Abstract
Site-specific modification of glycoproteins has wide application in both biochemical and biophysical studies. This method describes the conjugation of synthetic molecules to the N-terminus of a glycoprotein fragment: immunoglobulin G subclass 1 fragment crystallizable (IgG1 Fc) by native chemical ligation. The glycosylated IgG1 Fc is expressed in a glycosylation deficient yeast strain. The N-terminal cysteine is generated by the endogenous yeast protease Kex2 in the yeast secretory pathway. The N-terminal cysteine is then conjugated with a biotin thioester to produce a biotinylated, glycosylated IgG1 Fc using native chemical ligation.
Keywords: Glycoprotein, IgG1 Fc, site-specific chemical modification, native chemical ligation, N-terminal cysteine, biotin
1. Introduction
Site-specific chemical modification of proteins has wide application including the incorporation of labels, probes and post-translational modifications onto proteins for biochemical and biophysical studies, and the incorporation of synthetic modifications onto proteins to alter bioactivity and physical properties (1-7). N-terminal cysteines have been widely used for site-specific protein N-terminal modification using chemoselective ligations, such as native chemical ligation between N-terminal cysteines and thioesters (8-14), thiazolidine ligation between N-terminal cysteines and aldehydes (15, 16), and condensation between N-terminal cysteines and cyanobenzothiazole derivatives has been reported recently (17). However, production of expressed glycoproteins with N-terminal cysteines for site-specific glycoprotein modification has only recently been reported by our laboratory (7). Here, we present a detailed method for the production of N-terminal cysteine-containing glycoproteins in yeast for site-specific chemical modification.
In this method, a model glycoprotein: immunoglobulin G subclass 1 fragment crystallizable (IgG1 Fc) containing an N-terminal cysteine is expressed in a glycosylation deficient yeast strain. The N-terminal cysteine is generated by proteolytic removal of a propeptide leader sequence by the endogenous yeast protease Kex2 in the yeast secretory pathway. Next, the N-terminal cysteine is conjugated with a synthetic biotin thioester to produce a biotinylated, glycosylated IgG1 Fc using native chemical ligation as an example of glycoprotein modification. Biotinylated glycoproteins produced by this method can be used for western-blotting or site-specific protein immobilization, and these methods are compatible for use with a wide variety of thioester labels or synthetic modifications (18, 19).
2. Materials
2.1. Chemical Synthesis of a Biotin Thioester
Potassium ethylxanthate (Aldrich)
Acetone (Fisher Scientific)
tert-Butyl chloroacetate (Aldrich)
Diethyl ether (Fisher Scientific)
Ethanolamine (Aldrich)
Ethyl acetate (Fisher Scientific)
Biotin (Sigma)
DMF, dimethylformamide (Sigma-Aldrich)
DIC, 1,3-diisopropylcarbodiimide (Aldrich)
Methanol (Fisher Scientific)
Dichloromethane (Fisher Scientific)
TFA, trifluoroacetic acid (Aldrich)
Toluene (Fisher Scientific)
2.2. Construction of the pGAP-C-IgG1 Fc Expression Plasmid
pGAPzαA plasmid (Invitrogen). Store at −20 °C.
Restriction enzymes: Xho I and Not I (New England Biolabs Inc.). Store at −20 °C.
Antarctic phosphatase (New England Biolabs Inc.). Store at −20 °C.
Primers. Store at −20 °C.
MGC-12853 plasmid (Mammalian Gene Collection (20)). Store at −20 °C.
T4 DNA ligase (New England Biolabs Inc.). Store at −20 °C.
Top10F’ E. coli (Invitrogen). Store at −80 °C.
Zeocin (Invitrogen, 100 mg/mL). Store at −20 °C.
Low salt LB/Zeocin plates: 1% peptone, 0.5% yeast extract, 0.5% NaCl, 1.5% agar, 25 μg/mL Zeocin.
2.3. Expression and Purification of Glycosylated C-IgG1 Fc
Restriction enzymes: Avr II (New England Biolabs Inc.). Store at −20 °C.
YPD/Zeocin plates: 1% yeast extract, 2% peptone, and 2% glucose, 2% agar, 100 μg/mL Zeocin.
YPD/Zeocin medium: 1% yeast extract, 2% peptone, and 2% glucose, 100 μg/mL Zeocin.
Supplemented YPD medium: 1% yeast extract, 2% peptone, and 2% glucose, 4×10−5% biotin, 0.004% histidine, 1.34% yeast nitrogen base with ammonium sulfate without amino acids, 100 mM potassium phosphate buffer, pH 6.
Antifoam 204 (Sigma), sterilized by antoclave.
Sterile 20% glucose solution, sterilized by antoclave.
Protein G sepharose, fast flow (Sigma-Aldrich)
Phosphate buffer: 20 mM sodium phosphate, pH 7.0.
Tris buffer: 1 M Tris-base, pH 7.8.
Elution buffer: 100 mM glycine, pH 2.7.
Phenyl sepharose (Sigma-Aldrich)
Buffer A: 20 mM sodium phosphate, 1 M ammonium sulfate, pH 7.0.
DTT, dithiothreitol (Bio-rad laboratories)
Acetonitrile (J.T.Baker)
2.4. Ligation of the Biotin Thioester to the C-IgG1 Fc
Hydroxylamine buffer: 20 mM sodium phosphate, 200 mM hydroxylamine, pH 5.0.
Ligation buffer: 20 mM sodium phosphate, 5 mM betaine, pH 7.5.
Sodium 2-mercaptoethanesulfonate (Sigam-Aldrich)
2.5. Deglycosylation of Biotin Modified Glycosylated C-IgG1 Fc
PNGase F 500,000 units/ml (New England Biolabs). Stored at −20 °C.
3. Methods
This method describes the conjugation of biotin onto the N-terminus of an IgG1 Fc glycoprotein using native chemical ligation, which is a chemoselective ligation that joins N-terminal cysteine containing peptides and proteins to thioester containing molecules (8). In this method, the N-terminal cysteine-containing, glycosylated IgG1 Fc is produced by expression in a glycosylation deficient yeast strain, and then labeled with a synthetic biotin thioester which is synthesized as described below.
3.1. Chemical Synthesis of a Biotin Thioester, 2-(5-(2-oxohexahydro-thieno[3,4-d]imidazol-4-yl)pentanoylthio)acetic acid (6)
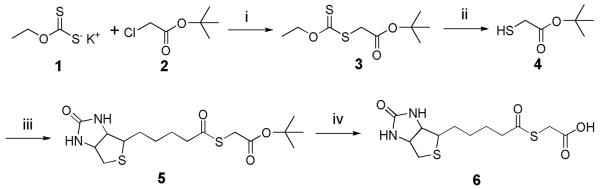
The carboxylic acid of biotin is coupled to tert-butyl 2-mercaptoacetate (21) using DIC. The tert-butyl protecting group is then removed by TFA to form a water soluble biotin thioester (Fig. 1; see Note 1; ref. 6).
Fig. 1.
Schematic of synthesis of the biotin thioester 6. (i) Acetone; (ii) Ethanolamine; (iii) Biotin, DMF, DIC; (iv) 95% TFA.
Potassium ethylxanthate 1 (5.0 g, 31.2 mmol) is suspended into 20 mL of acetone in a 50 mL round bottom flask at room temperature. tert-Butyl chloroacetate 2 (4.1 mL, 28.6 mmol) is then added into the flask dropwise with stirring (approximately 5 seconds per drop). The reaction is stirred at room temperature for 18 h and then the potassium chloride is removed by filtration through celite. The solvent is then removed by rotovap. 20 mL of diethyl ether is added into the residue and then washed with 5% NaHCO3, water and brine. Next the organic layer is separated and dried with anhydrous MgSO4. After 2 h drying, the MgSO4 is removed by filtration through celite and the solvent in the filtrate is removed by rotovap to give 4.7 g of tert-butyl 2-(ethoxycarbonothioylthio)acetate 3 as a clear oil. 1H NMR (400 MHz, CDCl3): δ 4.63 (q, 2H, J = 7.2), 3.83 (s, 2H), 1.47 (s, 9H), 1.42 (t, 3H, J = 7.2).
tert-Butyl 2-(ethoxycarbonothioylthio)acetate 3 (4.7 g, 19.9 mmol) is placed into a 15 mL round bottom flask with ethanolamine (1.2 g, 19.9 mmol). After 2 h stirring at room temperature, 10 mL of ethyl acetate is added into the reaction and then washed with 1 N HCl, water and brine. The organic layer is then separated and dried with anhydrous MgSO4. After 2 h drying, the MgSO4 is removed by filtration through celite and the solvent in the filtrate is removed by rotovap. The residue is vacuum distilled, and the distillate with a bp of 35-42 °C at 6 torr is collected to give 2.1 g of tert-butyl 2-mercaptoacetate 4 as a clear oil. 1H NMR (400 MHz, CDCl3): δ 3.17 (d, 2H, J = 8.0), 1.94 (t, 1H, J = 8.0), 1.47 (s, 9H).
Biotin (100 mg, 0.41 mmol) is dissolved in 10 mL DMF in a 25 mL round bottom flask. tert-Butyl 2-mercaptoacetate 4 (243 mg, 1.64 mmol) and DIC (207 mg, 1.64 mmol) were then added into the reaction. The reaction is stirred at room temperature for 24 h, and then the solvent is removed by rotovap. The residue is purified by flash chromatography using a mixture of methanol and dichloromethane (5:95). The resulting white solid 5 is then dissolved in 5 mL of 95% trifluoroacetic acid and stirred at room temperature for 30 min. The solvent is then removed by rotovap, and the residue is azeotroped three times with toluene and dried over night under vacuum to give 115 mg of the biotin thioester 6 as a white solid (see Note 2; ref. 6). 1H NMR (400 MHz, D2O): δ 4.60 (dd, 1H, J = 7.9, 5.0), 4.42 (dd, 1H, J = 7.9, 4.6), 3.56 (s, 2H), 3.33 (ddd, 1H, J = 9.1, 4.6, 4.6), 2.99 (dd, 1H, J = 12.9, 5.0), 2.77 (d, 1H, J = 12.9), 2.68 (t, 2H, J = 7.5), 1.75–1,39 (m, 6H); 13C NMR: δ 205.72, 177.55, 166.92, 63.61, 61.84, 56.85, 44.47, 41.34, 35.68, 29.31, 29.19, 26.49; MALDI-FTMS: calcd for C12H18N2O4S2 (MNa+) 341.0600, found 341.0601.
3.2. Construction of the pGAP-C-IgG1 Fc Expression Plasmid
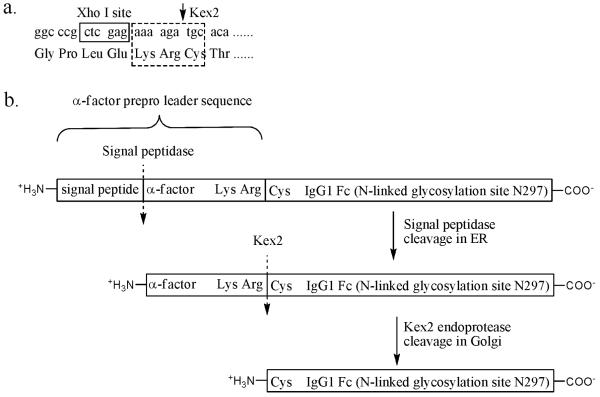
In this step, DNA encoding for the C-IgG1 Fc glycoprotein is inserted into a pGAPzαA yeast expression plasmid (Invitrogen) which contains a signal peptide and an α-factor prepro leader sequence to direct protein secretion. A cysteine residue is inserted into the primer complementary to the N-terminus of the protein such that it directly follows the lysine-arginine dipeptide that is cleaved by Kex2 protease. (Fig. 2)
Fig. 2.
(a) Using PCR primer to encode a cysteine residue at the N-terminal of C-IgG1 Fc right next to the KR that is cleaved by Kex2 protease. (b) C-IgG1 Fc expressed in yeast is fused to a α-factor prepro leader sequence that directs the C-IgG1 Fc protein into the secretory pathway. The α-factor, which directs C-IgG1 Fc to be secreted, is cleaved from the fusion protein by the Kex2 protease in Golgi to generate the N-terminal cysteine.
The pGAPzαA plasmid is double digested with Xho I and Not I restriction enzymes at 37 °C for 2 h and then treated with antarctic phosphatase at 37 °C for 0.5 h. The linearized product is gel purified and stored at −20 °C before usage.
The DNA fragment encoding the Fc region of human immunoglobulin heavy chain constant gamma 1 is amplified by polymerase chain reaction (PCR) using the primers 5′-ggcccgctcgagaaaagatgcacatgcccaccgtgcccagca-3′ and 5′-gggcccgcggcggccgctcatttacccggagacagggagag-3′ and plasmid MGC-12853 (Mammalian gene collection) as the template.
The PCR fragment from step 2 is gel purified and then double digested with Xho I and Not I restriction enzymes at 37 °C for 2 h. Then the digestion product is gel purified again.
The digestion product from step 3 and the linearized pGAPzαA plasmid from step 1 are ligated together by T4 DNA ligase at 37 °C for 2 h.
The ligated DNA is transformed into Top10F’ E. coli by electroporation and selected on low salt LB/Zeocin plates. Plasmid pGAP-C-IgG1 Fc is isolated from a positive clone and insertion of the correct C-IgG1 Fc encoding DNA is confirmed by DNA sequencing.
3.3. Expression and Purification of Glycosylated C-IgG1 Fc
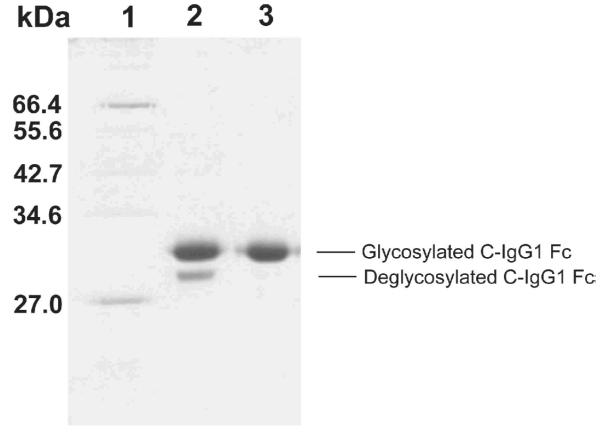
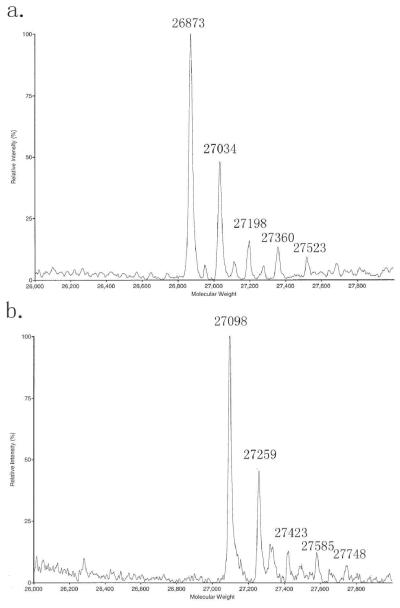
The glycosylated C-IgG1 Fc protein is expressed in an OCH1 deleted strain of SMD1168 Pichia pastoris (7, 22). The heterogeneously glycosylated C-IgG1 Fc is purified by a Protein G column followed by a phenyl sepharose column (Fig. 3). The heterogeneously glycosylated C-IgG1 Fc is characterized by ESI mass spectroscopy and the ESI mass spectrum indicates the presence of high mannose glycoforms containing 8-12 mannose residues (Fig. 4a).
Fig. 3.
Purification gel of C-IgG1 Fc. Lane 1, molecular weight markers; lane 2, C-IgG1 Fc after Protein G column purification; lane 3, glycosylated C-IgG1 Fc after phenyl sepharose column purification.
Fig. 4.
(a) ESI MS of heterogeneously glycosylated C-IgG1 Fc, calcd. masses for glycoforms containing 8-12 mannose residues: 26872, 27035, 27198, 27361, 27524 respectively, obs. 26873, 27034, 27198, 27360, 27523 respectively. (b) ESI MS of biotin modified heterogeneously glycosylated C-IgG1 Fc, calcd. masses for glycoforms containing 8-12 mannose residues: 27098, 27261, 27424, 27587, 27750 respectively, obs. 27098, 27259, 27423, 27585, 27748 respectively.
Plasmid pGAP-C-IgG1 Fc is linearized with restriction enzyme Avr II at 37 °C for 24 h. The linearized DNA is transformed into an OCH1 deleted strain of SMD1168 Pichia pastoris by electroporation and selected on YPD/Zeocin plates. The positive colonies containing human IgG1 Fc DNA are confirmed by PCR.
A positive colony is inoculated into 2 mL of YPD/Zeocin medium and is grown in a shaker at 25 °C for 3 days, then the 2 mL of dense culture is inoculated into a 50 mL culture of YPD/Zeocin medium and grown for another 3 days.
The 50 mL of dense culture is inoculated into a spinner flask containing 1 L of supplemented YPD medium (see Note 3) with 100 μL of sterile antifoam 204. During the yeast culture, the medium is aerated by addition of air through a sterilized glass sparger through a 0.2 micron filter at a rate of 1.5 L/min.
The 1 L of yeast culture is grown at 25 °C for 24 h to allow the culture to grow to density. After that, 50 mL of sterile 20% glucose solution is added into the culture every 24 h for 3 days.
The culture is harvested on the fourth day by centrifugation at 5400 g for 10 min at 4 °C. The culture supernatant is adjusted to pH 7 by 10 N NaOH solution and incubated at 4 °C for 2 h during which a precipitate often forms, and then filtered through filter paper using a Buchner filter.
A 5 mL bed volume Protein G sepharose column is pre-equilibrated with 50 mL of phosphate buffer, pH 7.0.
The filtered supernatant from step 5 is loaded onto the pre-equilibrated Protein G column, and then the column is washed with 50 mL of phosphate buffer, pH 7.0.
Prepare the collection tubes by addition of 0.1 mL of Tris buffer, pH 7.8 per mL of each fraction to be collected (see Note 4).
The C-IgG1 Fc is eluted with elution buffer and collected in the collection tubes. The resulting fractions are analyzed by SDS-PAGE gel. Fractions containing the C-IgG1 Fc are collected and dialyzed against buffer A at 4 °C overnight.
A 50 mL bed volume phenyl sepharose column is pre-equilibrated with 100 mL of buffer A.
The C-IgG1 Fc from step 9 is loaded onto the pre-equilibrated phenyl sepharose column.
A 600 mL of linear gradient starting with buffer A and ending with phosphate buffer, pH 7.0 is then used to elute the C-IgG1 Fc protein. Fractions (5 mL) are collected throughout the gradient and analyzed by SDS-PAGE gel. The fractions containing only glycosylated C-IgG1 Fc are pooled and dialyzed against phosphate buffer, pH 7.0 at 4°C overnight (see Note 5).
3.4. Ligation of the Biotin Thioester to the C-IgG1 Fc
In this step biotin is ligated onto the N-terminus of C-IgG1 Fc by native chemical ligation. The ligation product is characterized by ESI mass spectroscopy and the ESI mass spectrum indicates the formation of correctly ligated product (Fig. 4b).
Approximately 1.6 mL of glycosylated C-IgG1 Fc (protein concentration approx 1.5 mg/ mL) is placed into a 6000 MWCO dialysis bag and dialyzed against hydroxylamine buffer at room temperature for 8 h (see Note 6), and then dialyzed against ligation buffer at 4°C overnight.
The protein is transferred into an eppendorf tube for native chemical ligation.
200 μL of 40 mM biotin thioester (see Note 7; ref. 6) and freshly prepared 200 μL of 300 mM sodium 2-mercaptoethanesulfonate (see Note 8) are added into the tube. The reaction mixture is incubated at room temperature for 24 h.
The reaction mixture is dialyzed against phosphate buffer, pH 7.0 at 4 °C for 12 h to remove the excess thioester.
500 μL of biotin modified, glycosylated C-IgG1 Fc is reduced with 5 mM DTT, and then DTT and salts are removed using C18 reverse phase HPLC (10-90% acetonitrile in H2O, 0.1% TFA) and the resulting protein is analyzed by ESI mass spectroscopy.
3.5. Deglycosylation of Biotin Modified Glycosylated C-IgG1 Fc
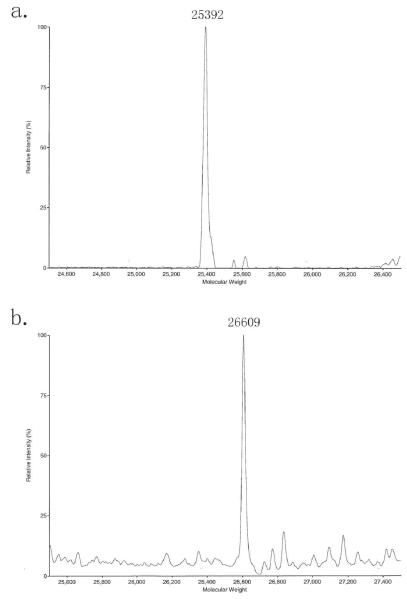
To simplify the mass spectrum of biotin modified C-IgG1 Fc, the biotin modified, glycosylated C-IgG1 Fc is deglycosylated by PNGase F and the resulting protein is analyzed by ESI mass spectroscopy (Fig. 5a). The mass spectrum can also be simplified by conversion to homogenous glycoforms (see Note 9).
Fig. 5.
(a) ESI MS of biotin modified deglycosylated C-IgG1 Fc, calcd. 25395, obs. 25392. (b) ESI MS of biotin modified Man5-C-IgG1 Fc, calcd. 26612, obs. 26609.
500 μL of biotin modified, glycosylated C-IgG1 Fc is placed into an eppendorf tube. 100 units of PNGase F (0.1 μL) are added into the tube and the reaction is incubated at room temperature for 12 h.
The reaction mixture is reduced with 5 mM DTT, and then DTT and salts are removed using C18 reverse phase HPLC (10-90% acetonitrile in H2O, 0.1% TFA) and the resulting protein is analyzed by ESI mass spectroscopy.
4. Notes
We use tert-butyl 2-mercaptoacetate in this protocol to produce a water soluble biotin thioester. Biotin in its protonated form is a fairly hydrophobic molecule, and biotin thioesters which do not contain an additional hydrophilic group are not water soluble. Deprotection of the tert-butyl ester in the intermediate biotin thioester produces a carboxylic acid that makes the resulting biotin thioester soluble in ligation buffer (pH 7.5). However, tert-butyl 2-mercaptoacetate is not commercially available. For molecules that are initially very water soluble, a commercially available thiol (such as methyl 3-mercaptopropionate or ethyl 3-mercaptopropionate) can be used to avoid the necessity of synthesizing tert-butyl 2-mercaptoacetate.
Thioesters are sensitive to basic conditions and can be slowly hydrolyzed in neutral aqueous conditions. Thus, synthesized thioesters should be sealed and stored in a freezer (23).
During the growth of the 1 L yeast culture, healthy yeast produce acidic metabolites that can decrease the pH of the medium. To stabilize the pH, the medium is supplemented with 100 mM phosphate buffer pH 6. The pH of the culture is monitored every day. If the pH falls below 6, concentrated ammonium hydroxide (28 -30%) can be used to adjust the pH back to 6.
The C-IgG1 Fc is collected in Tris buffer, pH 7.8 because the Tris buffer neutralizes the acidic elution buffer to prevent potential damage to C-IgG1 Fc by the acidic conditions.
1 L yeast culture can produce approximately 30 mg of C-IgG1 Fc that is not completely glycosylated. It contains approximately 10% nonglycosylated C-IgG1 Fc. Protein G affinity purification does not separate the glycosylated and nonglycosylated C-IgG Fc. Phenyl sepharose column purification can isolate the glycosylated C-IgG1 Fc. But after this purification step, we can only recover approximately 10 mg of glycosylated C-IgG1 Fc.
N-terminal cysteines are very reactive chemical moieties that may react with aldehyde-containg metabolites in the yeast culture medium to form thiazolidines. To remove these thiazolidines, the protein can be treated with methoxyamine or hydroxylamine prior to native chemical ligation reactions (24).
The biotin thioester is only partially soluble in water but completely soluble in basic pH. Because of this, the 40 mM biotin thioester stock solution is freshly made in the phosphate buffer pH 7.5 right before usage.
Sodium 2-mercaptoethanesulfonate solution is freshly prepared because the 2-mercaptoethanesulfonate might form disulfide bonds if the solution sits at room temperature for too long.
Although the biotin modified C-IgG1 Fc is heterogeneously glycosylated, the glycosylation on C-IgG1 Fc can be further manipulated to homogeneous glycoforms by in vitro enzymatic process. One example of conversion biotin modified heterogeneously glycosylated C-IgG1 Fc to homogeneous glycosylated biotin-Man5-C-IgG1 Fc by α-Mannosidase IA treatment has been done (Fig. 5b).
References
- 1.Hackenberger CPR, Schwarzer D. Chemoselective ligation and modification strategies for peptides and proteins. Angew. Chem. Int. Ed. 2008;47:10030–10074. doi: 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- 2.Flavell RR, Muir TW. Expressed protein ligation (EPL) in the study of signal transduction, ion conduction, and chromatin biology. Acc. Chem. Res. 2009;42:107–116. doi: 10.1021/ar800129c. [DOI] [PubMed] [Google Scholar]
- 3.Xiao J, Burn A, Tolbert TJ. Increasing Solubility of Proteins and Peptides by Site-Specific Modification with Betaine. Bioconjugate Chem. 2008;19:1113–1118. doi: 10.1021/bc800063k. [DOI] [PubMed] [Google Scholar]
- 4.Kochendoerfer GG. Site-specific polymer modification of therapeutic proteins. Curr. Opin. Chem. Biol. 2005;9:555–560. doi: 10.1016/j.cbpa.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Tolbert TJ, Wong C-H. Intein-Mediated Synthesis of Proteins Containing Carbohydrates and Other Molecular Probes. J. Am. Chem. Soc. 2000;122:5421–5428. [Google Scholar]
- 6.Tolbert TJ, Wong C-H. New methods for proteomic research: preparation of proteins with N-terminal cysteines for labeling and conjugation. Angew. Chem. Int. Ed. 2002;41:2171–2174. doi: 10.1002/1521-3773(20020617)41:12<2171::aid-anie2171>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Xiao J, Chen R, Pawlicki MA, Tolbert TJ. Targeting a homogeneously glycosylated antibody fc to bind cancer cells using a synthetic receptor ligand. J. Am. Chem. Soc. 2009;131:13616–13618. doi: 10.1021/ja9045179. [DOI] [PubMed] [Google Scholar]
- 8.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 9.Erlanson DA, Chytil M, Verdine GL. The leucine zipper domain controls the orientation of AP-1 in the NFAT.AP-1.DNA complex. Chem. Biol. 1996;3:981–991. doi: 10.1016/s1074-5521(96)90165-9. [DOI] [PubMed] [Google Scholar]
- 10.Tolbert TJ, Franke D, Wong CH. A new strategy for glycoprotein synthesis: ligation of synthetic glycopeptides with truncated proteins expressed in E. coli as TEV protease cleavable fusion protein. Bioorg. Med. Chem. 2005;13:909–915. doi: 10.1016/j.bmc.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Marsac Y, Cramer J, Olschewski D, Alexandrov K, Becker CF. Site-specific attachment of polyethylene glycol-like oligomers to proteins and peptides. Bioconjugate Chem. 2006;17:1492–1498. doi: 10.1021/bc0601931. [DOI] [PubMed] [Google Scholar]
- 12.Busch GK, Tate EW, Gaffney PR, Rosivatz E, Woscholski R, Leatherbarrow RJ. Specific N-terminal protein labelling: use of FMDV 3C pro protease and native chemical ligation. Chem. Commun. 2008:3369–3371. doi: 10.1039/b806727a. [DOI] [PubMed] [Google Scholar]
- 13.Xiao J, Tolbert TJ. Synthesis of Polymerizable Protein Monomers for Protein-Acrylamide Hydrogel Formation. Biomacromolecules. 2009;10:1939–1946. doi: 10.1021/bm900339q. [DOI] [PubMed] [Google Scholar]
- 14.Xiao J, Tolbert TJ. Synthesis of N-terminally linked protein dimers and trimers by a combined native chemical ligation-CuAAC click chemistry strategy. Org Lett. 2009;11:4144–4147. doi: 10.1021/ol9016468. [DOI] [PubMed] [Google Scholar]
- 15.Liu CF, Tam JP. Peptide segment ligation strategy without use of protecting groups. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6584–6588. doi: 10.1073/pnas.91.14.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam JP, Yu Q. A facile ligation approach to prepare three-helix bundles of HIV fusion-state protein mimetics. Org. Lett. 2002;4:4167–4170. doi: 10.1021/ol026932k. [DOI] [PubMed] [Google Scholar]
- 17.Ren H, Xiao F, Zhan K, Kim YP, Xie H, Xia Z, Rao J. A Biocompatible Condensation Reaction for the Labeling of Terminal Cysteine Residues on Proteins. Angew. Chem., Int. Ed. 2009;48:9658–9662. doi: 10.1002/anie.200903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn MJ. Detection of proteins on blots using the avidin-biotin system. Methods. Mol. Biol. 1994;32:227–232. doi: 10.1385/0-89603-268-X:227. [DOI] [PubMed] [Google Scholar]
- 19.Lesaicherre ML, Lue RY, Chen GY, Zhu Q, Yao SQ. Intein-mediated biotinylation of proteins and its application in a protein microarray. J. Am. Chem. Soc. 2002;124:8768–8769. doi: 10.1021/ja0265963. [DOI] [PubMed] [Google Scholar]
- 20.Strausberg RL, Feingold EA, Klausner RD, Collins FS. The mammalian gene collection. Science. 1999;286:455–457. doi: 10.1126/science.286.5439.455. [DOI] [PubMed] [Google Scholar]
- 21.Woulfe SR, Miller MJ. The synthesis of substituted [[3(S)-(acylamino)-2-oxo-1-azetidinyl]thio]acetic acids. J. Org. Chem. 1986;51:3133–3139. doi: 10.1021/jm00148a013. [DOI] [PubMed] [Google Scholar]
- 22.Cregg JM, Tolstorukov I, Kusari A, Sunga J, Madden K, Chappell T. Expression in the yeast Pichia pastoris. Methods Enzymol. 2009;463:169–189. doi: 10.1016/S0076-6879(09)63013-5. [DOI] [PubMed] [Google Scholar]
- 23.Tolbert TJ, Wong CH. Conjugation of glycopeptide thioesters to expressed protein fragments: semisynthesis of glycosylated interleukin-2. Methods Mol. Biol. 2004;283:255–266. doi: 10.1385/1-59259-813-7:255. [DOI] [PubMed] [Google Scholar]
- 24.Villain M, Vizzavona J, Rose K. Covalent capture: a new tool for the purification of synthetic and recombinant polypeptides. Chem. Biol. 2001;8:673–679. doi: 10.1016/s1074-5521(01)00044-8. [DOI] [PubMed] [Google Scholar]