Abstract
Mitragyna speciosa Korth is a medicinal plant indigenous to Thailand and Malaysia and has been known for its narcotic and coca-like effects. Many studies have been performed on the antinociceptive effect of the plant extracts of Thai origin; however, limited studies have been reported till date on M. speciosa extracts of Malaysian origin. Various concentrations of alkaloid (5–20 mg/kg), methanolic (50–200 mg/kg), and aqueous (100–400 mg/kg) extracts of Malaysian M. speciosa leaves were prepared and orally administered to nine groups of rats. Morphine (5 mg/kg, s.c.) and aspirin (300 mg/kg, p.o.) were used as control. Antagonism of the antinociceptive activity was evaluated by pretreatment with naloxone at a dose of 2 mg/kg (i.p.). Results showed that oral administration of the alkaloid (20 mg/kg), methanolic (200 mg/kg), and aqueous (400 mg/kg) extracts significantly prolonged the latency of nociceptive response compared with control groups in both hot plate and tail flick tests (P < 0.05). Antinociceptive action of the alkaloid (20 mg/kg), methanolic (200 mg/kg), and aqueous (400 mg/kg) extracts was significantly blocked by naloxone. In conclusion, these results suggest the presence of antinociceptive effect in various extracts of Malaysian M. speciosa leaves. In addition, the antinociceptive effective doses vary depending on the type of solvents used for extraction.
Keywords: Alkaloid, antinociceptive, aqueous, extract, methanol, Mitragyna speciosa
INTRODUCTION
Mitragyna speciosa Korth leaves have been used as a traditional medicine for decades in many southeast Asian countries, especially Thailand and Malaysia.[1] The plant has been used among the local population for its properties of alleviating pain from a cutting wound, reducing cough, and treating diarrhea.[2,3] Some native laborers and farmers also use the plant leaves to enhance working tolerance under the scorching sun (coca-like effect).[1,4] Smoking, chewing, or boiling leaves of M. speciosa has been reported to cause a narcotic-like effect in plant users.[3] Oral consumption of M. speciosa leaves has been reported as a substitution for morphine when weaning addicts off this drug.[1,3]
Till date, at least 22 alkaloids have been isolated from the leaves of M. speciosa.[5] Mitragynine, the dominant indole alkaloid found in this plant, has been isolated and quantified according to the geographic location and time of collection of samples.
Several in vivo and in vitro investigations of the antinociceptive activity of pure mitragynine, alkaloid and methanolic extracts of M. speciosa leaves have been conducted.[6–12] Interestingly, Watanabe et al.[13,14] reported that the antinociceptive effect of pure mitragynine was less potent than that of the alkaloid extract of M. speciosa. These studies, however, have been performed mostly on Thai plant material; limited studies have been reported so far on plant material of Malaysian origin.[15,16] Present study is important because the chemical constituents of M. speciosa of Malaysian origin differ from the chemical constituents found in extracts of M. speciosa of Thai origin. In addition, the aqueous extract of M. speciosa has been increasingly abused in Malaysia in the recent years.
This study was undertaken to determine the analgesic effect of alkaloid, methanolic, and aqueous extracts of Malaysian M. speciosa leaves in rats, using the hot plate and tail flick tests.
MATERIALS AND METHODS
Preparation of test materials
The morphine sulfate concentrate (ampoules of 10 mg/ml) was provided by the Pharmacy Section, Hospital of Universiti Sains Malaysia, Penang, Malaysia. Naloxone hydrochloride and acetylsalicylic acid anhydride were supplied by Sigma-Aldrich, London, United Kingdom. The methanol and alkaloid extracts of M. speciosa were dissolved in a mixture of propylene glycol, Tween 80, and distilled water in the ratio of 4:1:4 v/v. The aqueous extract was dissolved in distilled water. All the extract solutions as well as vehicle were prepared immediately before the experiments and administered orally to rats (maximum volume of administration of 5 ml/kg for rats), 30 minutes prior to undertaking the experiments. Also, aspirin was dissolved in distilled water and administered orally. Morphine sulfate concentrate was diluted in 0.9% sodium chloride and administered subcutaneously 15 minutes before starting the observation. Naloxone was also dissolved in distilled water and injected intraperitoneally, 10 minutes before test doses of oral administration.
Plant material
Fresh leaves of M. speciosa Korth (Rubiaceae) were collected from the states of Perlis and Perak, Malaysia. Authentication of the collected plants from Perlis was performed at School of Biological Science, Universiti Sains Malaysia. Authentication of plants collected from Perak was performed at School of Pharmaceutical Science, Universiti Kebangsaan Malaysia. The vouchers specimen (no.USM11074 and UKMB06509) were preserved for future reference.
Extract preparation
The M. speciosa Korth fresh leaves (10 kg), collected from Perlis, were oven-dried at 45–50°C for 3 days and powderized. The dried-powder leaves’ material (100 g) was macerated for 24 hours with methanol (2.5 l), using Soxhlet. The residue was diluted with deionized water in the ratio of 1:1 v/v and was concentrated using rotary evaporator (Buchi Rotary Evaporator R-110, BÜCHI Labortechnik AG, Flawil, Switzerland) resulting in a brownish powder yield of approximately 15 g. Alkaloid and aqueous extracts of M. speciosa were provided by Professor Emeritus Dato’ Moh’d Ikram Moh’d Said, Universiti Kebangsaan Malaysia. All the samples were analyzed by gas chromatography mass spectrometry (GCMS) (Hewlett Packard 6890 gas chromatography equipped with auto sampler, quadrupole mass spectrometer, and chemstation data system) to confirm the peak of mitragynine compound for each extract.
Animals
All experiments were performed on male Spraque-Dawley rats weighing between 130 and 180 g, kept in cages under room temperature, with free access to food and water. The animals were randomly divided into groups of five rats in each, and each rat was used just once during the experiments. The rats were acclimatized for 1 week in the laboratory holding room before the start of the experiment. All the experiments were carried out between 9:00 am and 1:00 pm each day to minimize the influence of the circadian rhythm. Study protocol was reviewed and approved by the Animal Ethical Committee, School of Pharmaceutical Science, Universiti Sains Malaysia.
Antinociceptive Activity
Hot plate test
The hot plate test was performed according to the method described by Woolfe and McDonald,[17] with slight modification.[18] The hot plate test was assessed by Incremental Hot/Cold plate (IITC Life Science Inc, Woodland Hills, CA, USA). The hot plate temperature was maintained at 55°C ± 1°C. Prior to treatment, only rats that showed response within 18 seconds were selected for this study. Rats (five per group) were orally administered alkaloid extract (5, 10, 20 mg/kg); methanol extract (50, 100, 200 mg/kg); and aqueous extract (100, 200, 400 mg/kg); aspirin (300 mg/kg, p.o.); morphine (5 mg/kg, s.c.); and vehicle (co solvent), respectively. Latency time of animal response to heat-induced pain, such as licking of hind paw or jumping, was measured every 15 minutes over a 60-minute period. Observation started 30 minutes after administration of the test substances, except for morphine, for which observation was started 15 minutes after administration. Cut-off time was set at 45 seconds to prevent tissue damage.[19]
Tail flick test
The tail flick test was assessed by the analgesiometer (IITC Life Science Inc., Woodland Hills, CA, USA) and the procedure used was that of D’Amour and Smith,[20] with modifications. The temperature of the tail flick was maintained at 45°C ± 1°C. Rat response to this focused heat stimulus (e.g., flicking or removing the inflicted tail) was referred to as latency time (seconds). Prior to treatment, a sensitivity test were conducted two times with a 15- minutes time interval, and rats that did not attempt to withdraw their tails within 4 seconds were discarded. The selected rats were randomized into control and test groups (n = 5). Similar dose regimens were provided as described in the hot plate test and the cut-off time was fixed at 10 seconds. Thirty minutes after administration of the test substances (15 minutes for morphine), latency time was measured at each 15-minute interval for 1 hour.
Antagonism of analgesic activity of extracts with naloxone
In this study, animals were pretreated with naloxone (2 mg/kg, i.p.), 10 minutes before the administration of the effective analgesic dose of each extract (20 mg/kg alkaloid extract, 200 mg/kg methanolic extract, 400 mg/kg aqueous extract) and of morphine (5 mg/kg). The antinociceptive activity was determined using both the hot plate and the tail flick tests, as described previously.
Statistical analysis
The data were expressed as the mean ± SEM. Statistical analysis was performed with one-way analysis of variance (ANOVA) for comparison of more than two groups followed by Dunnett's test. P < 0.05 was considered statistically significant during the analysis of data.
RESULTS
M. speciosa leaf extracts
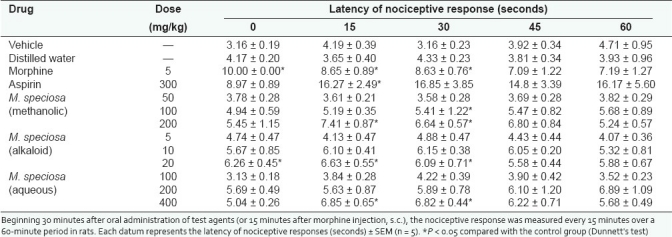
As summarized in Tables 1 and 2, significant analgesic effect, similar to morphine, was observed in rats treated with alkaloid extract (20 mg/kg), methanolic extract (200 mg/kg), and aqueous extract (400 mg/kg) of M. speciosa throughout the 90-minute observation in both hot plate and tail flick tests. In addition, aspirin showed no significant effect in either hot plate or tail flick tests. The data are shown in Tables 1 and 2.
Table 1.
Effect of the methanolic, alkaloid, and aqueous extracts of M. speciosa leaves as well as morphine on nociceptive response in hot plate test
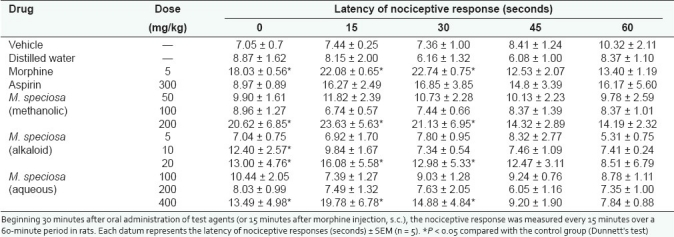
Table 2.
Effect of the methanolic, alkaloid, and aqueous extracts of M. speciosa leaves as well as morphine on nociceptive response in tail flick test
Antagonism of analgesic effect of extracts with naloxone
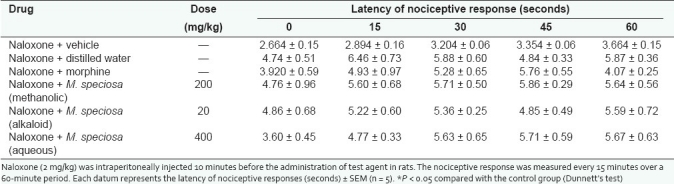
The antinociceptive action of alkaloid extract (20mg/kg), methanolic extract (200mg/kg), and aqueous extract (400mg/kg) of M.speciosa leaves were blocked by naloxone (2mg/kg, i.p.) for both the hot plate and the tail-flick tests as shown in Tables 3 and 4. Morphine (5mg/kg, s.c), a centrally acting narcotic opioid, was also antagonized using naloxone in all the experiments. No analgesic activity was observed in all naloxone pre-treated groups compared to the control group. This observation suggests that the antinociceptive activity was likely to be mediated via opioid receptors.
Table 3.
Effect of the naloxone on methanol and alkaloid extracts of M. speciosa and morphine in nociceptive response in hot plate test
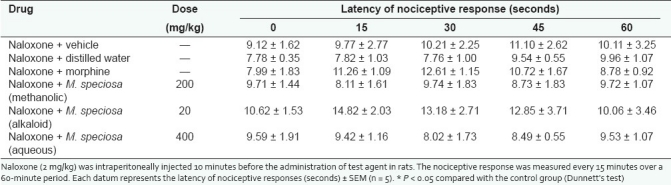
Table 4.
Effect of naloxone on methanol and alkaloid extracts of M. speciosa and morphine in nociceptive response in tail flick test
DISCUSSION
In our study, standardized extracts were used for analgesic screening. Analgesic effect was observed for all extracts tested at their respective doses for both the hot plate and the tail flick tests. Oral administration of alkaloid (20 mg/kg), methanolic (200 mg/kg), and aqueous (400 mg/kg) extracts in rats significantly prolonged the latency of nociceptive response at 30–60 minutes for both the hot plate and the tail flick tests (P < 0.05) as compared to treatment with vehicle. Similar analgesic response was also observed in morphine-treated rats. A study in Thailand reported otherwise for methanol and alkaloid extracts of M. speciosa for the tail flick test.[19] There was no analgesic effect at their respective studied doses (20 mg/kg for alkaloid extract and 100 mg/kg for methanol extract) after oral administration of the extracts. This phenomenon could be attributed to the differences in the chemical constituents of the extracts of different origin (Thailand) and/or quality of the extracts used for the study. In our study, the analgesic dose of the extracts ranged from 20 to 400 mg/kg, depending on the type of solvent used for extraction, which could be related to their respective mitragynine content, as evidenced by the fact that the mitragynine content in the aqueous, methanolic, and alkaloid extracts were in the increasing order of 0.45–0.6%, 8–10%, and 22–24%, respectively. In addition, Watanabe et al.[13,14] reported the alkaloid extract to be more potent than exclusively pure mitragynine. One possible reason for this is the existence of other compounds in the alkaloid extract that might have contributed to the analgesic effect. The analgesic action of the alkaloid (20 mg/kg), methanolic (200 mg/kg), and aqueous (400 mg/kg) extracts was also blocked by naloxone, as a nonselective and pure opioid antagonist in this experiment, thus suggesting the involvement of the central opioid system in mediating an antinociceptive activity.
CONCLUSION
The aqueous, methanolic, and alkaloid extracts of Malaysian M. speciosa leaves demonstrated analgesic activity at the higher dose levels of the three extracts, and the analgesic effects were blocked by naloxone, suggesting that opioid receptors partly mediate analgesic effects of the extracts. In addition, the experiment demonstrates that the antinociceptive effect of the three extracts studied varies depending on the type of solvent used for extraction, thus highlighting the importance of selecting the correct solvent for the proper preparation of herbal medication.
Acknowledgments
This project was funded by USM Research University Grant and Ministry of Science, Technology, and Innovation, Malaysia (MOSTI). The authors wish to thank Professor Emeritus Mohd Ikram Mohd Said for providing the aqueous and alkaloid extracts as well as Mr. Hilman and Mr.Zulkeflee for expert technical assistance. Special thanks goes to Mr.Narhari for checking the proof reading of the manuscript. A.S was supported by USM fellowship from Institute of Postgraduate Studies, USM.
Footnotes
Source of Support: USM Research University Grant and Ministry of Science, Technology, and Innovation, Malaysia (MOSTI).,
Conflict of Interest: None declared.
REFERENCES
- 1.Suwanlert S. A study of kratom eaters in Thailand. Bull Narc. 1975;27:21–7. [PubMed] [Google Scholar]
- 2.Burkil IH. Vol. 2. London: Governments of the Straits Settlements and Federated Malay States by the Crown Agents; 1935. A dictionary of the economic products of the Malay Peninsula; pp. 1480–3. [Google Scholar]
- 3.Jansen KL, Prast CJ. Ethnopharmacology of Kratom and the Mitragyna alkaloids. J Ethnopharmacol. 1988;23:115–9. doi: 10.1016/0378-8741(88)90121-3. [DOI] [PubMed] [Google Scholar]
- 4.Grewal KS. Observations on the pharmacology of mitragynine. J Pharmacol Exp Ther. 1932;46:251–71. [Google Scholar]
- 5.Shellard EJ, Houghton PJ, Resha M. The Mitragyna species of Asia. Part XXXI. The alkaloids of Mitragyna speciosa Korth from Thailand. Planta Med. 1978;34:26–36. [Google Scholar]
- 6.Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, et al. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996;59:1149–55. doi: 10.1016/0024-3205(96)00432-8. [DOI] [PubMed] [Google Scholar]
- 7.Macko E, Weisbach JA, Douglas B. Some observations on the pharmacology of mitragynine. Arch Int Pharmacodyn Ther. 1972;198:145–61. [PubMed] [Google Scholar]
- 8.Idid SZ, Saad LB, Yaacob H, Shahimi MM. Evaluation of analgesia induced by mitragynine, morphine and paracetamol on mice. ASEAN Review of Biodiversity and Environmental Conservation (ARBEC) Article IV. 1998:1–7. [Google Scholar]
- 9.Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, et al. Central antinociceptive effects of mitragynine in mice: Contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol. 1996;317:75–81. doi: 10.1016/s0014-2999(96)00714-5. [DOI] [PubMed] [Google Scholar]
- 10.Tohda M, Thongpraditchote S, Matsumoto K, Murakami Y, Sakai S, Aimi N, et al. Effects of mitragynine on cAMP formation mediated by delta-opiate receptors in NG108-15 cells. Biol Pharm Bull. 1997;20:338–40. doi: 10.1248/bpb.20.338. [DOI] [PubMed] [Google Scholar]
- 11.Thongpradichote S, Matsumoto K, Tohda M, Takayama H, Aimi N, Sakai S, et al. Identification of opioid receptor subtypes in antinociceptive actions of supraspinally administered mitragynine in mice. Life Sci. 1998;62:1371–8. doi: 10.1016/s0024-3205(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 12.Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from Rubicaceus Plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo) 2004;52:916–28. doi: 10.1248/cpb.52.916. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Yano S, Horie S, Yamamoto LT, Sakai S, Takayama H, et al. Pharmacological profiles of “Kratom” (Mitragyna speciosa), a Thai medical plant with special reference to its analgesic activity. In: Tongroach P, Watanabe H, Ponglux D, Suvanakoot U, Ruangrungsi N, editors. Advances in Research on Pharmacologically Active Substances from Natural Products. Chiang Mai: Chiang Mai Univ Bull; 1992. pp. 125–32. [Google Scholar]
- 14.Watanabe K, Yano S, Horie S, Yamamoto LT, Takayama H, Aimi N, et al. Pharmacological properties of some structurally related indole alkaloids contained in the Asian herbal medicines, hirsutine and mitragynine, with special reference to their Ca2+ antagonistic and opioid-like effects. In: Watanabe H, Shibuya T, Farnsworth NR, editors. Pharmacological Research on Traditional Herbal Medicines. Tokyo: Harwood Academic Press; 1999. pp. 163–77. [Google Scholar]
- 15.Shaik Mossadeq WM, Sulaiman MR, Tengku Mohamad TA, Chiong HS, Zakaria ZA, Jabit ML, et al. Anti-Inflammatory and antinociceptive effects of Mitragyna speciosa Korth methanolic extract. Med Princ Pract. 2009;18:378–84. doi: 10.1159/000226292. [DOI] [PubMed] [Google Scholar]
- 16.Kitajima M, Misawa K, Kogure N, Said IS, Horie S, Hatori Y, et al. A new indole alkaloid, 7-hydroxyspeciociliatine, from the fruits of Malaysian Mitragyna speciosa and its opioid agonistic activity. J Nat Med. 2006;60:28–35. [Google Scholar]
- 17.Woolfe G, McDonald AD. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) J Pharmacol Exp Ther. 1944;80:300–7. [Google Scholar]
- 18.Racz I, Zimmer A. Standards of mouse model phenotyping. Weinheim: Wiley-VCH Verlog GmbHand co.kG; 2006. Animal models of nociception; pp. 163–80. [Google Scholar]
- 19.Reanmongkol W, Keawpradub N, Sawangjaroen K. Effects of the extracts from Mitragyna speciosa Korth. leaves on analgesic and behavioral activities in experimental animals Songklanakarin. J Sci Technol. 2007;29:39–48. [Google Scholar]
- 20.D’Amour E, Smith DL. A method for determination of pain sensation. J Pharmacol Exp Ther. 1941;72:74–9. [Google Scholar]


