Abstract
The pars tuberalis (PT) is a distinct subdivision of the anterior pituitary gland that plays a central role in regulating seasonal prolactin release. In sheep, there is compelling evidence that seasonal changes in light, transformed into a melatonin signal, are interpreted by the PT to modulate the release of a factor which affects prolactin release. The identity of this factor(s) is unknown but has been preemptively called ‘tuberalin’. In the present study, we report on an initial immunocytochemical investigation where we have identified that many ovine PT cells are immunoreactive for the tachykinin substance P (SP). Few cells in the pars distalis immunoreact for SP. The SP-immunoreactive cells did not colocalize with β-luteinizing hormone. RT-PCR confirmed the presence of preprotachykinin A mRNA in the PT. We hypothesize that SP, and possibly other preprotachykinin A-derived tachykinins, may play a role in the seasonal regulation of prolactin secretion in sheep.
Keywords: Substance P, Neurokinin, Tachykinin, Prolactin, Seasonal rhythms
Introduction
Most animals exhibit circadian and seasonal rhythms. Circadian rhythms are regulated by the suprachiasmatic nucleus [1], but how and where seasonal rhythms are controlled remains unsolved. Almost all seasonal rhythms are driven by photoperiod. Photoperiodic information is translated by the pineal gland into a biochemical signal, melatonin, secreted during the night [2]. Thus, the duration of melatonin release is an accurate representation of the length of the night and, thereby, season [3]. Williams and Morgan [4] revealed melatonin binding sites in several areas of the rat brain but, most noticeably, in the pars tuberalis (PT). Several laboratories confirmed that the PT expressed melatonin binding sites in several species [5, 6], including sheep [7].
Different melatonin target sites regulate different rhythms. Strong evidence indicates that the melatonin target site controlling seasonal reproduction is in the brain [8, 9]. In contrast, the seasonal change in pelage growth that is driven by the seasonal prolactin cycle [10, 11] is regulated outside the brain. Studies on sheep have provided strong evidence that melatonin acts on the PT, and not the brain, to regulate seasonal prolactin secretion [12, 13]. Studies in hamsters bearing hypothalamic lesions showed that reproductive responses to changes in photoperiod were lost, but photoperiod-dependent rhythms in prolactin release persisted [14]. These studies form the basis of the ‘dual site’ hypothesis, which postulates that melatonin acts in the hypothalamus to drive reproductive rhythms but in the PT to regulate prolactin rhythms.
It is not known how the PT regulates the seasonal prolactin rhythm. In several rodent species there is evidence that thyroid-stimulating hormone (TSH) is produced by the PT [15–17], and recent research on birds [18] suggests that TSH may play a central role in photoperiod-driven processes in this class of vertebrates. In sheep, apart from gonadotropes confined to the ventral aspect [19–21], most PT cells are chromophobic and account for 90–95% of all PT cells [21]. Indeed, the phenotypic identity of the majority of cells in the PT of all species is unknown [19, 20, 22, 23]. This has led to speculation that the PT secretes a novel peptide, called ‘tuberalin’ [23–25]. However, as noted recently, ‘the identity of tuberalin remains elusive and to date no candidate molecules have been identified’ [26]. Several researchers have listed specific criteria that tuberalin should fulfill. It should be (1) evolutionarily conserved [26, 27], (2) a peptide [22, 24], (3) approximately 1 kDa [28] and (4) present in the pars distalis (PD) [29, 30]. The present report describes an initial finding that the PT contains an enriched population of substance P (SP)-immunoreactive cells. It is noteworthy that SP, which stimulates prolactin release in rodents [for a review, see ref. 31], meets all of the criteria for the elusive tuberalin.
Materials and Methods
Animal Management and Nutritional Treatment
Non-breeding season (June) Rambouillet X Columbia rams (n = 6) were injected intravenously with 25,000 IU heparin and killed with an anesthetic overdose. Animals were decapitated and perfused through the carotid arteries with 1 liter 1% sodium nitrite in 0.9% NaCl, then a 3-liter solution of cold 4% paraformaldehyde and 15% saturated picric acid in 0.1 M phosphate buffer (pH 7.4) and 1 liter 20% sucrose in 0.1 M phosphate buffer. The hypothalamus-PT complex and pituitaries were placed in the sucrose solution (for 24 h, at 4 °C), embedded in Tissue-Tek OCT (Miles Inc., Elkhart, N.J., USA) and frozen in liquid N2-cooled isopentane. Sections (20 μm) were mounted onto Silane-coated slides and kept at −80 °C. Procedures were approved by the University of Wyoming Animal Care Committee (IACUC No. A-3126-01).
Immunocytochemistry
A series of sections was washed in 0.01 M phosphate-buffered saline (pH 7.4; all washes 3 × 5 min at room temperature). Sections were incubated (for 72 h at 4 °C in a humidified chamber) in phosphate-buffered saline containing 1% Triton, 10% normal goat serum and a guinea pig SP antibody (T5019; Peninsula Laboratories, San Carlos, Calif., USA; 1:1,000) and, for dual labeling, a rabbit anti-ovine β-luteinizing hormone (βLH) antibody (1:10,000; AFP697071P, National Institute of Diabetes and Digestive and Kidney Diseases). Slides were washed, incubated in Texas Red goat anti-guinea pig IgG and biotinylated goat anti-rabbit (both Jackson Laboratories, West Grove, Pa., USA; 1:200, for 1 h at room temperature), washed, placed in FITC-streptavidin (Vector, Burlingame, Calif., USA; 1:200, for 1 h at room temperature), washed and mounted with Vectashield with DAPI (Vector). Pre-absorbing the SP antibody with 10 μg/ml SP (American Peptide Co., Sunnyvale, Calif., USA) resulted in no staining (data not shown). This antibody did not crossreact with 10 μg/ml neurokinin B. Additional controls included omission of the primary antibodies, and incubation in the primary antibodies followed by incubation with secondary antibodies raised in inappropriate species.
The percentage of immunoreactive cells relative to the total number of DAPI-labeled cells was calculated. As the PT is densely vascularized (fig. 1), it is not useful to express cell density relative to area. It is noteworthy that no SP-immunoreactive cells were evident in 1 animal, although βLH was identified; the ram was excluded from analysis. Data were statistically compared using Student’s paired t test and are presented as mean ± SEM.
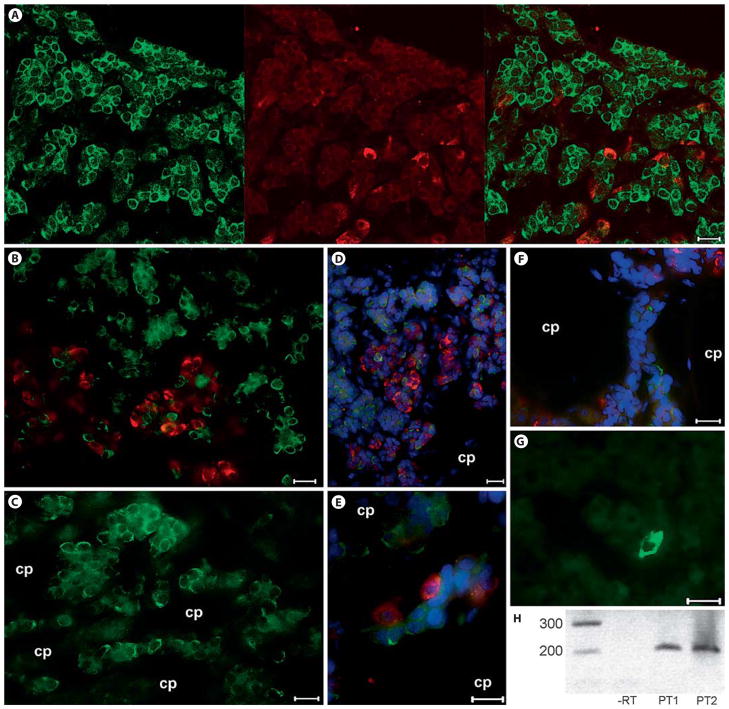
Fig. 1.
Photomicrographs and RT-PCR showing the presence of SP in the ovine PT. A, B, E βLH (red) and SP (green) do not colocalize in gonadotropes of the ram pituitary. C–F These SP-immunoreactive cells were frequently observed to surround hypophyseal portal capillaries. G Very few SP-immunoreactive cells were evident in the PD. H RT-PCR revealed distinct preprotachykinin A mRNA in 2 different sheep. If no RT was performed, no band was evident. Red = βLH; green = SP; blue = DAPI; cp = capillary. Scale bar = 10 μm.
Reverse Transcription PCR
RT-PCR was performed as previously described [32]. After the first-strand cDNA synthesis, samples were amplified for preprotachykinin A. Since the ovine preprotachykinin A mRNA sequence was not available from data bases, primers were identical to those previously described by others [33] for the bovine sequence (P01289). The sense primer, spanning exon 1 and exon 2, started at position 123 of the published nucleotide sequence, and the antisense primer, in exon 3, ends at position 323 (sense, GGGTCAGCTGCGAAATCC; antisense, CTTGGGTCTCCGAGCGATTACT). This fragment is present in all splice variants (α, β, γ and δ) of preprotachykinin A mRNA. Samples were visualized using a 0.7% agarose gel with ethidium bromide.
Results
SP-immunoreactive cells were abundant in the PT (58.1 ± 17.3%; fig. 1A–F) and sparse in the PD (0.9 ± 0.3%; p < 0.01; fig. 1G). These PT tachykinin-immunoreactive cells were often in close association with the hypophyseal vessels of the primary portal plexus that course through this pituitary subdivision (fig. 1). Many PT cells are characterized by an extremely thin cytoplasmic area relative to the nucleus, and these cells were often labeled for SP. No evidence of SP colocalization with βLH was detected, even in the ventral PT where gonadotropes are abundant (fig. 1A, B, D). RT-PCR for preprotachykinin A mRNA revealed a clear distinct band at the expected product size of approximately 200 base pairs (fig. 1H). This band was absent when PCR was performed without RT.
Discussion
The present study provides compelling evidence that SP is abundant in the ovine PT, whereas in the PD, comparatively few cells are immunoreactive for this tachykinin. This study concurs with our earlier investigation [34] in the ovine fetus that the PD contains few SP-immunoreactive cells. We have previously reported [35] how the PT, as the zona tuberalis, extends ventrally down the anterior face of the adenohypophysis, projecting ‘fingers’ into the PD. Thus, we cannot discount the possibility that these PD tachykinin-immunoreactive cells are of PT origin.
For the PT to produce a factor, which then acts on the lactotropes, the PT must release this factor into either the (1) hypophyseal portal system or (2) extracellular fluid, from which it then moves through the interstitial spaces to the PD. Only 2 preliminary studies have investigated whether tachykinins are released into the hypophyseal portal blood. In a preliminary investigation in the rat, SP levels increased in the hypophyseal portal blood relative to jugular blood following hypoxia, but data were variable [36]. It has been proposed that SP is not released into the hypophyseal portal system of the ewe [37]. Some caution is due as blood samples were collected during the breeding season. If SP is driving prolactin release, then it will be at its nadir during the breeding season in sheep. To emphasize this point, if gonadotropin-releasing hormone concentrations were analyzed in samples harvested sporadically during anestrus, over 90% would be below the sensitivity of the assay. There are no studies determining if a PT factor could be released in the extracellular fluid to act through a paracrine pathway.
SP acts preferentially through the NK-1 receptor but also the NK-2 receptor, with lower affinity for the NK-3 receptor [38]. SP binding has been shown on lactotropes in the rat [39]. Studies on rodents consistently report that brief (3–10 min) SP exposure stimulates prolactin secretion [for a review, see ref. 31]. In long-term ovariectomized ewes, a single 70-nmol intravenous SP injection had no effect on prolactin release [40]. These ewes were long-term ovariectomized with no estrogen replacement. The induction of NK-1 receptors is estrogen dependent [41], and there is evidence that tachykinins may not affect prolactin release in the ovariectomized rat [42]. Second, SP is rapidly degraded [37], and a 70-nmol dose may not have been sufficient to raise SP levels in the hypophyseal portal system. Indeed, SP administered directly into the hypophyseal portal circulation of the rat powerfully stimulated prolactin release, but when the same dose was administered intravenously, there was no effect [43]. Analysis of the rodent studies [43–49] suggests that SP concentrations must exceed 100 nM at the level of the pituitary gland to evoke a prolactin response. It is not known if this dose is physiological. An insufficient stimulus to lactotropes may also have been provided by SP infusion (1.5 pmol/kg/min) in men [50].
The PT is a distinct anatomical subdivision of the pituitary gland that remains an enigma. It is clearly an integrative site expressing high densities of insulin-like growth factor 1 [51], leptin [52] and melatonin receptors that potentially serve to modulate neuroendocrine activity. Although βTSH mRNA has been reported in the ovine PT, the βTSH protein has not yet been detected here [19, 21]. βTSH protein may be synthesized and released extremely rapidly, rendering it undetectable by our approach. Moreover, Hanon et al. [53] recently argued that ‘PT-derived TSH does not act as the pituitary paracrine signal between melatonin and prolactin secretion’. The present study provides evidence that the PT is an enriched source of SP. It is noteworthy that at least 3 other tachykinins are derived from the preprotachykinin A mRNA: neurokinin A, neuropeptide-γ and neuropeptide K may also be synthesized; neuropeptide K powerfully augments the SP-induced salivary response [54]. As these tachykinins may be coreleased, there are surprisingly few studies on the effect of 2 or more tachykinins on any system. Clearly, more research is required to establish whether PT-derived tachykinins play a role in seasonal hormone rhythms.
Acknowledgments
This research was supported by National Science Foundation grants No. 0745084 and P20RR015640 from the National Center for Research Resources. L.P. was funded by National Science Foundation grant No. EPS-0447681. Q.W. was supported by the Wyoming NASA Space Grant Consortium No. NNG05G165H. The contents of the study are solely the responsibility of the authors and do not necessarily represent the official views of the National Science Foundation, the National Center for Research Resources or the National Institutes of Health.
References
- 1.Ueyama T, Krout KE, Van Nguyen X, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nature Neurosci. 1999;2:1051–1053. doi: 10.1038/15973. [DOI] [PubMed] [Google Scholar]
- 2.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587–2590. [Google Scholar]
- 3.Arendt J. Melatonin and the Mammalian Pineal Gland. Cambridge: Chapman and Hall; 1995. [Google Scholar]
- 4.Williams LM, Morgan PJ. Demonstration of melatonin-binding sites on the pars tuberalis of the rat. J Endocrinol. 1988;119:R1–R3. doi: 10.1677/joe.0.119r001. [DOI] [PubMed] [Google Scholar]
- 5.Stanton TL, Siucia JA, Dubocovich ML, Krause DN. The area of 2-[125I] iodomelatonin binding in the pars tuberalis of the ground squirrel is decreased during hibernation. Brain Res. 1991;557:285–288. doi: 10.1016/0006-8993(91)90145-l. [DOI] [PubMed] [Google Scholar]
- 6.Weaver DR, Reppert SM. Melatonin receptors are present in the ferret pars tuberalis and pars distalis, but not in brain. Endocrinology. 1990;127:2607–2609. doi: 10.1210/endo-127-5-2607. [DOI] [PubMed] [Google Scholar]
- 7.Morgan PJ, Williams LM, Davidson G, Lawson W, Howell E. Melatonin receptors in the ovine pars tuberalis: characterization and autoradiographical localization. J Neuroendocrinol. 1989;1:1–4. doi: 10.1111/j.1365-2826.1989.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 8.Malpaux B, Skinner DC, Maurice F. The ovine pars tuberalis does not appear to be targeted by melatonin to modulate luteinizing hormone secretion, but may be important for prolactin release. J Neuroendocrinol. 1995;7:199–206. doi: 10.1111/j.1365-2826.1995.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 9.Malpaux B, Daveau A, Maurice-Mandon F, Duarte G, Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998;139:1508–1516. doi: 10.1210/endo.139.4.5879. [DOI] [PubMed] [Google Scholar]
- 10.Craven AJ, Ormandy CJ, Robertson FG, Wilkins RJ, Kelly PA, Nixon AJ, Pearson AJ. Prolactin signaling influences the timing mechanism of the hair follicle: analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology. 2001;142:2533–2539. doi: 10.1210/endo.142.6.8179. [DOI] [PubMed] [Google Scholar]
- 11.Curlewis JD, Sibbald AM, Milne JA, McNeilly AS. Chronic treatment with long-acting bromocriptine does not affect duration of the breeding season, voluntary food intake, body weight, or wool growth in the Scottish blackface ewe. Reprod Fertil Dev. 1991;3:25–33. doi: 10.1071/rd9910025. [DOI] [PubMed] [Google Scholar]
- 12.Lincoln G, Clarke IJ, Hut R, Hazlerigg DG. Characterizing a mammalian circannual pacemaker. Science. 2006;314:1941–1944. doi: 10.1126/science.1132009. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln GA, Clarke IJ. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo-pituitary disconnected rams: evidence for translation of the melatonin signal in the pituitary gland. J Neuroendocrinol. 1994;6:251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 14.Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the medio-basal hypothalamus spare the lactotropic, but block the gonadotropic response of the male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann M, Wittkowski W, Hoffmann K. Ultrastructural localisation of thyrotropin (TSH)-like immunoreactivity in specific secretory cells of the hypophyseal pars tuberalis in the Djungarian hamster, Phodopus sungorus. Cell Tissue Res. 1989;256:649–652. doi: 10.1007/BF00225616. [DOI] [PubMed] [Google Scholar]
- 16.Böckers TM, Sourgens H, Wittkowski W, Jekat A, Pera F. Changes in TSH-immunoreactivity in the pars tuberalis and pars distalis of the fetal rat hypophysis following maternal administration of propylthiouracil and thyroxine. Cell Tissue Res. 1990;260:403–408. doi: 10.1007/BF00318643. [DOI] [PubMed] [Google Scholar]
- 17.Wittkowski W, Bergmann M, Hoffmann K, Pera F. Photoperiod-dependent changes in TSH-like immunoreactivity of cells in the hypophysial pars tuberalis of the Djungarian hamster, Phodopus sungorus. Cell Tissue Res. 1988;251:183–187. doi: 10.1007/BF00215463. [DOI] [PubMed] [Google Scholar]
- 18.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 19.Skinner DC, Robinson JE. The pars tuberalis of the ewe: no effect of season or ovariectomy on the distribution, density or presence of immunoreactive cells. Cell Tissue Res. 1996;284:117–123. doi: 10.1007/s004410050572. [DOI] [PubMed] [Google Scholar]
- 20.Böckers TM, Bockmann J, Fauteck JD, Kreutz MR, Bock R, Wittkowski W. Pars tuberalis-specific cells in the ovine pituitary do express common alpha-chain of glyco-protein hormones: an in situ hybridization and immunocytochemical study. Eur J Endocrinol. 1994;131:540–546. doi: 10.1530/eje.0.1310540. [DOI] [PubMed] [Google Scholar]
- 21.Gross DS, Turgeon JL, Waring DW. The ovine pars tuberalis: a naturally occurring source of partially purified gonadotropes which secrete luteinizing hormone in vitro. Endocrinology. 1984;114:2084–2091. doi: 10.1210/endo-114-6-2084. [DOI] [PubMed] [Google Scholar]
- 22.Morgan PJ. The pars tuberalis: the missing link in the photoperiodic regulation of prolactin secretion? J Neuroendocrinol. 2000;12:287–295. doi: 10.1046/j.1365-2826.2000.00459.x. [DOI] [PubMed] [Google Scholar]
- 23.Wittkowski W, Bockmann J, Kreutz MR, Böckers TM. Cell and molecular biology of the pars tuberalis of the pituitary. Int Rev Cytol. 1999;185:157–194. doi: 10.1016/s0074-7696(08)60151-5. [DOI] [PubMed] [Google Scholar]
- 24.Morgan PJ, Webster CA, Mercer JG, Ross AW, Hazlerigg DG, MacLean A, Barrett P. The ovine pars tuberalis secretes a factor(s) that regulates gene expression in both lactotropic and nonlactotropic pituitary cells. Endocrinology. 1996;137:4018–4026. doi: 10.1210/endo.137.9.8756579. [DOI] [PubMed] [Google Scholar]
- 25.Guerra M, Rodriguez EM. Identification, cellular and subcellular distribution of 21 and 72 kDa proteins (tuberalins?) secreted by specific cells of the pars tuberalis. J Endocrinol. 2001;168:363–379. doi: 10.1677/joe.0.1680363. [DOI] [PubMed] [Google Scholar]
- 26.Johnston JD. Photoperiodic regulation of prolactin secretion: changes in intra-pituitary signalling and lactotroph heterogeneity. J Endocrinol. 2004;180:351–356. doi: 10.1677/joe.0.1800351. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln G. Melatonin modulation of prolactin and gonadotrophin secretion. Systems ancient and modern. Adv Exp Med Biol. 1999;460:137–153. doi: 10.1007/0-306-46814-x_16. [DOI] [PubMed] [Google Scholar]
- 28.Graham ES, Webster CA, Hazlerigg DG, Morgan PJ. Evidence for the biosynthesis of a prolactin-releasing factor from the ovine pars tuberalis, which is distinct from thyrotropin-releasing hormone. J Neuroendocrinol. 2002;14:945–954. doi: 10.1046/j.1365-2826.2002.00848.x. [DOI] [PubMed] [Google Scholar]
- 29.Morgan PJ, Barrett P, Davidson G, Lawson W. Melatonin regulates the synthesis and secretion of several proteins by pars tuberalis cells of the ovine pituitary. J Neuroendocrinol. 1992;4:557–563. doi: 10.1111/j.1365-2826.1992.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 30.Morgan PJ, Mercer JG. Control of seasonality by melatonin. Proc Nutr Soc. 1994;53:483–493. doi: 10.1079/pns19940059. [DOI] [PubMed] [Google Scholar]
- 31.Debeljuk L, Lasaga M. Tachykinins and the control of prolactin secretion. Peptides. 2006;27:3007–3019. doi: 10.1016/j.peptides.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Albertson AJ, Talbott H, Wang Q, Jensen D, Skinner DC. The gonadotropin releasing hormone type I receptor is expressed in the mouse cerebellum. Cerebellum. 2008 doi: 10.1007/s12311-008-0038-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Reibiger I, Aust G, Tscheudschilsuren G, Beyer R, Gebhardt C, Spanel-Borowski K. The expression of substance P and its neurokinin-1 receptor mRNA in the bovine corpus luteum of early developmental stage. Neurosci Lett. 2001;299:49–52. doi: 10.1016/s0304-3940(00)01763-8. [DOI] [PubMed] [Google Scholar]
- 34.Lutz L, Dufourny L, Skinner DC. Effect of nutrient restriction on the somatotropes and substance P-immunoreactive cells in the pituitary of the female ovine fetus. Growth Horm IGF Res. 2006;16:108–118. doi: 10.1016/j.ghir.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Skinner DC, Robinson JE. Melatonin-binding sites in the gonadotroph-enriched zona tuberalis of ewes. J Reprod Fertil. 1995;104:243–250. doi: 10.1530/jrf.0.1040243. [DOI] [PubMed] [Google Scholar]
- 36.Szkudlarek U, Cannon D, Corbally N, Traczyk WZ. Substance P in the blood plasma in hypophysial portal vessels. Acta Physiol Pol. 1986;37:92–99. [PubMed] [Google Scholar]
- 37.Clarke IJ, Jessop D, Millar R, Morris M, Bloom S, Lightman S, Coen CW, Lew R, Smith I. Many peptides that are present in the external zone of the median eminence are not secreted into the hypophysial portal blood of sheep. Neuroendocrinology. 1993;57:765–775. doi: 10.1159/000126435. [DOI] [PubMed] [Google Scholar]
- 38.Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- 39.Larsen PJ, Saermark T, Mau SE. Binding of an iodinated substance P analogue to cultured anterior pituitary prolactin- and luteinizing hormone-containing cells. J Histochem Cytochem. 1992;40:487–493. doi: 10.1177/40.4.1372633. [DOI] [PubMed] [Google Scholar]
- 40.Thomas GB, Cummins JT, Griffin N, Clarke IJ. Effect and site of action of hypothalamic neuropeptides on prolactin release in sheep. Neuroendocrinology. 1988;48:252–257. doi: 10.1159/000125019. [DOI] [PubMed] [Google Scholar]
- 41.Villablanca AC, Hanley MR. 17β-Estradiol stimulates substance P receptor gene expression. Mol Cell Endocrinol. 1997;135:109–117. doi: 10.1016/s0303-7207(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 42.Pisera D, Theas S, De Laurentiis A, Lasaga M, Duvilanski B, Seilicovich A. The hormonal status modulates the effect of neurokinin A on prolactin secretion in female rats. J Endocrinol. 1998;159:389–395. doi: 10.1677/joe.0.1590389. [DOI] [PubMed] [Google Scholar]
- 43.Abe H, Kato Y, Chihara K, Iwasaki Y, Imura H. Effects of drugs infused into a rat hypophysial portal vessel on prolactin and growth hormone release. Proc Soc Exp Biol Med. 1980;165:248–252. doi: 10.3181/00379727-165-40965. [DOI] [PubMed] [Google Scholar]
- 44.Rivier C, Brown M, Vale W. Effect of neurotensin, substance P and morphine sulfate on the secretion of prolactin and growth hormone in the rat. Endocrinology. 1977;100:751–754. doi: 10.1210/endo-100-3-751. [DOI] [PubMed] [Google Scholar]
- 45.Gullner HG, Yajima H, Herbert D, Owen WW. Growth hormone, prolactin and FSH release by the tachykinin dodecapeptide kassinin in the rat. Arch Int Pharmacodyn. 1982;256:4–9. [PubMed] [Google Scholar]
- 46.Vijayan E, McCann S. Effects of substance P and neurotensin on growth hormone and thyrotropin release in vivo and in vitro. Life Sci. 1980;26:321–327. doi: 10.1016/0024-3205(80)90344-6. [DOI] [PubMed] [Google Scholar]
- 47.Henriksen JS, Saermark T, Vilhardt H, Mau SE. Tachykinins induce secretion of prolactin from perifused rat anterior pituitary cells by interactions with two different binding sites. J Recept Signal Transduct Res. 1995;15:529–541. doi: 10.3109/10799899509045238. [DOI] [PubMed] [Google Scholar]
- 48.Mau SE, Witt MR, Saermark T, Vilhardt H. Substance P increases intracellular Ca 2+ in individual rat pituitary lactotrophs, somatotrophs, and gonadotrophs. Mol Cell Endocrinol. 1997;126:193–201. doi: 10.1016/s0303-7207(96)03988-3. [DOI] [PubMed] [Google Scholar]
- 49.Duvilanski BH, Pisera D, Seilicovich A, del Carmen Diaz M, Lasaga M, Isovich E, Velardez MO. Interaction between substance P and TRH in the control of prolactin release. J Endocrinol. 2000;166:373–380. doi: 10.1677/joe.0.1660373. [DOI] [PubMed] [Google Scholar]
- 50.Coiro V, Volpi R, Capretti L, Caiazza A, Caffarri G, Rossi G, Marchesi C, Chiodera P. Intravenously infused substance P is unable to change basal and TRH-stimulated PRL secretion in normal men. Horm Res. 1993;39:73–76. doi: 10.1159/000182699. [DOI] [PubMed] [Google Scholar]
- 51.Williams LM, Kelly D, Hannah LT, Morgan PJ. Localization of [ 125I]IGF-1 binding on the ovine pars tuberalis. J Neuroendocrinol. 1995;7:931–938. doi: 10.1111/j.1365-2826.1995.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 52.Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000;141:1515–1520. doi: 10.1210/endo.141.4.7433. [DOI] [PubMed] [Google Scholar]
- 53.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 54.Takeda Y, Krause JE. Neuropeptide K potently stimulates the salivary gland secretion and potentiates substance P-induced salivation. Proc Natl Acad Sci USA. 1989;86:392–396. doi: 10.1073/pnas.86.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]