotic Bax that can be up-regulated by tumor suppressor protein p53 in response to sensing DNA damage. Apoptosis can also be triggered through extracellular signals (or “death signals”) that bind to receptors on the cell’s surface and trigger caspase activation inside the cell. This receptor-mediated apoptosis is often triggered by cytokines such as TNF or TNF-related apoptosis-inducing ligand (TRAIL), or by the binding of the Fas receptor and Fas ligand.
Some other important players in apoptosis are the members of the Bcl-2 family of proteins including Bcl-2 and Bcl-xL. Both of these proteins are anti-apoptotic proteins that are found to be overexpressed in a wide variety of cancers. They are also playing a role in resistance to traditional cancer treatments. Survivin is another anti-apoptotic protein found to be overexpressed in cancer cells. If ways to down-regulate Bcl-2, Bcl-xL, and survivin, and ways to up-regulate p53, Bax, and caspases, can be found, these could help to trigger apoptosis in cancer cells.
For example, many spice-derived nutraceuticals have been shown to induce apoptosis in different types of cancer cells through a wide variety of mechanisms. Major ones are ajoene, allicin, allyl isothiocyanate, bergapten, β-carotene, α-sitosterol, canthaxanthin, capsaicin, carnosol, cineole, cinnamaldehyde, citral, crocin, curcumin, diallyl disulfide, diallyl sulfide, diallyl trisulfide, [6]-gingerol, kaempferol, limonene, lutein, myristicin, [6]-paradol, rosmarinic acid, S-allylcysteine, S-allylmercaptocysteine, sesamin, sesamolin, shogaol, sulforaphane, terpinen-4-ol, turmerone, and ursolic acid (Table 4) (70, 73–75, 78–93, 95, 96, 153–157). One of the most common targets of these nutraceuticals is Bcl-2, an anti-apoptotic protein. Research has shown that by down-regulating Bcl-2, apoptosis can be induced in cancer cells.
Table 4.
Induction of Apoptosis by Spice-Derived Nutraceuticals
| Nutraceuticals | Responsea,b |
|---|---|
| Ajoene | ↑ caspase-3, Bcl-2 cleavage (153) |
| Allicin | ↑ caspases-3, -8 and -9, cleaves PARP (86) |
| Allyl isothiocyanate | ↓ Bcl-2, ↓ Bcl-xL (154) |
| β-Carotene | ↓ Bcl-2, ↓ Bcl-xL (73) |
| β-Sitosterol | ↓ Bcl-2, ↓ PARP, ↓ phospholipase C-γ1, ↑ caspase-3 (74) |
| Capsaicin | ↓ STAT3, ↓ Bcl-2, ↓ Bcl-xL, ↓ survivin, ↑ caspase (70, 155) |
| Carnosol | ↓ Bcl-2 (75) |
| Citral | ↑ DNA fragmentation, ↑ caspase-3 (87) |
| Diallyl disulfide | ↑ caspase-3 (88, 89) |
| Diallyl sulfide | ↑ p53, ↑ Bax, ↓ survivin, ↓ Bcl-2 (78) |
| Diallyl trisulfide | ↑ caspase-3 (89) |
| [6]-Gingerol | ↓ Bcl-2 (79) |
| Kaempferol | ↑ caspase-3, ↑ AIF (90) |
| Limonene | ↓ Bcl-2, ↓ p53, ↑ Bax, ↑ caspase-9, ↑ caspase-3 (80, 91, 156) |
| Lutein | ↓ Bcl-2, ↑ Bax, ↑ p53, ↓ Bcl-2, ↑ Bax:Bcl-2 ratio (81, 96) |
| Myristicin | ↑ cytochrome C, ↑ caspase-3 (92) |
| [6]-Paradol | ↑ caspase-3 (93) |
| Rosmarinic acid | ↓ Bcl-2 (82) |
| S-Allylcysteine | ↓ Bcl-2 (83) |
| S-Allylmercaptocysteine | ↓ MT assembly, ⊥ mitosis, ↑ JNK, ↑ caspase-3 (157) |
| 6-Shogaol | ↑ DNA fragmentation, ↑ caspase-3 (95) |
| Sulforaphane | ↑ Fas ligand, ↑ caspase-3, ↑ caspase-8, ↑ caspase-9, ↓ Bcl-2, ↑ cytochrome C, ↑ PARP cleavage (84) |
| Ursolic acid | ↑ caspase-9, ↓ Bcl-2 (85) |
AIF, apoptosis inducing factor; JNK, c-Jun NH2-terminal kinase; MT, microtubule; PARP, poly (ADP-ribose) polymerase; STAT3, signal transducer and activator of transcription 3.
↑, up-regulate; ↓, down-regulate; ⊥, block/arrest.
AITC, a major component of garlic, induces apoptosis by a variety of mechanisms. Apoptosis with AITC was associated with cleavage of p22 BID protein to p15, p13, and p11 fragments and activation of JNK. In isothiocyanate-induced apoptosis, the caspase pathway has an essential role along caspase-8 and caspase-3; the JNK pathway a supporting role (72). Cells treated with AITC also show a significant reduction in the expression (31–68%) of anti-apoptotic protein Bcl-2 and approximately 58% reduction in Bcl-xL protein expression. Also there is significant reduction in expression of several proteins that regulate G2/M progression including cyclin B1, cell division cycle (cdc) 25B (44–48% reduction) and cdc25C (> 90% reduction), and cyclin dependent kinase 1 (CDK1) (32–50% reduction) (72). AITC in high doses can increase activity of phase II detoxification enzymes such as quinone reductase (QR) and GST in many rat tissues, while in moderate doses an increase in enzyme activity is mainly found in the bladder of rats (158). Induction of Phase II enzymes may contribute to lower incidences of bladder cancer. AITC causes induction of activities of ethoxyresor-ufin O-deethylase (EROD) and GST at dose levels which were protective towards benzo(a)pyrene-induced DNA damage (159). AITC can also cause increased acetylation of histones. AITC administration inhibits growth of PC-3 xenografts in vivo by inducing apoptosis and reducing mitotic activity (160).
Apigenin is a flavone mainly present in parsley and celery and possesses hydroxylized B ring, which makes it a natural and potent proteasome inhibitor and a tumor cell apoptosis inducer (161). The presence of C2-C3 double bond is associated with its growth inhibitory potential (162). Apigenin is implicated in inhibition of human cytochrome CYP1B1; this isoform metabolizes both polycyclic aromatic hydrocarbons and estrogenic compounds into carcinogenic by-products (163). The different ways through which apigenin induces apoptosis are listed below.
In human breast cancer cells, it inhibits proteasome function and through estrogen receptor (ER)-dependent and ER-independent pathways; it induces apoptosis in MCF-7 cells (164). In HER2/neu-overexpressing breast cancer cells, this phytoestrogen induces apoptosis by decreasing levels of HER2 protein, which in turn decreases levels of phosphorylated HER3, thus suppressing the signaling of HER2/HER3-phosphatidylinositide 3-kinase (PI3K)/Akt pathway (165).
In Jurkat T cells, apigenin activates caspase-3 and induces cleavage of poly (ADP-ribose) polymerase (PARP) (166). In human prostate cancer 22Rv1 cells, this flavonoid inhibits NF-κB/p65 transcriptional activity and induces p21/WAF-1 in a dose- and time-dependent manner. Besides this it was also found to increase expression of KIP1/p27, INK4a/p 16 and INK 4c/p18; it caused down modulation in the protein expression of cyclins D1, D2 and cyclin E along with cyclin dependent kinases such as cdk2, cdk4, and cdk6. It was found to cause a decrease in retinoblastoma phosphorylation at serine 780 (167). Apigenin also elevated the levels of insulin-like growth factor-1 (IGF-1) and inhibited the growth of human prostate xenograft in nude mice. ROS were generated with Apigenin by the action of NADPH oxidase, which resulted in rapid glutathione depletion, disruption of mitochondrial membrane potential and cytosolic release of cytochrome C and apoptosis. Apigenin led to a decrease in the levels of Bcl-xL and Bcl-2 and increase in Bax, which in turn triggered caspase-3, -7, -8, and -9 activation. It also concomitantly cleaved the inhibitor of the apoptosis protein, c-IAP2 (168). Apigenin also was found to induce sensitivity of SQ-5 spheroids (cells growing in three-dimensional structure, simulating the growth and microenvironment of in vivo tumors) to radiation. This action of apigenin along with induction of p21/WAF-1 and decreased expression of Bcl-2 also serves to increase radiosensitivity of certain tumors (169). In human esophageal adenocarcinoma cells this flavonoid was found to cause G2/M arrest through the up-regulation of GADD45β and 14-3-3 sigma, down-regulation of p53 at the mRNA and protein levels and up-regulation of PI3K and cleavage of caspase-3 and -9 causing p53-independent mitochondrial-mediated apoptosis (170). It also induced apoptosis via an increase in intracellular free calcium with calpain activation (171). Induction of apoptosis in certain types of cancer (e.g. prostate and breast) cells by apigenin is associated with its potential to inhibit fatty acid synthesis (172). Apigenin also exhibits proxidant effect causing oxidation of various thiols through the formation of various phenoxyl radicals, thus leading to abortive pathways leading to necrotic cell death (173). In solid tumors, apigenin inhibits hypoxia-activated pathways that lead to cancer progression especially the PI3K/Akt/glycogen synthase kinase-3 (GSK-3) pathways (174). Vitexin, natural derivative of apigenin, decreases mRNA levels of hypoxia inducible genes such as aldolase A, smad 3, enolase1, and collagen type III (175). In neuroblastomas this phytoestrogen acts by inducing caspase-dependent, p53-mediated apoptosis (162). It also induces glutathione depletion in human prostate tumor cells (176). It was observed that the combination of apigenin and gemcitabine enhanced anti-tumor activity through NF-κB and Akt activity suppression and apoptosis induction (177). Treatment with this flavonoid results in significant dose-related reduction in genotoxic activity of chemotherapeutic agents such as induced by mitomycin C and cyclophosphamide (178). Apigenin is also implicated in aromatase inhibition and could act as a chemopreventive agent in hormone-dependent cancers (179). Apigenin induces the expression of death receptor-5 and synergizes with exogenous soluble recombinant human TRAIL to selectively induce apoptosis in tumor cells but not in normal cells (180). Apigenin has been shown to decrease the differentiation of normal human keratinocyte cells by suppressing normal MAPK signal transduction and activator protein-1 (AP-1) transcription factor level (181).
Curcumin, the major polyphenol present in turmeric is a potent inducer of apoptosis in cancer cells. Curcumin induces up-regulation of pro-apoptotic proteins such as Bax, Bim, Bak, Puma and Noxa and down-regulates the expression of anti-apoptotic Bcl-2 and Bcl-xL (182, 183).
Both β-sitosterol and carnosol increase apoptosis through down-regulating Bcl-2 in human leukemia cells in vitro (74, 75). Curcumin down-regulates the expression of survivin and Bcl-2, while it up-regulates the expression of pro-apoptotic Bax leading to apoptosis in human multiple myeloma cells in vitro (77). Diallyl sulfide down-regulates the expression of both survivin and Bcl-2, but up-regulates the expression of Bax and p53 in mouse skin tumors (78). Eugenol prevents radiation-induced oxidative cell membrane damage resulting in formation of reactive oxygen species and induction of apoptosis in cells (184). [6]-Gingerol induces cell death with DNA fragmentation by inhibiting expression of Bcl-2 in promyelocytic leukemia cells in vitro (79). Lutein decreases the expression of Bcl-2 and increases the expression of Bax, stimulating apoptosis in esophageal carcinoma cells in vitro (81). In addition, mice fed lutein were found to have higher apoptotic activity in mammary tumors than control animals with an increase in expression of Bax and p53 and decrease in expression of Bcl-2 (96).
Rosmarinic acid induces apoptosis with corresponding suppression of Bcl-2 in human T-cell leukemia in vitro (82). S-allylcysteine inhibits Bcl-2 expression from hamster buccal pouch carcinogenesis, leading to apoptosis (83).
Many of the spice-derived nutraceuticals target cas-pases, which are essential for apoptotic cell death. Allicin activates caspases-3, -8, and -9 and increases PARP cleavage in cancer cells of both human and murine origin in vitro (86). Citral induces apoptosis in several hematopoietic cancer cell lines in vitro through activation of caspase-3 (87). Diallyl disulfide and diallyl trisulfide also induce apoptosis through the activation of caspase-3 (88, 89). Kaempferol induces apoptosis with significant DNA condensation with a marked increase in caspase-3 activity in human large-cell lung carcinoma cells in vitro (90). Myristicin triggers apoptosis through an accumulation of cytochrome C and caspase-3 activation in human neuroblastoma cells in vitro (92). [6]-Paradol causes proteolytic cleavage of caspase-3 to induce apoptosis in human squamous cell carcinoma in vitro (93). S-Allylmercapto-cysteine activates caspase-3 by binding directly to tubulin and disrupting the microtubule assembly in human colon cancer cells in vitro (157). Shogaol induces apoptotic cell death in human hepatoma p53 mutant cells through a caspase-dependent mechanism (95). Terpinen-4-ol induces caspase-dependent apoptosis in human melanoma cells in vitro (94).
A few of the compounds have been shown to both activate caspase activity and down-regulate expression of Bcl-2 or Bcl-xL. Ajoene activates caspase-3 and cleaves Bcl-2 in human myelocytic leukemia cells in vitro (153). Capsaicin down-regulates the expression of signal transducer and activator of transcription 3 (STAT3)-regulated gene products Bcl-2, Bcl-xL, and survivin, but activates caspases to induce apoptosis in multiple myeloma cells in vitro (70). Apoptosis in prostate PC-3 cells involves the mechanism of ceramide accumulation, and JNK and ERK activation in addition to ROS generation (155). Eugenol and related dimers induce cytotoxicity by way of interaction with the cell membranes of their phenoxyl and benzyl radicals (185). Limonene induces apoptosis by down-regulating expression of Bcl-2 and mutant p53 in human leukemia cells in vitro (80). In addition, limonene up-regulates Bax coupled with the release of cytochrome C from mitochondria leading to increased caspase-3 and caspase-9 (80, 91, 156). Gambogic acid (GA) is a derivative of the gamboge, a brownish residue from the Garcinia hanburry tree. It is a highly effective anticancer medication with low toxicity to normal tissue (186). According to studies seeking to explore the structure activity relation of GA, the 9, 10 carbon-carbon double bond of the alpha, beta-unsaturated ketone was responsible for biological activity (187). GA induces apoptosis by action with the transferrin receptor, thus increasing the action of TNF and inhibiting the NF-κB signaling pathway (188); besides GA is also implicated in increasing the cellular expression of apoptosis-regulated gene Bax, and decreasing the expression of apoptosis-regulated factor Bcl-2 (189). GA is an inhibitor of survivin and reverses docetaxel resistance in stomach cancer cells (190). GA also represses telomerase activity, by repressing human telomerase reverse transcriptase (hTERT) transcriptional activity via c-Myc and posttranscriptional modification of hTERT via Akt (191). Studies have been conducted both in vitro and in vivo showing that GA can pass the blood-brain barrier and reduce glioma mass by triggering the intrinsic mitochondrial pathway of apoptosis and antiangiogenesis (192).
Sulforaphane activates apoptosis in breast cancer cells in vitro through the activation of the Fas ligand, which in turn activates caspase-3 and caspase-8. In other breast cancer cell lines, sulforaphane is also able to decrease Bcl-2 activity while activating caspase-3 and caspase-9, leading to PARP cleavage and apoptosis (84). Ursolic acid induces apoptosis in human colon cancer cells in vitro through down-regulation of Bcl-2 and increased expression of caspase-9 (85).
A few nutraceuticals have demonstrated the capability to induce apoptosis in tumor cells, but their specific mechanism or molecular targets are unclear. Canthaxanthin inhibits cell growth and induces apoptosis in human colon adenocarcinoma and melanoma cells in vitro (193). Cineole induces apoptosis in human leukemia cells in vitro with morphological changes with apoptotic bodies appearing (194). Crocin causes tumor cell shrinkage, vacuole-like areas, pyknotic nuclei, and reduced cytoplasm consistent with apoptosis (195). Both sesamin and episesamin (an epimer of sesamin) induce apoptosis in human lymphoid leukemia cells in vitro, as demonstrated by such morphological changes as apoptotic bodies (196). Both sesamolin and turmerone induce apoptosis in human leukemia cells seen by DNA fragmentation into oligonucleosomal-sized fragments (197).
Effect of Nutraceuticals on Tumor Cell Proliferation
One of the hallmarks of cancer is aggressive proliferation of cells. In normal cells, proliferation is finely regulated by a balance of growth signals and anti-growth signals. However, cancer cells often encounter uncontrolled growth from both ends—not only do they acquire the capability to generate their own growth signals, they also become insensitive to anti-growth signals (198). There are many important factors that regulate the cell through its natural progression; however, certain factors can be up-regulated causing the cell to replicate uncontrollably. Some of these major factors are the cyclins, a family of proteins that regulate cell cycle progression, plus COX-2, and c-Myc. The most commonly affected cyclin is cyclin D1, which is an important cell cycle regulator that transitions the cell from G1 phase to S phase. In cancers, cyclin D1 overexpression has been linked to the development and progression of the disease. Deregulation of cyclin D1 degradation appears to be responsible for the increased levels of cyclin D1 in several cancers. COX-2 is a protein that is normally absent from cells, but appears rapidly in large amounts in pathological and often inflammatory situations. COX-2 was first described as being induced by a viral oncogene or tumor promoter, but it has also been shown to be inducible by a variety of growth factors and mitogens, making it relevant to the processes of cell growth and carcinogenesis (199). c-Myc is an oncoprotein that functions as a positive regulator of G1-specific cdk and, in particular, cyclin E/cdk2 complexes, which is necessary for cell cycle transit from G1 into S phase. c-Myc prevents cell cycle arrest in response to growth-inhibitory signals, differentiation stimuli, and mitogen withdrawal, and activation of c-Myc induces entry into the cell cycle in the absence of growth factors (200). Uncontrolled proliferation can often also be attributed to the down-regulation or inactivation of tumor suppressor protein p53. The latter acts as a checkpoint in the cell cycle, either preventing or initiating programmed cell death. It is believed that 50–55% of all cancers can be attributed, at least partially to inactivation of p53.
Several spice-derived nutraceuticals have been shown to halt cell proliferation by affecting cell cycle factors, both in vitro and in vivo. These nutraceuticals are AITC, apigenin, bergapten, β-carotene, β-sitosterol, capsaicin, carnosol, cinnamaldehyde, cinnamic acid, crocin, curcumin, diallyl disulfide, diallyl trisulfide, eugenol, geraniol, limonene, lutein, perillyl alcohol, phytic acid, quercetin, rosmarinic acid, S-allylmercaptocysteine, sesamin, sulforaphane, and ursolic acid (Table 5) (55, 59, 70, 73, 76, 82, 89, 97–107, 143, 156, 195, 201–205). Apigenin causes a decrease of c-Myc, cyclin D1, nuclear β-catenin and an increase of E-cadherin in the prostates of mice (97). In pancreatic cell lines, apigenin induced G2/M phase cell cycle arrest by reducing levels of cyclin A, cyclin B, phosphorylated forms of cdc2 and cdc25 which are proteins required for cell cycle transition (206). Eugenol suppresses COX-2 gene expression in mouse macrophages (143).
Table 5.
Inhibition of Proliferation by Spice-Derived Nutraceuticals
| Nutraceutical | Cell type/animal model | Responsesa |
|---|---|---|
| Allyl isothiocyanate | Human colon adenocarcinoma | ↓ α-tubulin-mitotic block (201) |
| Apigenin | Human prostate cancer cells | ↑ E-cadherin, ↓ nuclear translocation of β-catenin, ↓ c-Myc and cyclin D1 levels (97) |
| TRAMP mice | ↑ E-cadherin ↓ nuclear β-catenin, c-Myc, and cyclin D1 (97) | |
| Bergapten | Human hepatocellular carcinoma | ↓ cyclin B, ⊥ G2/M (103) |
| Human colon adenocarcinoma | ↓ cyclin A, ⊥ G2/M (73) | |
| β-Sitosterol | Human breast cancer | ⊥ G2/M (202) |
| Capsaicin | Multiple myeloma cells | ↓ cyclin D1, ⊥ G1, ↓ proliferation (70) |
| Carnosol | Human colonic adenocarcinoma | ⊥ G2/M phase, ↑ cyclin B1 (104) |
| Cinnamaldehyde | Human hepatoma cells | S-Phase arrest (76) |
| Cinnamic acid | Lung adenocarcinoma cells | ⊥ G1/G0 phase (203) |
| Crocin | Breast cancer cells | ↓ proliferation (195) |
| Curcumin | Human HNSCC, mouse melanoma cells | ↓ cyclin D1 (55, 99) ↓ NF-κB, ↓ COX-2, ↓ cyclin D1 (99) |
| Mouse skin tumors | ↓ tumor growth (99) | |
| Diallyl disulfide | Human gastric cancer cells | G2/M arrest, ↑ p38 MAPK (204) |
| Diallyl trisulfide | Human colon cancer cells | ↓ spindle formation, ↑ cyclin B1, mitotic arrest (89) |
| Eugenol | Human colon cancer cells | ↓ proliferation, ↓ mRNA expression of COX-2 (143) |
| LPS-stimulated mouse macrophage | ↓ COX-2 (143) | |
| Geraniol | Pancreatic adenocarcinoma cells | ⊥ G0/G1, ↑ p21 and p27, ↑ cyclin A, ↑ Cdk2 ↑ cyclin B1 (105) |
| Limonene | Human erythroleukemia cells | ⊥ G1 phase (156) |
| Lutein | Esophageal cancer cell line | ⊥ G0/G1, ↓ cyclin D1 (100) |
| Perillyl alcohol | Bcr/Abl-transformed leukemia cells | ↑ p21 and p27, ↑ cyclin A, ↑ cyclin B1, ↑ Cdk2 |
| Pancreatic adenocarcinoma cells | G0/G1 arrest (98, 105) | |
| Phytic acid | Human colon carcinoma cell lines | ↓ cell growth, ↓ cell proliferation, ↑ 53 and p21 gene, ⊥ G1 phase (106) |
| Quercetin | Human hepatoma cells | ⊥ G1, ↑ p21, ↑ p27, ↑ p53 (107) |
| Rosmarinic acid | Leukemia cells | ↓ cyclin D3, ↓ p21, ↑ p27 (82) |
| S-Allylmercapto-cysteine | Human colon cancer cell lines | ↓ growth, ⊥ G2-M, ↓ MT assembly (205) |
| Sesamin | Human breast cancer cells, lung cancer, renal cells, immortalized keratinocyte, melanoma and osteosarcoma | ⊥ G1 phase, ↓ cyclin D1 (101) |
| Sulforaphane | Human ovarian cancer cells | ↓ cyclin D1, cdk4, and cdk6 (102) |
| Ursolic acid | Non-specific cell types | ↓ cyclin D1 (59) |
↑, up-regulate; ↓, down-regulate; ⊥, block/arrest; COX-2, cyclooxygenase; HNSCC, head and neck squamous cell carcinoma; MAPK, mitogen-activated protein kinase; MT, microtubule; NF-κB, nuclear factor-κB; TRAMP, transgenic mouse prostate adenocarcinoma.
The majority of the nutraceuticals listed above affect cyclins and cyclin dependent kinases (cdk) causing cell cycle arrest. The most commonly affected cyclin is cyclin D1, which is an important cell cycle regulator that transitions the cell from G1 phase to S phase. Capsaicin down-regulates the expression of cyclin D1 causing multiple myeloma cells to arrest in G1 phase, thereby inhibiting proliferation (76). Lutein significantly down-regulates the expression of cyclin D1 and halts the cell cycle at G0/G1 phase in esophageal cancer in vitro (100). Sesamin was shown to down-regulate cyclin D1 expression in a wide variety of tumors including lung cancer, transformed renal cells, immortalized keratinocyte, melanoma, and osteosar-coma cells in vitro, as well induce growth arrest at G1 phase in human breast cancer cells in vitro (101). Sulforaphane down-regulates the expression of cyclin D1 and cell cycle transition regulators cdk4 and cdk6 in both mouse and human ovarian cancer cell lines in vitro (102). Ursolic acid down-regulates cyclin D1 expression in non-specific cell types in vitro (59).
Eugenol and curcumin target COX-2. Eugenol suppresses COX-2 gene expression in mouse macrophages in vivo. In addition, it inhibits the proliferation of human colon cancer cells in vitro by inhibiting COX-2 mRNA expression (143). Eugenol causes arrest of proliferating melanoma cells in the S phase of the cell cycle. Eugenol inhibits the E2F family of transcription factors; down-regulation of E2F1 is responsible for eugenol mediated growth inhibition of human melanoma cells (207). AITC, diallyl trisulfide, and S-allylmercaptocysteine (SA) all cause mitotic block by targeting tubulin. AITC disrupts α-tubulin in human adenocarcinoma cells in vitro resulting in mitotic block (201). Diallyl trisulfide directly modifies specific cysteine residues on β-tubulin molecules causing disruption of spindle formation and mitotic arrest in human colon cancer cells in vitro (89). SA binds directly to tubulin disrupting the MT assembly and thereby arresting cells in mitosis in human colon cancer cells in vitro (205). Apigenin has been shown to decrease both c-Myc and cyclin D1 in human colon cancer cells in vitro (97). It also inhibits geminin and Cdc6 at the mRNA and protein levels. Geminin and Cdc6 are proteins involved in replication of pancreatic cancer cell lines. It also decreased cell proliferation in Dunning rat prostate MAT Ly Lu model. Apigenin suppresses differentiation in normal human keratinocytes by decreasing AP-1 expression. It also inhibits increase in promoter activity associated with overexpression of protein kinase C-delta (PKCδ), constitutively active Ras, or MEKK1, which leads to a decrease in keratinocyte differentiation (208). Perillyl alcohol also targets c-Myc causing c-Myc-dependent apoptosis through a G0/G1 cell cycle arrest in transformed leukemia cells in vitro (98). Phytic acid inhibits cell growth and decreases proliferation through the up-regulation of p53 in human colon carcinoma cell lines, leading to arrest in G1 phase (106). Quercetin also induces cell cycle arrest at G1 by elevating p53 in human hepatoma cell line in vitro (107).
The remaining nutraceuticals cause cell cycle arrest at various stages of the cell cycle, many through effects exhibited on cyclins. Bergapten (5-methoxypsoralen) inhibits cyclin B1 in human hepatocellular carcinoma cells in vitro preventing cells from entering M phase (103). β-Carotene inhibits the growth of several human colon adenocarcinoma cell lines in vitro by reducing the expression of cyclin A causing cell cycle arrest at G2/M phase. β-Sitosterol induces cell cycle arrest at G2/M phase in human breast cancer cells in vitro (202). Carnosol causes accumulation of human colonic adenocarcinoma cells at G2/M phase along with an increase in cyclin B1 in vitro (104). Cinnamic acid causes cell cycle arrest at G1/G0 phase, therefore inhibiting the proliferation of lung adenocarcinoma cells in vitro (203). Crocin shows inhibition of growth in breast cancer cells in vitro (195). Diallyl disulfide induces G2/M phase arrest in human gastric cancer cell lines in vitro (204). The antiproliferative effect of gambogic acid results from its inhibition of the catalytic activity of topoisomerase (Topo) IIα; it binds to the ATPase domain of the enzyme Topo IIα (209). In human gastric carcinoma cells, GA causes a cell cycle arrest in the G2/M phase by decreasing the CDK7 mediated phosphorylation of CDC2/p34 (210). Geraniol induces a G0/G1 cell cycle arrest that coincides with a reduction in the levels of cyclin A, cyclin B1, and cyclin-dependent kinase 2 proteins in pancreatic adenocarcinoma cells in vitro. It also results in an increase in the expression of cyclin inhibitor proteins p21 and p27 (105). Limonene halts the cell cycle at G1 phase in human erythroleukemia cells in vitro (156). Rosmarinic acid inhibits proliferation of leukemia cells in vitro by suppressing the expression of cyclin D3 and p21 while up-regulating p27 (82).
Effect of Nutraceuticals in Tumor Cell Invasion
Invasion, also known as metastasis, involves the migration of cancer cells from their original site of origin to other parts of the body either via the bloodstream or lymphatic system. Some of the major factors influencing invasion, whether or not a tumor will metastasize, include the presence of MMPs and ICAM-1. MMPs are members of a family of enzymes that are instrumental in freeing cells from surrounding tissue, enabling them to move and spread. MMPs (specifically MMP-2 and MMP-9) are endopeptidases that degrade a wide range of basement membrane components, a process important for tumor invasion (211). It has been shown that if MMP-9 is blocked, colorectal cancer cells are unable to metastasize. This illustrates the importance of targeting MMP-9 to block its production and release, not only in colon cancer cell lines, but also in a variety of tumor cell lines. ICAM-1 is a type of intercellular adhesion molecule that is constantly present in low concentrations on the surface of leukocytes and endothelial cells. The concentration of ICAM-1 can be greatly increased by cytokine stimulation, such as from TNF or IL-1β. ICAM-1 protein expression on the cell surface has been positively correlated with metastatic potential of five human breast cancer cell lines. ICAM-1 mRNA levels have been shown to be elevated in breast tumor compared with adjacent normal tissue (212).
Several spice-derived nutraceuticals have been shown through a variety of mechanisms to inhibit MMP-2, MMP-9, and ICAM-1, thereby limiting the metastatic potential of tumors. These spice-derived nutraceuticals are allicin, AITC, apigenin, caffeic acid, carnosol, crocetin, curcumin, diallyl disulfide, [6]-gingerol, kaempferol, myricetin, phytic acid, piperine, quercetin, sulforaphane, ursolic acid, and vanillin (see Table 6) (64, 68, 108–113, 115–120, 213, 214). Allicin has been shown to inhibit the TNF-α induced expression of ICAM-1 in human endothelial cells (120) and kaempferol and apigenin have been shown to follow a similar mechanism in respiratory epithelial cells (119). Crocetin suppresses both ICAM-1 and MMPs in bovine endothelial cells (117).
Table 6.
Inhibition of Invasion by Spice-Derived Nutraceuticals
| Nutraceuticals | Responsea |
|---|---|
| Allicin | Inhibited TNF-α induced ICAM-1 expression in HUVECs (120). |
| Allyl isothiocyanate | Down-regulated mRNA level and activity of MMP-2/-9 in human hepatoma SK-Hep1 cells (108). |
| Apigenin | Inhibited TNF-α induced ICAM-1 expression (119). |
| Caffeic acid | Inhibited MMP-9 activity in human hepatocellular carcinoma cell line (109). |
| Carnosol | Suppressed expression and activation of MMP-9 in mouse melanoma B16/F10 cells (110). |
| Crocetin | Suppressed advanced glycation end product-induced ICAM-1 expression in bovine endothelial cells (117). |
| Curcumin | Down-regulated MMP-2 expression and activity and expression of integrin receptors, FAK, and MT1-MMP in HEp2 cells (115). |
| Diallyl disulfide | Inhibited activity of MMP-2 and MMP-9 in HUVECs (64). |
| [6]-Gingerol | Suppressed expression and enzymatic activity of MMP-2/MMP-9 in human breast cancer cells (213). |
| Kaempferol | Inhibited TNF-α induced ICAM-1 expression (119). |
| Myricetin | Inhibited expression and activity of MMP-2 in colorectal cancer cells (116). |
| Phytic acid | Modulated integrin dimerization, cell surface expression and integrin-associated signaling pathway and secretion of MMP-9 in human breast cancer MDA-MB-231 cells (214). |
| Piperine | Inhibited the matrix metalloproteinase production in B16F-10 melanoma cells (68). |
| Quercetin | Decreased the expressions of MMP-2 and MMP-9 in PC-3 cells (111). |
| Sulforaphane | Inhibited the activation of MMPs (118). |
| Ursolic acid | Down-regulated MMP-9 in HT1080 human fibrosarcoma cell line (112). |
| Vanillin | Inhibited invasion and migration of cancer cells and inhibited enzymatic activity of MMP-9 secreted by the cancer cells (113). |
FAK, focal adhesion kinase; HBMECs, human brain microvascular endothelial cells; ICAM-1, intracellular adhesion molecule-1; MMP, matrix metalloproteinases; MT1-MMP, membrane type 1-matrix metalloproteinases; PMA, phorbol 12-myristate 13-acetate; TNF-α, tumor necrosis factor-α; VCAM, vascular cell adhesion molecule.
The remaining nutraceuticals inhibit the expression MMP-2, MMP-9, or both. AITC suppresses both MMP-2 and MMP-9 at both protein and mRNA levels in human hepatoma cells in vitro (108). It also results in elevated tissue levels of tissue inhibitor of metalloproteinases (TIMP). In the Dunning rat prostate MAT Ly Lu model, treatment with apigenin was found to increase the intensity of connexin 43-mediated gap junctional coupling and decrease cell motility. In breast cancer cells, the HER2-HER3-PI3K-Akt pathway inhibition by apigenin also plays an important role in inhibition of the multiplication, survival, adhesion, and motility of cancer (165). Apigenin also has a role to play in inhibiting metastasis of lung melanoma cells by inhibiting vascular cell adhesion molecule-1 (VCAM-1) expression in a dose-dependent manner (215). In vitro studies have shown that apigenin inhibited invasiveness of tumor cells; the possibility is that it would prevent tumor penetration of healthy tissues in vivo (216). Caffeic acid shows a strong inhibitory effect on MMP-9 activity in non-specific cell types in vitro (109). Carnosol results in a reduction of MMP-9 levels in mouse melanoma cells in vitro through the down-regulation of NF-κB and AP-1 (110). Diallyl disulfide was shown to have an inhibitory effect on the activation of MMP-2 and MMP-9 in human umbilical vein endothelial cells in vitro (64). [6]-Gingerol inhibits cell adhesion, invasion, motility and activities of MMP-2 and MMP-9 in human breast cancer cell lines in vitro (213). Myricetin inhibits MMP-2 protein expression and enzyme activity in colorectal carcinoma cells in vitro (116). Phytic acid inhibits cancer cell adhesion, migration, and invasion in human breast cancer cells in vitro. This inhibited invasion is mediated through the modulation of integrin dimerization, cell surface expression, and the integrin-associated signaling pathway (214). Piperine inhibits MMP production in melanoma cells in vitro preventing the collagen matrix invasion in a dose-dependent manner (68). Quercetin decreases the expressions of MMP-2 and MMP-9 in a dose-dependent manner in prostate cancer cells in vitro (111). Ursolic acid induces down-regulation of MMP-9 gene in human fibrosarcoma cells in vitro (112).
Both sulforaphane and vanillin have shown in vivo effects in mice. Sulforaphane inhibits the activation of MMPs, thereby inhibiting lung metastasis induced by melanoma cells in mice. This led to a 95.5% inhibition of lung tumor nodule formation and 94.06% increase in the life span of metastatic tumor-bearing animals (118). Vanillin reduces numbers of lung metastasized colonies in mice with mammary adenocarcinoma when administered orally (113). In addition, vanillin inhibits invasion and migration of cancer cells and inhibits enzymatic activity of MMP-9 secreted by the cancer cells in vitro (113).
Effect of Nutraceuticals in Tumor Cell Angiogenesis
Angiogenesis is a physiological process where new blood vessels grow from pre-existing ones. Angiogenesis is a normal, natural process that occurs during growth and wound healing; however, angiogenesis is also a marker that a tumor had changed from a dormant to malignant state. The growth of human tumors and development of metastases depends on the de novo formation of blood vessels (217). Angiogenesis enhances tumor growth by delivering oxygen and nutrients to its interior via new blood flow. Some pro-angiogenic factors include IL-8, TNF, fibroblast growth factor-2 (FGF-2), and platelet-derived growth factor (PDGF). However, one of the most important factors in angiogenesis is VEGF, which has been shown to be a potent stimulator of angiogenesis in vitro (217). By limiting VEGF in tumors, the blood supply to the tumor is reduced by potentially causing blood vessels to die as well as preventing the growth of new ones. Inhibition of the VEGF tyrosine kinase signaling pathway blocks new blood vessel formation in growing tumors, leading to stasis or regression of tumor growth. Due to this, finding agents that can down-regulate or inhibit the expression of VEGF or its signaling pathway in tumor cells may be very helpful in preventing increased tumor growth and metastasis.
Currently, there is only one FDA-approved pharmaceutical on the market, Avastin (bevacizumab), a mono-clonal antibody directed against VEGF for patients with metastatic colorectal cancer. However, some major side effects of Avastin include gastrointestinal perforation, fatal pulmonary hemorrhage, and complications in wound healing (www.avastin.com) and it costs around $4,400 a month for treatment.
Many spice-derived nutraceuticals have been shown to down-regulate the expression of VEGF in vitro and others have been shown to prevent new blood vessel formation in vivo. These nutraceuticals are AITC, alliin, caffeic acid, capsaicin, curcumin, diallyl disulfide, diallyl sulfide, gingerol, lutein, perillyl alcohol, phytic acid, quercetin, rosmarinic acid, and sulforaphane (see Table 7) (58, 64, 96, 110, 121–123, 126, 127, 147, 218–221).
Table 7.
Inhibition of Angiogenesis by Spice-Derived Nutraceuticalsa
| Nutraceuticals | Responses |
|---|---|
| Allyl isothiocyanate | Acted as angiogenesis inhibitors through the down-regulation of VEGF and proinflammatory cytokines such as IL-1β, IL-6, GM-CSF, and TNF-α and up-regulation of IL-2 and TIMP (218). |
| Apigenin | Inhibited expression of HIF-1 and VEGF in different cancer cells under both normoxic and hypoxic conditions (219). |
| Alliin | Inhibited both FGF2 and VEGF secretion from human fibrosarcoma cells. Inhibited FGF2-induced EC tube formation and angiogenesis in CAM model (121). |
| Caffeic acid | Suppressed STAT3 phosphorylation, HIF-1α expression, vascularization and STAT3-inducible VEGF gene expression in Caki-I bearing mice (122). |
| Capsaicin | Inhibited VEGF-induced chemotactic motility, and capillary-like tube formation of primary cultured human endothelial cells (123). Reduced both VEGF-induced vessel sprouting in rat aortic ring assay and VEGF-induced vessel formation in the mouse Matrigel plug assay (123). |
| Curcumin | Reduced the overexpression of COX-2 and serum VEGF in hepatocellular carcinoma cell-implanted nude mice (220). |
| Diallyl sulfide | Reduced serum level of VEGF in B16F-10 melanoma bearing C57BL/6 mice (64). |
| Gingerol | Blocked VEGF-induced capillary-like tube formation in endothelial cells and inhibited sprouting in endothelial cells in the rat aorta and formation of new blood vessel in the mouse cornea (126). |
| Gambogic acid | Inhibited VEGF-induced proliferation, migration, invasion, tube formation, and microvessel growth of HUVECs (221). Inhibited tumor angiogenesis in xenograft prostate tumor model (221). |
| Lutein | Lowered angiogenic activity in mouse mammary tumor model (96). |
| Perillyl alcohol | Decreased the release of VEGF from cancer cells but stimulated the expression of Ang2 by endothelial cells (127). |
| Phytic acid | Decreased tumor microvessel density and inhibited tumor-secreted VEGF levels in prostate cancer animal model (147). |
| Rosmarinic acid | Inhibited angiogenesis, VEGF expression and IL-8 release in HUVECs (110). |
| Sulforaphane | Inhibited NF-κB-regulated VEGF expression in human prostate cancer PC-3 C4 cells (58). |
Ang2, angiopoietin 2; CAM, chick chorioallantoic membrane; COX-2, cyclooxygenase-2; EC, endothelial cell; FGF2, fibroblast growth factor 2; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIF-1, hypoxia inducible factor-1; HUVECs, human umbilical vein endothelial cells; IL-1β, interleukin-1β; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3; TIMP, tissue inhibitor of metalloproteinase; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
AITC is highly potent in the down-regulation of VEGF and IL-1β, IL-6, and GM-CSF, which are proinflammatory cytokines; it also elevates levels of IL-2 and TIMP, which are potent antiangiogenic factors (218). Also AITC down-regulates nitric oxide and TNF-α production, and inhibits tumor specific angiogenesis (218). Apigenin prevents the activation of downstream target gene VEGF and angiogenesis in hypoxic solid tumors (189). It also inhibits expression of hypoxia inducible factor (HIF) (219). In ovarian tumor cells, apigenin inhibits VEGF and HIF-1 via the PI3K/AKT/p70S6K1 and HDM2/p53 pathways (222). It also inhibits tube formation by human umbilical vein endothelial cells. Apigenin has an inhibitory effect on HGF (hepatocyte growth factor)-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and integrin β4 functions. Alliin had been shown to significantly reduce VEGF secretion in human fibrosarcoma cells (121). Diallyl disulfide reduces the expression and secretion of VEGF in human promyelocytic leukemia cells in vitro (125). [6]-Gingerol blocks capillary-like tube formation by endothelial cells in response to VEGF secretion in human endothelial cells in vitro (126). Phytic acid significantly reduces VEGF in both pancreatic cancer cell lines and melanoma cell lines in vitro (129, 130). Gambogic acid inhibits VEGFR2 signaling, thus inhibiting angiogenesis and prostate tumor growth (221). Rosmarinic acid reduces VEGF expression in human umbilical vein endothelial cells in vitro (128). Sulforaphane inhibits gene expression of NF-κB-regulated VEGF in human prostate cancer cells in vitro (58).
A few of the spice-derived nutraceuticals have been shown to reduce VEGF in vivo. Alliin has been shown to reduce the secretion of VEGF in the chick chorioallantoic membrane (CAM) assay (121). Caffeic acid inhibits the expression of VEGF in mice renal carcinoma (122). Diallyl sulfide significantly reduces serum levels of VEGF in melanoma cells in mice (64). In addition, a few nutraceuticals have been shown to prevent angiogenesis by diminishing the growth of new blood vessel formation in vivo. These nutraceuticals are capsaicin, curcumin, lutein, perillyl alcohol, and quercetin. Capsaicin has been shown to inhibit VEGF-induced vessel sprouting in the CAM assay and rat aortic ring assay in vivo (123). Curcumin treatment results in the decrease in neocapillary density in nude mice with hepatocellular carcinoma (220). Lutein-fed mice were found to have lower angiogenic activity in mammary tumors in comparison to mice that were not supplemented with lutein (96). Perillyl alcohol prevents new blood vessel growth in the in vivo CAM assay (127). Quercetin displays an antiangiogenic effect in the CAM assay in vivo (223).
Conclusion
This review has illustrated the effects of nutraceuticals in spices on the initiation and progression of cancer. By analogy, including spice or spice-derived nutraceutical in one’s diet should help prevent cancer. In addition, these nutraceuticals show great potential in being used as agents to aid in cancer treatment, either alone or in combination with existing standard of care. However, it is important to note that the majority of these nutraceuticals have only been tested under pre-clinical conditions, either in vitro or in animal models. Clinical trials or further studies with these nutraceuticals must be done in order to elucidate and understand their efficacy in the treatment and prevention of human cancer. This does not mean that these nutraceuticals shouldn’t be used as an option in cancer treatment, as the beauty of using plant-derived nutraceuticals is that there is generally little to no cytotoxicity that occurs resulting in minor to no side effects in patients. What are the doses required to exert these effects and whether they could be obtained by consuming spices or whether they could only be obtained by using supplements, are the questions unclear at present. Whether long-term consumption of the spices by people from certain countries is directly linked to lower incidence of cancer and other chronic diseases, is supportive but not proven.
In addition, the spices mentioned in this article and their respective nutraceuticals that play a role in halting the various phases of tumorigenesis, support the idea that a diet high in plant-based foods may help prevent and treat disease. Many of the spices mentioned in this article have been regarded in a variety of cultures as having health benefits for centuries, such as garlic and ginger. However, some of the spices have begun to receive more and more attention due to their nutraceutical composition and their effectiveness in disease prevention and treatment. Although over 100 pilot clinical trials have been done with spices and their components in human subjects, more evidence is still needed to demonstrate their potential in prevention and treatment. The possibility for spices to exhibit multiple effects and for ability to counteract treatment regimens exists just like any other drug. Unlike conventional drugs, however, spices have been consumed by healthy people for centuries.
Acknowledgments
This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
We thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
References
- 1.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brower V. Nutraceuticals: poised for a healthy slice of the healthcare market? Nat Biotechnol. 1998;16:728–731. doi: 10.1038/nbt0898-728. [DOI] [PubMed] [Google Scholar]
- 4.Zeisel SH. Regulation of “nutraceuticals”. Science (New York, NY) 1999;285:1853–1855. doi: 10.1126/science.285.5435.1853. [DOI] [PubMed] [Google Scholar]
- 5.Miyajima Y, Kikuzaki H, Hisamoto M, Nikatani N. Antioxidative polyphenols from berries of Pimenta dioica. BioFactors (Oxford, England) 2004;21:301–303. doi: 10.1002/biof.5520220159. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues VM, Rosa PT, Marques MO, Petenate AJ, Meireles MA. Supercritical extraction of essential oil from aniseed (Pimpinella anisum L) using CO2: solubility, kinetics, and composition data. J Agric Food Chem. 2003;51:1518–1523. doi: 10.1021/jf0257493. [DOI] [PubMed] [Google Scholar]
- 7.Eigner D, Scholz D. Ferula asafoetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 8.Grayer RJ, Kite GC, Goldstone FJ, Bryan SE, Paton A, Putievsky E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry. 1996;43:1033–1039. doi: 10.1016/s0031-9422(96)00429-3. [DOI] [PubMed] [Google Scholar]
- 9.Conforti F, Statti G, Uzunov D, Menichini F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) coutinho seeds. Biol Pharm Bull. 2006;29:2056–2064. doi: 10.1248/bpb.29.2056. [DOI] [PubMed] [Google Scholar]
- 10.Musenga A, Mandrioli R, Ferranti A, D’Orazio G, Fanali S, Raggi MA. Analysis of aromatic and terpenic constituents of pepper extracts by capillary electrochromatography. J Sep Sci. 2007;30:612–619. doi: 10.1002/jssc.200600456. [DOI] [PubMed] [Google Scholar]
- 11.Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem. 2005;53:57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 12.Richter J, Schellenberg I. Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography. Anal Bioanal Chem. 2007;387:2207–2217. doi: 10.1007/s00216-006-1045-6. [DOI] [PubMed] [Google Scholar]
- 13.Marongiu B, Piras A, Porcedda S. Comparative analysis of the oil and supercritical CO2 extract of Elettaria cardamomum (L.) Maton. J Agric Food Chem. 2004;52:6278–6282. doi: 10.1021/jf034819i. [DOI] [PubMed] [Google Scholar]
- 14.Saleh MM, Zwaving JH, Malingre TM, Bos R. The essential oil of Apium graveolens var. secalinum and its cercaricidal activity. Pharm Weekbl Sci. 1985;7:277–279. doi: 10.1007/BF01959202. [DOI] [PubMed] [Google Scholar]
- 15.Fejes S, Blazovics A, Lugasi A, Lemberkovics E, Petri G, Kery A. In vitro antioxidant activity of Anthriscus cerefolium L. (Hoffm.) extracts. J Ethnopharmacol. 2000;69:259–265. doi: 10.1016/s0378-8741(99)00171-3. [DOI] [PubMed] [Google Scholar]
- 16.Tazaburo K, Masao K. Studies on the constituents of Anthriscus sylvestris Hoffm. on the components of the flower and leaves. Yakugaku Zasshi. 1979;99:602–606. doi: 10.1248/yakushi1947.99.6_602. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto S, Miyazawa M, Kameoka H. Volatile flavor components of chive (Allium schoenoprasum L.) J Food Sci. 1983;48:1858–1859. [Google Scholar]
- 18.Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem. 2003;51:4344–4348. doi: 10.1021/jf034169i. [DOI] [PubMed] [Google Scholar]
- 19.Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 20.Smallfield BM, van Klink JW, Perry NB, Dodds KG. Coriander spice oil: effects of fruit crushing and distillation time on yield and composition. J Agric Food Chem. 2001;49:118–123. doi: 10.1021/jf001024s. [DOI] [PubMed] [Google Scholar]
- 21.Beis SJ, Goshman LM, Newkirk GL. Risk factors for metformin-associated lactic acidosis. WMJ. 1999;98:56–57. [PubMed] [Google Scholar]
- 22.Khalaf AF. Toxicological efficacy of some indigenous dill compounds against the flesh fly, Parasarcophaga dux Thomson. J Egypt Soc Parasitol. 2004;34:227–237. [PubMed] [Google Scholar]
- 23.Barazani O, Fait A, Cohen Y, Diminshtein S, Ravid U, Putievsky E, Lewinsohn E, Friedman J. Chemical variation among indigenous populations of Foeniculum vulgare var. vulgare in Israel. Planta Med. 1999;65:486–489. doi: 10.1055/s-2006-960824. [DOI] [PubMed] [Google Scholar]
- 24.Zeller A, Rychlik M. Character impact odorants of fennel fruits and fennel tea. J Agric Food Chem. 2006;54:3686–3692. doi: 10.1021/jf052944j. [DOI] [PubMed] [Google Scholar]
- 25.Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum) Ultrason Sonochem. 2006;13:54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;65:1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Park IK, Choi KS, Kim DH, Choi IH, Kim LS, Bak WC, Choi JW, Shin SC. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae) Pest Manag Sci. 2006;62:723–728. doi: 10.1002/ps.1228. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Maroto MC, Perez-Coello MS, Gonzalez Vinas MA, Cabezudo MD. Influence of drying on the flavor quality of spearmint (Mentha spicata L.) J Agric Food Chem. 2003;51:1265–1269. doi: 10.1021/jf020805l. [DOI] [PubMed] [Google Scholar]
- 30.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Q, Zhang G, Sun X, Liu X. Study on chemical constituents of the essential oil from Myristica fragrans Houtt. by supercritical fluid extraction and steam distillation. Zhong Yao Cai. 2004;27:823–826. [PubMed] [Google Scholar]
- 32.Yuan ZM, Wang J, Lv J, Jia TZ. Comparing analysis of components in volatile oils of nutmeg and prepared nutmeg by GC-MS. Zhongguo Zhong Yao Za Zhi. 2006;31:737–739. [PubMed] [Google Scholar]
- 33.Ly TN, Hazama C, Shimoyamada M, Ando H, Kato K, Yamauchi R. Antioxidative compounds from the outer scales of onion. J Agric Food Chem. 2005;53:8183–8189. doi: 10.1021/jf051264d. [DOI] [PubMed] [Google Scholar]
- 34.Wang HX, Ng TB. Isolation of allicepin, a novel antifungal peptide from onion (Allium cepa) bulbs. J Pept Sci. 2004;10:173–177. doi: 10.1002/psc.509. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues MR, Krause LC, Caramao EB, dos Santos JG, Dariva C, Vladimir de Oliveira J. Chemical composition and extraction yield of the extract of Origanum vulgare obtained from sub- and supercritical CO2. J Agric Food Chem. 2004;52:3042–3047. doi: 10.1021/jf030575q. [DOI] [PubMed] [Google Scholar]
- 36.Gnayfeed MH, Daood HG, Illes V, Biacs PA. Supercritical CO(2) and subcritical propane extraction of pungent paprika and quantification of carotenoids, tocopherols, and capsaicinoids. J Agric Food Chem. 2001;49:2761–2766. doi: 10.1021/jf001292q. [DOI] [PubMed] [Google Scholar]
- 37.Lopez MG, Sanchez-Mendoza IR, Ochoa-Alejo N. Comparative study of volatile components and fatty acids of plants and in vitro cultures of parsley (Petroselinum crispum (Mill) nym ex hill) J Agric Food Chem. 1999;47:3292–3296. doi: 10.1021/jf981159m. [DOI] [PubMed] [Google Scholar]
- 38.Krist S, Stuebiger G, Unterweger H, Bandion F, Buchbauer G. Analysis of volatile compounds and triglycerides of seed oils extracted from different poppy varieties (Papaver somniferum L.) J Agric Food Chem. 2005;53:8310–8316. doi: 10.1021/jf0580869. [DOI] [PubMed] [Google Scholar]
- 39.Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J Agric Food Chem. 2000;48:1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- 40.Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 41.Carmona M, Zalacain A, Pardo JE, Lopez E, Alvarruiz A, Alonso GL. Influence of different drying and aging conditions on saffron constituents. J Agric Food Chem. 2005;53:3974–3979. doi: 10.1021/jf0404748. [DOI] [PubMed] [Google Scholar]
- 42.Raal A, Orav A, Arak E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat Prod Res. 2007;21:406–411. doi: 10.1080/14786410500528478. [DOI] [PubMed] [Google Scholar]
- 43.Lampronti I, Saab AM, Gambari R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int J Oncol. 2006;29:989–995. [PubMed] [Google Scholar]
- 44.Crews C, Hough P, Brereton P, Godward J, Lees M, Guiet S, Winkelmann W. Quantitation of the main constituents of some authentic sesame seed oils of different origin. J Agric Food Chem. 2006;54:6266–6270. doi: 10.1021/jf0603578. [DOI] [PubMed] [Google Scholar]
- 45.Pino JA, Marbot R, Vazquez C. Volatile components of tamarind (Tamarindus indica L.) grown in Cuba. J Essent Oil Res. 2004;16:318–320. [Google Scholar]
- 46.Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agric Food Chem. 2005;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- 47.Hudaib M, Speroni E, Di Pietra AM, Cavrini V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J Pharm Biomed Anal. 2002;29:691–700. doi: 10.1016/s0731-7085(02)00119-x. [DOI] [PubMed] [Google Scholar]
- 48.Braga ME, Leal PF, Carvalho JE, Meireles MA. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J Agric Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- 49.Sinha AK, Verma SC, Sharma UK. Development and validation of an RP-HPLC method for quantitative determination of vanillin and related phenolic compounds in Vanilla planifolia. J Sep Sci. 2007;30:15–20. doi: 10.1002/jssc.200600193. [DOI] [PubMed] [Google Scholar]
- 50.Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr. 2002;976:265–275. doi: 10.1016/s0021-9673(02)00376-x. [DOI] [PubMed] [Google Scholar]
- 51.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 52.Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- 53.Medeiros R, Passos GF, Vitor CE, Koepp J, Mazzuco TL, Pianowski LF, Campos MM, Calixto JB. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br J Pharmacol. 2007;151:618–627. doi: 10.1038/sj.bjp.0707270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DH, Kim CH, Kim MS, Kim JY, Jung KJ, Chung JH, An WG, Lee JW, Yu BP, Chung HY. Suppression of age-related inflammatory NF-kappaB activation by cinnamaldehyde. Biogerontology. 2007;8:545–554. doi: 10.1007/s10522-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 55.Marin YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S, Ryu JH. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17:274–283. doi: 10.1097/CMR.0b013e3282ed3d0e. [DOI] [PubMed] [Google Scholar]
- 56.Berchtold CM, Chen KS, Miyamoto S, Gould MN. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-kappaB pathway. Cancer Res. 2005;65:8558–8566. doi: 10.1158/0008-5472.CAN-04-4072. [DOI] [PubMed] [Google Scholar]
- 57.Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, Park JW, Park EK, Shin HI, Kim SH. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56:210–215. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 58.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 59.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- 60.Taylor P, Noriega R, Farah C, Abad MJ, Arsenak M, Apitz R. Ajoene inhibits both primary tumor growth and metastasis of B16/BL6 melanoma cells in C57BL/6 mice. Cancer Lett. 2006;239:298–304. doi: 10.1016/j.canlet.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Lang A, Lahav M, Sakhnini E, Barshack I, Fidder HH, Avidan B, Bardan E, Hershkoviz R, Bar-Meir S, Chowers Y. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin Nutr. 2004;23:1199–1208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep. 2005;57:390–394. [PubMed] [Google Scholar]
- 64.Thejass P, Kuttan G. Antiangiogenic activity of Diallyl Sulfide (DAS) Int Immunopharmacol. 2007;7:295–305. doi: 10.1016/j.intimp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Lee YY, Hung SL, Pai SF, Lee YH, Yang SF. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Tripathi S, Maier KG, Bruch D, Kittur DS. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res. 2007;138:209–213. doi: 10.1016/j.jss.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 67.Surh YJ, Park KK, Chun KS, Lee LJ, Lee E, Lee SS. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger. J Environ Pathol Toxicol Oncol. 1999;18:131–139. [PubMed] [Google Scholar]
- 68.Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol. 2004;4:1795–1803. doi: 10.1016/j.intimp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Weglarz L, Wawszczyk J, Orchel A, Jaworska-Kik M, Dzierzewicz Z. Phytic acid modulates in vitro IL-8 and IL-6 release from colonic epithelial cells stimulated with LPS and IL-1beta. Dig Dis Sci. 2007;52:93–102. doi: 10.1007/s10620-006-9320-0. [DOI] [PubMed] [Google Scholar]
- 70.Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, Aggarwal BB. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Min JM, Cui JR, Zhang LH, Wang K, Valette A, Davrinche C, Wright M, Leung-Tack J. Z-ajoene induces apoptosis of HL-60 cells: involvement of Bcl-2 cleavage. Nutr Cancer. 2002;42:241–247. doi: 10.1207/S15327914NC422_14. [DOI] [PubMed] [Google Scholar]
- 72.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 73.Palozza P, Serini S, Maggiano N, Angelini M, Boninsegna A, Di Nicuolo F, Ranelletti FO, Calviello G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis. 2002;23:11–18. doi: 10.1093/carcin/23.1.11. [DOI] [PubMed] [Google Scholar]
- 74.Park C, Moon DO, Rhu CH, Choi BT, Lee WH, Kim GY, Choi YH. Beta-sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol Pharm Bull. 2007;30:1317–1323. doi: 10.1248/bpb.30.1317. [DOI] [PubMed] [Google Scholar]
- 75.Dorrie J, Sapala K, Zunino SJ. Carnosol-induced apoptosis and downregulation of Bcl-2 in B-lineage leukemia cells. Cancer Lett. 2001;170:33–39. doi: 10.1016/s0304-3835(01)00549-3. [DOI] [PubMed] [Google Scholar]
- 76.Wu SJ, Ng LT, Lin CC. Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 2005;77:938–951. doi: 10.1016/j.lfs.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Liu B, Bai QX, Chen XQ, Gao GX, Gu HT. Effect of curcumin on expression of survivin, Bcl-2 and Bax in human multiple myeloma cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:762–766. [PubMed] [Google Scholar]
- 78.Kalra N, Arora A, Shukla Y. Involvement of multiple signaling pathways in diallyl sulfide mediated apoptosis in mouse skin tumors. Asian Pac J Cancer Prev. 2006;7:556–562. [PubMed] [Google Scholar]
- 79.Wang CC, Chen LG, Lee LT, Yang LL. Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo. 2003;17:641–645. [PubMed] [Google Scholar]
- 80.Guo XM, Lu Q, Liu ZJ, Wang LF, Feng BA. Effects of D-limonene on leukemia cells HL-60 and K562 in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:692–695. [PubMed] [Google Scholar]
- 81.Pei YX, Heng ZC, Duan GC, Zhang ZZ, Wang MC, Hu CL, Gao DL. Effects and mechanism of lutein on apoptosis of esophageal carcinoma EC9706 cells. Zhongguo Zhong Yao Za Zhi. 2007;32:332–334. 354. [PubMed] [Google Scholar]
- 82.Kolettas E, Thomas C, Leneti E, Skoufos I, Mbatsi C, Sisoula C, Manos G, Evangelou A. Rosmarinic acid failed to suppress hydrogen peroxide-mediated apoptosis but induced apoptosis of Jurkat cells which was suppressed by Bcl-2. Mol Cell Biochem. 2006;285:111–120. doi: 10.1007/s11010-005-9064-8. [DOI] [PubMed] [Google Scholar]
- 83.Balasenthil S, Rao KS, Nagini S. Apoptosis induction by S-allylcysteine, a garlic constituent, during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Cell Biochem Funct. 2002;20:263–268. doi: 10.1002/cbf.967. [DOI] [PubMed] [Google Scholar]
- 84.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 85.Tan J, Shen ZX, Geng W. Ursolic acid induces apoptosis in colon cancer HT-29 cells. Zhonghua Zhong Liu Za Zhi. 2006;28:99–102. [PubMed] [Google Scholar]
- 86.Oommen S, Anto RJ, Srinivas G, Karunagaran D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur J Pharmacol. 2004;485:97–103. doi: 10.1016/j.ejphar.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 87.Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005;71:484–488. doi: 10.1055/s-2005-864146. [DOI] [PubMed] [Google Scholar]
- 88.Lu HF, Sue CC, Yu CS, Chen SC, Chen GW, Chung JG. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004;42:1543–1552. doi: 10.1016/j.fct.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 89.Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem. 2005;280:41487–41493. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- 90.Leung HW, Lin CJ, Hour MJ, Yang WH, Wang MY, Lee HZ. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol. 2007;45:2005–2013. doi: 10.1016/j.fct.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 91.Ji J, Zhang L, Wu YY, Zhu XY, Lv SQ, Sun XZ. Induction of apoptosis by d-limonene is mediated by a caspase-dependent mitochondrial death pathway in human leukemia cells. Leuk Lymphoma. 2006;47:2617–2624. doi: 10.1080/00268970600909205. [DOI] [PubMed] [Google Scholar]
- 92.Lee BK, Kim JH, Jung JW, Choi JW, Han ES, Lee SH, Ko KH, Ryu JH. Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells. Toxicol Lett. 2005;157:49–56. doi: 10.1016/j.toxlet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Keum YS, Kim J, Lee KH, Park KK, Surh YJ, Lee JM, Lee SS, Yoon JH, Joo SY, Cha IH, Yook JI. Induction of apoptosis and caspase-3 activation by chemopreventive [6]-paradol and structurally related compounds in KB cells. Cancer Lett. 2002;177:41–47. doi: 10.1016/s0304-3835(01)00781-9. [DOI] [PubMed] [Google Scholar]
- 94.Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone M, Salvatore G, Mondello F, Arancia G, Molinari A. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J Invest Dermatol. 2004;122:349–360. doi: 10.1046/j.0022-202X.2004.22236.x. [DOI] [PubMed] [Google Scholar]
- 95.Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH, Lu FJ, Lin YS, Kuo SH, Chen CH. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J Agric Food Chem. 2007;55:948–954. doi: 10.1021/jf0624594. [DOI] [PubMed] [Google Scholar]
- 96.Chew BP, Brown CM, Park JS, Mixter PF. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003;23:3333–3339. [PubMed] [Google Scholar]
- 97.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 98.Clark SS. Perillyl alcohol induces c-Myc-dependent apoptosis in Bcr/Abl-transformed leukemia cells. Oncology. 2006;70:13–18. doi: 10.1159/000091181. [DOI] [PubMed] [Google Scholar]
- 99.LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 100.Pei YX, Heng ZC, Duan GC, Wang MC. The mechanisms and effects of lutein on inducing the cell differentiation of human esophagus cancer EC9706. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:629–632. [PubMed] [Google Scholar]
- 101.Yokota T, Matsuzaki Y, Koyama M, Hitomi T, Kawanaka M, Enoki-Konishi M, Okuyama Y, Takayasu J, Nishino H, Nishikawa A, Osawa T, Sakai T. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–1453. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chaudhuri D, Orsulic S, Ashok BT. Antiproliferative activity of sulforaphane in Akt-overexpressing ovarian cancer cells. Mol Cancer Ther. 2007;6:334–345. doi: 10.1158/1535-7163.MCT-06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YM, Wu TH, Chen SF, Chung JG. Effect of 5-methoxypsoralen (5-MOP) on cell apoptosis and cell cycle in human hepatocellular carcinoma cell line. Toxicol In Vitro. 2003;17:279–287. doi: 10.1016/s0887-2333(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 104.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 105.Wiseman DA, Werner SR, Crowell PL. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21(Cip1) and p27(Kip1) in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther. 2007;320:1163–1170. doi: 10.1124/jpet.106.111666. [DOI] [PubMed] [Google Scholar]
- 106.Saied IT, Shamsuddin AM. Up-regulation of the tumor suppressor gene p53 and WAF1 gene expression by IP6 in HT-29 human colon carcinoma cell line. Anticancer Res. 1998;18:1479–1484. [PubMed] [Google Scholar]
- 107.Hung H. Dietary quercetin inhibits proliferation of lung carcinoma cells. Forum Nutr. 2007;60:146–157. doi: 10.1159/000107165. [DOI] [PubMed] [Google Scholar]
- 108.Hwang ES, Lee HJ. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp Biol Med (Maywood) 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 109.Park WH, Kim SH, Kim CH. A new matrix metalloproteinase-9 inhibitor 3,4-dihydroxycinnamic acid (caffeic acid) from methanol extract of Euonymus alatus: isolation and structure determination. Toxicology. 2005;207:383–390. doi: 10.1016/j.tox.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 110.Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 111.Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol Cell Biochem. 2006;287:109–116. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- 112.Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, Kim KW. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771–778. doi: 10.1038/sj.onc.1201587. [DOI] [PubMed] [Google Scholar]
- 113.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 114.Su CC, Chen GW, Lin JG, Wu LT, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006;26:1281–1288. [PubMed] [Google Scholar]
- 115.Mitra A, Chakrabarti J, Banerji A, Chatterjee A, Das BR. Curcumin, a potential inhibitor of MMP-2 in human laryngeal squamous carcinoma cells HEp2. J Environ Pathol Toxicol Oncol. 2006;25:679–690. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i4.70. [DOI] [PubMed] [Google Scholar]
- 116.Ko CH, Shen SC, Lee TJ, Chen YC. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol Cancer Ther. 2005;4:281–290. [PubMed] [Google Scholar]
- 117.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107:25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 118.Thejass P, Kuttan G. Antimetastatic activity of Sulforaphane. Life Sci. 2006;78:3043–3050. doi: 10.1016/j.lfs.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 119.Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 120.Mo SJ, Son EW, Rhee DK, Pyo S. Modulation of TNF-alpha-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003;26:244–251. doi: 10.1007/BF02976837. [DOI] [PubMed] [Google Scholar]
- 121.Mousa AS, Mousa SA. Anti-angiogenesis efficacy of the garlic ingredient alliin and antioxidants: role of nitric oxide and p53. Nutr Cancer. 2005;53:104–110. doi: 10.1207/s15327914nc5301_12. [DOI] [PubMed] [Google Scholar]
- 122.Jung JE, Kim HS, Lee CS, Park DH, Kim YN, Lee MJ, Lee JW, Park JW, Kim MS, Ye SK, Chung MH. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis. 2007;28:1780–1787. doi: 10.1093/carcin/bgm130. [DOI] [PubMed] [Google Scholar]
- 123.Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, Kim KW, Gho YS, Kwon YG. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004;64:644–651. doi: 10.1158/0008-5472.can-03-3250. [DOI] [PubMed] [Google Scholar]
- 124.Chen WH, Chen Y, Cui GH. Effects of TNF-alpha and curcumin on the expression of VEGF in Raji and U937 cells and on angiogenesis in ECV304 cells. Chin Med J (Engl) 2005;118:2052–2057. [PubMed] [Google Scholar]
- 125.Fan ZL, Qi ZH, Xie Y. Effect of diallyl disulfide on the expression and secretion of VEGF in HL-60 cells. Zhonghua Xue Ye Xue Za Zhi. 2006;27:626–629. [PubMed] [Google Scholar]
- 126.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 127.Loutrari H, Hatziapostolou M, Skouridou V, Papadimitriou E, Roussos C, Kolisis FN, Papapetropoulos A. Perillyl alcohol is an angiogenesis inhibitor. J Pharmacol Exp Ther. 2004;311:568–575. doi: 10.1124/jpet.104.070516. [DOI] [PubMed] [Google Scholar]
- 128.Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;239:271–280. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 129.Rizvi I, Riggs DR, Jackson BJ, Ng A, Cunningham C, McFadden DW. Inositol hexaphosphate (IP6) inhibits cellular proliferation in melanoma. J Surg Res. 2006;133:3–6. doi: 10.1016/j.jss.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 130.McMillan B, Riggs DR, Jackson BJ, Cunningham C, McFadden DW. Dietary influence on pancreatic cancer growth by catechin and inositol hexaphosphate. J Surg Res. 2007;141:115–119. doi: 10.1016/j.jss.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 131.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 133.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 134.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 136.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 137.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 138.Cortes JR, Perez GM, Rivas MD, Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J Immunol. 2007;179:3881–3887. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- 139.Souza MC, Siani AC, Ramos MF, Menezes-de-Lima OJ, Henriques MG. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie. 2003;58:582–586. [PubMed] [Google Scholar]
- 140.Woo ER, Pokharel YR, Yang JW, Lee SY, Kang KW. Inhibition of nuclear factor-kappaB activation by 2′, 8″-biapigenin. Biol Pharm Bull. 2006;29:976–980. doi: 10.1248/bpb.29.976. [DOI] [PubMed] [Google Scholar]
- 141.Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Vinodhkumar R, Devaki T. Capsaicin modulates pulmonary antioxidant defense system during benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother Res. 2008;22:529–533. doi: 10.1002/ptr.2393. [DOI] [PubMed] [Google Scholar]
- 142.Jeyabal PV, Syed MB, Venkataraman M, Sambandham JK, Sakthisekaran D. Apigenin inhibits oxidative stress-induced macromolecular damage in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinogenesis in Wistar albino rats. Mol Carcinog. 2005;44:11–20. doi: 10.1002/mc.20115. [DOI] [PubMed] [Google Scholar]
- 143.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, Nam Han Y, Lee SK. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 144.Girdhani S, Bhosle SM, Thulsidas SA, Kumar A, Mishra KP. Potential of radiosensitizing agents in cancer chemoradiotherapy. J Cancer Res Ther. 2005;1:129–131. doi: 10.4103/0973-1482.19585. [DOI] [PubMed] [Google Scholar]
- 145.Young SC, Wang CJ, Hsu JD, Hsu JL, Chou FP. Increased sensitivity of Hep G2 cells toward the cytotoxicity of cisplatin by the treatment of piper betel leaf extract. Arch Toxicol. 2006;80:319–327. doi: 10.1007/s00204-005-0051-3. [DOI] [PubMed] [Google Scholar]
- 146.Dange P, Sarma H, Pandey BN, Mishra KP. Radiation-induced incidence of thymic lymphoma in mice and its prevention by antioxidants. J Environ Pathol Toxicol Oncol. 2007;26:273–279. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i4.40. [DOI] [PubMed] [Google Scholar]
- 147.Singh A, Singh SP, Bamezai R. Modulatory potential of clocimum oil on mouse skin papillomagenesis and the xenobiotic detoxication system. Food Chem Toxicol. 1999;37:663–670. doi: 10.1016/s0278-6915(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 148.Krishnaswamy K, Raghuramulu N. Bioactive phytochemicals with emphasis on dietary practices. Indian J Med Res. 1998;108:167–181. [PubMed] [Google Scholar]
- 149.Rompelberg CJ, Steenwinkel MJ, van Asten JG, van Delft JH, Baan RA, Verhagen H. Effect of eugenol on the mutagenicity of benzo[a]pyrene and the formation of benzo[a]pyrene-DNA adducts in the lambda-lacZ-transgenic mouse. Mutat Res. 1996;369:87–96. doi: 10.1016/s0165-1218(96)90052-x. [DOI] [PubMed] [Google Scholar]
- 150.Sukumaran K, Kuttan R. Inhibition of tobacco-induced mutagenesis by eugenol and plant extracts. Mutat Res. 1995;343:25–30. doi: 10.1016/0165-1218(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 151.Jeng JH, Hahn LJ, Lu FJ, Wang YJ, Kuo MY. Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts. J Dent Res. 1994;73:1050–1055. doi: 10.1177/00220345940730050601. [DOI] [PubMed] [Google Scholar]
- 152.Yokota H, Hashimoto H, Motoya M, Yuasa A. Enhancement of UDP-glucuronyltransferase, UDP-glucose dehydrogenase, and glutathione S-transferase activities in rat liver by dietary administration of eugenol. Biochem Pharmacol. 1988;37:799–802. doi: 10.1016/0006-2952(88)90164-5. [DOI] [PubMed] [Google Scholar]
- 153.Ahmed N, Laverick L, Sammons J, Zhang H, Maslin DJ, Hassan HT. Ajoene, a garlic-derived natural compound, enhances chemotherapy-induced apoptosis in human myeloid leukaemia CD34-positive resistant cells. Anticancer Res. 2001;21:3519–3523. [PubMed] [Google Scholar]
- 154.Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol Cancer Ther. 2005;4:1250–1259. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- 155.Sanchez AM, Malagarie-Cazenave S, Olea N, Vara D, Chiloeches A, Diaz-Laviada I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis. 2007;12:2013–2024. doi: 10.1007/s10495-007-0119-z. [DOI] [PubMed] [Google Scholar]
- 156.Gao D, Xiao Z, Lu AE. Proliferation inhibition and apoptosis induction of K562 cells by D-limonene. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:1120–1122. [PubMed] [Google Scholar]
- 157.Xiao D, Pinto JT, Soh JW, Deguchi A, Gundersen GG, Palazzo AF, Yoon JT, Shirin H, Weinstein IB. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH(2)-terminal kinase 1 activation. Cancer Res. 2003;63:6825–6837. [PubMed] [Google Scholar]
- 158.Munday R, Munday CM. Selective induction of phase II enzymes in the urinary bladder of rats by allyl isothiocyanate, a compound derived from Brassica vegetables. Nutr Cancer. 2002;44:52–59. doi: 10.1207/S15327914NC441_7. [DOI] [PubMed] [Google Scholar]
- 159.Laky B, Knasmuller S, Gminski R, Mersch-Sundermann V, Scharf G, Verkerk R, Freywald C, Uhl M, Kassie F. Protective effects of Brussels sprouts towards B[a]P-induced DNA damage: a model study with the single-cell gel electrophoresis (SCGE)/Hep G2 assay. Food Chem Toxicol. 2002;40:1077–1083. doi: 10.1016/s0278-6915(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 160.Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- 161.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 163.Chaudhary A, Willett KL. Inhibition of human cytochrome CYP 1 enzymes by flavonoids of St. John’s wort. Toxicology. 2006;217:194–205. doi: 10.1016/j.tox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 164.Long X, Fan M, Bigsby RM, Nephew KP. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol Cancer Ther. 2008;7:2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Way TD, Lin JK. Role of HER2/HER3 co-receptor in breast carcinogenesis. Future Oncol. 2005;1:841–849. doi: 10.2217/14796694.1.6.841. [DOI] [PubMed] [Google Scholar]
- 166.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 167.Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006;5:843–852. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- 168.Morrissey C, O’Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RW. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe N, Hirayama R, Kubota N. The chemopreventive flavonoid apigenin confers radiosensitizing effect in human tumor cells grown as monolayers and spheroids. J Radiat Res (Tokyo) 2007;48:45–50. doi: 10.1269/jrr.0635. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–2053. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 171.Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- 172.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 173.Miyoshi N, Naniwa K, Yamada T, Osawa T, Nakamura Y. Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch Biochem Biophys. 2007;466:274–282. doi: 10.1016/j.abb.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 174.Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 175.Choi HJ, Eun JS, Kim BG, Kim SY, Jeon H, Soh Y. Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells. Mol Cells. 2006;22:291–299. [PubMed] [Google Scholar]
- 176.Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44:1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee SH, Ryu JK, Lee KY, Woo SM, Park JK, Yoo JW, Kim YT, Yoon YB. Enhanced anti-tumor effect of combination therapy with gemcitabine and apigenin in pancreatic cancer. Cancer Lett. 2008;259:39–49. doi: 10.1016/j.canlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 178.Siddique YH, Beg T, Afzal M. Antigenotoxic effect of apigenin against anti-cancerous drugs. Toxicol In Vitro. 2008;22:625–631. doi: 10.1016/j.tiv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 179.van Meeuwen JA, Nijmeijer S, Mutarapat T, Ruchirawat S, de Jong PC, Piersma AH, van den Berg M. Aromatase inhibition by synthetic lactones and flavonoids in human placental microsomes and breast fibroblasts—a comparative study. Toxicol Appl Pharmacol. 2008;228:269–276. doi: 10.1016/j.taap.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 180.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther. 2006;5:945–951. doi: 10.1158/1535-7163.MCT-05-0431. [DOI] [PubMed] [Google Scholar]
- 181.Balasubramanian S, Eckert RL. Keratinocyte proliferation, differentiation, and apoptosis—differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol Appl Pharmacol. 2007;224:214–219. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 184.Pandey BN, Mishra KP. Modification of thymocytes membrane radiooxidative damage and apoptosis by eugenol. J Environ Pathol Toxicol Oncol. 2004;23:117–122. doi: 10.1615/jenvpathtoxoncol.v23.i2.40. [DOI] [PubMed] [Google Scholar]
- 185.Atsumi T, Fujisawa S, Satoh K, Sakagami H, Iwakura I, Ueha T, Sugita Y, Yokoe I. Cytotoxicity and radical intensity of eugenol, isoeugenol or related dimers. Anticancer Res. 2000;20:2519–2524. [PubMed] [Google Scholar]
- 186.Yang Y, Yang L, You QD, Nie FF, Gu HY, Zhao L, Wang XT, Guo QL. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256:259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 187.Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, Drewe J, Cai SX. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12:309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 188.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol. 2005;11:3655–3659. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang T, Wei J, Qian X, Ding Y, Yu L, Liu B. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262:214–222. doi: 10.1016/j.canlet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 191.Zhao Q, Yang Y, Yu J, You QD, Zeng S, Gu HY, Lu N, Qi Q, Liu W, Wang XT, Guo QL. Posttranscriptional regulation of the telomerase hTERT by gambogic acid in human gastric carcinoma 823 cells. Cancer Lett. 2008;262:223–231. doi: 10.1016/j.canlet.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 192.Qiang L, Yang Y, You QD, Ma YJ, Yang L, Nie FF, Gu HY, Zhao L, Lu N, Qi Q, Liu W, Wang XT, Guo QL. Inhibition of glioblastoma growth and angiogenesis by gambogic acid: an in vitro and in vivo study. Biochem Pharmacol. 2008;75:1083–1092. doi: 10.1016/j.bcp.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 193.Palozza P, Maggiano N, Calviello G, Lanza P, Piccioni E, Ranelletti FO, Bartoli GM. Canthaxanthin induces apoptosis in human cancer cell lines. Carcinogenesis. 1998;19:373–376. doi: 10.1093/carcin/19.2.373. [DOI] [PubMed] [Google Scholar]
- 194.Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol Rep. 2002;9:757–760. [PubMed] [Google Scholar]
- 195.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 196.Miyahara Y, Hibasami H, Katsuzaki H, Imai K, Komiya T. Sesamolin from sesame seed inhibits proliferation by inducing apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 2001;7:369–371. [PubMed] [Google Scholar]
- 197.Aratanechemuge Y, Komiya T, Moteki H, Katsuzaki H, Imai K, Hibasami H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int J Mol Med. 2002;9:481–484. [PubMed] [Google Scholar]
- 198.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 199.Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001;134:1137–1150. doi: 10.1038/sj.bjp.0704365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 201.Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- 202.Awad AB, Williams H, Fink CS. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr Cancer. 2001;40:157–164. doi: 10.1207/S15327914NC402_12. [DOI] [PubMed] [Google Scholar]
- 203.Jin G, Zhang T, Wang T, Yang LP. Inhibition of alpha-interferon and cinnamic acid on proliferation of human lung cancer cell. Ai Zheng. 2002;21:860–862. [PubMed] [Google Scholar]
- 204.Yuan JP, Ling H, Zhang MX, Liu Y, Song Y, Su Q. Diallyl disulfide-induced G2/M arrest of human gastric cancer MGC803 cells involves activation of p38 MAP kinase pathways. Ai Zheng. 2004;23:169–172. [PubMed] [Google Scholar]
- 205.Shirin H, Pinto JT, Kawabata Y, Soh JW, Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR, Weinstein IB. Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res. 2001;61:725–731. [PubMed] [Google Scholar]
- 206.Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH, Jr, Iwamura T, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5:76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280:5812–5819. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- 208.Balasubramanian S, Zhu L, Eckert RL. Apigenin inhibition of involucrin gene expression is associated with a specific reduction in phosphorylation of protein kinase Cdelta Tyr311. J Biol Chem. 2006;281:36162–36172. doi: 10.1074/jbc.M605368200. [DOI] [PubMed] [Google Scholar]
- 209.Qin Y, Meng L, Hu C, Duan W, Zuo Z, Lin L, Zhang X, Ding J. Gambogic acid inhibits the catalytic activity of human topoisomerase IIalpha by binding to its ATPase domain. Mol Cancer Ther. 2007;6:2429–2440. doi: 10.1158/1535-7163.MCT-07-0147. [DOI] [PubMed] [Google Scholar]
- 210.Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, Zhang HW, Tan Z, Wang X. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis. 2007;28:632–638. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 211.Noujaim D, van Golen CM, van Golen KL, Grauman A, Feldman EL. N-Myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene. 2002;21:4549–4557. doi: 10.1038/sj.onc.1205552. [DOI] [PubMed] [Google Scholar]
- 212.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 213.Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19:313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 214.Tantivejkul K, Vucenik I, Shamsuddin AM. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: II. Effects on integrins and focal adhesions. Anticancer Res. 2003;23:3681–3689. [PubMed] [Google Scholar]
- 215.Piantelli M, Rossi C, Iezzi M, La Sorda R, Iacobelli S, Alberti S, Natali PG. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol. 2006;207:23–29. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- 216.Czyz J, Madeja Z, Irmer U, Korohoda W, Hulser DF. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int J Cancer. 2005;114:12–18. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]
- 217.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5 (Suppl 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 218.Thejass P, Kuttan G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by down-regulating nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) production. Nitric Oxide. 2007;16:247–257. doi: 10.1016/j.niox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 219.Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X, Jiang BH. Apigenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 220.Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin Hemorheol Microcirc. 2005;33:127–135. [PubMed] [Google Scholar]
- 221.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 223.Tan WF, Lin LP, Li MH, Zhang YX, Tong YG, Xiao D, Ding J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur J Pharmacol. 2003;459:255–262. doi: 10.1016/s0014-2999(02)02848-0. [DOI] [PubMed] [Google Scholar]