Abstract
Women experience menopause differently across the world, in terms of their symptomology. Many experience symptoms of menopause like hot flashes, joint pain and loss of libido. Estrogen replacement is the prescribed therapy for most of the sexual dysfunction observed in menopausal women. Many women are reluctant to use exogenous hormone therapy for treatment of menopausal symptoms and are turning to botanical and dietary supplements for relief. In the present study IND-HE (friedelin rich fraction) was studied for estrogenic activity as well as its effect on sexual behavior in overiectomized female Wistar rats.
The rats were divided into 4 groups of six rats each. The Group 1 received distilled water, Group II - IND-HE (75 mg/kg p. o.), Group III - IND-HE (100 mg/kg p. o.) and Group IV received estrogen (estradiol) (1 mg/kg in olive oil suspension, s.c. bi-weekly). The treatment period was 8 weeks. On 1 day, one month and two month of treatment the sexual behavior was studied. At the end of the treatment the blood was withdrawn from retro-orbital plexus. The animals were sacrificed and uterus was removed, weighed and histology was studied. In different group of rats estrous cycle was studied which indicate estrogenic activity and for progestogenic activity of deciduoma formation was studied.
The result indicated that IND-HE (75 and 100 mg/kg p.o.) improved sexual behavior parameters. IND-HE (75 and 100) significantly (P< 0.01) decreased darting and hopping latency. The darting frequency and hopping frequency was significantly (P< 0.01) improved in IND-HE (75 and100 mg/kg p.o.) as well as estrogen group. Lordosis interval (LI) was increased significantly in estrogen group after 1st month (P< 0.05), and after 2nd month (P< 0.01). IND-HE (100) treatment showed increase in LI after 1st month (P< 0.05) remained during 2nd month (P< 0.01). While IND-HE (75) treatment increased LI only after 2nd month (P< 0.05).IND-HE (75 and 100 mg/kg p.o.) showed estrogenic activity as indicated by vaginal cornification, increase in uterine weight and rise in serum estrogen.
Keywords: Cissus quandragularis, estrogenic activity, female sexual behavior
INTRODUCTION
Female sexual dysfunction (FSD), defined as persistent or recurring reduction of sex drive, aversion to sexual activity, difficulty in becoming aroused, inability to reach orgasm and pain during sexual intercourse, is a multicausal disease with many determinants.[1,2] It is a new, rapidly expanding area of sexual medicine. It is a multicausal and multidimensional disorder, which results in significant personal distress, adversely impacting on life quality and interpersonal relationship. Current epidemiologic data reveal that up to 43% of women have some type of sexual dysfunction.[3] Approximately 10 million American women aged 50–74 years have the complaints of diminished vaginal lubrication, pain, and discomfort with intercourse, decreased arousal and difficulty in achieving orgasm. Lauman et al, found that sexual dysfunction is more prevalent in women than in men, and is associated with various psychodemographic characteristics such as age, education and poor physical and emotional health. The most frequently reported causes of FSD in postmenopausal women result from hormonal changes in postmenopausal women and psychological problems such as depression, stress and fatigue.[4]
According to the results of the Women's Health Initiative (WHI), many women are reluctant to use exogenous hormone therapy for the treatment of menopausal symptoms and are turning to botanical and dietary supplements (BDS) for relief.[5] Cissus quadrangularis (Vitaceae), a climbing shrub, characterized by a thick quadrangular fleshy stem, is an edible plant found in hotter parts of India, Sri Lanka, Malaya, Java and West Africa. Commonly known as the “bone setter,” the plant is referred to as “Asthisamdhani” in Sanskrit and “Hadjod” in Hindi because of its ability to join bones.[6] The plant has been documented in Ayurveda for its medicinal uses in gout, syphilis, venereal disease, piles, leukorrhea and as an aphrodisiac and in the Siddha system of medicine for the treatment of piles, diarrhea and dysentery and in diseases of kapham. The multifarious medicinal claims of C. quadrangularis by the Gond tribals of Raisen district, India, have been reported. The stem juice is used to treat scurvy and irregular menstruation, the plant juice in otorrhea and epistaxis while the root is specific for bone fracture.[7] The root is reported as the most useful for the fractures of bones, with the same effects as plaster externally.[8] The phytochemical analysis of the plant showed the presence of Vitamin C, β-carotene, two symmetric tetracyclic triterpenoids, β-sitosterol, α-amyrin, α-amyrone and three stilbene derivatives and quandragularins A, B, C.[9,10] In addition to vitamin C, it also contains a high amount of carotene A, anabolic steroidal substance and calcium.[11]
Another species, Cissus repens, contains five lignans and eight triterpenoids, isolated from the aerial part. Triterpenoids isolated were friedelin and epifriedelanol.[12] Plants like Maytenus ilicifolia and Eleutherococcus senticosus contain friedelin.[13,14] These plants are reported to have estrogenic activity.[15,16]
The objective of study was to investigate estrogenic activity of friedelin rich fraction isolated from C. quadrangularis and its effect on female sexual behavior.
MATERIALS AND METHODS
Preparation of IND-HE
Collection and authentication
The plant was collected from the districts of Madurai, Coimbatore and Tanjore in India. The plant material was authenticated at Agharkar Research Institute at Pune.
The aerial parts of C. quandragularis were cut into pieces and washed thoroughly in running water to free the adhering soil. This material was dried under shade (30°C, 45% σ humidity). The dried material was then extracted with ethanol (70%) at room temperature for 8 hours in a circulation bed and the filtrate was made free of suspended foreign matters. This filtrate was concentrated under vacuum at 45°C to obtain a paste, which was then redissolved in 0.01 M sodium acetate buffer and filtered free of sediments. The clear liquid was loaded in a counter current column (tubular) and extracted with N-butanol. The butanol layer was separated and concentrated to powder. This powder was dissolved in demineralized water and passed through polymer adsorbent column Amberlite XAD-7 (ROHM and HAAS) (Philadelphia, USA). The column was washed thoroughly with 2% w/v sodium chloride solution followed by demineralized water and eluted in a gradient manner with ethanol:water mixture and the fractions were collected. The elutants were characterized by comparing with various standards (Sigma Aldrich) using High Performance Liquid Chromatography (HPLC). Among the standards, the peak of the elutant matched with peak of friedelin. The fractions containing maximum quantity of compound identical with friedelin were concentrated under vacuum at 40°C and the powder was recrystallized in alcohol.
HPLC details
Column: Kromosil reverse phase C-18 (250 × 4.6 mm with 5μ particle size). Mobile phase gradient was (A) water and (B) acetone according to following profile: at 0 minute (75% A and 25% B), at 20 minutes (65% A and 35% B). Flow rate: 1 ml/minute. Detector: UV (205 nm). The final product (standardized 2.5% friedelin) from C. quandragularis was labeled as IND-HE. In the HPLC chromatogram, the main peak was obtained at 4.2 minutes. The fraction showed the presence of steroids and glycosides.
Chemicals
Estradiol benzoate (Himedia Laboratories, India), hydroxy progesterone (Sigma, USA), ketamine (Aneket, Neon laboratories, India), xylaxine (Xylaxin, Indian immunologicals Ltd., India), anesthetic ether (TKM Pharma, India), friedelin (Sigma, USA) were purchased. All the chemicals were of analytical grade and solvents were of HPLC grade.
Animals
Female Wistar rats of weight 120 ± 5 g and adult mice of either sex (20–25 g) were purchased from National Toxicology Centre, Pune, India, and used for the study. They were maintained at a temperature of 25 ± 1°C and relative humidity of 45–55% under 12-hour light:dark cycle. The animals had free access to food pellets (Chakan Oil Mills, Pune, India) and water. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune, India, constituted according to guidelines of Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA).
Preparation of drug solution
Four hundred milligrams of IND-HE was dissolved in 4 ml of distilled water to prepare stock solution of 100 mg/ml. Appropriate dilutions were made for the preparation of lower doses to administer the drug solution according to the body weight of rats.
Acute oral toxicity of IND-HE
Healthy adult Swiss mice of either sex weighing between 20 and 25 g were used for acute oral toxicity study. The study was carried out according to Organization for Economic Co-operation and Development (OECD) guideline no. AOT-425.[17] The mice were observed for 2 hours for behavioral, neurological and autonomic profiles and for any lethality or death for the next 48 hours.
Experiment I
Pharmacologic methods
Ovariectomy was performed in the rats having body weight of 50–55 g. For ovariectomy, the animals were anesthetized with an intraperitoneal injection of 5% ketamine hydrochloride (80 mg/kg body weight). Bilateral dorsal incisions were made on the back and both the ovaries were identified. The ovarian blood vessels were clamped and the ovaries were excised. The muscle layer was tied and skin incision was sutured.[18] After the recovery, the rats had free access to food and water. The rats were divided into four groups of six rats in each. Group I received distilled water, Groups II and III received IND-HE (75 mg/kg) and (100 mg/kg) p.o., while Group IV received estrogen (estradiol) (1 mg/kg in olive oil suspension, s.c. biweekly). The treatment period was 8 weeks. On day 1, and at the end of the first and second months of the studies, the sexual behavior was studied.
Effect on sexual behavior
Male Wistar rats (200–250 g), previously experienced in sexual behavior, were used for the study. Rectangular wooden box with Plexiglas front and wire mesh top was used as the apparatus. One male rat was placed 5 minutes in it before the introduction of female. The observations were made for 30 minutes. The study was carried out at 22:00–24:00 hours under dim white light (30 lux). Sexual behavior in female rats was studied by the methods described.[19] Sexual behavior was divided into prospective and receptive components.
Sexual behavior parameters
The following parameters were studied:
darting (prospective): a short run where female abruptly stops presenting her posterior to male;
hopping (prospective): a short jump with stiff legs followed by immobility and presentation;
lordosis (receptive): behavior posture assumed by female to allow mounting by male;
orientational activities: anogenital grooming.
Serum estrogen estimation
On the last day of the treatment, blood was withdrawn from retroorbital plexus. Blood was centrifuged to separate the serum. About 10 µl of serum was used for the estimation of serum estrogen (E2) by Clia:immulite (USA), a fully automated immunoassay analyzer.
Uterus weight
On the last day, the animals were sacrificed, uteri were carefully removed, freed of adipose tissue and uterine weights (in mg) were determined.
Histopathology of uterus
The uterus was removed and stored for 24 hours in 10% formalin. The specimen was dehydrated by placing it three times in xylene (1 hour each) and later in alcohol 70, 90 and 100% strength, respectively, each for 2 hours. The infiltration and impregnation was carried out by treating with paraffin wax twice, each time for 1 hour. Paraffin wax was used to prepare paraffin L moulds. Specimens were cut into sections of 3–5 μm thickness and were stained with hematoxylin and eosin. The specimen was mounted on the slide by use of Distrene Pthalate Xylene (D.P.X).
Experiment II
Estrous cycle in female rats
Immature female Wistar rats weighing about 55 ± 5 g were ovariectomized. They were allowed to recover. After recovery, the rats were divided into four groups of six rats in each. Group I received distilled water, Groups II and III received IND-HE (75 mg/kg p.o. and 100 mg/kg p.o., respectively) and Group IV received estrogen (estradiol benzoate) (1 mg/kg in olive oil suspension, s.c. biweekly). The compounds were dosed, e.g., twice daily on two following days at 10:00 AM and 5:00 PM. At 5:00 PM on the third day and at 10:00 AM on the fourth day, vaginal smears were prepared using cotton swabs moistened with saline. The smears were transferred to a glass slide and stained for 10 minutes with 5% aqueous methylene blue solution. The smears were observed microscopically and scored as: 0, diestrus smear, mainly leukocytes, few epithelia cells; 1, mixture of leukocytes and epithelial cells; 2, proestrus smear, nucleated or nucleated plus cornified cells; 3, estrus smear, cornified cells only. Only animals showing score 2 or 3 were considered to be positive.[20]
Experiment III
Deciduoma formation
Different groups of adult female Wistar rats (200–250 g), were ovariectomized and 1 week later treated with 0.5 μg estradiol/animal once daily subcutaneously for 4 days. The rats were divided into four groups of six rats in each group. Group I was vehicle control group and received distilled water, Groups II and III received IND-HE (75 mg/kg p.o. and 100 mg/kg p.o., respectively), while Group IV received the standard progesterone (Sigma, USA) (1 mg per animal s.c. biweekly.). The treatment period was 9 days. The animals were lightly anesthetized with anesthetic ether. The uterus was exposed on the fifth day of progesterone/test drug treatment and 1.0 mg histamine dihydrochloride was injected into the lumen of one horn. The animals were sacrificed after the last treatment. Both the uterine horns were removed and weighed. The degree of deciduoma formation was evaluated by the percent increase in the weight of the histamine-injected uterine horn as compared with the control horn.[21]
Statistical analysis
Data for each of the parameter were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test using Graph Pad, Prism software, version 4.03.
RESULTS
Yellow crystals (yield 2.5 w/v) obtained were named as IND-HE. The high-performance liquid chromatogram [Figure 1] of IND-HE showed the presence of friedelin as a major peak at 4.2 minutes. Oral administration of IND-HE (5000 mg/kg) did not cause mortality or any signs of clinical abnormality in both the groups. The LD50 of IND-HE was more than 5000 mg/kg body weight.
Figure 1.
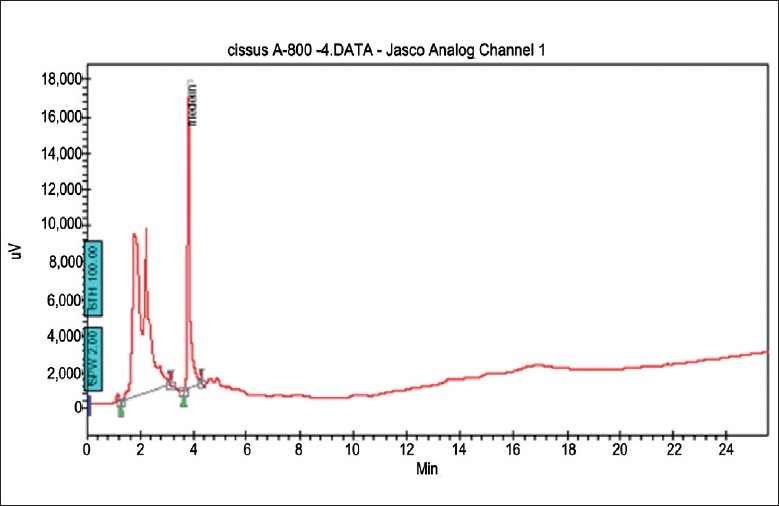
HPLC tracing of IND-HE (standardized to friedelin)
Sexual behavior study
Darting latency
The time taken to initiate the short run made by female rats in front of male exposing their posterior was considered as darting latency (DL). The DL observed in control group was 1143 ± 102 seconds while in estrogen, IND-HE (100) and IND-HE (75) groups, it was 1470 ± 123, 1598± 42 and 1413 ± 113 seconds, respectively, on the first day of the treatment. The decrease was significant from the first month onward in the entire three treatment groups (P < 0.001) as compared to vehicle treated group. After 1 month period, DL in vehicle, estrogen, IND-HE (100) and IND-HE (75) was 1143 ±112, 156 ± 11.1, 329 ± 95, 213 ± 37.8 seconds, respectively, and at the end of second month it was further significantly (P < 0.001) decreased in treatment groups except the control group, to 1381 ± 68, 32 ± 5.9, 37 ± 7, 34.5 ± 2.4 seconds, respectively [Figure 2a].
Figure 2.
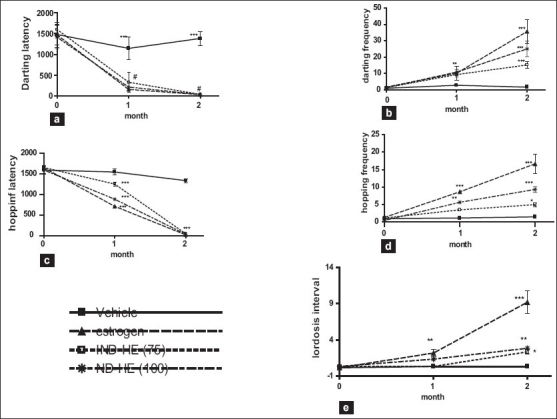
Data represented are mean number of observations ± SEM of sexual behavior in ovariectomized female rats (n = 6) analyzed by two way ANOVA followed by Bonferroni post hoc test. (a) Darting latency, (b) Darting frequency, (c) Hopping latency, (d) Hopping frequency, (e) Lordosis interval.*P < 0.05, **P < 0.01, ***P < 0.001
Darting frequency
The number of darting recorded for 30 minutes period drugs in control group was 1 ± 0.25 and that of estrogen, IND-HE (100) and IND-HE (75) group was 1.67± 0.3, 1.33 ± 0.42, 1.33± 0.21, while darting frequency (DF) after the first month was 2.8 ± 0.6, 10.16 ± 1.7, 9.33 ± 1.3, 10.8 ± 0.65 and after the second month it was 1.67 ± 0.33, 35.6 ± 2.9, 15.1 ± 2.0, 25 ± 1.93, respectively. The results indicated that DF significantly increased after first (P < 0.01) and second months (P < 0.001) in estrogen and IND-HE groups [Figure 2b].
Hopping latency
Hopping is characterized by a short jump with stiff legs followed by immobilityand a presenting behavior. Hoping latency (HL) seen in control rats was observed to be 1591 ±81.6 seconds, while in estrogen, IND-HE (100) and IND-HE (75) groups, it was 1647 ± 54.1, 1652 ± 38.2, 1592 ± 101 seconds, on the first day of treatment. HL after the first month was 1545 ± 68.2, 709 ± 35.7, 1249 ±41.4, 882 ± 41 seconds and after the second month was 1333 ± 48.6, 16.6 ± 2.7, 45.16± 5.7, 33 ± 2.3 seconds in the above said four treatment groups, respectively. The results indicated that HL decreased significantly (P < 0.001) after the first and second months of treatment [Figure 2c].
Hopping frequency
Initially, control group exhibited 1.0 ± 0.2 hops, estrogen, IND-HE (75 and 100) treated groups showed 1.3 ± 0.2, 1.16 ± 0.16, 0.5 ± 0.2 hops, respectively. Treatment with IND-HE (75 and 100) increased the number of hops significantly after the first month. The number of hops were control 1.16 ± 0.3, estrogen 8.6 ± 0.49 (P < 0.001), IND-HE (75) 3.5 ± 0.3 (ns), IND-HE (100) 5 ± 0.5 (P < 0.01). HF increased significantly in all three groups after the second month. The HF was control 1.5 ±0.2, estrogen 16.6 ± 2.7 (P < 0.001), IND-HE (75) 45.16 ± 5.7 (P < 0.05), IND-HE (100) 33 ± 2.3 (P < 0.01) [Figure 2d].
Lordosis interval
The lordosis interval (LI) observed in control group was 0.167 ± 0.1 seconds, while that in estrogen, IND-HE (100) and IND-HE (75) groups, it was 0.167 ± 0.16, 0.34± 0.21 and 10.34 ± 0.21 seconds, respectively. The increase was significant from the first month onward in the entire three treatment groups (P < 0.001) as compared to vehicle treated group. After a month period, LI in vehicle, estrogen, IND-HE (100) and IND-HE (75) groups was 0.34 ±0.2, 2.16 ± 0.47, 0.34 ±0.21, 0.34 ±0.21, respectively, and at the end of second month it significantly (P < 0.001) increased to 9.16 ± 1.5, 2.3 ± 1.5, 2.8 ± 0.3 seconds in estrogen, IND-HE (75) and IND-HE (100) groups, respectively, while LI remained the same in control group. The results indicated that LI increased significantly in estrogen group after the first month (P < 0.05) and after the second month (P < 0.01). IND-HE (100) treatment showed increase in LI after the first month (P < 0.05), remained the same during second month (P < 0.01), while IND-HE (75) treatment increased LI only after the second month (P < 0.05) [Figure 2e].
Vaginal cornification
Control group and estradiol (1 mg/kg in olive oil suspension, s.c. biweekly) treated rats showed estrous score of 0 and 3, respectively. Oral administration of IND-HE (75 and 100 mg/kg p.o.) showed estrous score of 1 and 3, respectively. The score was given according to maximum numbers of animals in a given phase [Table 1]. Oral administration of IND-HE (75 and 100 mg/kg p.o.) in ovariectomized female rats showed cornified and nucleated epithelial cells.
Table 1.
Data represented are scores of the vaginal cornification study in ovariectomized rats treated with control (vehicle treated), standard estrogen (1 mg/kg in olive oil), IND-HE (75 mg/kg, p.o.) and IND-HE (100 mg/kg, p.o.)

Serum estrogen
The serum estradiol (pg/ml) in control group, IND-HE (75 mg/kg), IND-HE (100 mg/kg) and estrogen group was 15.83 ± 1.8, 46.67 ± 6.5, 52.67 ± 0.9 and 105.9 ± 5.3, respectively. The results showed significant increase in serum estradiol with estrogen (P < 0.01), IND-HE (75 mg/kg) (P < 0.05) as well as IND-HE (100 mg/kg) (P < 0.05) as compared to the control group [Figure 3]. The rise in serum estradiol was 89.17, 31.04 and 36.84% in estrogen group, IND-HE (100 mg/kg) and IND-HE (75 mg/kg) groups, respectively.
Figure 3.
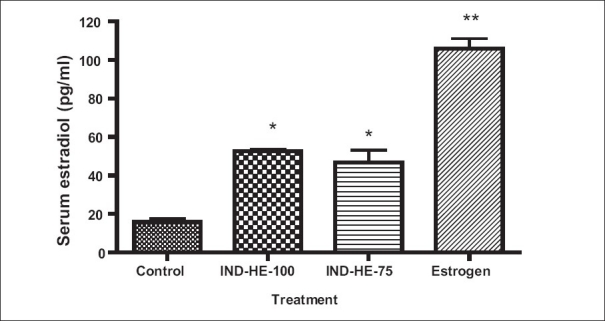
Data represented are mean number of observations ± SEM of serum estradiol (pg/ml) in ovariectomized female rats (n = 6) analyzed by one way ANOVA followed by Bonferroni post hoc test. *P < 0.05, **P < 0.01
Effect on uterus weight
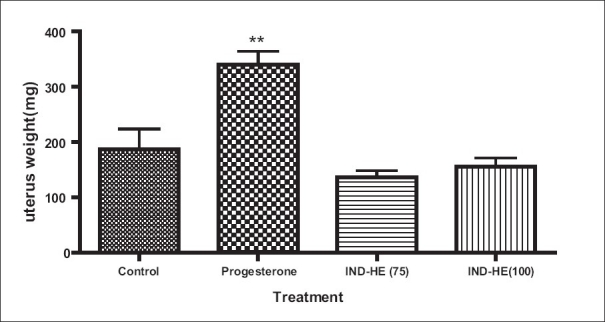
The mean uterus weight in control ovariectomized, IND-HE (75 mg/kg p.o.), IND-HE (75 mg/kg p.o.) and estrogen group was 90.1 ± 8.79 mg, 191.0 ± 6.59 mg, 270.3 ± 52.36 mg, 404.5 ± 34.73 mg. The results showed significantly increased (P < 0.01) mean uterus weight in IND-HE (100) and estrogen treated groups [Figure 4].
Figure 4.
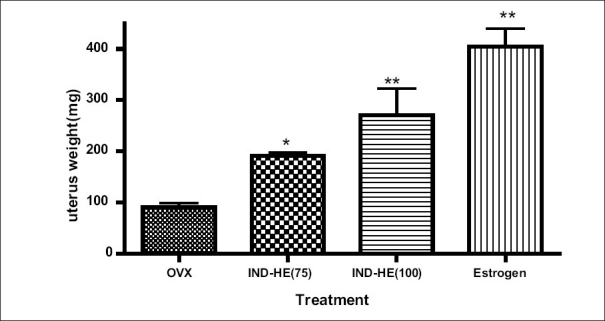
Data represented are mean number of observations ± SEM of uterus weight (mg) in ovariectomized female rats (n = 6) analyzed by one way ANOVA followed by Bonferroni post hoc test. *P < 0.05, **P < 0.01
Effect on deciduoma formation
Administration of histamine (1 mg/kg) in the uterine horn of hydroxyprogesterone (0.04 mg/animal s.c.) treated rats showed increased weight of uterine horn due to deciduoma formation compared to control group (P < 0.01). There was no significant increase in uterus weight in IND-HE (75) and IND-HE (100) groups. The results thus indicated absence of progesterone like activity in IND-HE (75) and IND-HE (100) [Figure 5].
Figure 5.
Data represented are mean number of observations ± SEM of uterus weight (mg) in ovariectomized female rats (n = 6) analyzed by one way ANOVA followed by Bonferroni post hoc test. **P < 0.01
Histopathology
Treatment with estrogen showed marked proliferation of uterine glands, the cells were normal and no inflammation was observed. Both the doses of IND-HE showed sparse proliferation of glands, normal cellular arrangement and no inflammation [Table 2] [Figure 6].
Table 2.
Effect of estrogen (1 mg/kg), IND-HE (100 mg/kg) and IND-HE (75 mg/kg) on histology of uterus (400×)
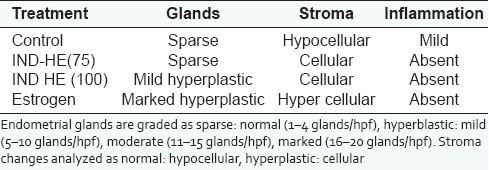
Figure 6.
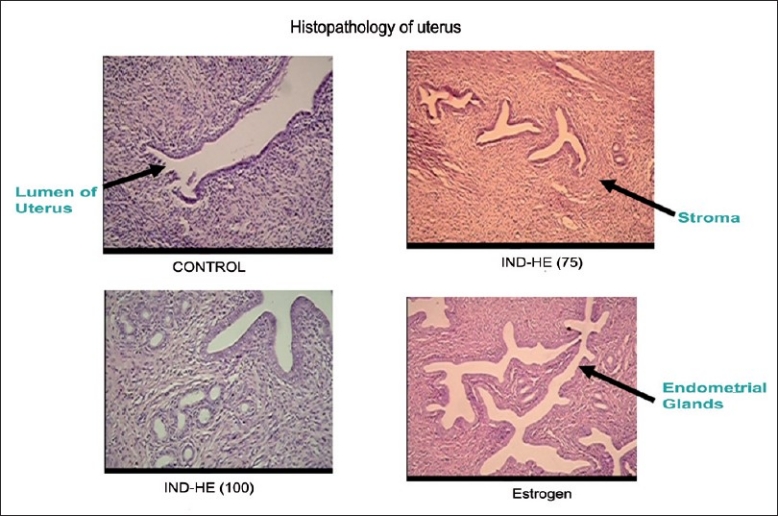
Effect of estrogen (1 mg/kg), IND-HE (100 mg/kg) and IND-HE (75 mg/kg) on histology of uterus (400×)
DISCUSSION
In the present study we examined the estrogenic effects of fraction containing maximum amount of friedelin (a phytoestrogen) and its faciltatory effect on appetitive and consummatory elements of sexual behavior in ovariectomized rats.
FSD is a new, rapidly expanding area of sexual medicine. It is multicausal and multidimensional disorder, which results in significant personal distress, adversely impacting on life quality and interpersonal relationship. The most frequently reported causes of FSD in postmenopausal women are hormonal changes in postmenopausal women and psychological problems such as depression, stress and fatigue. Postmenopausal estrogen deficiency causes a decrease in genital vasocongestion and lubrication, atrophy of the vaginal epithelium and dyspareunia due to vaginal dryness.[22] In animals, estrogen deficiency causes neuropathy in the distribution of the pudendal nerve. The same changes may occur in estrogen-deficient women, resulting in decreased sensation in the clitoral and vulvar area. Vaginal dryness and dyspareunia are responsive to estrogen replacement therapy (ERT) via restoration of vaginal cells, pH and blood flow.[23]
Ovariectomy in rats declines all parameters of sexual behavior as evident in Figure 1. In many postmenopausal women, the lack of estrogen produces loss of libido and a subsequent decrease in their nerve cells in and immediately surrounding the ventromedial nucleus (VMN) of the hypothalamus that is absolutely essential for female-typical lordosis behavior in animals.[24] Treatment with IND-HE improved the proceptive part of sexual behavior especially in the second month of treatment. Lordosis behavior also significantly improved.
Vaginal cytology assay is particularly used to determine the estrogenic activity of the synthetic estrogens,[25,26] xenoestrogens[27] and phytoestrogens.[26,28] It is a sensitive, simple and inexpensive method to predict the estrogenic activity. The assay can be performed in either immature or ovariectomized rodents.[29,30] The vagina is covered by a mucosa with a malpighian pluristratified epithelium with marked sensitivity to sex steroids, particularly to estrogens. This specific action of estrogens is due to the presence of specific receptors, ER-α and ER-β.[31,32] In ovariectomized rats treated for 3 months with estradiol (0.5 mg/day/animal, via food), vaginal cornification was regularly observed, whereas the sham-treated animals have an unestrous vaginal smear.[33] There are many studies about the effects of phytoestrogens on genital tracts of women and female animals. Dietary supplementation with phytoestrogens led to increased vaginal cytological maturation in women.[34] In the present study, IND-HE treatment in ovariectomized rats also induced vaginal cornification indicating estrogenic activity, which is further confirmed by increase in uterus weight and increase in serum estrogen level in ovariectomized rats. The improvement in the sexual activity by IND-HE was comparable to estrogen, which suggests that IND-HE improves sexual behavior in ovariectomized animals due to its estrogenic activity.
Vaginal estrogens may be a good choice in women whose sexual problems are due to vaginal dryness and dyspareunia. Though ERT is a good choice for the FSD, it may induce androgen deficiency. Moreover, estrogen causes other side effects such as venous thromboembolism, flushing, vomiting, coronary events, strokes, endometrial hyperplasia, esophagitis and breast cancer, making them less desirable among patients.[35] Nowadays, focus has been driven toward seeking alternative regimes like herbs which improve the hormonal imbalances without causing any side effects.
The changes in absolute weight of the uterus of animals used with experiment are consolidated in Figure 4. As expected, uteri of ovariectomized + estrogen group were significantly heavier than those of ovariectomized control. The weight of the uterus in IND-HE treated animals significantly increased compared to ovariectomized controls. Increased weight of uteri confirmed that IND-HE possesses uterotrophic effect. It is abundantly clear from histopathology of uterus that E2 and IND-HE treatment was able to sustain the growth of uterus without having any adverse effects.
CONCLUSION
Thus we can conclude that IND-HE exhibits mild to moderate estrogenic activity. Estrogen treatment increases excitability and reproductive behavior. Sexual behavior in IND-HE and estrogen treated animals showed remarkable similarity.
It is thus apparent that IND-HE improved sexual behavior in ovariectomized rats due to its estrogenic activity. IND-HE therefore stands a chance as a potential candidate for hormonal replacement therapy in postmenopausal woman.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Walton B, Thorton T. Female sexual dysfunction. Curr Womens Health Rep. 2003;3:319–26. [PubMed] [Google Scholar]
- 2.Modelska K, Milián M. Treatment of female sexual dysfunction in postmenopausal women: What is the evidence? Rev Gynaecol Pract. 2004;4:121–32. [Google Scholar]
- 3.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Aslan E, Fynes M. Female sexual dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:293–305. doi: 10.1007/s00192-007-0436-3. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 6.Sivarajan V, Balachandran I. Ayurvedic drugs and their plant sources. In: Rao GP, Iyengar MA, editors. Current Sci. Vol. 67. New Delhi/Mumbai/Calcutta: Oxford and IBH Publishing Co. Pvt. Ltd.; 1994. p. 507. [Google Scholar]
- 7.Shirwaikar A, Khan S, Malini S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. J Ethnopharmacol. 2003;89:245–50. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni K, Nadkarni A. Mumbai: Popular Prakashan; 1994. Dr. KM Nadkarni's Indian Materia Medica. [Google Scholar]
- 9.Chopra R. Calcutta: Academic Publishers; 1958. Indigenous drugs of India. [Google Scholar]
- 10.Attawish A, Chavalittumrong P, Chivapat S, Chuthaputti A, Rattanajarasroj S, Punyamong S. Subchronic toxicity of Cissus quadrangularis linn. Songklanakarin J Sci Technol. 2002;24:39–51. [Google Scholar]
- 11.Deka D, Lahon L, Saikia J, Mukit A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius-ulna of dog: A preliminary study. Indian J Pharmacol. 2009;26:44. [Google Scholar]
- 12.Yue-Hu W, Zhong-Kai Z, Hong-Ping H, Suo G, Ning-Chuan K, Ming D, et al. Lignans and Triterpenoids from Cissus repens (Vitaceae) Acta Bot Yunnanica. 2006;28:433. [Google Scholar]
- 13.Queiroga CL, Silva GF, Dias PC, Possenti A, de Carvalho JE. Evaluation of the antiulcerogenic activity of friedelan-3 -ol and friedelin isolated from Maytenus ilicifolia (Celastraceae) J Ethnopharmacol. 2000;72:465–8. doi: 10.1016/s0378-8741(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 14.Tang W, Eisenbrand G. Chinese drugs of plant origin. Berlin: Springer Berlin; 1992. [Google Scholar]
- 15.Montanari T, Bevilacqua E. Effect of Maytenus ilicifolia Mart. on pregnant mice. Contraception. 2002;65:171–5. doi: 10.1016/s0010-7824(01)00301-8. [DOI] [PubMed] [Google Scholar]
- 16.Kropotov AV, Kolodnyak OL, Koldaev VM. Effects of Siberian ginseng extract and ipriflavone on the development of glucocorticoid-induced osteoporosis. Bull Exp Biol Med. 2002;133:252–4. doi: 10.1023/a:1015834717178. [DOI] [PubMed] [Google Scholar]
- 17.Paris: Environment Directorate, OECD; 2001. Organization for Economic Co-operation and Development. Guidance Document on Acute Oral Toxicity Testing. [Google Scholar]
- 18.Wang JW, Xu SW, Yang DS, Lv RK. Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int. 2007;18:1641–50. doi: 10.1007/s00198-007-0412-2. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano F, Julia-Guilloteau V. Neurophysiology of female genital sexual.Women's sexual function and dysfunction: Study, diagnosis and treatment. Informa HealthCare. 2006:168. [Google Scholar]
- 20.Allen E, Doisy E. The induction of a sexually mature condition in immature females by injection of the ovarian follicular hormone. Am J Physiol. 1924;69:577. [Google Scholar]
- 21.Astwood E, Fevold H. Action of progesterone on the gonadotropic activity of the pituitary. Am J Physiol. 1939;127:192. [Google Scholar]
- 22.Sarrel PM. Effects of hormone replacement therapy on sexual psychophysiology and behavior in postmenopause. J Womens Health Gend Based Med. 2000;9:25–32. doi: 10.1089/152460900318830. [DOI] [PubMed] [Google Scholar]
- 23.Nathorst-Böös J, Wiklund I, Mattsson LA, Sandin K, von Schoultz B. Is sexual life influenced by transdermal estrogen therapy. A double blind placebo controlled study in postmenopausal women? Acta Obstet Gynecol Scand. 1993;72:656–60. doi: 10.3109/00016349309021160. [DOI] [PubMed] [Google Scholar]
- 24.Pfaff DW. Patterns of steroid hormone effects on electrical and molecular events in hypothalamic neurons. Mol Neurobiol. 1989;3:135–54. doi: 10.1007/BF02935628. [DOI] [PubMed] [Google Scholar]
- 25.Ashby J, Odum J, Paton D, Lefevre PA, Beresford N, Sumpter JP. Re-evaluation of the first synthetic estrogen, 1-keto-1, 2, 3, 4-tetrahydrophenanthrene, and bisphenol A, using both the ovariectomised rat model used in 1933 and additional assays. Toxicol Lett. 2000;115:231–8. doi: 10.1016/s0378-4274(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 26.Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H. Ability of xeno-and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: Estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol. 2000;73:1–10. doi: 10.1016/s0960-0760(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 27.Stroheker T, Chagnon MC, Pinnert MF, Berges R, Canivenc-Lavier MC. Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: A comparative study. Reprod Toxicol. 2003;17:421–32. doi: 10.1016/s0890-6238(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 28.Malaivijitnond S, Chansri K, Kijkuokul P, Urasopon N, Cherdshewasart W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J Ethnopharmacol. 2006;107:354–60. doi: 10.1016/j.jep.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Mathur R, Vinita S, Prakash AO. Effect of Pueraria tuberosa DC on the oestrous cycle of adult rats. Acta Eur Fertil. 1984;15:393–4. [PubMed] [Google Scholar]
- 30.Gebrie E, Makonnen E, Debella A, Zerihun L. Phytochemical screening and pharmacological evaluations for the antifertility effect of the methanolic root extract of Rumex steudelii. J Ethnopharmacol. 2005;96:139–43. doi: 10.1016/j.jep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Johnson M, Everitt B. Cambridge, MA: Blackwell Pub; 2007. Essential reproduction. [Google Scholar]
- 32.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor {beta} Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 33.Wuttke W, Jarry H, Westphalen S, Christoffel V, Seidlová-Wuttke D. Phytoestrogens for hormone replacement therapy? J Steroid Biochem Mol Biol. 2002;83:133–47. doi: 10.1016/s0960-0760(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox G, Wahlqvist ML, Burger HG, Medley G. Oestrogenic effects of plant foods in postmenopausal women. BMJ. 1990;301:905–6. doi: 10.1136/bmj.301.6757.905-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]


