Abstract
Hormonal induction of growth-arrested 3T3-L1 preadipocytes rapidly activates expression of CCAAT/enhancer-binding protein (C/EBP) β. Acquisition of DNA-binding activity by C/EBPβ, however, is delayed until the cells synchronously enter the S phase of mitotic clonal expansion (MCE). After MCE, C/EBPβ activates expression of C/EBPα and peroxisome proliferator-activated receptor γ, which then transcriptionally activate genes that give rise to the adipocyte phenotype. A-C/EBP, which possesses a leucine zipper but lacks functional DNA-binding and transactivation domains, forms stable inactive heterodimers with C/EBPβ in vitro. Infection of 3T3-L1 preadipocytes with an adenovirus A-C/EBP expression vector interferes with C/EBPβ function after induction of differentiation. A-C/EBP inhibited events associated with hormone-induced entry of S-phase of the cell cycle, including the turnover of p27/Kip1, a key cyclin-dependent kinase inhibitor, expression of cyclin A and cyclin-dependent kinase 2, DNA replication, MCE, and, subsequently, adipogenesis. Although A-C/EBP blocked cell proliferation associated with MCE, it did not inhibit normal proliferation of 3T3-L1 preadipocytes. Immunofluorescent staining of C/EBPβ revealed that A-C/EBP prevented the normal punctate nuclear staining of centromeres, an indicator of C/EBPβ binding to C/EBP regulatory elements in centromeric satellite DNA. The inhibitory effects of A-C/EBP appear to be due primarily to interference with nuclear import of C/EBPβ caused by obscuring its nuclear localization signal. These findings show that both MCE and adipogenesis are dependent on C/EBPβ.
Keywords: 3T3-L1 adipocytes, nuclear localization, adipogenesis, obesity, cell cycle
Obesity gives rise to adipocyte hyperplasia through recruitment and proliferation of preadipose cells present in the vascular stroma of adipose tissue (1–3). This hyperplasia is mimicked ex vivo by the adipogenic differentiation program of 3T3-L1 preadipocytes induced by treatment with the appropriate hormonal inducers. After hormonal induction, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle, undergo mitotic clonal expansion (MCE), and then express genes that produce the adipocyte phenotype (4–7). A large body of evidence has shown that peroxisome proliferator-activated receptor γ (PPARγ) and several members of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors play essential roles in the differentiation program.
These transcription factors function in a signaling cascade that culminates in the activation of a large number of adipocyte genes (6, 8, 9). C/EBPβ is rapidly expressed on hormonal induction, however, but only acquires DNA-binding activity after a long lag period (≈12 h) as the cells synchronously reenter the cell cycle traversing the G1–S checkpoint and initiate MCE (10). Once DNA-binding activity is acquired, C/EBPβ transcriptionally activates the C/EBPα and PPARγ genes by interacting with C/EBP regulatory elements in their proximal promoters (11–14). C/EBPα and PPARγ serve as pleiotropic transcriptional activators that coordinately induce expression of adipocyte genes.
Preadipocytes exit the cell cycle having undergone approximately two rounds of MCE. The expression of C/EBPα, which is antimitotic, occurs as the cells exit the cell cycle and is thought to be responsible for terminating MCE. This antimitotic action of C/EBPα has been demonstrated both for adipocyte differentiation of 3T3-L1 preadipocytes (15–17) and for hepatocyte proliferation in culture (18–20) and in vivo (19, 21). C/EBPα appears to arrest proliferation of hepatocytes by inhibiting cyclin-dependent kinases (Cdks) (22, 23). In contrast, C/EBPβ has been shown to be promitotic. Partial hepatectomy in mice carrying a targeted disruption of the C/EBPβ gene resulted in impaired liver regeneration (24, 25). Recent evidence in our laboratory (26) showed that C/EBPβ–/– MEFs were unable to reenter the cell cycle and undergo MCE or adipogenesis when treated with differentiation inducers.
The present study was conducted to determine the effect on adipogenesis of a dominant-negative C/EBP (A-C/EBP) that blocks C/EBPβ DNA binding by dimerizing with its leucine zipper (27). We provide evidence that A-C/EBP prevents entry of C/EBPβ into the nucleus of 3T3-L1 preadipocytes and thereby blocks MCE and adipogenesis.
Materials and Methods
Cell Culture and Differentiation. 3T3-L1 preadipocytes were cultured in DMEM supplemented with 10% (vol/vol) calf serum. To induce differentiation, 2-day postconfluent 3T3-L1 (designated day 0) were fed DMEM containing 10% FBS, 1 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine until day 2. Cells then were fed DMEM supplemented with 10% FBS and 1 μg/ml insulin for 2 days, after which they were fed every other day with DMEM containing 10% FBS.
C/EBP Adenoviral Expression Vectors and Infection. A-C/EBP (27) as a NdeI–HindIII DNA fragment was cloned into Advector, a modified version of pACCMV.pLpA with a FLAG epitope and a NdeI–HindIII cloning site (28). This adenoviral vector expresses A-C/EBP under control of the CMV promoter, whereas the control viral vector lacks a A-C/EBP insert. Adenoviral stocks were dialyzed into 10 mM Tris (pH 8), 2 mM MgCl2, and 10% glycerol, and 4 × 104 optical particle units of control or A-C/EBP viral particles per cell were added to monolayers at 95% confluence. Viral titers of the control and A-C/EBP adenoviral viruses were similar. After 2 days of infection, cells were induced to differentiate with virus-free medium as described above.
Immunoblotting. At various times after induction of differentiation, cell monolayers were washed with cold PBS and scraped into lysis buffer (1% SDS/60 mM Tris·Cl, pH 6.8). Lysates were heated at 100°C for 10 min and clarified by centrifugation, and equal amounts of protein were separated by SDS/PAGE. Proteins were transferred to Immobilon-P membranes (Millipore) and immunoblotted with antibodies to cyclin D1, cyclin B1, cyclin A, Cdk2, Kip1/p27, C/EBPβ, Flag, C/EBPα, 422/aP2, and PPARγ. Antibodies to the C-terminal regions of C/EBPβ and C/EBPα and to 422/aP2 were prepared as described (10, 29), the antibody to the N-terminal region of C/EBPβ was provided by David Ron (New York University Medical School, New York), and the antibody to PPARγ was provided by Mitchell Lazar (University of Pennsylvania, Philadelphia). Antibodies to cyclin D1, cyclin B1, cyclin A, and Cdk2 were obtained from Santa Cruz Biotechnology. Antibody to p27/Kip1 was from BD Pharmingen, and antibody against Flag was from Sigma.
BrdUrd Labeling and Immunofluorescence Microscopy. For BrdUrd labeling, 3T3-L1 cells plated on glass coverslips were induced to differentiate by using the standard differentiation protocol and 18 h after induction (during S phase) were pulse-labeled for 2 h with 30 μg/ml BrdUrd and then shifted to normal medium. On day 3, coverslips were fixed in 70% ethanol for 30 min and incubated in 100% methanol for 10 min at room temperature, after which they were treated for 30 min with 1.5 M HCl, blocked with 0.5% Tween 20 in PBS for 5 min, incubated with anti-BrdUrd primary antibody (1:500) in the same buffer for 2 h at room temperature, and incubated with FITC-conjugated secondary antibody (1:200) with 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 1 h at room temperature. After each step, cells were washed with PBS three times, antifade solution (Molecular Probes) was added, and the coverslips were mounted on slides for confocal microscopy. Immunostaining with antibody against C/EBPβ was conducted as described (10). Antibody against Flag was included for double labeling.
Oil Red O Staining. Cells were washed three times with PBS and then fixed for 2 min with 3.7% formaldehyde. Oil red O (0.5% in isopropanol) was diluted with water (3:2), filtered through a 0.45-μm filter, and incubated with the fixed cells for 1 h at room temperature. Cells were washed with water, and the stained fat droplets in the cells were visualized by light microscopy.
Immunoprecipitation. Coimmunoprecipitation was performed by using anti-Flag M2-Agarose Affinity Gel (Sigma) according to the manufacturer's protocol. Briefly, virally transduced cells were harvested 20 h after induction of differentiation. After washing with cold PBS, cells (one 10-cm dish) were lysed in 1 ml of lysis buffer (50 mM Tris·HCl, pH 7.4/150 mM NaCl/1 mM EDTA/1% Triton X-100). Cell lysates were clarified by centrifugation. To pull down the Flag protein complex, 40 μl of anti-Flag gel suspension was coincubated with 1 ml of cell lysate for 2 h on a roller shaker at 4°C. The resin was washed three times with 0.5 ml of TBS and eluted with sample buffer for gel electrophoresis and immunoblotting. To assess the fraction of C/EBPβ associating with A-C/EBP-Flag, an equivalent amount of supernatant along with the eluted sample (normalized by dilution) were subjected to SDS/PAGE and immunoblotting with anti-C/EBPβ and anti-Flag.
Quantitation of Genomic DNA. Quantitation of genomic DNA was performed by using a FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Molecular Probes) according to the manufacturer's protocol. Ninety-six hours after induction of differentiation, 3T3-L1 cells were harvested and stained with Hoechst 33258. DyNA Quant 200 (Hoefer) was used to measure the fluorescence.
Results
Adipogenesis Is Blocked by A-C/EBP. To assess the effect of expression of a dominant-negative C/EBP on differentiation of 3T3-L1 preadipocytes, preadipocytes were infected with an adenoviral vector expressing A-C/EBP. In A-C/EBP, the basic region critical for DNA binding is replaced by acidic amino acids and the transactivation domain is eliminated producing a molecule that heterodimerizes with the entire B-ZIP region of C/EBPβ and forms a stable coiled-coil complex lacking DNA-binding activity (27). A-C/EBP inhibits the DNA binding of C/EBP family members but not other B-ZIP transcription factors (30). The A-C/EBP vector contained a Flag-tag epitope in the N terminus of the A-C/EBP sequence. Nearly 95% confluent 3T3-L1 preadipocytes were transduced with the adenoviral A-C/EBP vector. An empty adenoviral construct served as a negative control. The transduction efficiency, assessed by immunostaining with anti-Flag antibody, was found to be ≈80%. Two-day postconfluent preadipocytes were induced to differentiate with 1-methyl-3-isobutyl-xanthine, dexamethasone, and insulin (MDI). Eight days after treatment with differentiation inducers, the cells were subjected to Oil red O staining (Fig. 1A). Preadipocytes treated with empty virus differentiated well, as evidenced by the accumulation of cytoplasmic triglyceride stained with Oil red O, whereas preadipocytes infected with adenovirus encoding A-C/EBP did not. It is likely that the small number of cells that differentiated had not been transduced by A-C/EBP.
Fig. 1.
Effect of dominant-negative A-C/EBP on the differentiation of 3T3-L1 preadipocytes. Nearly confluent 3T3-L1 preadipocytes were infected with adenoviruses either lacking an insert (control) or containing an insert that encodes A-C/EBP, a dominant-negative C/EBP. Exposure to the viruses was continued for 2 days, at which time (2 days postconfluent) the preadipocytes were induced to differentiate by using the standard protocol. (A) Eight days after induction, the cell monolayers were stained with Oil red O. (B) Four days after induction, cell lysates were immunoblotted with antibody specific against C/EBPα, PPARγ, and 422/aP2.
Immunoblotting of cell lysates prepared 4 days after treatment with differentiation inducers revealed that expression of adipocyte markers (C/EBPα, PPARγ, and 422/aP2) was virtually abolished in cells infected with the adenoviral A-C/EBP vector. In contrast, cells infected with the control virus exhibited high levels of expression of adipocyte markers (Fig. 1B). The inhibition of marker gene expression can be attributed to the inhibition of C/EBPβ and not C/EBPα, because expression of C/EBPα (and PPARγ) occurs much later in the differentiation program than C/EBPβ (8, 31).
Inhibition of MCE by A-C/EBP. The possibility was considered that A-C/EBP interferes with MCE, a relatively early step in the differentiation program. Normally, when growth-arrested 3T3-L1 preadipocytes are induced to differentiate, they synchronously reenter the cell cycle and undergo approximately two rounds of division (referred to as MCE) before terminal differentiation. These mitoses precede expression of the adipocyte genes that give rise to the adipocyte phenotype. The effect of expression of the A-C/EBP or control adenoviral vector growth-arrested cells on cell number was assessed 96 h after induction of differentiation. Untreated preadipocytes and those treated with control vector underwent an ≈4-fold increase in cell number compared to growth-arrested preadipocytes that had not been induced to differentiate (Fig. 2A). In contrast, preadipocytes treated with the A-C/EBP vector and induced to differentiate exhibited only an ≈1.5-fold increase. Thus, MCE was markedly inhibited by expression of A-C/EBP. The small increase in cell number that did occur is likely due to the fact that some cells (≈20%) were not transduced by the viral vector (see above).
Fig. 2.
Effect of A-C/EBP on MCE. 3T3-L1 preadipocytes were infected with A-C/EBP or control adenoviruses and induced as in Fig. 1. Three days after induction of differentiation, cell number was determined (A), genomic DNA was quantified (B), and incorporation of BrdUrd into cellular DNA was determined by using a monoclonal anti-BrdUrd antibody and FITC-conjugated secondary antibody (C). Cells were then stained with DAPI and photographed with a fluorescence microscope.
These findings were corroborated by quantifying changes in genomic DNA (Fig. 2B). Thus, untransduced preadipocytes or those transduced with the control vector underwent an ≈4-fold increase in DNA content by 96 h after induction of differentiation, whereas preadipocytes transduced with the A-C/EBP viral vector underwent only an ≈1.6-fold increase in DNA content (Fig. 2B).
To verify that DNA synthesis was blocked in virtually the entire cell population, DNA synthesis was measured by BrdUrd labeling. Eighteen hours after induction of differentiation, preadipocytes harboring the control or A-C/EBP viral vectors were pulse-labeled for 2 h with BrdUrd. Seventy-two hours after induction of differentiation, cells were immunostained with anti-BrdUrd. As shown in Fig. 2C, most preadipocytes transduced with the control adenoviral vector (when compared with DAPI-stained cells; Fig. 2C) exhibited BrdUrd incorporation into nuclear DNA. In contrast, preadipocytes harboring the A-C/EBP viral vector exhibited little BrdUrd incorporation and or clonal expansion when compared with DAPI-stained cells (Fig. 2C). Taken together, these results indicate that blocking C/EBPβ function with A-C/EBP blocked DNA synthesis and, thereby, MCE.
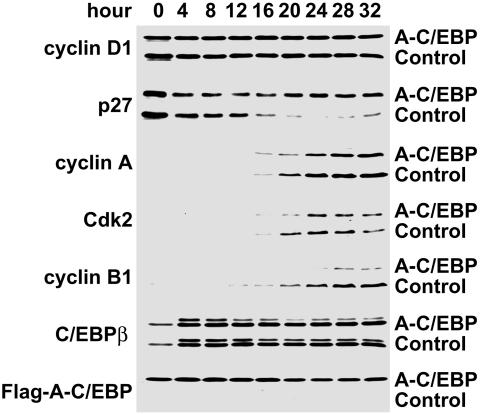
Effect of A-C/EBP on the Expression of Cell Cycle Markers During MCE. To determine the effect of A-C/EBP on the expression of cell cycle markers, lysates from cells harboring the A-C/EBP or control adenoviral vectors were harvested every 4 h after induction of differentiation and subjected to immunoblotting analysis with antibodies against cyclin D1, p27/kip1, Cdk2, cyclin A, and cyclin B1. The expression of A-C/EBP and endogenous C/EBPβ was also verified by immunoblotting. As illustrated in Fig. 3, A-C/EBP was expressed constitutively and had no effect on C/EBPβ, whose expression was constant during the 28-h (4–32 h) period after the induction of differentiation. Likewise, A-C/EBP had no effect on the expression of cyclin D1 during this period. It should be noted that during this 32-h time window, i.e., at ≈12–14 h, preadipocytes synchronously enter S phase of the cell cycle (7). The usual down-regulation of p27/Kip1, however, was almost completely blocked. This is of importance because Cdk2/cyclin A is normally held “in check” by inhibitory interaction with p27/Kip1 until this inhibitor is down-regulated at the G1–S checkpoint. Previous studies in our laboratory have shown that the degradation of p27 is required for preadipocytes to transit the G1–S checkpoint and proceed through the MCE phase of adipocyte differentiation (32). In addition, the expression of both cyclin A, and Cdk2 were delayed and partially inhibited by A-C/EBP. Another dramatic effect of A-C/EBP is the decreased and delayed expression of cyclin B1, which serves as a regulatory subunit of cdc2 kinase and is required for the G2–M transition (33, 34).
Fig. 3.
Effect of A-C/EBP on the expression of cell-cycle markers. 3T3-L1 preadipocytes were infected with control or A-C/EBP adenoviruses and induced to differentiate as in Fig. 1. Cell lysates were prepared every 4 h after induction differentiation. Cell lysates were subjected to SDS/PAGE and immunoblotted with antibodies against cyclin D1, cyclin B1, p27/Kip1, cyclin A, Cdk2, C/EBPβ, and Flag (recognizing Flag-A-C/EBP fusion protein).
Interaction of A-C/EBP with C/EBPβ. Coimmunoprecipitation experiments were conducted to verify the interaction between A-C/EBP and C/EBPβ. Cell lysates were prepared from 3T3-L1 preadipocytes containing the A-C/EBP adenoviral expression vector 20 h after treatment with differentiation inducers. Anti-Flag agarose affinity gel was used to immunoprecipitate the “Flag-tagged” A-C/EBP fusion protein from the cell lysates. Immunoblotting of precipitates and supernates fraction was performed with antibody directed against the N-terminal region of C/EBPβ. As shown in Fig. 4, 70–80% of the C/EBPβ protein coprecipitated with A-C/EBP in lysates from preadipocytes harboring the A-C/EBP expression vector, whereas none was immunoprecipitated from lysates of preadipocytes harboring the control vector. The residual C/EBPβ remaining in the supernatant of cells harboring the A-C/EBP vector is most likely due to the ≈20% of cells that failed to take up the adenoviral vector. These findings show that A-C/EBP forms a stable complex with C/EBPβ in lysates from 3T3-L1 preadipocytes infected with the A-C/EBP expression vector.
Fig. 4.
Interaction of endogenous C/EBPβ with A-C/EBP. 3T3-L1 preadipocytes were infected with A-C/EBP or control adenoviruses and induced as in Fig. 1. Twenty hours after induction, cell lysates were prepared and subjected to immunoprecipitation by using anti-Flag agarose beads. After three washes, supernates and precipitates were separated by SDS/PAGE and immunoblotted with anti-C/EBPβ and anti-flag antibodies.
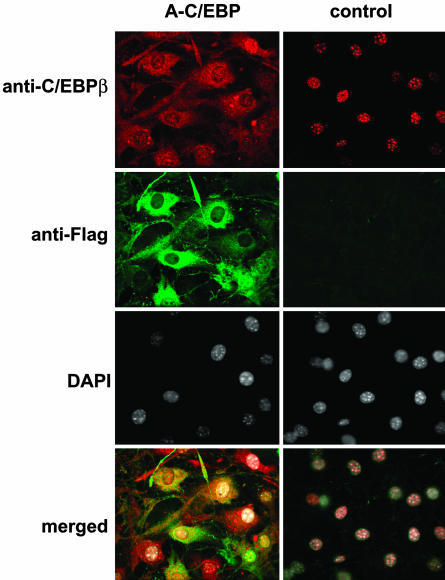
A-C/EBP Prevents Centromeric and Nuclear Localization of C/EBPβ. C/EBPβ is rapidly (≤2 h) expressed after hormonal induction of differentiation. At this point in the differentiation program, C/EBPβ is located in the nucleus but has not yet acquired DNA-binding activity (10). Acquisition of DNA-binding activity is delayed until 12–14 h after induction and becomes maximal after ≈24 h (10). When DNA-binding activity is acquired, C/EBPβ associates with centromeres where it binds to repetitive consensus C/EBP regulatory elements in centromeric satellite DNA (10). This event is coincident with the synchronous entry of S phase at the onset of MCE. As illustrated in Fig. 5, association of C/EBPβ with centromeres, as evidenced by its punctate immunostaining pattern (at 24 h after hormonal induction), is largely blocked by A-C/EBP. Also illustrated in Fig. 5 is the centromeric association of C/EBPβ that occurs in preadipocytes harboring the control (empty) adenoviral vector. These cells exhibit the normal punctate staining of nuclear C/EBPβ, which is localized exclusively in nuclei of 3T3-L1 cells and is coincident with DAPI staining.
Fig. 5.
A-C/EBP prevents nuclear localization and centromeric localization of C/EBPβ. 3T3-L1 preadipocytes on coverslips were infected with control and A-C/EBP (Flag-tagged) adenoviruses as in Fig. 1 and then induced to differentiate. Cells were immunostained with antibodies to C/EBPβ and Flag and were stained with DAPI.
Unlike cells infected with the control vector, those infected with the A-C/EBP vector exhibited colocalization of C/EBPβ and Flag-tagged A-C/EBP almost entirely in the cytoplasmic compartment (Fig. 5). Thus, A-C/EBP appeared to sequester C/EBPβ in the cytoplasm by forming heterodimers in which the nuclear localization signals are obscured. This is consistent with the fact that there are two conserved nuclear localization signals in the basic region of C/EBPβ (35). Presumably, the acidic region of A-C/EBP heterodimerizes with the B-ZIP basic region and forms a stable coiled-coil extension of the leucine zipper, thereby sequestering the nuclear localization signal.
Discussion
The present study provides compelling evidence that C/EBPβ is an obligate transcription factor for both MCE and adipogenesis in 3T3-L1 preadipocytes. We show that expression of a dominant-negative C/EBP (A-C/EBP) that forms stable heterodimers with C/EBPβ (ref. 27 and Fig. 4) disrupts both of these processes (Figs. 1 and 2). Failure to undergo MCE is indicated by the inability of preadipocytes harboring a A-C/EBP expression vector to enter the S phase of the cell cycle as evidenced by a prevention of events early in the cell cycle, most notably a failure to down-regulate the Cdk2/cyclin A inhibitor, p27/Kip1 (Fig. 3). Previous studies in this laboratory (32, 36) showed that blocking initiation of the proteolytic degradation of p27/Kip1 by calpain also prevents MCE and differentiation of 3T3-L1 cells. As a consequence of its effects on entry of the cell cycle at the G1–S checkpoint, A-C/EBP prevented the normal differentiation-induced DNA synthesis (Fig. 2 B and C) and cell proliferation (Fig. 2 A). Finally, it was shown that by heterodimerization with A-C/EBP, C/EBPβ fails to translocate into the nucleus (Fig. 5). Presumably, the formation of functional C/EBPβ homodimers is prevented by obscuring the two nuclear localization signals located in its basic DNA-binding domain by formation of a stable coiled-coil extension of the leucine zipper with the acidic region of A-C/EBP. This notion is supported by the fact that both A-C/EBP and C/EBPβ can be coimmunoprecipitated from lysates of 3T3-L1 cells harboring a A-C/EBP expression vector (Fig. 4).
Our findings are consistent with growing evidence that C/EBPβ and MCE play critical roles in progression of the adipocyte differentiation program. This evidence includes the observations that (i) inhibition of MEK or Cdk2 in 3T3-L1 preadipocytes (7), (ii) disruption of the gene encoding cAMP-response element-binding protein (in mouse embryo fibroblasts), a factor required for the transcriptional activation of the C/EBPβ gene (37), or (iii) disruption of the gene encoding C/EBPβ in mouse embryo fibroblasts (26) prevents both MCE and adipogenesis. Together these findings indicate that C/EBPβ is indispensable for both of these processes.
Acknowledgments
We thank Dr. David Johns (Department of Neurosurgery, Johns Hopkins University School of Medicine) for his advice and assistance in the preparation of adenoviral vectors. This work was supported by grants from the National Institutes of Health to M.D.L. (DK38418 and DK060787) and Q.-Q.T. (K01-DK61355).
Abbreviations: Cdk, cyclin-dependent kinase; C/EBP, CCAAT/enhancer-binding protein; DAPI, 4′,6-diamidino-2-phenylindole; MCE, mitotic clonal expansion; MDI, 1-methyl-3-isobutyl-xanthine, dexamethasone, and insulin; PPARγ, peroxisome proliferator-activated receptor γ.
References
- 1.Shepherd, P. R., Gnudi, L., Tozzo, E., Yang, H., Leach, F. & Kahn, B. B. (1993) J. Biol. Chem. 268, 22243–22246. [PubMed] [Google Scholar]
- 2.Gnudi, L., Shepherd, P. R. & Kahn, B. B. (1996) Proc. Nutr. Soc. 55, 191–199. [DOI] [PubMed] [Google Scholar]
- 3.Van, R. L., Bayliss, C. E. & Roncari, D. A. (1976) J. Clin. Invest. 58, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernlohr, D. A., Bolanowski, M. A., Kelly, T. J. & Lane, M. D. (1985) J. Biol. Chem. 260, 5563–5567. [PubMed] [Google Scholar]
- 5.Cornelius, P., MacDougald, O. A. & Lane, M. D. (1994) Annu. Rev. Nutr. 14, 99–129. [DOI] [PubMed] [Google Scholar]
- 6.MacDougald, O. A. & Lane, M. D. (1995) Annu. Rev. Biochem. 64, 345–373. [DOI] [PubMed] [Google Scholar]
- 7.Tang, Q.-Q., Otto, T. C. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. (2000) Genes Dev. 14, 1293–1307. [PubMed] [Google Scholar]
- 9.Hwang, C., Loftus, T., Mandrup, S. & Lane, M. (1997) Annu. Rev. Cell Dev. Biol. 13, 231–259. [DOI] [PubMed] [Google Scholar]
- 10.Tang, Q.-Q. & Lane, M. D. (1999) Genes Dev. 13, 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christy, R. J., Kaestner, K. H., Geiman, D. E. & Lane, M. D. (1991) Proc. Natl. Acad. Sci. USA 88, 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang, Q.-Q., Jiang, M. S. & Lane, M. D. (1999) Mol. Cell. Biol. 19, 4855–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, Y., Qi, C., Korenberg, J. R., Chen, X. N., Noya, D., Rao, M. S. & Reddy, J. K. (1995) Proc. Natl. Acad. Sci. USA 92, 7921–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, S. L., Robinson, C. E. & Gimble, J. M. (1997) Biochem. Biophys. Res. Commun. 240, 99–103. [DOI] [PubMed] [Google Scholar]
- 15.Umek, R. M., Friedman, A. D. & McKnight, S. L. (1991) Science 251, 288–292. [DOI] [PubMed] [Google Scholar]
- 16.Lin, F.-T., MacDougald, O. A., Diehl, A. M. & Lane, M. D. (1993) Proc. Natl. Acad. Sci. USA 90, 9606–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timchenko, N., Wilde, M., Nakanishi, M., Smith, J. & Darlington, G. (1996) Genes Dev. 10, 804–815. [DOI] [PubMed] [Google Scholar]
- 18.Diehl, A. M., Johns, D., Yang, S. Q., Lin, H. Z., Yin, M. & Lawrence, J. (1996) J. Biol. Chem. 271, 7343–7350. [DOI] [PubMed] [Google Scholar]
- 19.Timchenko, N. A., Harris, T. E., Wilde, M., Bilyeu, T. A. & Burgess-Beusse, B. L. (1997) Mol. Cell. Biol. 17, 7353–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks-Taylor, L. R. & Darlingto, G. J. (1995) Nucleic Acids Res. 23, 4726–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flodby, P., Barlow, C., Kylefjord, H., Ahrlund-Richter, L. & Xanthopoulos, K. G. (1996) J. Biol. Chem. 271, 24753–24760. [DOI] [PubMed] [Google Scholar]
- 22.Wang, H., Iakova, P., Wilde, M., Welm, A., Goode, T., Roesler, W. J. & Timchenko, N. A. (2001) Mol. Cell 8, 817–828. [DOI] [PubMed] [Google Scholar]
- 23.Iakova, P., Awad, S. S. & Timchenko, N. A. (2003) Cell 113, 495–506. [DOI] [PubMed] [Google Scholar]
- 24.Buck, M., Poli, V., Geer, P. V. D., Chojkier, M. & Hunter, T. (1999) Mol. Cell 4, 1087–1092. [DOI] [PubMed] [Google Scholar]
- 25.Taub, R., Greenbaum, L. E., Li, W., Cressman, D. E., Peng, Y., Ciliberto, G. & Poli, V. (1998) J. Clin. Invest. 102, 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang, Q.-Q., Otto, T. C. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwel, P., Tanaka, S., Penkov, D., Zhang, W., Olive, M., Moll, J., Vinson, C., Di Liberto, M. & Ramirez, F. (2000) Mol. Cell. Biol. 20, 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonovich, M., Olive, M., Reed, E., O'Connell, B. & Vinson, C. (2002) Cancer Gene Ther. 9, 62–70. [DOI] [PubMed] [Google Scholar]
- 29.MacDougald, O. A., Cornelius, P., Liu, R. & Lane, M. D. (1995) J. Biol. Chem. 270, 647–654. [DOI] [PubMed] [Google Scholar]
- 30.Vinson, C., Myakishev, M., Acharya, A., Mir, A. A., Moll, J. R. & Bonovich, M. (2002) Mol. Cell. Biol. 22, 6321–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh, W.-C., Cao, Z., Classon, M. & McKnight, S. L. (1995) Genes Dev. 9, 168–181. [DOI] [PubMed] [Google Scholar]
- 32.Patel, Y. M. & Lane, M. D. (2000) J. Biol. Chem. 275, 17653–17660. [DOI] [PubMed] [Google Scholar]
- 33.Nurse, P. (1990) Nature 344, 503–508. [DOI] [PubMed] [Google Scholar]
- 34.Innocente, S. A., Abrahamson, J. L., Cogswell, J. P. & Lee, J. M. (1999) Proc. Natl. Acad. Sci. USA 96, 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, S. C., Angerer, N. D. & Johnson, P. F. (1997) Gene Expression 6, 371–385. [PMC free article] [PubMed] [Google Scholar]
- 36.Patel, Y. M. & Lane, M. D. (1999) Proc. Natl. Acad. Sci. USA 96, 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, J.-W., Klemm, J. D., Vinson, C. & Lane, M. D. (2003) J. Biol. Chem., in press. [DOI] [PubMed]