Abstract
Aim:
As per traditional claims, root, bark, leaf and flower of the plant Cassia occidentalis Linn. (Caesalpiniaceae) have been reported to possess antidiabetic activity. Based on this traditional indication, the aim of this study was to evaluate the antidiabetic activity of ethanolic extract of C. occidentalis in normal and alloxan induced diabetic rats.
Materials and Methods:
Ethanolic extract of the whole plant of C. occidentalis was orally tested at doses of 100 and 200 mg/kg for evaluating the hypoglycemic effect in normal and alloxan-induced diabetic rats. In addition, changes in body weight, serum cholesterol, triglyceride and total protein levels, assessed in the ethanol extract treated diabetic rats were compared with diabetic control and normal animals. Histopathologic observations during 21 days of treatment were also evaluated.
Results:
Ethanolic extract of C. occidentalis produced a significant reduction in fasting blood glucose levels in the normal and alloxan-induced diabetic rats at doses of 100 and 200 mg/kg body weight. Treatment with ethanolic extract of C. occidentalis in normal and alloxan-induced diabetic rats led to a dose-dependent fall in blood sugar levels. Significant differences were observed in serum lipid profiles (cholesterol and triglyceride), serum protein and changes in body weight in ethanolic extract treated diabetic animals, when compared with the diabetic control and normal animals. Concurrent histopathologic studies of the pancreas of these animals showed comparable regeneration by ethanolic extract, which were earlier necrosed by alloxan.
Conclusion:
Ethanolic extract of C. occidentalis exhibited significant antidiabetic activity in normal and alloxan-induced diabetic rats. The rats also showed improvement in parameters like body weight and lipid profiles and also, histopathologic studies showed regeneration of β-cells of pancreas and so it might be of value in the treatment of diabetes.
Keywords: Antidiabetic activity, Cassia occidentalis, histopathology, alloxan
INTRODUCTION
In conventional therapy, type I diabetes is treated with exogenous insulin and type 2 with oral hypoglycemic agents (sulfonylureas, biguanides, etc.).[1] In traditional practice, medicinal plants are used in many countries to control diabetes mellitus.[2] Diabetes mellitus is a chronic metabolic disorder resulting from insulin deficiency, characterized by hyperglycemia, altered metabolism of carbohydrates, protein and lipids, and an increased risk of vascular complication.[3]
Diabetes mellitus has recently been identified by Indian Council of Medical Research (ICMR) as one of the refractory diseases for which satisfactory treatment is not available in modern allopathic system of medicine and suitable herbal preparations are to be investigated. A large number of plant preparations have been reported to possess antidiabetic activity over the last several decades. Researchers in India have documented the use of over 150 plants in various families with hypoglycemic activity.[4]
Cassia occidentalis Linn. (COL) Family Caesalpiniaceae is a common weed scattered from the foothills of Himalayas to West Bengal, South India, Burma, and Sri Lanka.[5] The plant is a diffuse (usually annual) undershrub with loosely spreading branches 60–150 cm long, found throughout India, up to an altitude of 1500 m.[6] Different parts of this plant have been reported to possess anti-inflammatory,[7] antihepatotoxic,[8] antibacterial[9] and antiplasmodial activities.[10] They possess purgative, tonic, febrifugal, expectorant and diuretic properties. The plant is also used to cure sore eyes, hematuria, rheumatism, typhoid, asthma and disorder of hemoglobin and is also reported to cure leprosy. An infusion of the bark is given in diabetes. A wide range of chemical constituents isolated from C. occidentalis including sennoside,[11] anthraquinone glycoside,[12] fatty oils, flavonoids, glycosides,[13] gallactomannan, polysaccharides and tannins.[14] Therefore, the aim of this study was to evaluate the antidiabetic potential of ethanolic extract of C. occidentalis (COL) on fasting blood sugar levels and biochemical parameters such as serum cholesterol, total protein and triglyceride. Histologic examination was also carried out on hematoxylin-eosin stained sections of pancreatic tissue.
MATERIALS AND METHODS
Plant material
The plant of C. occidentalis (COL) was collected from Kaaripatti, Salem district, Tamil Nadu, with the help of a field botanist. The plant of COL had been authenticated by Prof. A. Balasubramanion, horticulturist, director of ABS Botanical Conservation, Research and Training Centre, Kaaripatti, Salem district, Tamil Nadu, India (Ref. no. ABSRTC/08/A-4069). The whole plant was dried initially under shade. It was preserved in tightly closed container and powdered as per the requirements.
Preparation of extracts
The dried whole plant was subjected to size reduction to a coarse powder by using dry grinder and passed through sieve. About 150 g of this powder was packed into soxhlet apparatus and extracted with ethanol for 72 hours (percentage yield 1.49%). The solvent was recovered by distillation in vacuo and the extract was stored in a desiccator and used for subsequent experiments.
Preliminary phytochemical screening
Ethanolic extract of COL was subjected to various qualitative tests for the identification of various plant constituents present in this species.[15,16]
Animals
Healthy adult male Wistar albino rats between 2 and 3 months of age and weighing about 150–200 g were used for the study. The animals were housed in polypropylene cages, maintained under standard conditions (12 hours light:2 hours dark cycle; 25 ± 30°C; 35–60% humidity). They were fed with standard rat pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The Institutional Animal Ethical Committee of VNS, Bhopal, Madhya Pradesh, India (778/03/c/CPCSEA) approved the study.
Sample collection
Blood samples were collected by retro-orbital plexus puncture method from overnight fasted rats under light ether anesthesia and blood glucose levels were estimated using Accu-chek Active™ glucose strips in Accu-chek Active Test Meter.
Acute toxicity study
Normal healthy rats were divided into five groups of six animals each. Different doses (100, 250, 500, 750 and 1000 mg/kg body weight) of ethanolic extract of plant COL were administered orally. The rats were observed continuously for 2 hours for behavioral, neurological and autonomic profiles and after 24 and 72 hours for any lethality.[17]
Antihyperglycemic studies
Normal fasted rat model
For normoglycemic study, albino rats were divided into four groups (n = 6) and were orally administered 2% gum acacia solution, 0.5 g/kg of metformin,[18] ethanolic extract 100 and 200 mg/kg body weight, respectively. The blood glucose levels were measured just prior to and 2, 4 and 6 hours after drug administration.[19]
Alloxan-induced diabetic rat model
Diabetes was induced in rats by injecting 120 mg/kg of alloxan monohydrate intraperitoneally in 0.9% w/v NaCl to overnight fasted rats. The rats were then kept for the next 24 hours on 10% glucose solution, in their cases to prevent hypoglycemia.[20] After 72 hours of injection, rats with marked hyperglycemia (fasting blood glucose > 250 mg/dl) were selected and used for study. The selected diabetic animals were divided into four groups (n = 6). Also, one more group of normal non-alloxanized animals was added in the study. Group I (normal control or non-alloxanized rats); group II (untreated diabetic control rats) received a single oral dose of 0.5 ml/100 g of the vehicle; group III diabetic rats were treated orally with metformin (0.5 g/kg) as the reference drug. Groups IV and V treated orally with ethanolic extract at doses of 100 and 200 mg/kg body weight, respectively. Treatment was continued for 21 consecutive days and fasting blood glucose levels were estimated on days 0, 1, 7, 14 and 21 in all these groups of animals.[21]
Estimation of biochemical parameters
On day 21, blood was collected from retro-orbital plexus of the overnight fasted rats under light ether anesthesia and kept aside for half an hour for clotting. Serum was separated by centrifuging the sample at 6000 rpm for 20 minutes. The serum was analyzed for total protein (Biuret method),[22] Cholesterol oxidase and Para-amino- phenazone (CHOD-PAP method)[23] and Glycerol phosphate oxidase (GPO method).[24]
Histopathologic studies
The whole pancreas of each animal from all the groups was subjected to histopathologic study.The whole pancreas was removed after sacrificing the animal under ether anesthesia and was collected in 10% formalin solution and immediately processed by the paraffin technique. Sections of 5 μm thickness were cut and stained by hematoxylin and eosin (H and E) for histologic examination.[25]
Statistical analysis
All the values of body weight, fasting blood sugar, and biochemical estimations were expressed as mean ± standard error of mean (SEM). The results are analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Dunnet's test. P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Effect on blood glucose level in normal fasted rats
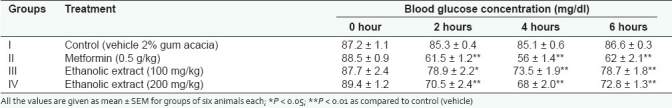
Effect of the ethanolic extract on the fasting blood sugar level of normal rats is shown in Table 1. Treatment with ethanolic extract in normal fasted rats at doses of 100 and 200 mg/kg resulted in significant decrease in the elevated blood glucose levels (P < 0.05, P < 0.01, respectively) as compared to the control group. In normal rats, treatment with ethanolic extract of COL led to a dose-dependent fall in blood sugar levels.
Table 1.
Effect of ethanolic extract of COL on blood glucose level in normal rats
Effect on fasted blood glucose level in alloxan-induced diabetic rats
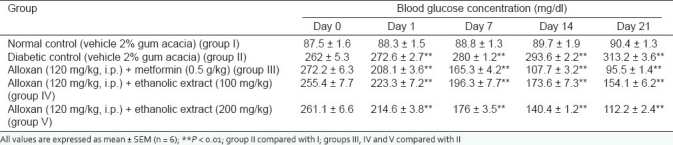
Effect of the ethanolic extract on the fasting blood sugar level of alloxan-induced diabetic rats is shown in Table 2. Treatment with ethanolic extract in diabetic rats at doses of 100 and 200 mg/kg resulted in significant (P < 0.01) and dose-dependent decrease in the elevated blood glucose levels as compared to the control.
Table 2.
Effect of 3-week treatment with ethanolic extract of COL on blood glucose level in alloxan (120 mg/kg, i.p.) induced diabetic rats
Effect on biochemical parameters
Ethanolic extract treated diabetic rats exhibited significant (P < 0.01) difference in serum lipid profiles (cholesterol and triglyceride), serum protein [Table 3] at doses of 100 and 200 mg/kg, when compared with the diabetic control and normal animals.
Table 3.
Effects of ethanolic extract of COL on some biochemical parameters in alloxan-induced diabetic rats (on 21st day)

Effect on body weight of animals
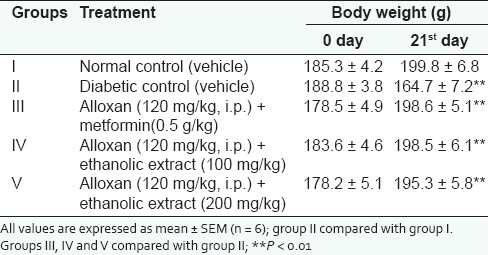
Significant (P < 0.01) difference was observed with regard to changes in body weight [Table 4] in ethanolic extract treated diabetic animals at the doses of 100 and 200 mg/kg as compared to diabetic control and normal animals.
Table 4.
Effect of ethanolic extracts of COL on body weight in alloxan (120 mg/kg, i.p.) induced diabetic rats
Histopathologic studies
Figures 1a–e depicts the islets of the pancreas of rats in different groups. Photomicrographs (a) of normal healthy control group show normal acini and normal cellular population in the islets of langerhans. However, in the alloxan only treated rat, there is extensive damage of the islets of langerhans and they appear to be irregular (b). Treatment of diabetic rats with metformin shows moderate expansion of cellular population and size of islet cells (c). However, ethanolic extract treatment in diabetic rats shows partial restoration of normal cellular population and size of islet cells. Treatment of diabetic rats with ethanolic extract at the dose of 200 mg/kg shows more restoration of normal cellular population and size of islet in comparison to the dose of 100 mg/kg (d, e).
Figure 1.
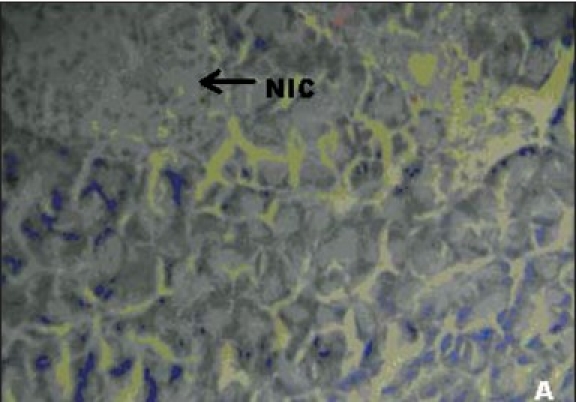
(A) Photomicrographs of normal healthy control group's rat showing normal globules of acini with normal islet cells (NIC), (H&E, ×400)
Figure 2.
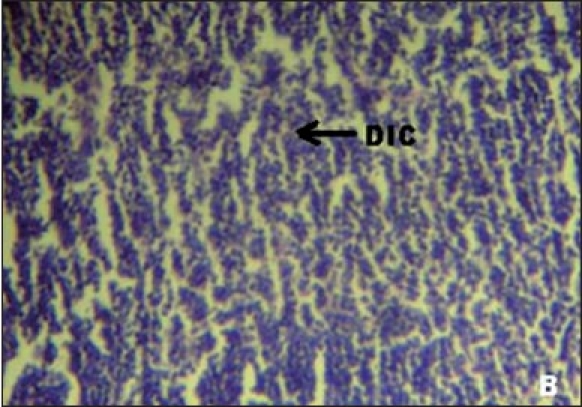
(B)Photomicrographs of diabetic control group's rat showing damaged islet cells (DIC), (H&E, ×400)
Figure 3.
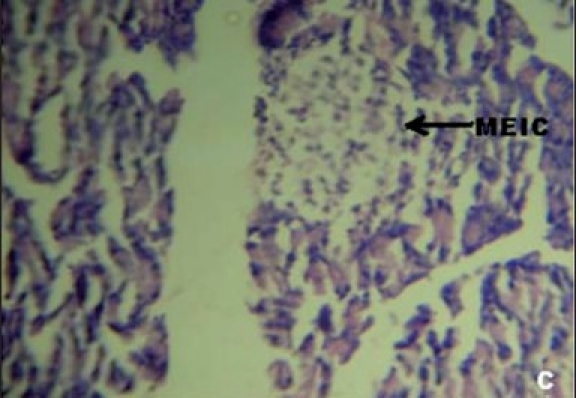
(C) Photomicrographs of standard (Metformin 0.5 g/mg) treated group's rat showing moderate expansion of islet cells (MEIC), (H&E, ×400)
Figure 4.
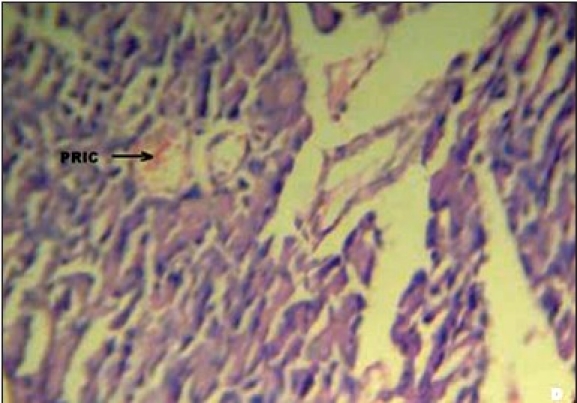
(D) Photomicrographs of ethanolic extract (100 mg/kg) treated group's rat showing partial restoration of islet cells (PRIC), (H&E, ×400)
Figure 5.
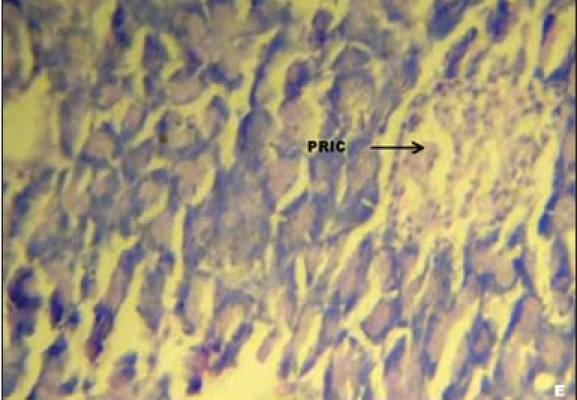
(E) Photomicrographs of ethanolic extract (200 mg/kg) treated group's rat showing partial restoration of islet cells (PRIC), (H&E, ×400)
The result obtained from the data indicates that ethanolic extract of COL exhibited significant antidiabetic activity in normal and alloxan-induced hyperglycemic rats. Ethanol extract administration resulted in a marked decrease in blood glucose levels at both dose levels, i.e., 100 and 200 mg/kg. Alloxan-induced diabetic rats administered ethanolic extract at a dose of 100 mg/kg showed 12.64, 23.20, 32.07 and 39.76% decline in the blood glucose levels on 1, 7, 14 and 21 days, respectively, whereas ethanolic extract at a dose of 200 mg/kg resulted in 17.75, 32.56, 46.23 and 56.96% decline in the blood glucose level on 1, 7, 14 and 21 days, respectively. They can also improve the condition of diabetes as indicated by parameters like body weight, serum cholesterol, serum triglyceride and total protein.
It is now established that there is a gradual decrease in β-cell function and mass that may occur in individuals at high risk of developing type II diabetes. To prevent loss of β-cell function and mass, β-cell stabilization or regeneration must occur.[26] The renewal of β-cells in diabetes has been studied in several animal models. Epicatechin has been shown to act by β-cell regeneration.[27] Similarly, Vinca rosea extracts also cause regeneration of β-cell in alloxan-induced diabetic rats.[28]
Progression of type II diabetes is mainly due to loss of pancreatic β-cell function, which results in increased impairment of patient's ability to produce insulin in response to increased blood glucose.[29] Metformin directly improves insulin action and is effective only in the presence of insulin.[30] It is to be seen whether the antidiabetic effect of COL may be due to increased insulin secretion, similar to that observed in metformin.
In our studies, damage of pancreas was observed in alloxan-treated diabetic control rats [Figure 1b] Metformin treated group showed regeneration of β-cells [Figure 1c]. The comparable regeneration was also shown by ethanolic extracts of COL [Figure 1d and e]. Photomicrographs reinforce healing of pancreas by ethanolic extract of COL as a plausible mechanism of their antidiabetic activity.
Preliminary phytochemical screening of COL revealed the presence of alkaloids, glycosides, proteins and amino acids, sterols, carbohydrates, phenolic compounds, flavonoids, saponins and tannins. The antidiabetic activity of COL may be due to the presence of flavonoids. It is reported that flavonoid components are associated with active biological principles of most medicinal plants having hypoglycemic and antidiabetic properties.[31] However, the extract should further be subjected to bioactivity guided drug discovery to isolate the lead compound responsible for antidiabetic activity and to explore the possible mechanism of action.
CONCLUSIONS
It can be concluded that ethanolic extract of C. occidentalis exhibited significant antidiabetic activity in normal and alloxan-induced diabetic rats. The extract also resulted in improvement in parameters like body weight and lipid profile as well as regeneration of β-cells of pancreas and so it might be of value in the treatment of diabetes.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Andrew JK. New York: Churchill Living Stone; 2001. Diabetes; p. 1. [Google Scholar]
- 2.Alarcon-Aguilara FJ, Roman Ramos R, Flores-Saenz JL. Plant medicinales usadas en el control de la diabetes mellitus. Cienc. 1993;44:361–3. [Google Scholar]
- 3.Barar FS. New Delhi: S. Chand and Company Ltd; 2004. Essentian of Pharmacotherapeutics; p. 340. [Google Scholar]
- 4.Patel PM, Patel KN, Patel NM, Goyal RK. Development of HPTLC method for estimation of charantin in herbal formulations. Pharmacognosy Mag. 2006;8:224–226. [Google Scholar]
- 5.Gupta AK. Quality standard of Indian medicinal plants. Indian Council of Medical Research. 2003;1:47–8. [Google Scholar]
- 6.The wealth of India, A dictionary of Indian Raw Material and Industrial Products, Council of Scientific and Industrial Research, New Delhi. 1998 [Google Scholar]
- 7.Kuo SC, Chen SC, La CF, Teng CM, Wang JP. Studies on the anti-inflammatory and antiplatelet activities of constituents isolated from the roots and stem of Cassia occidentalis L. Chinese Pharma J. 1996;48:291–302. [Google Scholar]
- 8.Saraf S, Dixit VK, Tripathi SC, Patnaik GK. Antihepatotoxic Activity of Cassia occidentalis. Pharm Biol. 1994;32:178–83. [Google Scholar]
- 9.Samy RP, Ignacimuthu S. Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J Ethnopharmacol. 2000;69:63–71. doi: 10.1016/s0378-8741(98)00156-1. [DOI] [PubMed] [Google Scholar]
- 10.Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, et al. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Christ B, Poppinghaus T, Wirtz-Peitz F. Isolation and structural definition of a new sennoside from Cassia senna L. Arzneimittelforschung. 1978;28:225–31. [PubMed] [Google Scholar]
- 12.Lal J, Gupta PC. Two new anthraquinones from the seeds of Cassia occidentalis. Experientia. 1974;30:850–1. [Google Scholar]
- 13.Purwar C, Rai R, Srivastava N, Singh J. New flavonoid glycosides from Cassia occidentalis. Section B - organic chemistry including medicinal chemistry. Indian J Chem. 2003;42:434–6. [Google Scholar]
- 14.Kudav NA, Kulkarni AB. Chemical investigation of Cassia occidentalis. Indian J Chem. 1974;12:1042–4. [Google Scholar]
- 15.Kokate CK. New Delhi: Vallabh Prakashan; 1994. Practical Pharmacognosy; pp. 107–13. [Google Scholar]
- 16.Harborne JB. London: Chapman and Hall; 1998. Phytochemical Methods; pp. 60–6. [Google Scholar]
- 17.Turner MA. New York: Academic Press; 1965. Screening methods in Pharmacology; p. 26. [Google Scholar]
- 18.Trivedi NA, Mazumdar B, Bhatt JD, Hemavathi KG. Effect of shilajit on blood glucose and lipid profile in alloxan induced diabetic rats. Indian J Pharmacol. 2004;36:373–6. [Google Scholar]
- 19.Somani R, Kasture S, Singhai AK. Antidiabetic potential of Butea monosperma in rats. Fitoterapia. 2006;77:86–90. doi: 10.1016/j.fitote.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Jarald EE, Joshi SB, Jain DC. Antidiabetic activity of aqueous extract and non polysaccharide fraction of Cynodon dactylon Pers. Indian J Exp Biol. 2008;46:660–7. [PubMed] [Google Scholar]
- 21.Nagappa AN, Thakurdesai PA, Venkat Rao N, Singh J. Antidiabetic activity of Terminalia catappa Linn fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 22.Burkhardt RT, Batsakis JG. An interlaboratory comparison of serum total protein analysis. Am J Clin Pathol. 1978;70:508–10. [PubMed] [Google Scholar]
- 23.Demacker PN, Hessels M, Toenhake-Dijkstra H, Baadenhuijsen H. Precipitation methods for high density lipo-protein cholesterol measurement compared, and final evaluation under routine operating conditions of a method with a low sample-to-reagent ratio. Clin Chem. 1997;43:663–8. [PubMed] [Google Scholar]
- 24.McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–42. [PubMed] [Google Scholar]
- 25.Luna LC. New York: Mc Graw Hill Book Co; 1990. Manual of histological screening methods of Armed forces Institute of Pathology; p. 125. [Google Scholar]
- 26.Robert R. Henry, MD.Clinical Impact of Therapies Directed at Beta-cell Preservation. Resurrecting the Beta Cell in Type 2 Diabetes: Clinical Impact of Therapies Directed at Beta-cell Preservation. Available from: http://cme.medscape.com/viewarticle/544820_3 .
- 27.Chakravarthy BK, Gupta S, Gode KD. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (-)-epicatechin. Life Sci. 1982;31:2693–7. doi: 10.1016/0024-3205(82)90713-5. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Suryawanshi SA. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol. 2001;39:748–59. [PubMed] [Google Scholar]
- 29.Haberman AB. Addressing Unmet Type 2 diabetes Needs. Gen Clin Res Diag. 2008;34:34–35. [Google Scholar]
- 30.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755–72. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 31.Wollenweber LE, Cody V, Middleton EJ, Harborne JB, Beretz A. Plant flavanoids in biology and medicine II: Biochemical, cellular and medicinal properties. Prog Clin Biol Res. 1988;45:45–55. [Google Scholar]


