Abstract
The in vitro and in vivo antitrypanosomal effects of the ethanol extract of Senna occidentalis leaf were investigated. The crude extract exhibited an in vitro activity against Trypanosoma brucei brucei as it completely eliminated parasites’ motility within 10 minutes postincubation with 6.66 mg/ml of effective extract concentration. The extract was further used to treat experimentally T. brucei brucei infected rats at concentrations of 100 and 200 mg/kg body weight, beginning on day 5 post infections (p.i.). At the termination of the experiment on Day 11 p.i., the extract significantly (P < 0.05) kept the parasitemia lower than was recorded in the infected untreated rats. All the infected animals developed anemia, the severity of which was significantly (P < 0.05) ameliorated by the extract treatment. The infection caused significant (P < 0.05) increases in serum alanine and aspartate aminotransferases as well as serum urea and creatinine levels. However, treatment of infected animals with the extract significantly (P < 0.05) prevented the trypanosome-induced increase in these biochemical indices. Furthermore, the T. brucei infection caused hepatomegaly and splenomegaly that were significantly (P < 0.05) ameliorated by the extract administration. It was concluded that orally administered ethanol extract of S. occidentalis leaf possessed anti-T. brucei brucei activity and could ameliorate the disease-induced anemia and organ damage.
Keywords: Anemia, antitrypanosomal effect, Senna occidentalis, Trypanosoma brucei brucei
INTRODUCTION
Pathogenic trypanosome infections of domestic animals are still a major scourge in sub-Saharan Africa and they largely account for the low livestock productivity of the continent,[1] thus making it an important priority for biomedical and public agencies, agricultural sector and the scientific community.[2] The disease caused by the Trypanosoma brucei subgroup is associated with anemia, hepatocellular degeneration and glomerulonephritis,[3] which is largely attributed to the large amount of free radicals and superoxides generated by the trypanosomes that attack membrane polyunsaturated fatty acids and proteins, resulting in cellular injuries and consequently affecting vital tissues and organs of the infected animals.[1,4,5] There is little or no hope for the production of antitrypanosomal vaccine in the near future because of antigenic variation exhibited by the parasites. This coupled with the limitations of the present trypanocidal drugs such as toxicity, drug resistance, and being inconvenient to administer to local populations increase the need for urgent search of more effective plant-derived therapeutic agents against the disease.[6] More so, several semi-synthetic and synthetic drug derivatives were originally isolated from natural compounds.[7,8] Reports on ethnopharmacology revealed several medicinal plants as potent trypanocides.[9–12] These suggest the need for exploring medicinal plants for efficient and cheaper trypanocides.
Senna occidentalis (Linn.) (formerly Cassia occidentalis) is a weed of the leguminosae family, and is distributed throughout the tropical and subtropical regions of the world. It can be found in open pastures and in fields cultivated with cereals such as soybean, corn, sorghum and others; thus, during the harvest it is almost impossible to prevent this plant from mixing with the cultivated crops.[13,14] The antibacterial and antimalarial activities of leaves and rootbark extracts of this plant have been reported.[15–18] However, information on either in vitro and/or in vivo antitrypanosomal activities of any part of this plant has never been documented. In this article, we report for the first time, the in vitro and in vivo antitrypanosomal activities of ethanolic extract of S. occidentalis leaves. We also evaluate the extract's ability to ameliorate the associated trypanosome-induced pathologic changes.
MATERIALS AND METHODS
Plant Material
Ripe S. occidentalis leaves were collected in August 2008 from the Botanical garden of the Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria. S. occidentalis was identified at the species level at the Herbarium unit of the same department. The voucher herbarium specimen was deposited with number 1047. The leaves were thoroughly washed and air-dried for 2 weeks to a constant weight. The dried leaves were pounded to fine powder with mortar and pestle, and then stored in dry containers until needed.
Experimental animals
The protocol employed met the guidelines of the Good Laboratory Practice (GLP) regulations of World Health Organization. Apparently healthy white albino rats of both sexes weighing between 140 and 260 g were used for the work and were obtained from the Nigerian Institute of Trypanosomiasis and Onchorcerciasis Research, Kaduna, Nigeria. The animals were kept in well-ventilated laboratory cages with 12 hours day/night cycles. The rats were maintained on a commercial poultry feed (ECWA Feeds, Jos, Nigeria) and drinking water ad libitum.
Extract preparation
The ethanolic extract of S. occidentalis leaf was prepared by soaking 50 g of the powder in 150 ml of 95% ethanol and shaking on Wrist action shaker for 6 hours. The preparation was left to stand for another 24 hours and then filtered through a Whatmann's filter paper. The filtrate was concentrated to dryness at 40°C under reduced pressure on a rotary evaporator and stored in a refrigerator at −4°C until required.
Test organism
Trypanosoma brucei brucei (Federe strain) was obtained from Department of Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. Parasites harvested from the blood of a donor rat at peak parasitemia (109parasites/ml) were diluted with phosphate buffered saline and then used for both the in vitro studies and infection of experimental animals.
In vitro Screening for Antitrypanosomal Activity
Different concentrations of the extract ranging from 2.5 to 20 mg/ml were prepared. The in vitro antitrypanosomal activity was assessed in triplicates in 96-well microtiter plates (Flow laboratories Inc., Mclean, VA, USA). In the wells of the microtiter plates, aliquots of 20 μl of each extract concentration were incubated with 40 μl of the infected blood, achieving effective extract concentrations of 6.66, 3.33, 1.67 and 0.83 mg/ml in the reaction mixtures. For control, the extract was replaced with phosphate buffered saline. Parasite count was then monitored on a glass slide (covered with a covering slip) and observed under a microscope at ×400 magnification. The percentage of motile parasites was counted at 10 minutes intervals for 1 hour. Cessation or drop in motility of the parasites in extract-treated blood compared to that of parasite-loaded control blood without extract was taken as a measure of antitrypanosomal activity.[11]
In vivo Activity of the Ethanol Extract of S. occidentalis Against T. brucei brucei
To investigate the effect of the extract on T. brucei brucei infection, 30 albino rats (140–260 g) of both sexes were divided into six groups of five rats in each and treated as follows.
Uninfected (normal) control: The rats in this group were neither infected with the parasites nor treated with the extract.
Extract control: This group of rats was also left uninfected but orally treated with 200 mg/kg body weight of the extract.
Infected untreated control: The five rats in this group were each infected by intraperitoneal injection of about 105 T. brucei per 100 g b.w. and were not further treated.
Infected + 100 mg/kg b.w.: Rats in this group were infected similar to that of the infected control group above. However, on day 5 post infections (p.i.), when parasitemia approximately reached 108 trypanosomes/ml, they were given daily oral treatment of 100 mg/kg b.w. of the extract.
Infected + 200 mg/kg b.w.: The rats in this group were also similarly infected but on day 5 p.i., when parasitemia approximately reached 108 trypanosomes/ml, daily oral treatment with 200 mg/kg b.w. of the extract was commenced.
Infected + diminal: Infection of the animals in this group with the parasites was carried out as described above and then treated with 80 mg/kg b. w. of Diminal®(Eagle Chemical Company Ltd, Ikeja, Nigeria; diminal contains 445 mg diminazene aceturate + 555 mg phenazone/g) starting from Day 5 p.i.
The parasites were then monitored daily using the rapid matching counting method[19] and the experiment terminated on Day 11 p.i. The preinfection and terminal (on Day 11 p.i.) packed cell volumes (PCVs) of all groups of rats were determined by the microhematocrit method.
Serum biochemical parameters
Serum harvested from the blood of all animals during cardiac puncture on Day 11 p.i. was used to measure alanine aminotransferase and aspartate aminotransferase (ALT and AST) activities using commercial reagent kits (Randox Laboratories, Ireland), while urea and creatinine concentrations were determined by the diacetylmonoxime and Jaffe's reactions respectively, as described by Kaplan et al.[20] Liver, kidney and spleen of all the rats were also collected and weighed to ascertain the organ:body weight ratio for all groups of rats.
Statistical analysis
The results were presented as mean ± standard deviation and students’ ‘t’-test was used to compare paired means and a difference was considered statistically significant when P < 0.05.
RESULTS
In vitro Screening
The ethanol extract of S. occidentalis exhibited an in vitro anti-T. brucei brucei activity in a dose-dependent fashion. While no parasitic motility was observed at 10 minutes postincubation with 6.66 mg/ml of the effective extract concentration, 75% of the parasites were still motile in the control with 18% being active even at 60 minutes postincubation [Figure 1].
Figure 1.
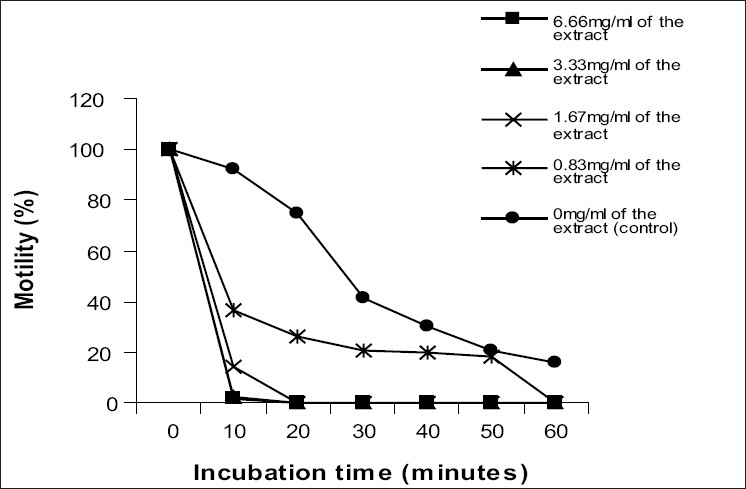
In vitro antitrypanosomal effects of ethanolic extract of S. occidentalis leaves
In vivo Trypanosuppressive Action of the Extract
Figure 2 presents the effect of different intraperitoneal doses of the extract on the course of T. brucei brucei infection in rats. Compared with the infected untreated rats, treatment with the extract suppressed the multiplication of the parasites in a dose-dependent manner; whereas a higher trypanosuppresive effect was observed in the diminal- treated infected group on Day 11 p.i.
Figure 2.
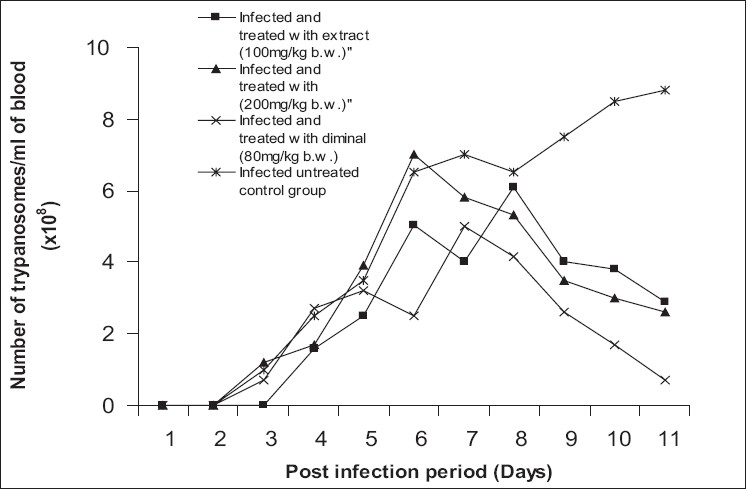
In vivo antitrypanosomal activity of different doses of ethanolic extract of S. occidentalis leaves
Effect of the extract on the parasite-induced anemia and organ damage
The T. brucei brucei infections in this work caused significant (P < 0.05) drop in PCV of infected rats, indicative of anemia. However, the anemia observed in the infected untreated controls is significantly (P < 0.05) more severe than that of the extract- and diminal-treated animals [Table 1]. The results of the indices on hepatic and renal functions analyzed in this experiment are presented in Table 2. Infection, without treatment, caused significant increases (P < 0.05) in the activities of serum ALT and AST which were significantly (P < 0.05) ameliorated by the administration of 200 mg/kg b.w. of the extract and diminal. Trypanosome-induced increases in both serum urea and creatinine concentrations were also significantly (P < 0.05) prevented by the extract and diminal treatments. More so, the hepatomegaly and splenomegaly observed as increase in liver:body weight and spleen:body weight ratios, respectively, in the infected animals were significantly (P < 0.05) higher in the untreated rats than in the extract-treated [Table 3] rats. The kidney:body weight ratio was affected by neither the infection nor the extract administration.
Table 1.
Effect of oral administration of different doses of ethanolic leaf extract of S. occidentalis on PCVs (mean ± SD) in T. brucei brucei infected rats (n = 5)
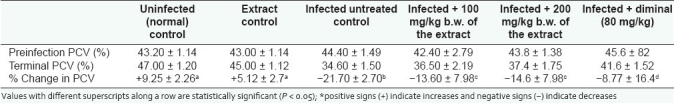
Table 2.
Effect of oral administration of different doses of ethanolic leaf extract of S. occidentalis on hepatic and renal function (mean ± SD) of T. brucei brucei infected rats (n = 5)
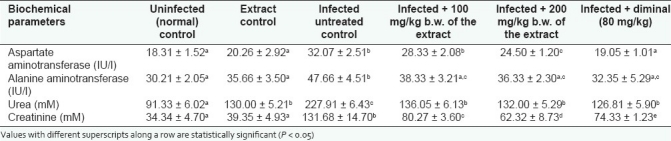
Table 3.
Effect of oral administration of different doses of ethanolic leaf extract of S. occidentalis on relative organ weights (Mean ± SD) of T. brucei brucei infected rats (n=5)

DISCUSSION
The antibacterial and antimalarial activities of S. occidentalis leaf extracts have been demonstrated.[15,16] So far, information on the trypanocidal action of this plant does not appear in the literature. This study is the first attempt to demonstrate the in vitro and in vivo activities of an extract of S. occidentalis against T. brucei and also the ameliorative effects of the extract on the disease-induced pathologic changes.
The observed in vitro antitrypanosomal activity of S. occidentalis leaves’ ethanolic extract is not surprising since previous reports[10,21–23] have clearly demonstrated that plants of different families could possess antitrypanosomal activity. Since an extract with high in vitro activity may show no in vivo antitrypanosomal activity and vice versa, due to the biotransformation of plant materials that may convert active therapeutic molecules to inactive ones, we further tested the extract for in vivo activity so that a conclusive statement can be made on the antitrypanosomal action of this plant.
The S. occidentalis ethanolic extract trypanosuppressive activity in a dose-dependent manner supports earlier reports that some plant extracts contain potent trypanocides.[9–12] Although the exact mechanism for the observed in vivo trypanosuppressive effect is not known, it is obvious that the extract contains some phytochemicals that could interfere with the survival of the parasites in vivo. However, this work did not involve structural elucidation but the presence of alkaloids, flavonoids, tannins and anthraquinones have been reported in this extract[24] and previous reports attributed the antitrypanosomal activity of a number of tropical plants to the flavonoids (azaanthraquinone), highly aromatic planar quaternary alkaloids, barbarine and harmaine.[25,26] It is thus possible that the observed trypanosuppressive action of this extract could be due to the presence of one or more of these bioactive compounds.
The acute anemia recorded in the T. brucei infected rats is a consistent feature of trypanosome infections[5,27] and the extract treatment was able to significantly (P < 0.05) ameliorate the disease-induced anemia. Perhaps the ability of the extract to lower the parasitemia could translate into the amelioration of the trypanosome-induced anemia since the degree of parasitemia has been linearly linked to the severity of anemia.[27] It is also possible that this extract possesses some antioxidant activities that could scavenge T. brucei generated free radicals which are implicated in the development of anemia during trypanosomiasis.[3,5,27–29] We have previously reported the amelioration of trypanosome-induced anemia and organ damage by some antioxidant vitamins.[3,5]
While previous reports focus on establishing the in vitro and/or in vivo antitrypanosomal action of medicinal plants only, this investigation further assesses the ability of the extract to reduce the trypanosome-associated pathologic changes. The trypanosome-induced hepatocellular damage, monitored by hepatic cell leakage of ALT and AST, was invariably eliminated by the extract treatment. Furthermore, the damage to renal structures as reflected by an increase in serum concentrations of urea and creatinine during infection was also significantly (P < 0.05) prevented by the extract administration. These could all be attributed to the ability of this extract to reduce the degree of parasitemia and/or due to the presence of some antioxidant activities in it, consequently decreasing the damage to hepatic and renal cells. The extract-treated uninfected rats did not show much difference in the biochemical parameters from the normal rats. This is indicative of the relative safety of the extract administration in the animals.
Hepatomegaly and splenomegaly consistently reported[5,30] in trypanosomiasis were also observed in this investigation. The enlargement of liver and spleen is caused by the activation and expansion of the reticuloendothelial system during trypanosome infection. The extract administration also prevented, to a lesser extent, the disease-induced hepatomegaly and splenomegaly. This observation could further lend credence to the above results on biochemical parameters and that the ethanol extract of S. occidentalis could ameliorate trypanosome-associated organ damage.
We concluded that some ethanol-extractable phytochemicals from S. occidentalis leaves possess both in vitro and in vivo antitrypanosomal activities and tend to ameliorate the trypanosome-induced anemia and organ damage. The findings as a whole further support the fact that cheap, easily available and relatively safe/nontoxic trypanocides could be developed from a tropical plant. We are presently working on detailed bioassay-directed fractionation to identify and structurally elucidate the bioactive compound(s) responsible for the results observed herein.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Umar IA, Igbalajobi FI, Toh ZA, Gidado A, Shugaba A, Buratai LB. Effects of oral administration of repeated doses of vitamin E on some biochemical indices in rats infected with Trypanosoma brucei brucei. West Afr J Biol Sci. 2001;12:1–7. [Google Scholar]
- 2.Aksoy S. Control of tsetse flies and trypanosomes using molecular genetics. Vet Parasitol. 2003;115:125–45. doi: 10.1016/s0304-4017(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 3.Umar IA, Rumah BL, Bulus SL, Kamla AA, Jobin A, Asueliman BI, et al. Effects of intraperitoneal administration of vitamins C and E or A and E combinations on the severity of Trypanosoma brucei brucei infection in rats. Afr J Biochem Res. 2008;2:88–91. [Google Scholar]
- 4.Igbokwe IO. Mechanism of cellular injury in African Trypanosomiasis. Vet Bull. 1994;64:611–5. [Google Scholar]
- 5.Umar IA, Ene O, Okodaso D, Kimeng E, Stancheva G, Omage JJ, et al. Amelioration of anaemia and organ damage by combined intraperitoneal administration of Vitamins A and C to Trypanosoma brucei brucei infected rats. Afr J Biotechnol. 2007;6:2083–6. [Google Scholar]
- 6.Wurochekke AU, Chechet G, Nok AJ. In vitro and in vivo antitrypanosomal activity of the leaf of Lawsonia inermis against Trypanosoma brucei brucei infection in mice. J Med Sci. 2004;4:236–9. [Google Scholar]
- 7.Soejarto DD. Biodiversity prospecting and benefit sharing: Perspective from field. J Ethnopharmacol. 1996;51:1–5. doi: 10.1016/0378-8741(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 8.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 9.Igweh AG, Onabanjo AO. Chemotherapeutic effects of Annona senegalensis in T. brucei brucei infection in mice. Ann Trop Med Parasitol. 1989;89:527–34. doi: 10.1080/00034983.1989.11812382. [DOI] [PubMed] [Google Scholar]
- 10.Nok AJ, Esievo KA, Londjet I, Arowosafe S, Onyenekwe PC, Gimba CE, et al. Trypanocidal potential of Azadirachta indica: In vivo activity of leaf extract against T. brucei brucei. J Clin Biochem Nutr. 1993;15:113–8. [Google Scholar]
- 11.Atawodi SE, Alafiatayo AA. Assessment of the phytochemical and antitrypanosomal properties of some extracts of leaves, stem and root bark of Landolphia sp., P. Beauv. J Ethnopharmacol. 2007;114:207–11. doi: 10.1016/j.jep.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim MA, Njoku GC, Sallau AB. In vivo activity of stembark aqueous extract of Khaya senegalensis against Trypanosoma brucei. Afr J Biotechnol. 2008;7:661–3. [Google Scholar]
- 13.Lar J, Gupta PC. Anthraquinone glycosides from the seeds of Cassia occidentalis Linn. Experientia. 1973;29:142–3. [Google Scholar]
- 14.Barbosa-Ferreira M, Dagli ML, Maiorka PC, Górniak SL. Sub acute intoxication by Senna occidentalis seeds in rats. Food Chem Toxicol. 2005;43:497–503. doi: 10.1016/j.fct.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Tona L, Ngimbi NP, Tsakala M, Mesia K, Cimanga K, Apers S, et al. Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. J Ethnopharmacol. 1999;68:193–203. doi: 10.1016/s0378-8741(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 16.Samy RP, Ignacimuthu S. Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J Ethnopharmacol. 2000;69:63–71. doi: 10.1016/s0378-8741(98)00156-1. [DOI] [PubMed] [Google Scholar]
- 17.Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okond’ahoka, Cimanga K, et al. In vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol. 2001;95:47–57. [PubMed] [Google Scholar]
- 18.Chukwujekwu JC, Coombes PH, Mulholland DA, Vanstaden J. Emordin, an antibacterial anthraquinone from the roots of Cassia occidentalis. South Afr J Bot. 2006;72:295–7. [Google Scholar]
- 19.Herbert WJ, Lumsden WH. Trypanasoma brucei: A rapid “matching” method for estimating the host's parasitaemia. Exp Parasitol. 1976;40:427–31. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan LA, Szabo LL, Opherin EK. 3rd ed. Philadelphia: Lea and Febliger; 1908. Enzymes in clinical chemistry: Interpretation and Techniques; pp. 182–4. [Google Scholar]
- 21.Asuzu IU, Chineme CN. Effects of Morinda lucida leaf extract on T. brucei brucei infection in mice. J Ethnopharmacol. 1990;30:307–13. doi: 10.1016/0378-8741(90)90109-7. [DOI] [PubMed] [Google Scholar]
- 22.Owolabi OA, Makanga B, Thomas EW, Molyneux DH, Oliver RW. Trypanocidal potentials of Africa woody plants. In vitro trials of Khaya grandifolia seed extracts. J Ethnopharmacol. 1990;30:227–31. doi: 10.1016/0378-8741(90)90012-i. [DOI] [PubMed] [Google Scholar]
- 23.Atawodi SE, Bulus T, Ibrahim S, Ameh DA, Nok AJ, Mamman M, et al. In vitro trypanocidal effects of methanolic extracts of some Nigeria savannah plants. Afr J Biotechnol. 2003;2:317–21. [Google Scholar]
- 24.Ogunkunle ATJ, Ladejobi TA. Ethnobotanical and phytochemical studies on some species of Senna in Nigeria. Afr J Biotechnol. 2006;5:2020–3. [Google Scholar]
- 25.Hopp KH, Cunningham LV, Bromel MC, Schermeister LJ, Khalil SK. In vitro antitrypanosomal activity of certain alkaloids against Trypanosoma lewisi. Lloydia. 1976;39:375–7. [PubMed] [Google Scholar]
- 26.Nok AJ. Azaanthraquinone inhibits respiration and in vitro growth of long slender blood stream forms of T. congolense. Cell Biochem Funct. 2001;20:205–12. doi: 10.1002/cbf.948. [DOI] [PubMed] [Google Scholar]
- 27.Umar IA, Toh ZA, Igbalajobi FI, Igbokwe IO, Gidado A. The effect of orally administered vitamins C and E on the severity of anaemia in T. brucei-infected rats. Trop. 1999;18:71–7. [Google Scholar]
- 28.Igbokwe IO, Esievo KA, Saror DI, Obagaiye OK. Increased susceptibility of erythrocytes to in vitro peroxidation in acute Trypanosoma brucei infection in mice. Vet Parasitol. 1994;55:279–86. doi: 10.1016/0304-4017(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 29.Igbokwe IO, Umar IA, Omage JJ, Ibrahim ND, Kadima KB, Obagaiye OK, et al. Effect of acute Trypanosoma vivax infection on cattle erythrocyte glutathione and susceptibility to in vitro peroxidation. Vet Parasitol. 1996;63:215–24. doi: 10.1016/0304-4017(95)00887-x. [DOI] [PubMed] [Google Scholar]
- 30.Morrison WI, Murray M, Sayer PD. Pathogenesis of tissue lesions in T. brucei infections. In: Losos G, Chouinard A, editors. Pathogenicity of trypanosomes, Proceeding of a workshop held in Nairobi. Ottawa: Kenya IDRC; 1978. pp. 171–7. [Google Scholar]


