Abstract
Insulin transport across the epithelial cell layer in the small intestine was studied using insulin/transferrin conjugates with and without the presence of P(MAA-g-EG) microparticles in contact with a co-culture of Caco-2/HT29-MTX cells. The insulin/transferrin conjugate was shown to increase transport relative to pure insulin by a factor of 7, achieving an apparent permeability of 37 × 109 cm•s−1. The presence of P(MAA-g-EG) microparticles increased conjugate transport by a factor of 14 times relative to insulin, achieving an apparent permeability of 72.8 × 109 cm•s−1. The presence of the microparticles in solution was found to improve conjugate transport by nearly 100% with little to no change in cell monolayer integrity.
Introduction
Bioavailability of orally administered therapeutic proteins depends on several factors such as the degree of protein degradation and residence time at the site of absorption. However, one of the most significant factors which affects absorption into the bloodstream is transport across the epithelial cell layer. The purpose of the epithelial cell layer is to absorb only required nutrients such as vitamins and minerals and to expel unrecognized or unwanted entities such as toxins or viruses. Additionally, tight junctions between the cells will only permit the transport of molecules with radii <11 Ǻ 1. Because of its ability to limit transport of large molecules, it is necessary to create strategies to significantly increase epithelial transport in order to effectively deliver therapeutic proteins.
We have successfully developed strategies to overcome the inherent challenges to oral protein delivery such as protein degradation and the narrow absorption window in the small intestine by incorporation into complexation hydrogels. Hydrogels are three-dimensional, hydrophilic polymer networks which can imbibe water and swell under certain conditions. Yamagata et al.2 demonstrated the potential of the hydrogel carriers to protect proteins by encapsulating insulin within poly(methacrylic acid-graft-ethylene glycol) microparticles and achieving preservation of over 80% of the loaded insulin after being treated for 1 h in gastric fluid. In contrast, only 20% of free insulin remained intact after the same treatment with gastric fluid. Also, increased residence time at the site of absorption has been achieved by the addition of polymers which are known to be mucoadhesive3 and through the implementation of polymer tethers. Serra et al.4 showed that the addition of poly(ethylene glycol) (PEG) tethers to a poly(acrylic acid) (PAA) hydrogel system increased the mucoadhesive capacity of the microparticles, generating a work of adhesion of approximately 130 × 10−3 mJ, or five times that of a pure PAA system. Madsen and Peppas5 were able to show that the calcium binding effect of the anionic hydrogels near the epithelial cell layer helped to reversibly loosen the cellular tight junctions and consequently allowed increased paracellular transport of therapeutic proteins. Finally, Morishita et al.6 demonstrated the overall effectiveness and potential of the hydrogel system by performing in vivo studies in which insulin was administered in P(MAA-g-EG) carriers to diabetic rats, resulting in a bioavailability of 12.8%, a significant increase over insulin administered alone. Hydrogels have been shown to effectively protect the protein in the stomach, to create extended residence time in the small intestine for protein release, and even to enhance paracellular transport of therapeutic proteins, altogether demonstrating the promise of complexation hydrogels as carriers for the oral delivery of proteins. The use of complexation hydrogels addresses many of the challenges of oral protein delivery, but further design strategies are required to significantly increase cellular transport in order to increase bioavailability in the bloodstream. A large portion of the design strategies to improve epithelial transport focuses on paracellular protein transport, or protein transport between the cells. System designs based on large molecule absorption through the paracellular route contain an inherent disadvantage. Because of naturally poor protein transport across the epithelial cell layer, the monolayer must be disrupted or loosened to allow for increased protein transport. The monolayer disruption is typically nonspecific to the protein of interest, translating to an increased probability of toxins or viruses entering. An alternative to protein absorption through the paracellular route is to absorb the protein through the cell itself, utilizing the transcellular route for absorption. Transcellular transport can be utilized to be specific to the protein and may result in increased bioavailability as well. In our laboratory, we have been able to successfully synthesize and characterize protein-transporter conjugates which utilize the specific targeting mechanisms of ligand-receptor interactions for use with complexation hydrogels to ultimately deliver a significant amount of protein to the bloodstream through the transcellular route.7 The goal of this work was to synthesize insulin/transferrin conjugates for evaluation of cellular transport characteristics using a Caco-2/HT29-MTX cellular model. This work achieves several objectives beyond the scope of the prior work from our laboratory on the subject.7 As evident in the results and discussion section, this work addresses new factors related to the insulin/transferrin oral delivery system such as conjugate size related to diffusion, the use of a co-culture for more reliable diffusion and permeability results, and determina- tion of a more realistic microparticle concentration for cellular studies, resulting in an increased understanding of their effect on transcellular transport versus the previous work on the subject.
The addition of the transporter transferrin to the therapeutic protein insulin allows for specific targeting of the bioconjugate entity as well as potentially increased transport. Transferrin is a glycoprotein which is normally involved in iron transport. The transferrin receptor is expressed on human intestinal epithelial cells. When evaluating the potential of a novel oral dosage form for oral delivery, it is necessary to examine cellular interactions with the therapeutic entity using cellular models. The cellular model used for evaluation of insulin/transferrin bioconjugates was a co-culture consisting of both absorptive enterocyte-like Caco-2 cells which are commonly used to determine molecule permeability8–11 and mucus-producing HT29-MTX goblet cells.12 The advantage of using a Caco-2/HT29-MTX co-culture versus a simpler Caco-2 cellular model is in observing the effect that intestinal mucus will play in the diffusion of the large insulin/transferrin conjugate molecules, resulting in a more accurate depiction of overall permeability. A system consisting solely of Caco-2 cells used to measure permeability of large molecules the size of the insulin/transferrin conjugates would disregard the significant effect that diffusion through the mucus has on permeability. The Caco-2/HT29-MTX co-culture was used to compare the permeability and transport of insulin/transferrin conjugates to native insulin. Additionally, cellular studies were performed in the presence of the P(MAA-g-EG) microparticles to give insight into the effect of complexation hydrogels on the transport processes of the epithelial cell monolayer. Based on the results of the cellular transport studies, the overall rate of transport was quantified as an overall apparent permeability, Papp, for the study. By comparing the apparent permeability values for each set of conditions, conclusions were drawn regarding the effect of protein-transporter conjugation and the presence of complexation hydrogels on epithelial transport of insulin.
Materials and Methods
Hydrogel Synthesis
P(MAA-g-EG) was prepared using a free radical UV polymerization in solution. P(MAA-g-EG) was prepared by mixing methacrylic acid (MAA, Sigma-Aldrich Inc., St. Louis, MO) with poly(ethylene glycol) monomethyl ether monomethacrylate with a approximate molecular weight of 1000 Da (PEGMA1000, Polysciences Inc., Warrington, PA) in a 1:1 molar ratio of MAA/ethylene glycol. The crosslinker used within the polymer network was poly(ethylene glycol) dimethacrylate with an approximately molecular weight of 1000 (PEGDMA1000, Polysciences Inc., Warrington, PA). The amount of crosslinker added to each monomer mixture was equal to 1 mol% of the total amount of monomer. In order to eventually initiate the polymerization, the photoinitiator 1-hydroxycyclohexyl phenyl ketone (Irgacure 184, Sigma-Aldrich Inc., St. Louis, MO) was added to the polymerization mixture in the amount of 1 wt% of the total monomer added. A solvent mixture consisting of 50:50 by weight deionized water (Milli-Q Plus system, Millipore) and ethanol (AAPER Alcohol, Shelbyville, KY) was added to the polymer mixture in a 1:1 ratio by weight relative to monomer present in the prepolymer mixture. The presence of the solvent solution is essential to prevent autopolymerization as well as to produce a workable thin polymer film.
To ensure all components dissolved and went into a homogenous solution, the polymer mixture was sonicated for 15 minutes. After sonication, nitrogen was bubbled through the polymer solution within a nitrogen environment to eliminate oxygen. Oxygen is a free radical scavenger and significant oxygen levels could prematurely end the polymerization process. After removal of oxygen by nitrogen purging, the polymer mixture was poured between two glass slides (153 × 153 × 3 mm) separated by a Teflon spacer (0.7 mm) while still in a nitrogen environment. The glass slide apparatus containing the polymer solution spread into a thin film was then placed under a UV light source while still in a nitrogen environment. The solution was allowed to polymerize under the light source within an intensity range of 16–17 mW/cm2 for 30 minutes. After the polymerization was completed, the polymer gels were removed from the nitrogen environment, separated from the glass slides, and placed in deionized water. The polymer films were washed in the deionized water for 7 days to remove excess monomer and contaminants. After washing was completed, the polymers were dried in a vacuum oven at approximately 30° C for 2 days. After completely drying the polymer films, they were crushed into microparticles using a mortar and pestle to sizes less than 75 microns. The sieved microparticles were then stored in a vial within a desiccator to prevent moisture entering until further use.
Synthesis of Insulin-Transferrin Conjugates
The method for protein conjugation outlined in this section was originally developed by Carlsson et al13. The protein conjugation method was first utilized for the conjugation of insulin to transferrin by Shah and Shen14. The conjugation scheme consists of using N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) as a protein crosslinker to conjugate insulin (Ins) to transferrin (Tf) to form insulin-transferrin (Ins-Tf) heteroconjugates. To start the reaction, the n-terminal primary amines of the insulin chains were effectively “blocked” by reaction of insulin with dimethylmaleic anhydride (DMMA, Fluka/Sigma Aldrich Inc., St. Louis, MO), excluding possible reactive sites for SPDP. To begin the reaction, 10.5 mg of DMMA was added to a stock solution of insulin (Sigma Aldrich Inc., St. Louis, MO) and reacted for approximately one hour. During the reaction period, the pH was maintained within the pH range of 6.8–6.9 by adding small volumes of a 1 M Na2CO3 (Fisher Scientific, Fair Lawn, NJ) solution. After the reaction had finished, the insulin-DMMA intermediate was purified by dialysis (MWCO 3,500, Spectrum Laboratories Inc., Rancho Dominguez, CA) for at least 24 hours to remove unreacted DMMA.
Insulin-DMMA was reacted with 6.0 mg of SPDP (Pierce Biotechnology Inc., Rockford, IL) to add disulfide bonds to the remaining unblocked primary amine at B29-Lysine. The reaction was performed at 4°C under constant stirring within a pH range of 8.8–9.0. The pH was maintained by adding small volumes of 1 M Na2CO3. Upon completion of the reaction time period, the insulin-PDP product was purified by dialysis (MWCO 3,500) at 4°C for at least 24 hours to remove unreacted SPDP reagent. In addition to the modification of insulin, transferrin (Sigma Aldrich Inc., St. Louis, MO) was also reacted with SPDP to form PDP groups on the transferrin molecule. To initiate the reaction, 8.0 mg of SPDP was added to a solution containing 120 mg of human holo-transferrin in PBS buffer (pH 7.0). The reaction was held at approximately 4°C and allowed to react for 2 hours. The transferrin-PDP product was purified through dialysis (MWCO 12,000–14,000, Spectrum Laboratories Inc., Rancho Dominguez, CA) in a solution of PBS buffer (pH 8.0) overnight to remove unreacted SPDP. After formation of PDP groups on transferrin, the modified protein was reduced by the addition of 1 M dithiothreitol (DTT, Sigma Aldrich Inc., St. Louis, MO). The transferrin-PDP was reduced using DTT for one hour, forming a product with attached sulfohydryl groups (Tf-SH). The transferrin-SH product was purified by dialysis for at least 24 hours to remove unreacted DTT.
Upon formation of transferrin-SH, the product was purified by elution from D-Salt Dextran Desalting Columns (10 mL, Pierce Biotechnology Inc., Rockford, IL). To initiate the final step in the conjugation reaction, the product solutions of Ins-PDP and Tf-SH were combined and allowed to react under constant stirring. The reaction was allowed to proceed for approximately 90 minutes before it was stopped by the addition of n-ethylmaleimide (NEM, ACROS Organics, Geel, Belgium), which reacts with free thiol groups15 and prevents further crosslinking. The final insulin-transferrin product was purified by dialysis (MWCO 12,000–14,000) for at least 48 hours to remove unreacted reagents. To determine the concentration of the conjugate solution, the product was analyzed using HPLC (Waters Corporation, Milford, MA). After analysis, the conjugate solution was refrigerated until further use.
Development of Caco-2/HT29-MTX Monolayers
To form the Caco-2/HT29-MTX monolayer, both Caco-2 and HT29-MTX cells were initially cultured in culturing flasks (75 cm2, VWR Scientific, West Chester, PA) with 10 mL of culture media (DMEM, Biofluids Inc., Rockville, MD) containing additional supplements. Cultivation was performed at a seeding density of 2.5 × 105 cells per flask for the Caco-2 cells and 1.25 × 105 cells per flask for the HT29-MTX cells. During cultivation, the cells were incubated at 37° C, 95% relative humidity, and 5% CO2 while the culture media was replaced every other day until the cells reached 70–80% confluency. After reaching the desired confluency, both types of cells were passaged at least once prior to combination of the cell types. After passaging, the Caco-2 and HT29-MTX cells were transferred at the desired seeding density to experimental wells in a 1:1 ratio as to obtain a 50% Caco-2/50% HT29-MTX experimental co-culture monolayer.
For the transport studies, a co-culture of Caco-2/HT29-MTX cells was grown in 6-well Transwell® plates (4.71 cm2/well, Costar Corning Inc., Corning, NY). The cell density in the experimental wells was approximately 3 × 105 cells/well with the composition being 50% of each cell type. The co-culture was grown in the culture medium for approximately 21–23 days until the monolayer achieved a constant transepithelial electrical resistance (TEER) as measured by a chopstick electrode (World Precision Instrument, Sarasota, FL). Typically, a constant TEER value indicates that the monolayer has formed tight junctions16,17. During the formation of the monolayer, the culture media was changed every other day and the TEER was monitored regularly.
Upon the formation of tight junctions, the transport studies began by allowing the cells to equilibrate with Hank’s Balanced Salt Solution (HBSS, HyClone, Logan, UT), the experimental medium for the study. HBSS is typically listed having a pH of 7.1, which closely mimics the conditions at the targeted site of delivery in the upper ileum. Though the pH of the small intestine varies throughout its length, the mucoadhesive nature of the hydrogels ensures that delivery should occur early in transit and in the upper ileum4. The HBSS also contained Ca2+ ions to ensure regulation of intracellular Ca2+ ions, which are instrumental in maintaining the integrity of the tight junctions. After addition of the HBSS, the TEER of the cell monolayer was monitored for the next hour with samples regularly withdrawn. The conclusion of the equilibration period was marked by the achievement of a constant TEER, indicating adjustment to the HBSS solution as well as the ability to begin the transport studies.
Protein Transport Across the Cell Monolayer
For the studies in which only protein was present in the apical chamber, insulin or insulin-transferrin was dissolved in warm HBSS at a concentration of 0.2 mg/mL. To begin the study, the previous HBSS solution was removed from the apical chamber. Approximately 1.5 mL of the solution containing either the insulin or insulin-transferrin was placed in the apical chamber. The Transwell® plates were then placed in an incubator at 37° C. Samples of 100 µL were withdrawn from the basolateral chambers at time points of 0, 0.5, 1, 2, and 3 hours after the addition of protein solution to the well. The TEER of the monolayer was also measured at the withdrawal of each sample. Throughout the course of the transport study, all measurements were taken while maintaining the Transwell® plate at 37° C, ensuring no changes in the integrity of the tight junctions or in the amount of transcellular transport as both vary significantly as temperature varies. The samples were placed in small vials and refrigerated until they could be analyzed. Analysis of protein concentration of the apical chamber was performed by HPLC and protein concentration in the basolateral chamber was determined by use of an ELISA kit.
Protein Transport Across the Cell Monolayer in the Presence of P(MAA-g-EG) Microparticles
In addition to transport studies using only proteins, more transport studies were performed to determine the effect of the presence of P(MAA-g-EG) microparticles on the cellular transport of both insulin and insulin/transferrin conjugates.
As with the previous transport studies, solutions of either insulin or insulin-transferrin conjugates were prepared at a concentration of 0.2 mg/mL in warm HBSS. After removing the HBSS used for equilibration of the monolayer from the apical chamber, approximately 1.5 mL of either of the protein solutions was added to refill the apical chamber with sample. Immediately after addition of the protein solutions to the apical side, dry P(MAA-g-EG) microparticles were added to the apical chamber at a concentration of 1 mg/mL. Sampling of both the apical and basolateral chambers occurred in the same manner as previously described and all samples were analyzed by either HPLC (apical samples) or using an ELISA kit (basolateral samples).
Results and Discussion
Protein Transport Across the Cell Monolayer
Solutions of either insulin or insulin-transferrin conjugates were investigated through transport studies. In the studies, samples were withdrawn from the basolateral chamber at regular time points to determine the amount of protein transported over time. The transport profiles were fit using a linear regression model and the slope of the fit was used to calculate apparent permeability values, Papp, for each of the protein samples.
In order to calculate an apparent permeability for protein transport, the following equation was used:
where Q(t) is the cumulative amount of protein (mg) transported at time t, A is the area of the cell monolayer (cm2), and CA0 is the initial protein concentration in the apical (donor) compartment (mg/cm3). The term dQ(t)/dt is represented by the slope of the linear fit to the data plotting cumulative protein transported (Q(t)) versus time (t). It should be noted that the lines drawn on Figures 1, 3, 4, and 6 are slope lines representative of the dQ(t)/dt term in determining apparent permeability values. The lines are only a reflection of average protein transport over the entire course of the study, not a predictive model intended to represent the data trends. In fact, certain types of transport, particularly transcellular transport, may not transport protein linearly but instead in a non-linear fashion based on the timing of cellular processes. Apparent permeability values were calculated over the course of the entire study versus between each data point to provide an overall comparison of protein transport within the different transport mechanisms that may be occurring.
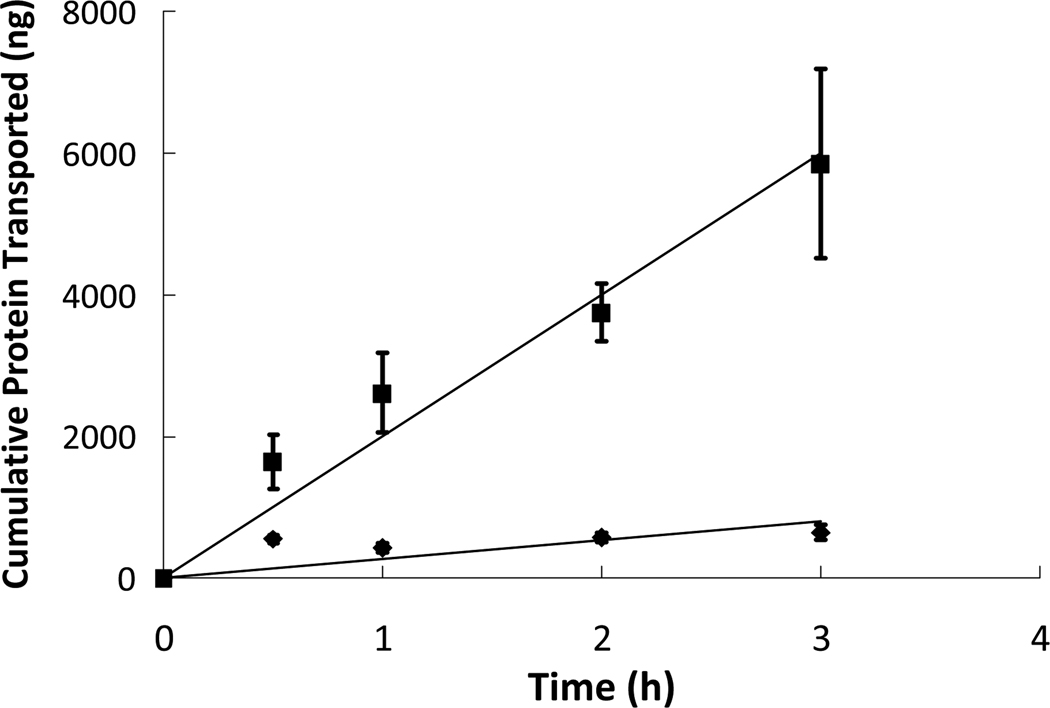
Figure 1.
Insulin ( ) and insulin-transferrin conjugates (
) and insulin-transferrin conjugates ( ) were placed in the apical chamber at a concentration of 0.2 mg/mL and were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
) were placed in the apical chamber at a concentration of 0.2 mg/mL and were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
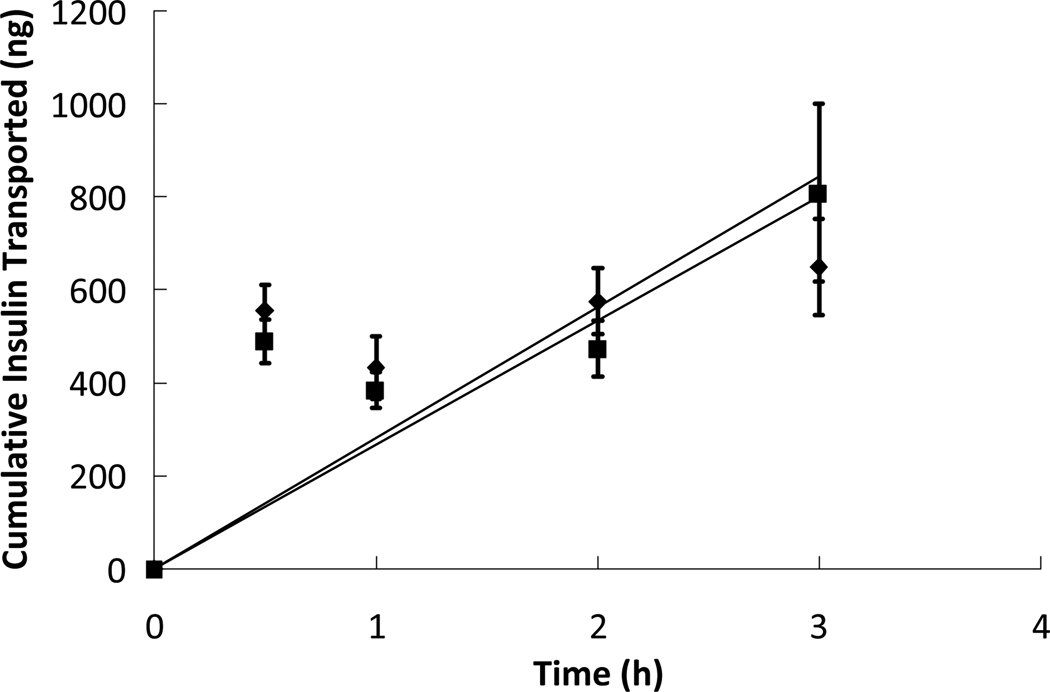
Figure 3.
Insulin was placed in the apical chamber without the presence of P(MAA-g-EG) microparticles ( ) and while in the presence of P(MAA-g-EG) microparticles (
) and while in the presence of P(MAA-g-EG) microparticles ( ) at a protein concentration of 0.2 mg/mL and a microparticle concentration of 1 mg/mL. The insulin was allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
) at a protein concentration of 0.2 mg/mL and a microparticle concentration of 1 mg/mL. The insulin was allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
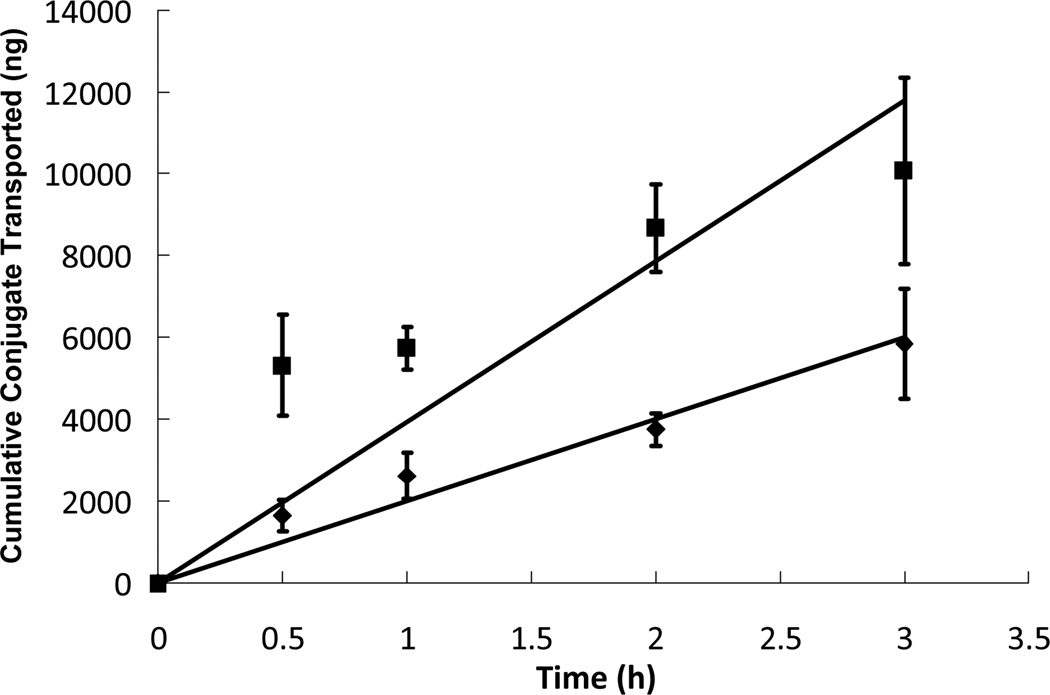
Figure 4.
Insulin-transferrin was placed in the apical chamber without the presence of P(MAA-g-EG) microparticles ( ) and while in the presence of P(MAA-g-EG) microparticles (
) and while in the presence of P(MAA-g-EG) microparticles ( ) at a protein concentration of 0.2 mg/mL and a microparticle concentration of 1 mg/mL. The insulin-transferrin conjugates were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
) at a protein concentration of 0.2 mg/mL and a microparticle concentration of 1 mg/mL. The insulin-transferrin conjugates were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
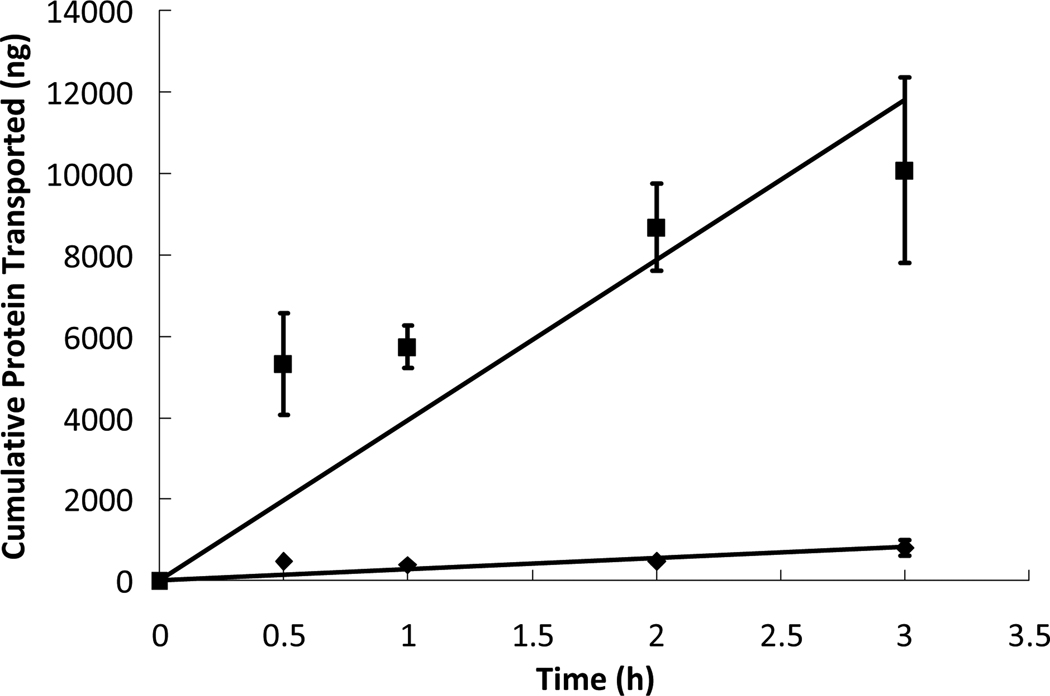
Figure 6.
Insulin ( ) and insulin-transferrin conjugates (
) and insulin-transferrin conjugates ( ) were placed in the apical chamber at a protein concentration of 0.2 mg/mL while in the presence of P(MAA-g-EG) microparticles at a particle concentration of 1 mg/mL and were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
) were placed in the apical chamber at a protein concentration of 0.2 mg/mL while in the presence of P(MAA-g-EG) microparticles at a particle concentration of 1 mg/mL and were allowed to diffuse through a cell monolayer of Caco-2/HT29-MTX for 3 hours. The protein concentrations in the basolateral chamber were measured at time points of 0.5, 1, 2, and 3 hours using ELISA and compared to standards of known concentrations. n = 9 ± SD
As an experimental control, a solution of insulin dissolved in HBSS was added to the apical chamber and was allowed to interact with the cell monolayer. The apparent permeability of the insulin relative to the cell monolayer was determined to be 4.95 × 109 cm/s using the equation shown above. The extent of transport as well as the apparent permeability of the insulin control served as a standard of comparison for the experimental protein solutions. To determine the effect of the conjugation of a transporter protein to insulin, the transport of a solution of insulin-transferrin conjugates across the cell monolayer was measured and analyzed. In comparison to the insulin control, the transport occurred to a much greater extent and also in a much more linear fashion. The linearity of the transport can most likely be attributed to the timing of cellular mechanisms involved in transcellular transport, leading to a slower but more linear transport profile. The apparent permeability, Papp, of the insulin-transferrin conjugates across the Caco-2/HT29-MTX monolayer was calculated and found to be 37.0 × 109 cm/s. The transport profiles of the insulin control and insulin-transferrin are shown in Figure 1. To compare the protein transport studies, the most simple and direct method is to compare the apparent permeability values between the different protein solutions. According to the values, the insulin-transferrin molecule has more than seven times the permeability compared to unmodified insulin. Increasing transport of a potential therapeutic more than sevenfold has dramatic potential ramifications on the bioavailability of the drug in the bloodstream. Based on the results of the transport studies conducted using solutions containing only protein, the transport of an orally administered therapeutic protein can be greatly increased by conjugating the drug to a transporter molecule such as transferrin.
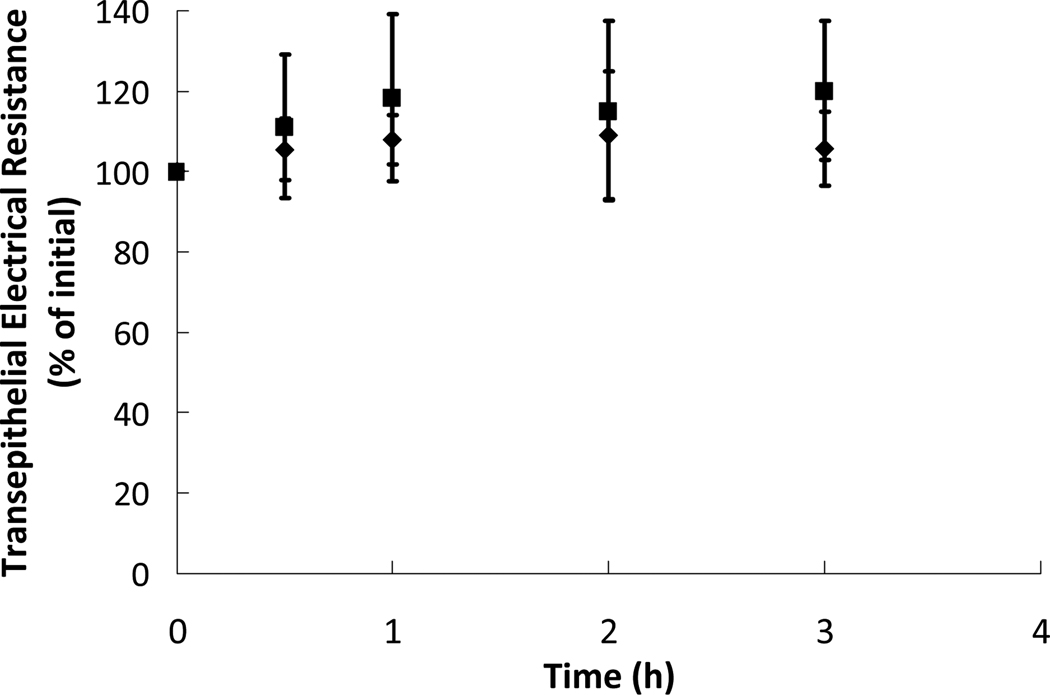
While performing the transport studies, it was important to monitor the integrity of the cell monolayer and the tight junctions as to ensure that administration of such therapeutics would not cause the epithelial cell layer to have a lessened ability to expel dangerous viruses or toxins. Over the course of each transport study, the transepithelial electrical resistance was measured as a means of monitoring tight junction integrity. The TEER values throughout both transport studies are shown as Figure 2. As evident in Figure 2, the TEER of the Caco-2/HT29-MTX monolayer stayed near 100% of the initial value throughout the course of each transport study. The absolute TEER values for all the co-cultures in the transport studies were recorded in the range of 85–95 Ω*cm2. It should be noted that Caco-2/HT29-MTX co-cultures have been shown to have lower TEER values relative to pure Caco-2 cell monolayers18. However, it should also be noted that the typical TEER values of Caco-2/HT29-MTX co-cultures more closely represent the TEER values of the human intestine, known to range from 50–100 Ω*cm2.19 The maintenance of steady TEER values in these experiments indicates that the proteins (insulin and insulin-transferrin) do not have a disruptive effect on the cell monolayer, ensuring their presence upon administration will not cause illness due to uptake of dangerous toxins or viruses through a weakened epithelial cell barrier.
Figure 2.
Transepithelial electrical resistances (TEER) of cell monolayers of Caco-2/HT29-MTX co-cultures over the course of a 3 hour transport study using insulin ( ) and insulin-transferrin conjugates (
) and insulin-transferrin conjugates ( ) at a protein concentration of 0.2 mg/mL. The values are percentages of the TEER as compared to the initial values before the study began. n = 9 ± SD
) at a protein concentration of 0.2 mg/mL. The values are percentages of the TEER as compared to the initial values before the study began. n = 9 ± SD
Protein Transport Across the Cell Monolayer in the Presence of P(MAA-g-EG) Microparticles
To determine the effect of the presence of polymer microparticles on cellular insulin transport, a prepared solution of insulin dissolved in HBSS with added P(MAA-g-EG) microparticles was added to the apical chamber and was allowed to interact with the cell monolayer. The apparent permeability, Papp, of the insulin in the presence of polymer microparticles was determined to be 5.20 × 109 cm/s. The apparent permeability of insulin in the presence of polymer microparticles is slightly higher than the value obtained for insulin alone, indicating that the presence of the microparticles may have a slight permeation enhancing effect for the paracellular transport of insulin. However, while the apparent permeability value is slightly higher for transport in the presence of microparticles, the difference in amount of protein transported is not significant between the cases. Therefore, the presence of microparticles at this concentration has little to no effect on insulin transport. The transport profiles of insulin with and without the presence of microparticles are shown in Figure 3. A higher concentration of microparticles in solution would have most likely caused a significant increase in insulin transport due to concurrently increased calcium binding capacity, further loosening the tight junctions of the cell monolayer as shown in previous work from our laboratory5.
Similar transport studies were also conducted which used solutions of insulin-transferrin conjugates in the presence of P(MAA-g-EG) microparticles to determine if the microparticles had a more pronounced effect on insulin transport. The apparent permeability of the insulin-transferrin conjugates across the Caco-2/HT29-MTX monolayer in the presence of polymer microparticles was calculated and found to be 72.8 × 109 cm/s. In comparison to the transport of insulin-transferrin conjugates alone, the permeability of the conjugate nearly doubled in the presence of polymer microparticles. The transport profiles of insulin-transferrin with and without the presence of microparticles are shown in Figure 4. Though it is not completely clear whether the presence of the microparticles enhanced transcellular transport of the conjugate or simply opened up paracellular pathways, it is evident that transport was greatly increased. The previous study showed that the presence of polymer microparticles had little to no effect on insulin transport at the given microparticle concentration. However, at the same concentration, the microparticles were able to enhance insulin-transferrin transport by nearly 100%. The drastic difference in enhanced transport suggests that P(MAA-g-EG) microparticles act as better enhancers for transport that has been hypothesized to be transcellular versus that which has been thought to be paracellular. In this study, while improved conjugate transport could be attributed to newly opened paracellular pathways, the absence of improved transport in the previous case seems to negate this idea. Our hypothesis is that the microparticles facilitated transcellular transport, possibly through calcium binding, the consequent loss of intracellular calcium, and the resultant effect which lowered calcium has on transcytotic processes. However, to rigorously determine the effect of the microparticles on the transport mechanism of insulin-transferrin conjugates, further investigation into the cellular transport mechanisms of intestinal cells as well as more in-depth public knowledge about the intracellular transferrin mechanisms would be required.
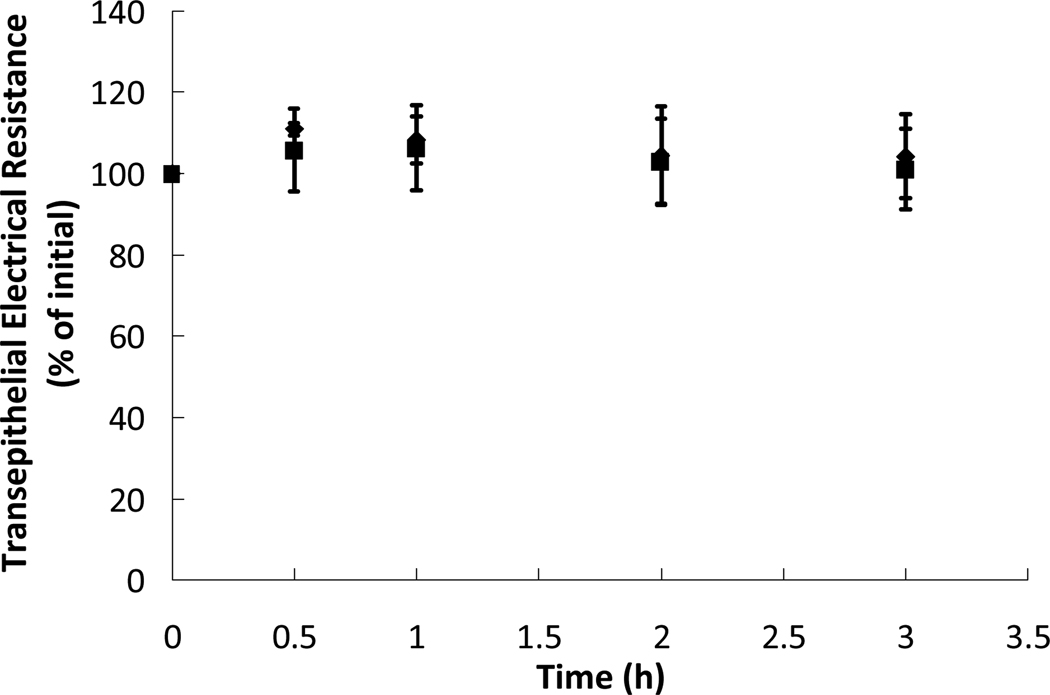
To gain a greater understanding of the effect of microparticles on the integrity of the tight junctions and the monolayer, the transepithelial electrical resistance (TEER) was measured over the course of the transport studies. The TEER values throughout the two transport studies are shown as Figure 5. As can be seen in Figure 5, the TEER of the Caco-2/HT29-MTX monolayer stayed near 100% of the initial value throughout the course of both transport studies. Steady TEER values throughout the studies indicate little to no disruption of the monolayer in the presence of both proteins and P(MAA-g-EG) microparticles. While previous studies have shown that the TEER values and the tight junctions will be affected by the presence of polymer microparticles20,21, the microparticles used in this study were likely not introduced at a high enough concentration to achieve the significant disruptive effect that had been observed in previous studies.
Figure 5.
Transepithelial electrical resistances (TEER) of cell monolayers of Caco-2/HT29-MTX co-cultures over the course of a 3 hour transport study using insulin ( ) and insulin-transferrin conjugates (
) and insulin-transferrin conjugates ( ) at protein concentrations of 0.2 mg/mL. Also present in the apical chamber were P(MAA-g-EG) microparticles at a concentration of 1 mg/mL. The values are percentages of the TEER as compared to the initial values before the study began. n = 9 ± SD
) at protein concentrations of 0.2 mg/mL. Also present in the apical chamber were P(MAA-g-EG) microparticles at a concentration of 1 mg/mL. The values are percentages of the TEER as compared to the initial values before the study began. n = 9 ± SD
Combinatorial Effect of Transferrin Conjugation and Presence of Microparticles
It is also useful to determine the effect of transferrin conjugation on insulin transport while in the presence of microparticles. The transport profiles of insulin and insulin-transferrin in the presence of microparticles can be compared in Figure 6. Simply replacing insulin with insulin-transferrin conjugates at the same concentration but in the presence of microparticles increased the apparent permeability by a factor of nearly fourteen. Assuming that a formulation of insulin-transferrin conjugates loaded into P(MAA-g-EG) microparticles could deliver the same amount of protein to the site of absorption as an insulin-loaded microparticle, the benefit of having potentially fourteen times the cellular transport would translate into a much more effective drug with significantly higher bioavailability.
To compare all of the transport studies quantitatively, it is important to compare the apparent permeability values. The apparent permeability values for all of the transport studies can be directly examined in Table 1. Based on the permeability values, the insulin-transferrin conjugate alone achieves more than seven times the transport of insulin alone. Meanwhile, the addition of P(MAA-g-EG) microparticles increases insulin-transferrin transport by nearly 100%. Due to the ability of the microparticles to enhance conjugate transport, the insulin-transferrin conjugate achieves nearly fourteen times the transport that insulin does in the presence of the microparticles. The progress achieved in this work is significant relative to previous work from the laboratory in the area7. While the protein (insulin) and transporter ligand (transferrin) used are the same, the conjugates in this work were constructed to be slightly smaller (i.e. lower insulin to transferrin ratio) than the previous work to improve epithelial transport. The conjugate size can have a profound effect on the diffusion of the entity through the mucosa of the small intestine. Thus, the use of a smaller conjugate has resulted in increased transport characteristics relative to studies performed previously in our laboratory7. The effect of conjugate size is magnified by the use of a co-culture of Caco-2/HT29-MTX cells; the use of these cells provides a more accurate depiction of condition in the small intestine as well as more accurate conditions for diffusion due to the mucus secreted by the HT29-MTX cells. A simple Caco-2 cell study is acceptable for determining permeability of small molecules, but the diffusion in the mucus of the intestine cannot be ignored for large molecules such as the insulin-transferrin conjugate, thus requiring the need for the co-culture permeability study as contrasted with the previous work from our laboratory7. Also, this study is the first from our laboratory to accurately measure the effect of the microparticles on overall transport of insulin-transferrin conjugates. In previous studies, the concentration of particles used was too high to the point of adversely affecting cell integrity. Using a more realistic concentration yields no loss of cell integrity but instead a possible enhancement to transcellular transport. Moreover, the concentration of microparticles used in the transport study more accurately reflects the estimated amount of particles per approximate area of the small intestine. All of these factors combined result in significant research findings that further the possibility of insulin-transferrin systems being used in future drug delivery designs.
Table 1.
Apparent permeability values for each protein and particle solution from transport studies.
| Transport Sample | Apparent Permeability (Papp) |
|---|---|
| Insulin (control) | 4.95 × 109 cm/s |
| Insulin with P(MAA-g-EG) Particles | 5.20 × 109 cm/s |
| Insulin-Transferrin Conjugate | 37.0 × 109 cm/s |
| Conjugate with P(MAA-g-EG) Particles | 72.8 × 109 cm/s |
Considering all of the transport studies performed in this work, modification of therapeutic proteins by conjugation to transporter molecules remains an attractive option for increasing epithelial transport and achieving high bioavailability.
Conclusions
Cellular evaluation of the insulin-transferrin conjugate was performed using a co-culture Caco-2/HT29-MTX cell model. The conjugate was investigated by conducting transport studies on both the insulin-transferrin conjugate and an insulin control with and without the presence of microparticles. The results of the transport studies indicated a sevenfold increase in transport by replacing insulin with insulin-transferrin conjugates. Also, the presence of P(MAA-g-EG) microparticles enhanced transport for the insulin-transferrin conjugate by nearly 100% while having minimal effect on pure insulin transport, indicating that the polymer microparticles may assist in transcytotic processes. Finally, the transport studies also demonstrated that a 14-fold increase in transport can be achieved by switching from insulin in the presence of polymer microparticles to insulin-transferrin conjugates in the presence of polymer microparticles. The specific targeting and increased transport of the insulin-transferrin conjugate in combination with the protection and site-specific release capabilities of P(MAA-g-EG) microparticles combine synergistically to form a potential dosage form which could effectively deliver therapeutics to the small intestine and across the epithelium, resulting in high bioavailability in the bloodstream.
Acknowledgements
This research was supported by grants from the (US) National Institutes of Health (EB-000246) and the (US) National Science Foundation-IGERT Fellowship for JPS.
REFERENCES
- 1.Fasano A. Novel approaches for oral delivery of macromolecules. J. Pharm. Sci. 1998;87(11):1351–1356. doi: 10.1021/js980076h. [DOI] [PubMed] [Google Scholar]
- 2.Yamagata T, Morishita M, Kavimandan NJ, Nakamura K, Fukuoka Y, Takayama K, Peppas NA. Characterization of insulin protection properties of complexation hydrogels in gastric and intestinal enzyme fluids. J. Control. Release. 2006;112(3):343–349. doi: 10.1016/j.jconrel.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17(16):1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 4.Serra L, Domenech J, Peppas NA. Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems. Eur. J. Pharm. Biopharm. 2006;63(1):11–18. doi: 10.1016/j.ejpb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20(18):1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 6.Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J. Control. Release. 2004;97(1):115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin-transferrin conjugates. Biomaterials. 2006;27(20):3846–3854. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Artursson P, Lindmark T, Davis SS, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm. Res. 1994;11(9):1358–1361. doi: 10.1023/a:1018967116988. [DOI] [PubMed] [Google Scholar]
- 9.Artursson P, Borchardt RT. Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm. Res. 1997;14(12):1655–1658. doi: 10.1023/a:1012155124489. [DOI] [PubMed] [Google Scholar]
- 10.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliver. Rev. 2001;46(1–3):27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 11.Ungell A-LB. Caco-2 replace or refine? Drug Discov. Today: Technologies. 2004;1(4):423–430. doi: 10.1016/j.ddtec.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Walter E, Blake SJ, Roessler J, Hilfinger JM, Amidon GL. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro-in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996;85(10):1070–1076. doi: 10.1021/js960110x. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson J, Drevin H, Axen R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem. J. 1978;173:723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv. Drug Deliver. Rev. 2003;55(11):1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Faulstich H, Zobeley S, Heintz D, Drewes G. Probing the phalloidin binding site of actin. FEBS Lett. 1993;318(3):218–222. doi: 10.1016/0014-5793(93)80515-v. [DOI] [PubMed] [Google Scholar]
- 16.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am. J. Physiol. Renal Physiol. 1998;274(1):F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. Cell Physiol. 1987;253(6):C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 18.Wood KM, Stone GM, Peppas NA. The effect of complexation hydrogels on insulin transport in intestinal epithelial cell models. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.05.032. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balimane PV, Chong S. Cell culture-based models for intestinal permeability: a critique. Drug Discov. Today. 2005;10(5):335–343. doi: 10.1016/S1359-6446(04)03354-9. [DOI] [PubMed] [Google Scholar]
- 20.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with Caco-2 cultures. Eur. J. Pharm. Biopharm. 2004;57(3):447–455. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: In vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J. Biomed. Mater. Res. Part A. 2003;67A(2):609–617. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]