Abstract
Rationale
The hypocretin (hcrt) system has been implicated in addiction-relevant effects of several drugs but its role in nicotine dependence has been little studied.
Objectives
These experiments examined the role of the hcrt system in nicotine reinforcement.
Methods
Rats were trained for nicotine self-administration (NSA) on fixed-ratio schedules. The effects of acute, pre-session treatments with the hcrtR1 antagonist SB334867 and the hcrtR1/2 antagonist almorexant were examined on NSA maintained on a fixed-ratio 5 (FR5) schedule. Gene expression for the hcrt system (mRNA for hcrt, hcrtR1 and hcrtR2) was measured in animals following NSA on a FR1 schedule for a 19-day period.
Results
The hcrtR1 antagonist SB334867 and the hcrtR1/2 antagonist almorexant both reduced NSA dose-dependently (significantly at doses of 30 and 300 mg/kg respectively); SB334867 did not affect food-maintained responding whereas almorexant (at the 300 mg/kg) did. Tissue from animals collected 5 hours after self-administration showed an increase in hcrtR1 mRNA in the arcuate nucleus compared to control subjects. In tissue collected immediately after a similar 19-day self-administration period, mRNA for hcrtR1 was decreased in the rostral lateral hypothalamus compared to controls.
Conclusions
These data confirm a previous report (Hollander et al. 2008) that the hypocretin receptor hcrtR1 is activated in nicotine reinforcement, and in addition show that both the arcuate nucleus and lateral hypothalamus are sites at which hcrt receptor mechanisms may influence reinforcement. Different patterns of mRNA expression at different times after NSA suggest that changes in the hcrt system may be labile with time.
Keywords: hypocretin, nicotine, reinforcement, self-administration, SB334867, almorexant
Introduction
The hypocretin/orexin neuropeptides (de Lecea et al. 1998; Sakurai et al. 1998), hypocretin-1/-2 or orexin-A/-B, are expressed in a small population of neurons in the lateral hypothalamus (LH) and perifornical area (PFA) of the CNS and project extensively throughout the brain (Nambu et al. 1999; Peyron et al. 1998) where they interact with two G-protein coupled receptors, hcrtR1/hcrtR2 or OX1R/OX2R, with different affinities. These receptors also have widespread differential distribution in brain (de Lecea et al. 2002; Marcus et al. 2001; Trivedi et al. 1998). Hypocretins1 have been linked to a number of functions including feeding, physical activity and energy expenditure, arousal, the regulation of sleep and narcolepsy (de Lecea et al. 2002; Horvath and Gao 2005; Kilduff and Peyron 2000; Kotz 2006; Paneda et al. 2005; Sakurai 2007; Siegel 2004; Sutcliffe and de Lecea 2002; Winsky-Sommerer et al. 2005).
Recent experiments have implicated hypocretin-1 (hcrt-1) mechanisms in the addiction-relevant effects of cocaine and morphine. Hcrt mechanisms influence neural plasticity within the ventral tegmental area (VTA), behavioral sensitization to cocaine, cocaine self-administration and reinstatement (Borgland et al. 2009; Borgland et al. 2006; Boutrel et al. 2005; España et al. 2010). Hcrt neurons respond to chronic morphine and morphine withdrawal, and the latter is attenuated in hcrt knock-out mice (Georgescu et al. 2003). Microinjection of hcrt-1 and hcrt-2 into the VTA increases dopamine (DA) and its metabolites in the synaptic field in the nucleus accumbens, and intra-VTA infusion of the selective hcrtR1 antagonist SB-334867-A suppresses conditioned preference for an environment paired to morphine effects; dependence-related opiate effects are abolished in mice in which the prepro-hcrt gene is knocked out (Narita et al. 2006). Activation of LH hcrt neurons measured by Fos expression is significantly correlated to conditioned preferences for food, cocaine or morphine, and extinguished preferences for opioids are reinstated by activation of LH hcrt neurons or VTA hcrt receptors (Harris et al. 2005).
It has been proposed that LH hcrt neurons are relevant in reward processing per se whereas those in the PFA may be associated with arousal and stress (Harris and Aston-Jones 2006). This is consistent with the link between addiction, and the activation of CRF mechanisms (de Lecea et al. 2006; Koob 2006; 2008). A recent review summarizes work in this area (Aston-Jones et al. 2009).
Nicotine reinforces tobacco use. Given that the effects of nicotine appear to include arousal and attentional improvements, hypocretin mechanisms are potential candidates as substrates in part because hcrt projection areas include the VTA and pontine regions such as the pedunculopontine tegmental nucleus (PPTg), both of which are loci at which self-administered nicotine acts to produce reinforcing effects (Corrigall et al. 1994; Lança et al. 2000). In addition, hcrt mechanisms may influence other brain regions and contribute broadly to the effects of nicotine relevant to addiction (Corrigall 2009). However, few investigations have been reported. Of these, several have documented the effects of experimenter-administered nicotine, including an increase in the expression of hcrt precursor and receptor mRNA and hcrt peptides following chronic high-dose nicotine (Kane et al. 2000) and an increase in the fraction of hcrt-containing neurons in LH/PFA expressing Fos following acute nicotine (Pasumarthi et al. 2006). This increase was particularly present in hcrt-containing neurons projecting to the basal forebrain – potentially mediating nicotine effects on attention – and to the paraventricular nucleus of the dorsal thalamus (PVT), possibly mediating nicotine-induced arousal via circuitry from the PVT to prefrontal cortex (Pasumarthi and Fadel 2008). In addition, nicotine and hcrt excite the same thalamocortical synapses and improve performance in an attentional demand task (Lambe et al. 2005).
The role of the hypocretin system in nicotine’s reinforcing effects has received little attention. One study recently reported that the selective hcrtR1 antagonist SB334867, administered systemically, reduced the self-administration of nicotine but not food-maintained responding, and decreased the nicotine-produced reduction in brain-reward threshold (Hollander et al. 2008). In this study, SB334867 also reduced NSA when delivered locally into the insular cortex. The role of hcrtR2 mechanisms in nicotine’s reinforcing effects has not yet been studied.
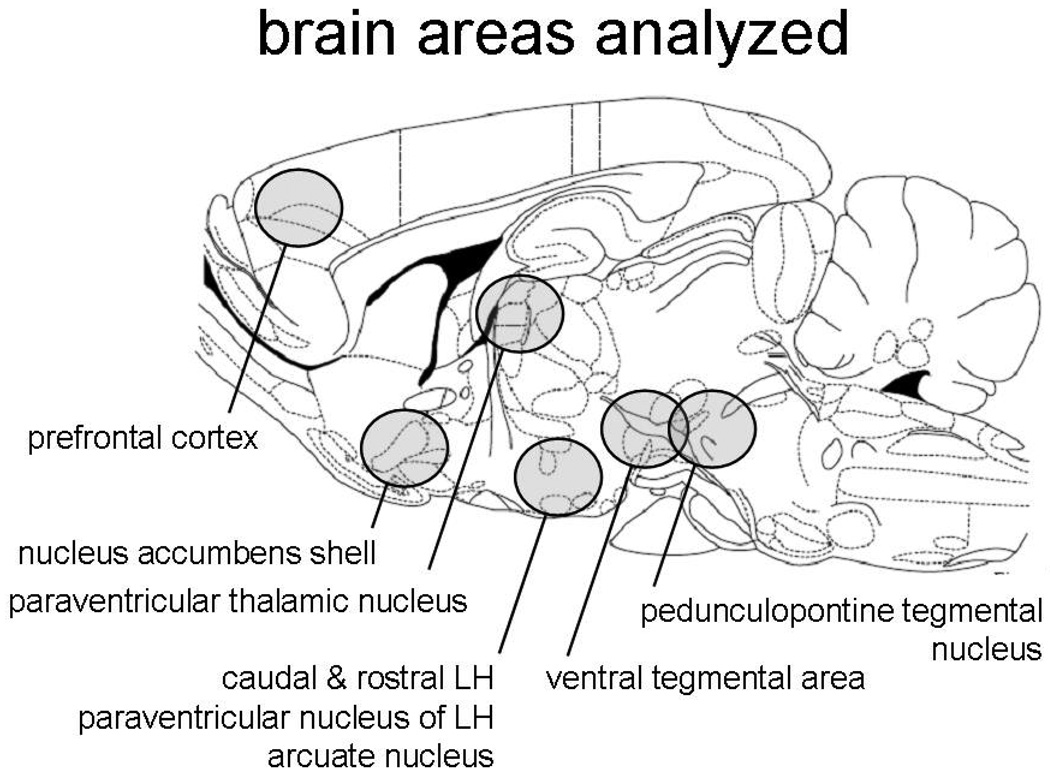
In the experiments reported here, we have examined the effects of the selective hcrtR1 antagonist SB334867 and the hcrtR1/2 antagonist almorexant on NSA and food-maintained responding in laboratory rats. Comparing these two drugs allows a preliminary assessment of whether a hcrtR1/2 antagonist has any efficacy above and beyond that produced by a selective hcrtR1 antagonist. To the extent that it does, it may suggest a role for hcrtR2 systems in NSA. In addition, we have examined the expression of mRNA for the hcrt system (hcrt, hcrtR1, hcrtR2) in several brain regions at 2 time points following a 4-week period of intravenous NSA. The brain regions chosen for examination (shown in Fig 2b) have a previously demonstrated role in hcrt-related behaviors and synthesize hcrt and/or hcrt receptors.
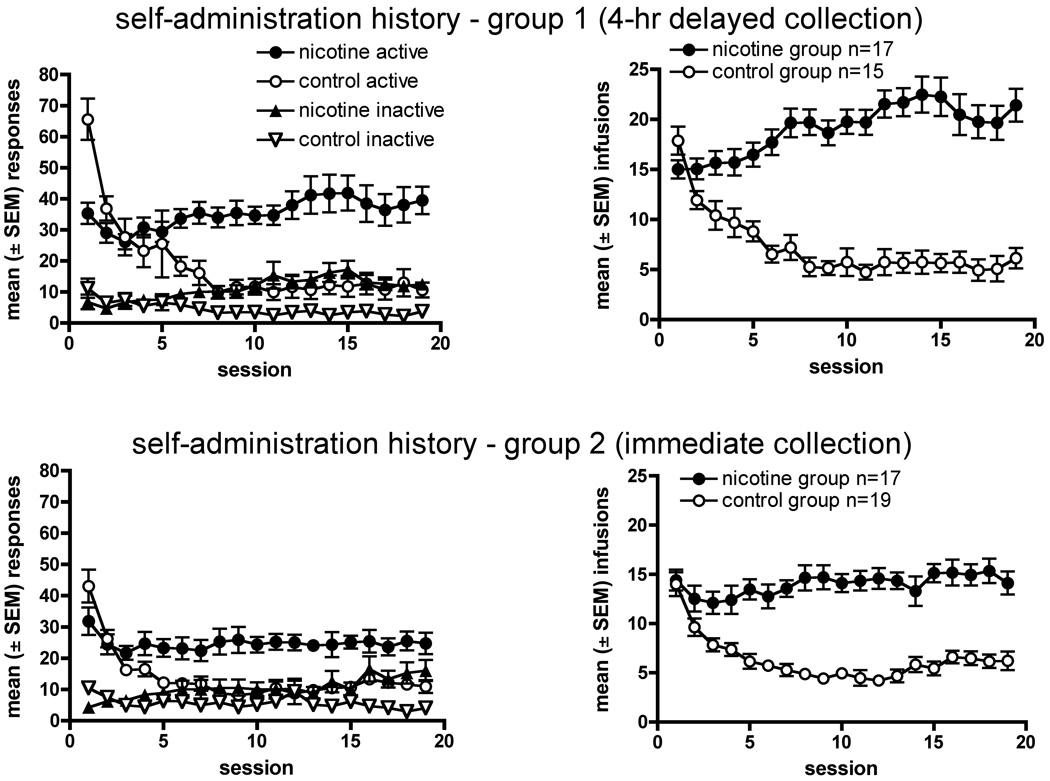
Fig 2.
(a) The 4 panels show the NSA data over 19-day periods for the 2 groups of animals used in the gene expression studies. Left-hand panels for each group show the total number of lever presses. The right-hand panels show the number of nicotine infusions obtained. The top two panels show data from the group in which brain tissue was collected 5 hr after the last NSA session, while the bottom two panels show data from the group in which tissue was collected immediately (1–11 min) after the final session. (b) Schematic shows the brain regions that were collected by micro-dissection in group 1.
Materials and methods
Subjects
Groups of experimentally-naïve male Long Evans rats weighing 300–400 g were maintained under a restricted feeding regimen throughout the entire experiment (approx. 18 g/day rat chow) to limit excessive body weight gain. Each rat was individually housed in a temperature- and humidity-controlled colony room with unlimited access to water under a reversed 12h light/dark cycle (lights off at 10:00 am). Animal husbandry and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation and University of Minnesota, and were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals.
Apparatus
Experimental sessions occurred in sixteen identical operant-conditioning chambers. The front panel contained two response levers, a stimulus light over each response lever, and an aperture for delivery of 45-mg food pellets. Each chamber was enclosed in a sound-attenuating box equipped with an exhaust fan that provided masking noise. An infusion pump for delivery of nicotine infusions was placed on top of the sound-attenuating box. In all experiments, presses on the left (active) lever produced a 45-mg food pellet or an infusion of 0.03 mg/kg nicotine (see below); presses on the other (inactive) lever were recorded but had no programmed consequence (except for controls in the gene expression study, for which both levers had no programmed consequences).
Food Training
All rats were initially trained to lever press for food pellets. During this phase, each response on the active lever produced a single 45-mg food pellet. Once trained (100 pellets earned within 1 hr for 3 consecutive sessions), rats were either implanted with a jugular catheter or further trained to respond for food as described below. During training and all subsequent phases of the experiment, sessions were conducted Monday through Friday.
Surgery
For intravenous self-administration, each rat was implanted with a chronic indwelling jugular catheter under intramuscular droperidol (2.0 mg/kg) and fentanyl (0.04 mg/kg) anesthesia. A silastic catheter (0.51 mm I.D. × 0.94 mm O.D.) was inserted into the right jugular vein and advanced to the junction of the vena cava and the right atrium and sutured to tissue surrounding the vein. The catheter was tunneled subcutaneously to the back where it exited between the scapulae and attached to a guide cannula mounted in a harness assembly on the back of the rat. A stainless steel spring tether attached to the guide cannula allowed connection to a fluid swivel for nicotine administration. Rats were allowed to recover for at least four days after surgery, during which each rat received daily intravenous (IV) infusions of a heparinized glycerol/saline solution (25% glycerol, 25 units/ml heparin) and antibiotic (rocephin, 5.25 mg) into the jugular catheter. To help maintain catheter patency throughout the remainder of the experiment, catheters were flushed Monday through Thursday with the heparinized glycerol/saline solution, and “locked” on Fridays with a glycerol/saline containing 50% glycerol and urokinase (0.67 mg/ml of heparinized saline). Infusions of methohexital (0.1 ml, 50 mg/ml, IV) were administered occasionally to determine catheter patency (production of ataxia) if malfunctions were suspected.
Behavioral training for tests of SB334867 and almorexant
Nicotine self-administration was established with a unit dose of 0.03 mg/kg/infusion which is commonly used in self-administration research with rats, and is mid-range on the dose-effect curve (Corrigall and Coen 1989; Ross et al. 2007). Rats were initially given access to nicotine infusions (delivered in 1 sec) during 1-hr sessions under a fixed-ratio (FR) 1 schedule, wherein each press on the active lever produced a nicotine infusion. A 1-min timeout followed each infusion, during which responses on both levers were recorded but had no programmed consequence. Once NSA was well-established under this schedule (at least 8 infusions per session for 5 consecutive sessions), the response requirement was gradually increased to FR 5 over several sessions (typically 2–3 weeks). Training under the terminal FR 5 schedule continued for at least 10 sessions and until NSA was stable (at least 8 infusions per session and no visually-evident trend in infusion rates for five consecutive sessions), at which drug pretreatments began. Similar training criteria are commonly used in studies of NSA (Corrigall and Coen 1989; LeSage et al. 2004; Ross et al. 2007). The mean number of NSA sessions to meet stability criteria were 40 (±4.5 SEM) and 66 (±8.1 SEM) for groups treated with SB334867 and almorexant, respectively. For measurement of gene expression (see below), all rats received 19 sessions of NSA prior to sacrifice.
For control groups responding for food, once food-maintained responding was well-established under the FR 1 schedule of food delivery (at least 50 pellets earned per session for 5 consecutive sessions), the response requirement and timeout were gradually increased to FR5 and 1 min respectively (identical to the self-administration schedule) over several sessions (2–3 weeks). Training under the FR 5 schedule continued until response rate stabilized (at least 40 pellets earned per session and no trend in response rate for 5 consecutive sessions), at which point drug pretreatments began. Sessions were 1 hour in duration. Food pellets were 45 mg Rodent Dustless Precision Pellets (Formula PJAI, TestDiet, Richmond, IN).
The effects of SB334867 (10, 18 and 30 mg/kg i.p. at a volume of 4 ml/kg) and almorexant (100 and 300 mg/kg p.o. at a volume of 5 ml/kg) and vehicle injections (see below) were assessed on Tuesdays and Fridays, provided that response rates during the previous session were within the range of stable baseline response rates. In some cases, rats failed to meet these criteria (e.g., following the highest test dose or in the event of a catheter leak or occlusion). When this occurred, drug testing was suspended until criteria were met for at least three consecutive sessions. Antagonist doses and routes of administration were selected from prior studies in rats in which the compounds showed effectiveness in general behavioral measures and feeding (SB334867; Haynes et al. 2000; Rodgers et al. 2001) and in studies of alertness (almorexant; Brisbare-Roch et al. 2007). Drug and vehicle administration occurred 30 min prior to sessions for SB334867 (Duxon et al. 2001; Harris et al. 2005) and 2 hrs prior for almorexant (Brisbare-Roch et al. 2007). Rats were administered each dose of an antagonist once in a mixed order that was counterbalanced across rats. Different groups of animals were used to test each antagonist in both self-administration and food-maintained responding.
Behavioral training for gene expression studies
For these studies, animals had access to the same dose of nicotine (0.03 mg/kg/infusion delivered in 1 sec), however the schedule remained at FR1 (timeout 1 min) rather than being increased to FR5. This was done to attempt to minimize animal-to-animal variability in acquisition and total nicotine exposure. Control subjects had the identical surgical and behavioral history as the nicotine subjects, but their responding in the experimental chambers only produced infusion-related cues (no infusion was actually administered). Experimental sessions occurred for 19 days, at which point animals were sacrificed either immediately after the session (i.e., 1 to 11 min) or 5 hours later. The latter time is approximately 5 half-lives for nicotine, a time at which plasma levels would be expected to be low. The first session occurred on either Monday or Tuesday, with the start date counterbalanced across groups. Accordingly, rats were sacrificed on either Thursday or Friday, with the day of sacrifice counterbalanced across groups. Tissue from pre-selected brain regions was collected by tissue punch micro-dissection on dry ice as previously described (Kotz et al. 1997). The samples were frozen and stored at −70 °C for subsequent analysis. The regions collected for measurement were chosen based upon the presence of hcrt or hcrt receptors, and their previously demonstrated involvement in appetite, arousal, addiction and/or physical activity (Borgland et al. 2006; Kotz 2006). Brain punches were taken using 0.5 or 1 mm diameter punching tools, from 1–2 mm coronal sections corresponding to the region of interest, using a standard brain atlas as a guide (Paxinos and Watson 1998).
One-Step Real Time RT-PCR
The primers for preproorexin, OX1R, OX2R and the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, GADPH, were created using MacVector 7.2 (Accerlys, San Diego, CA.) (table 1). One-step real time RT-PCR was performed using 100 ng of total RNA, and the reagents provided in the Roche RNA Amplification Kit SYBR Green I, and a Roche LightCycler (Roche Applied Science, Indianapolis, IN.). RT-PCR was performed as follows: reverse transcription for 30 min at 42°C, denaturation for 30 sec at 95°C, followed by 35 cycles of cDNA amplification consisting of a 15 sec denaturation at 95°C, primer annealing for 20 sec at 60°C (preproorexin) or 59°C (OX1R, OX2R and GADPH), and product elongation for 15 sec at 72°C. Data acquisition was taken at the end of each amplification cycle at a temperature slightly lower than the temperature required to melt the PCR product, 84°C (OX1R), 82°C (OX2R) and 79°C (preproorexin and GADPH). Amplification products from PCR were purified (QIAquick PCR Purification Kit, Valencia, CA), determined by electrophoresis in a 3% Nuseive gel, and then verified by capillary electrophoresis.
Table 1.
summary of changes in hcrt system following nicotine self-administration. In Group 1, tissue was collected 5 hr after the last NSA session; in Group 2, tissue collection occurred immediately after the last session.
| Group 1 | |||
|---|---|---|---|
| brain region | hcrt | hcrtR1 | hcrtR2 |
| cLH | correlated with
|
ns | correlated with
|
| rLH | ns | ns | ns |
| PPTg | ns | correlated with
|
|
| VTA | ns | ns | |
| PVA | ns | ns | |
| NAccSh | ns | ns | |
| PFC | ns | ns | |
| PVN | ns | ns | |
| ARC | nicotine > control (p=0.01) | ns | |
| Group 2 | |||
| brain region | hcrt | hcrtR1 | hcrtR2 |
| cLH | ns | correlated with
|
|
| rLH | nicotine < control (p<0.05) | ns | |
| PPTg | ns | ns | |
| VTA | |||
| PVA | |||
| NAccSh | |||
| PFC | |||
| PVN | |||
| ARC | ns | ns | |
Abbreviations: cLH, caudal lateral hypothalamus; rLH, rostral lateral hypothalamus; PPTg, pedunculopontine tegmental nucleus; VTA, ventral tegmental area; PVA, paraventricular thalamic nucleus; NAccSh, nucleus accumbens shell; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; ARC, arcuate nucleus
Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline containing 25 units/ml heparin. The pH of the solution was adjusted to 7.4 with dilute NaOH. Nicotine doses are expressed as the base. SB334867 (provided by Eli Lilly Co) was mixed in dimethyl sulfoxide (DMSO) and sonicated for approximately 30 min. Immediately prior to administration of each dose, Hydroxypropyl Beta Cyclodextrin (HBC) and water were added to form a vehicle solution of 10% DMSO, 10% HBC, and 80% sterile water. Almorexant (ACT-078573, provided by Actelion Pharmaceuticals Inc) was dissolved in a vehicle 0.25% hydroxypropyl methylcellulose solution. Receptor activity for the antagonists has been documented (Brisbare-Roch et al. 2007; Porter et al. 2001).
Data Analysis
The main dependent variables were the number of reinforcers earned per session and relative mRNA expression levels (corrected for GADPH). All data are presented as mean values; error bars represent the standard error of the mean. Hypocretin antagonist data were analyzed using a one-way repeated measures analysis of variance (ANOVA) with Tukey’s Multiple Comparison post hoc tests where appropriate. mRNA levels were compared in NSA and control groups using unpaired t-tests with Welch’s correction where appropriate and Pearson’s R was used for regression analyses. Data was considered significant when p < 0.05.
Results
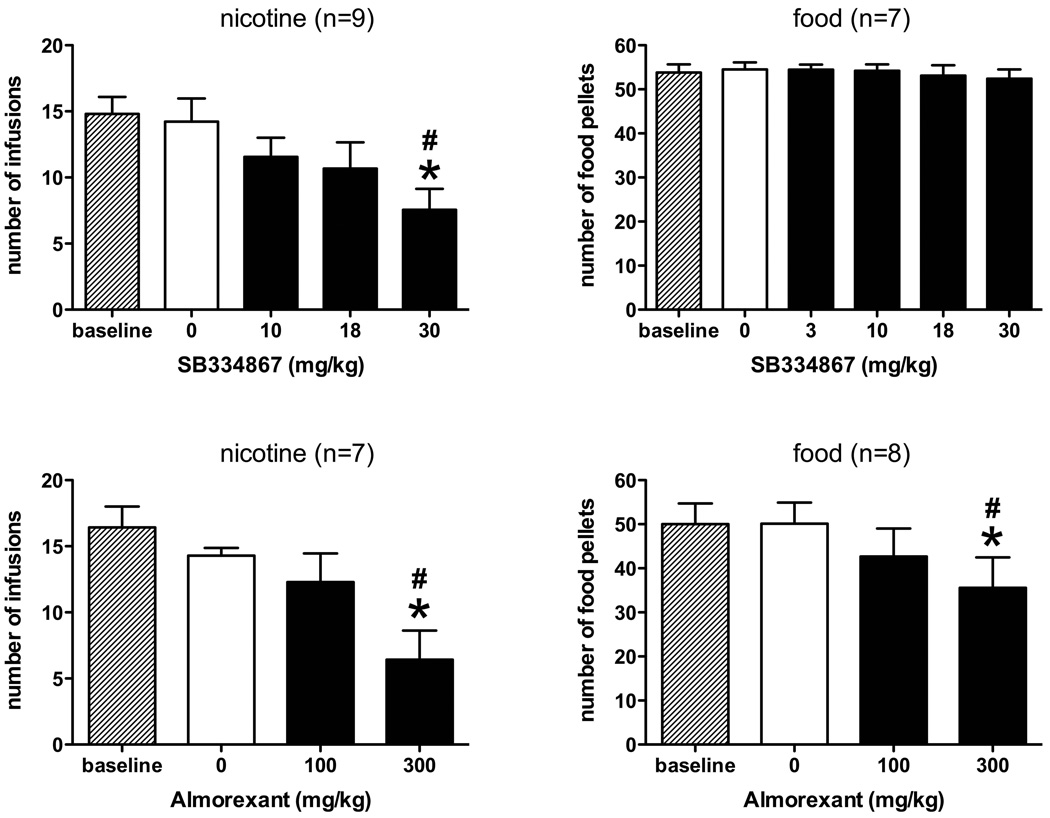
The selective hcrtR1 antagonist SB334867 produced a dose-dependent and significant reduction in NSA maintained on a FR5 schedule (Fig 1 upper panels; F4,44 = 7.07, p < 0.0005). In contrast to its effect on NSA, the same doses of the antagonist did not alter food-maintained responding on the same schedule of reinforcement. In addition, the complex vehicle used in these experiments was also without effect in either behavioral test.
Fig 1.
Effects of pre-session treatment with the selective hcrtR1 antagonist SB334867 (upper panels) and the mixed hcrtR1/2 antagonist almorexant (lower panels) on NSA and food-maintained responding. #Different from baseline, p<0.05. *Different from vehicle, p<0.05
The mixed hcrtR1/2 antagonist almorexant had a different pattern of effect in that it reduced both self-administration and food-maintained responding on an FR5 schedule to a similar extent (nicotine: F3,27 = 8.28, p < 0.002; food: F3,31 = 2.81, p = < 0.05; Fig 1 lower panels). Although the mean reduction in NSA was greater than that in food-maintained responding, the difference in the reduction produced by almorexant between groups was not statistically significant. Small but non-significant effects of vehicle treatments were evident in the NSA data, but not in food-maintained responding.
The self-administration history for the animals maintained on a FR1 schedule for the gene expression experiments is shown in Fig 2a. As expected, responding in the saline control groups rapidly extinguished, whereas responding maintained by nicotine delivery stabilized or increased moderately over the 4-week period. Responding on the active lever in the NSA groups was somewhat greater in the first group (in which tissue was collected 5 hours after the last NSA session, Fig 2a upper panels) than in the second (tissue collected immediately after the last session, Fig 2a lower panels), resulting in somewhat greater nicotine intake. Also as expected, responding on the inactive lever was small in all groups.
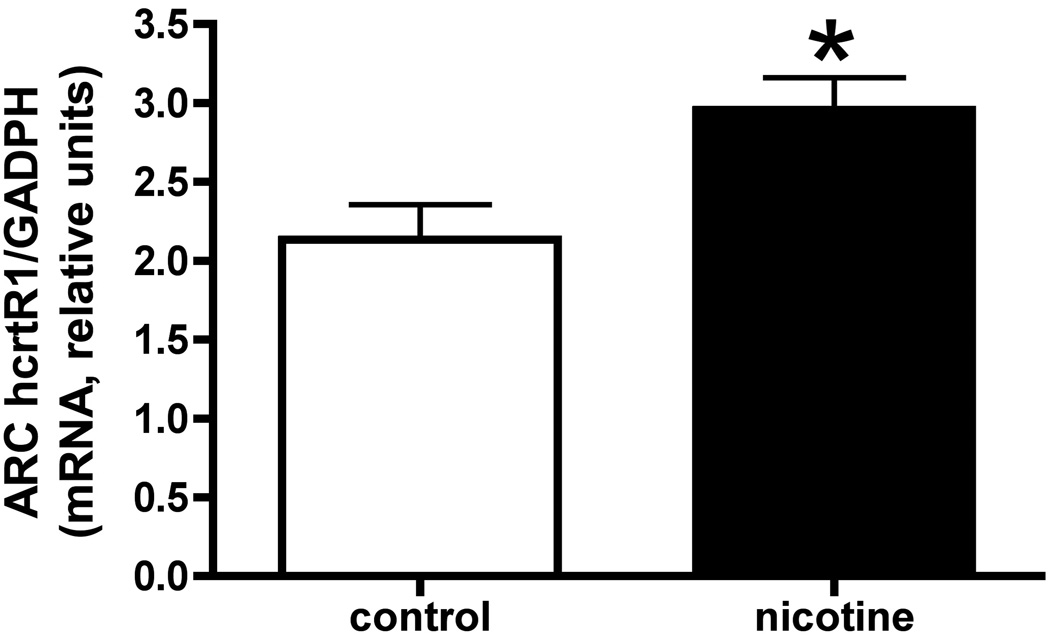
Fig 2b shows the areas that were selected for analysis of mRNA in group 1. At sacrifice, the plasma nicotine levels measured from these animals were in the range of < 2–8 ng/ml. In this group, the only significant difference observed in the mean values between the nicotine and control subjects was an increase in hcrtR1 mRNA in the arcuate nucleus (ARC) in the nicotine subjects (Fig 3(a) [t=2.74, df=26, p < 0.02]). In addition, however, there were some significant correlations, specifically (i) in the cLH, between each of hcrt and hcrtR2 and both nicotine intake (hcrt: r = 0.56, p < 0.05; hcrtR2: r = 0.58, p < 0.05) and the extent of lever pressing (hcrt: r = .62, p < 0.05; hcrtR2: r = 0.59, p < 0.05), and (ii) in the PPTg, between lever pressing and hcrtR2 mRNA (r = 0.60, p < 0.05). These findings are summarized in Table 1.
Fig 3.
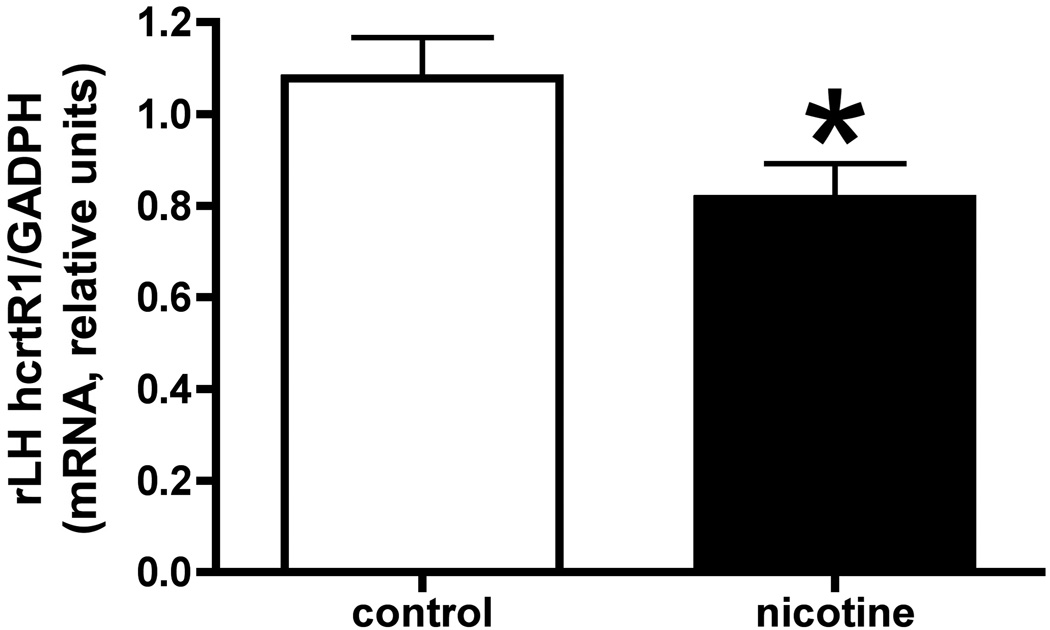
(a) This figure shows mRNA values for hcrtR1 in the arcuate nucleus (ARC) in NSA and control animals from group 1 (*, p<0.05). (b) mRNA values for hcrtR1 in the rostral lateral hypothalamus (rLH) in NSA and control animals from group 2 (*, p<0.05). Data in both are presented as the mean values; error bars are s.e.m.
In group 2, tissue collection occurred immediately (i.e., 1 to 11 min) after the final self-administration session, at which time the plasma nicotine levels ranged from 56–247 ng/ml. The single significant difference in mean values between nicotine and control animals was a decrease in the rLH hcrtR1 in the former compared to the latter (Fig 3(b) [t = 2.23, df = 23, p < 0.05]. In addition, nicotine intake was correlated with hcrtR1 in the cLH (Table 1 [r = 0.70, p < 0.01]).
Discussion
Both the selective hcrtR1 antagonist SB334867 and the mixed hcrtR1/2 antagonist almorexant reduced NSA, the former without an effect on food-maintained responding. Our findings with SB334867 qualitatively replicate a previous report (Hollander et al. 2008), although the doses of SB334867 in our study were substantially higher. Notably, the doses we used were in the same range as those reported in other studies with this antagonist, including studies examining reinstatement of alcohol- or drug-seeking behavior induced by cues, stress or chemical stimulation of LH neurons, extinction responding and operant responding for alcohol and cocaine (Aston-Jones et al. 2008; Borgland et al. 2009; Boutrel et al. 2005; España et al. 2010; Harris et al. 2005; Lawrence et al. 2006; Richards et al. 2008). The higher doses in our study compared to Hollander et al. may be due to strain differences or the greater nicotine intake which in turn is likely due to our use of food deprivation (motivation to self-administration of a wide range of drugs is directly related to the degree of food deprivation; Comer et al. 1995).
The mixed hcrtR1/2 antagonist almorexant, at a dose that was equally effective in reducing NSA as SB334867, also caused a reduction in food-maintained responding. The similarity in effects on NSA between the two drugs provides preliminary evidence that the effects of almorexant on NSA may be primarily due to hcrtR1 antagonism. However, the contribution of hcrtR2 needs to be examined directly with selective antagonists as has been done for other reinforcers (e.g., Smith et al. 2009).
The absence of effect of SB334867 on food-reinforced behavior is consistent with reports that the same dose (30 mg/kg) of the antagonist is without effect on responding for sucrose pellets on a progressive-ratio (PR) schedule in food-deprived rats (España et al. 2010), and that a similar dose (20 mg/kg) is without effect on responding for food pellets on a PR schedule (Borgland et al. 2009), for 5% sucrose on a FR3 schedule (Richards et al. 2008), and for water (Lawrence et al. 2006). In contrast, the same 20 mg/kg dose reduced responding for high-fat pellets (Nair et al. 2008), and a 30 mg/kg dose reduced free-feeding and feeding stimulated by overnight fast (Haynes et al. 2000). SB334867 appears to reduce feeding by advancing satiety temporally rather than by changing the structure of feeding; 30 mg/kg of SB334867, given 30 min before testing, advanced satiety in deprived animals to approximately 20 min after the start of feeding compared to about 40 min in controls (Rodgers et al. 2001). Visual inspection of their data shows consumption of approx 6 gm of wet mash prior to the onset of SB334867-evoked satiety, far more than the approximate 2.5 gm of pellets consumed by animals in our study. In the Nair et al study, the time-out period was short (20 sec) compared to our study, and the pellets were a palatable high-fat formulation; in the first 15 min of the sessions, animals consumed 40–45 pellets, almost as many as in our 1-hr sessions, yet there was no significant effect of SB334867 over this time. These high-fat pellets (7-fold greater fat content than the ones used in our study) have the potential to produce substantially greater satiation. Hence we believe that the absence of an effect of SB334867 on food-maintained responding in our study is due to the failure to reach satiation in the limited access, relatively short duration sessions employed. The interaction of SB334867 with satiation mechanisms is consistent with the observation that a 20 mg/kg dose suppresses responding for palatable, high-fat but not normal, pellets (Bonci and Borgland 2009).
The reduction of food-maintained responding by almorexant suggests that antagonism of both hcrt receptors is more effective at influencing mechanisms of feeding. Although almorexant is in clinical development as a sleeping aid (Brisbare-Roch et al. 2007), we did not observe somnolence in the animals over the test period. However, the antagonist was administered at a time of presumed high motivational state (approx 23 hr of food, or nicotine, deprivation), a fact which may have militated against manifestation of somnolence. Alternatively, Rodgers et al. (2002) have advanced the idea that the hcrt system may be involved in alertness/wakefulness to support foraging for food, and almorexant may particularly influence this dimension of behavior. In addition, other factors, such as pharmacokinetic differences between the two antagonists, may contribute to the differential effects on food-maintained responding.
The changes observed in the hcrt system after NSA were limited, perhaps reflecting the relatively small number of CNS areas selected for study, intended to provide a sample of regions linked to (i) reward/reinforcement (mesocorticolimbic regions) and (ii) hcrt-containing neurons and a range of their projections (LH, ARC, PVA, PVN, PPTg) Nonetheless, they are informative. In tissue collected from animals 5 hours after the last NSA session, the increase in mRNA for hcrtR1 following nicotine in the arcuate nucleus (ARC), a major gateway to appetite regulation, suggests that nicotine might in turn alter neuropeptide Y and pro-opiomelanocortin mechanisms and appetitive systems. Given that the change in ARC was in message for hcrtR1, the effect of SB334867 on NSA may derive in part from action within this brain region.
Other changes in tissues collected 5 hours post NSA were correlations with nicotine intake and lever presses in the cLH (hcrt and hcrtR2) and a correlation with lever presses in the PPTg. The former are not surprising given that hcrt-containing neurons are located in the LH and other studies have found changes in Fos expression in LH neurons after experimenter-administered nicotine (Pasumarthi et al. 2006).
Correlations in PPTg samples are of interest since this pontine region has been implicated in NSA (Alderson et al. 2006; Lança et al. 2000) and nicotine reward (Laviolette et al. 2002). The PPTg, which receives limbic and sensory input, influences burst firing (Grace et al. 2007) and conditioned responses (Pan and Hyland 2005) of midbrain dopaminergic neurons. Hcrt input to the region (Brischoux et al. 2008; Greco and Shiromani 2001; Marcus et al. 2001; Nambu et al. 1999; Peyron et al. 1998) may therefore participate in the organization of reinforced behavior such as lever pressing. Certainly hcrt can activate neurons in the PPTg (Kim et al. 2009). Were this hcrtR2-mediated, as suggested by the correlation observed here, the effect of almorexant on both NSA and food-maintained behavior could reflect an effect on the ability to marshal the needed circuitry to reinforce a complex task. However, correlations such as these need to be viewed with caution given that they derive from a relatively small sample size.
In tissue collected immediately after the last self-administration session, significant main-effect changes were again limited to hcrtR1. Based on this limited sample, it appears that prominent changes in the hcrt system related to NSA are in this receptor. However, as already noted, the role of hcrtR2 needs to be determined directly by future studies using new hcrtR2 antagonists (Dugovic et al. 2009; Malherbe et al. 2009).
The absence of overlap in the findings from the two groups raises the possibility that changes in mRNA for hcrt and its receptors are quite labile. This is not to discount the possibility that changes may also be related to the state of nicotine exposure, that is, changes in LH which are evident only immediately post-session might be related to the presence of nicotine, whereas changes in ARC that are manifest only several hours following NSA might be related to early withdrawal. In addition, nicotine intake over the 19-day period was not identical in the two groups, a common occurrence in self-administration studies.
The present findings are in need of extension with additional tools, not only hcrtR2 selective antagonists but also other schedules for NSA such as PR schedules which measure the motivational strength of the behavior and which can yield different results in pharmacological testing (Coen et al. 2009); further in this vein, recent data shows that SB334867 reduces cocaine self-administration on a PR but not a FR schedule (España et al. 2010). It would also be informative to use various degrees of exposure to nicotine and post-exposure times to explore both dose-sensitivity and withdrawal. Nonetheless, these data contribute additional convincing evidence that voluntary nicotine self-administration interacts with the hcrt system, and that it does so broadly, leading to changes in several brain regions. Notable by their absence in the present study are changes in the VTA or NAccSh, whereas potential appetitive interactions were observed. A dichotomy in hcrt function between reward and arousal has been proposed, in which arousal-related effects bypass the VTA/accumbens circuit but engage others, including pontine PPTg mechanisms (Harris and Aston-Jones 2006). In view of the correlations in the PPTg sample, further investigation of hcrt mechanisms in the pontine region may be fruitful. In addition, further studies with intracranial microinfusions of hcrt antagonists during NSA sessions would help to locate CNS regions in which hcrt mechanisms influence nicotine reinforcement.
Acknowledgements
This research was supported by a grant from the Academic Health Center of the University of Minnesota (Corrigall PI), NIDA grant DA020136 (LeSage PI), and funding from the Department of Veterans Affairs (Kotz PI). We are grateful to Dr. David McKinzie of Eli Lilly and Company for the gift of SB334867, and to Drs. Francois Jenck and Catherine Brisbare-Roch of Actelion Pharmaceuticals Limited for the gift of almorexant. In addition, we acknowledge the expert technical assistance of Martha A. Grace, Jennifer A. Teske, Andrew Kotz and Mark Margosian.
Footnotes
We use the hypocretin nomenclature here
References
- Alderson HL, Latimer MP, Winn P. Intravenous self-administration of nicotine is altered by lesions of the posterior, but not anterior, pedunculopontine tegmental nucleus. Eur J Neurosci. 2006;23:2169–2175. doi: 10.1111/j.1460-9568.2006.04737.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56 Suppl 1:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56 Suppl 1:107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- Coen KM, Adamson KL, Corrigall WA. Medication-related pharmacological manipulations of nicotine self-administration in the rat maintained on fixed- and progressive-ratio schedules of reinforcement. Psychopharmacology (Berl) 2009;201:557–568. doi: 10.1007/s00213-008-1321-6. [DOI] [PubMed] [Google Scholar]
- Comer SD, Turner DM, Carroll ME. Effects of food deprivation on cocaine base smoking in rhesus monkeys. Psychopharmacology (Berl) 1995;119:127–132. doi: 10.1007/BF02246152. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Hypocretin mechanisms in nicotine addiction: evidence and speculation. Psychopharmacology (Berl) 2009 Jun 16; doi: 10.1007/s00213-009-1588-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36:85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JRS. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Gao X-B. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexins/hypocretins on pedunculopontine tegmental neurons in rats: an in vitro study. Peptides. 2009;30:191–209. doi: 10.1016/j.peptides.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 Suppl 1:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav. 2006;88:294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Levine AS, Billington CJ. Effect of naltrexone on feeding, neuropeptide Y and uncoupling protein gene expression during lactation. Neuroendocrinology. 1997;65:259–264. doi: 10.1159/000127183. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lança AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci. 2002;22:8653–8660. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, Roche O, Rogers-Evans M, Wettstein JG, Moreau JL. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. Br J Pharmacol. 2009;156:1326–1341. doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Winsky-Sommerer R, Boutrel B, de Lecea L. The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drug News Perspect. 2005;18:250–255. doi: 10.1358/dnp.2005.18.4.908659. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. Academic Press, Academic Press; 1998. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Ishii Y, Halford JC, Blundell JE. Orexins and appetite regulation. Neuropeptides. 2002;36:303–325. doi: 10.1016/s0143-4179(02)00085-9. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]