Abstract
Salvia splendens (Labiatae) is widely used in Indian traditional medicine for the control of diabetes mellitus. In this study, the hypoglycemic effects produced by the acute and subacute administration of various extracts of S. splendens were investigated. Both the aqueous extract (SSAE) and the methanolic extract (SSME) from the aerial parts resulted in significant reductions of glycemia in streptozotocin (STZ)-induced diabetic rats after oral administration at a dose of 100 and 200 mg/kg, respectively. On oral administration, aqueous and methanolic extracts showed statistically significant (P < 0.001) effect by reducing the effect of glycemia in STZ-induced diabetic rats. These findings suggest the significant antihyperglycemic potential of the S. splendens extracts in ameliorating the diabetic conditions in diabetic rats. No significant effects were found in the normal rats.
Keywords: Acute and subacute antidiabetic studies, glibenclamide, Salvia splendens, streptozotocin-induced diabetic rats
INTRODUCTION
Diabetes mellitus is a group of metabolic diseases characterized by high blood sugar (glucose) levels that result from defects in insulin secretion, or action, or both. Normally, blood glucose levels are tightly controlled by insulin, a hormone produced by the pancreas. Insulin lowers the blood glucose level. When the blood glucose elevates (for example, after eating food), insulin is released from the pancreas to normalize the glucose level. In patients with diabetes, the absence or insufficient production of insulin causes hyperglycemia. Diabetes is a chronic medical condition, meaning that although it can be controlled, it lasts for a lifetime. Over time, diabetes can lead to blindness, kidney failure, and nerve damage. These types of effects are the result of damage to small vessels, referred to as microvascular disease. Diabetes is also an important factor in accelerating the hardening and narrowing of the arteries (atherosclerosis), leading to strokes, coronary heart disease, and other large blood vessel diseases. This is referred to as macrovascular disease. Instances of diabetes have increased and it is expected that there will be 300 million people with diabetes worldwide, by the year 2025. Diabetes mellitus occurs in several forms. Approximately 10% of diabetic patients have type-1 diabetes mellitus, an autoimmune disease that destroys insulin-producing beta cells in the pancreas, leading to a decrease in the concentration of insulin in the body, and the remainder has type-2 (noninsulin-dependent diabetes mellitus). Type-2 diabetes mellitus is a metabolic disorder characterized by a progressive decline in insulin action. The beta cells normally compensate for insulin resistance by secreting greater amount of insulin in order to maintain glucose homeostasis. In noninsulin-dependent diabetes mellitus, the beta cell function becomes impaired due to insulin resistance, leading to deterioration in glucose homeostasis and a subsequent development of impaired glucose tolerance.[1]
Insulin and oral hypoglycemic agents are the most widely used drugs for lowering the blood glucose in diabetes, but these drugs also have various side effects such as hypoglycemia, weight gain (sulfonyl urea), and lactic acidosis (biguanides), and all of these drugs can cause liver and renal damage. Therefore, in recent years, considerable attention has been directed toward identification of plants with antidiabetic activity, which may be used for human consumption. More than 800 plants are used as traditional remedies for the treatment of diabetes throughout the world.[2]
The Lamiaceae (Labiatae) family comprises 200 genera and 3000 species. One of the largest genera of the family, Salvia L., is represented by over 900 species and some species of salvia have been cultivated worldwide for use in folk medicines and for culinary purposes. The plants are typically 30–150 cm tall, herbaceous or suffruticose, and perennial, rarely biennial, or annual, with attractive flowers in various colors.
The name salvia comes from the Latin word “salvare” which means to heal. Salvia species have been used since ancient times for more than 60 different ailments ranging from aches to epilepsy, and mainly to treat colds, bronchitis, tuberculosis, hemorrhage, and menstrual disorders.
The main secondary metabolite constituents of Salvia species are terpenoids and flavonoids. The aerial parts of these plants contain flavonoids, triterpenoids, and monoterpenes, particularly in the flowers and leaves, while diterpenoids are found mostly in the roots. However, some American Salvia species contain diterpenoids in the aerial parts, and in certain Salvia species, triterpenoids and flavones are present in the roots.[3–5]
Salvia has always been a greatly esteemed medicinal herb in view of its multifarious curative effects. Many compounds isolated from salvia extracts are associated with antiseptic, antibacterial, antifungal, antiviral, anti-inflammatory, antioxidant, hypoglycemic, antispasmodic, cytotoxic, and antitumor activities. In this study, the hypoglycemic effects produced by the acute and subacute administration of various extracts of Salvia splendens were investigated.
MATERIALS AND METHODS
Plant material
The plant was collected from the Dept. of Horticulture, Palandu, Ranchi, and the plant was identified and authenticated by H. J. Chowdary, Joint Director, Botanical Survey of India, Botanical Garden, Howrah. The plant material was dried under shade, powdered, and passed through a 40-mesh sieve.
Preparation of aqueous extract
The treatment procedure of the plant extract consist of adding 1% acetic acid to 50 g of dry material in the ratio of 1:15, which yielded 750 ml of solution. The process of extraction was continued for 8 hours at room temperature, using mechanical stirrer. Extract was filtered with the paper and 650 ml of the solution was obtained. The solution was treated with dichloromethane for three times, using 500 ml of dichloromethane each time. Then, the solution was treated with ethyl acetate for two times, using 500 ml ethyl acetate each time. The remaining 400 ml of solution was dried in the lyophylisator. Finally, 2 g of the powder obtained was tested if positive for anthocyanins and this powder extract (SSAE) was used for pharmacological study.[6]
Preparation of the methanolic extract
The powdered plant material was extracted with methanol in a soxhlet apparatus for 72 hours. After extraction, the solvent was filtered and then evaporated under reduced pressure. The obtained crude methanolic extract (SSME) was used for phytochemical screening and pharmacological studies. Phytochemical screening and the preliminary chemical examination of the methanolic extract revealed the presence of anthocyanins, flavonoids, terpenoids, glycosides, reducing sugars.
Assessment of antidiabetic activity
Animals
Albino rats of 150–200 g body weight were used in study. Animals were procured from Laboratory Animal house of Birla Institute of Technology, Mesra. All the animal experiments were strictly complied with the approval of institutional ethical committee. All the animals were kept in polyacrylic cages and maintained under standard housing conditions (room temperature 24–27°C and humidity 60–65%) with 12:12 light:dark cycles. Food was provided in the form of dry pellets and water ad libitum.
Chemicals
Streptozotocin (STZ) was purchased from Sigma-Aldrich Himedia. Glibenclamide was a generous gift sample from Themis laboratories, Wagle estate, Thane, India. All other reagents used were of analytical grade.
Treatment protocol
Six animals were taken as normal controls. They were treated with the vehicle 5% Tween 80 at a dose of 0.5 ml/100 g body weight throughout the period of the oral hypoglycemic study.
Induction of diabetes
The animals were rendered diabetic by a single intraperitoneal injection of STZ, freshly prepared in 0.1 M citrate buffer (pH 4.5) at a dose of 50 mg/kg body weight.[7] The STZ-injected animals were then given 5% glucose solution for 24 hours following STZ injection to prevent initial drug-induced hypoglycemic mortality. After 72 hours of STZ injection, blood was drawn from the retro-orbital plexus of the rats and fasting blood sugar was estimated by using a glucometer[8–12] (ACCU CHEK-Roche Diagnostic Gmbh, Berlin, Germany).
The animals having fasting blood glucose above 200–250 mg/dl were included for the treatment below. Treatment was started 7 days after the induction of diabetes.
Group-1: diabetic controls received 5% v/v of Tween 80 orally at a dose of 0.5 ml/100 g body weight
Group-2: standard received glibenclamide at a dose of 2.5 mg/kg body weight orally
Group-3: received SSME at a dose of 100 mg/kg body weight orally
Group-4: received SSME at dose of 200 mg/kg body weight orally
Group-5: received SSAE at dose of 100 mg/kg body weight orally
All the animals were kept in 18 hours fasting condition with water given ad libitum prior to the commencement of the experiment.
Estimation of blood glucose
Fasting blood samples were collected from the retro-orbital plexus and blood glucose level was determined using a glucometer.
For acute antidiabetic study,[13,14] blood samples were collected and fasting blood glucose level was determined at 0, 1/2, 1, 2, 4, 8, and 24 hours.
For subacute antidiabetic study,[13,14] blood samples were collected and fasting blood glucose level was determined on 3rd, 5th, 7th, and 14th days.
RESULTS AND DISCUSSION
Administration of STZ caused rapid destruction of pancreatic beta cells in rats, which led to impaired glucose-stimulated insulin release and insulin resistance, both of which are marked features of type-2 diabetes. The blood glucose lowering effect of plant extracts is generally dependent upon the degree of pancreatic beta cell destruction and is useful in moderate STZ-induced diabetes. The lesser the degree of pancreatic beta cell destruction, the more useful is the herb in treating diabetes in animals.
As shown in Table 1, in acute study, the maximum percent blood glucose reduction [60.65% at 100 mg/kg (SSAE); 52.94% at 100 mg/kg and 56.07% at 200 mg/kg (SSME)] in diabetic rats was observed at 8th hour. The results of antidiabetic effect of S. splendens were compared with the reference standard drug glibenclamide (65.71% at 2.5 mg/kg body weight). [Figure 1]
Table 1.
Acute hypoglycemic effect of S. splendens extracts on STZ-induced diabetic rats
Figure 1.
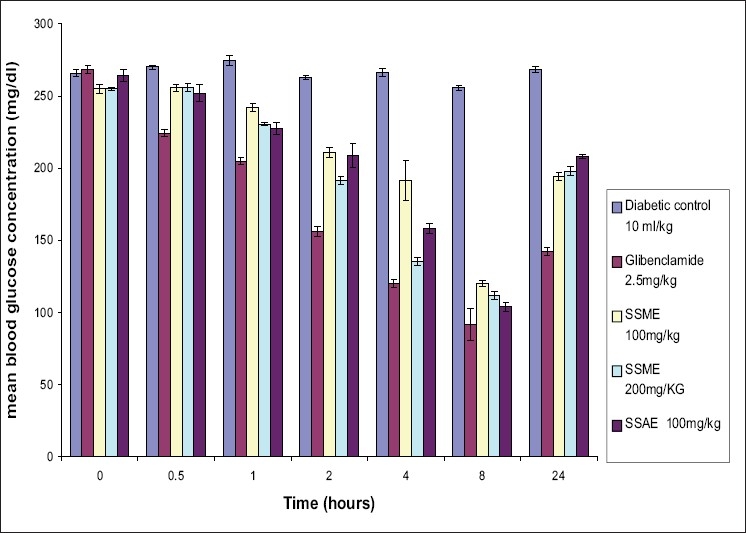
Acute hypoglycemic effect of Salvia splendens extracts on Streptozotocin induced diabetic rats
As shown in Table 2, in subacute study, the maximum percent blood glucose reduction [63.38% at 100 mg/kg (SSAE); 61.30% at 100 mg/kg and 61.17% at 200 mg/kg (SSME)] in diabetic rats was observed on the 14th day. The results of antidiabetic effect of S. splendens were compared with the reference standard drug glibenclamide (64.75% at 2.5 mg/kg body weight).[Figure 2]
Table 2.
Subacute hypoglycemic effect of S. splendens extracts on STZ-induced diabetic rats
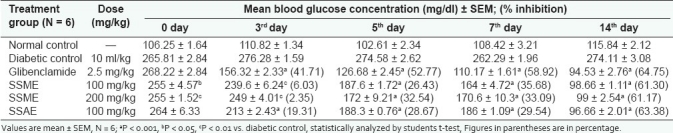
Figure 2.
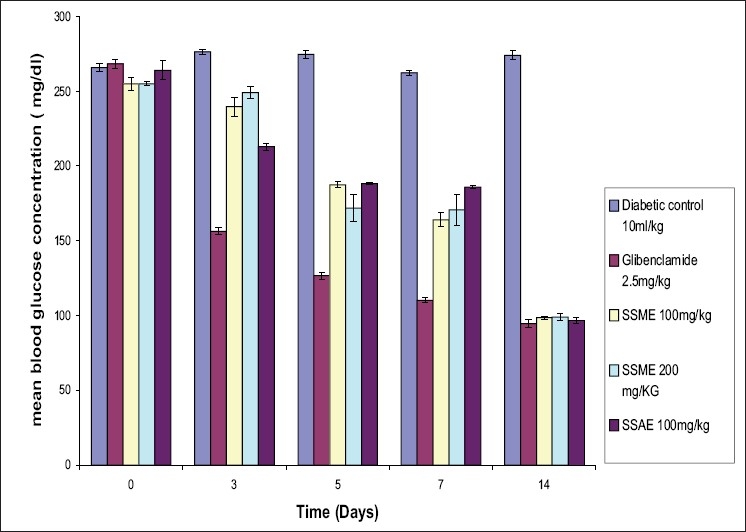
Sub acute hypoglycaemic effect of Salvia splendens extracts on Streptozotocin induced diabetic rats
This study established the antihyperglycemic activity of the methanolic and aqueous extracts of S. splendens. Both the extracts significantly reduced the blood glucose levels in STZ-induced diabetic rats.
CONCLUSION
The hypoglycemic activity of aqueous and methanolic extracts of S. splendens might be due to the presence of high amounts of anthocyanins and phenolic and flavonoid compounds.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Farswan M, Mazumder PM, Percha V. Protective effect of Cassia glauca Linn. on the serum glucose and hepatic enzymes level in streptozotocin induced NIDDM in rats. Indian J Pharmacol. 2009;41:19–22. doi: 10.4103/0253-7613.48887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sage boasts beauty, aroma. [last cited on 2010 Jan 10]. Available from: http://www.peakonline.com .
- 3.Salvia splendens update. [last cited on 2010 Jan 10]. Available from: http://Brunelle@rpa.net .
- 4.Scarlet sage. [last cited on 2010 Jan 10]. Available from: http://www.wikipedia.com .
- 5.Topçu G. Bioactive triterpenoids from Salvia species. J Nat Prod. 2006;69:482–7. doi: 10.1021/np0600402. [DOI] [PubMed] [Google Scholar]
- 6.Jong-Yuh C, Mei-Fen S. Potential hypoglycemic effects of Chlorella in streptozotocin induced diabetic mice. Life Sci. 2005;77:980–90. doi: 10.1016/j.lfs.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Orhan DD, Aslan M, Sendogdu N, Ergun F, Yesilada E. Evaluation of the hypoglycemic effect and antioxidant activity of three viscum album sub species (European mistletoe) in Streptozotocin induced diabetic rats. J Ethnopharmacol. 2005;98:95–102. doi: 10.1016/j.jep.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Tas S, Sarandol E, Ziyanok S, Aslan K, Dirican M. Effects of green tea on serum paraoxonase/ arylesterase activities in Streptozotocin induced diabetic rats. Nutr Res. 2005;25:1061–74. [Google Scholar]
- 9.Kumar SG, Shetty AK, Baiah SK, Salimath PV. Antidiabetic property of Fenugreek seed mucilage and spent turmeric in Streptozotocin induced diabetic rats. Nutr Res. 2005;25:1021–5. doi: 10.1007/s11130-005-5104-5. [DOI] [PubMed] [Google Scholar]
- 10.Gerhard VH. 2nd edition. Germany: springer-verlag Berlin Heidelberg; 2002. Drug discovery and evaluation. [Google Scholar]
- 11.Hnatyszyn O, Miño J, Ferraro G, Acevedo C. The hypoglycemic effect of Phyllanthus sellowianus fractions in streptozotocin –induced diabetic mice. Phytomedicine. 2002;9:556–9. doi: 10.1078/09447110260573209. [DOI] [PubMed] [Google Scholar]
- 12.Xia T, Wang Q. Antihyperglycemic effect of Cucurbita ficifolia fruit extract in Streptozotocin induced diabetic rats. Fitoterapia. 2006;77:530–3. doi: 10.1016/j.fitote.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Sriplang K, Adisakwattana S, Rungsipipat A, Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin induced diabetic rats. J Ethnopharmacol. 2007;109:510–4. doi: 10.1016/j.jep.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Arulselvan P, Subramanian SP. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic beta cells in experimental diabetes in rats. Chem Biol Interact. 2007;165:155–64. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]



