Abstract
Hemichorea-hemiballism syndrome (HCHB) is a relatively rare cause of unilateral chorea in diabetic patients and is due to non ketotoic hyperglycaemia. Characteristic magnetic resonance (MR) findings include T1 hyperintensity in the contralateral putamen without any significant signal alteration on other conventional MR sequences. We report susceptibility weighted imaging (SWI) findings in a case of HCHB syndrome.
Keywords: Diabetes, hemichorea-hemiballism, putamen, susceptibility
Introduction
There are various theories regarding the cause of T1 hyperintensity in putamen in hemichorea-hemiballism syndrome (HCHB) syndrome. Out of these, ischemia is considered the most appropriate cause. We present susceptibility weighted imaging (SWI) findings in case of HCHB syndrome that supports this hypothesis. There is only one series of three cases describing SWI findings in HCHB syndrome.[1]
Case Report
A 45-year-old male, known case of Type 2 diabetes mellitus (DM) for two years, presented with complaints of abnormal movements (choreoathetoid) in the upper right and lower limb. Patient was conscious and well oriented. Magnetic Resonance (MR) study of the brain was performed using Siemens Magnetom Avanto1.5-T scanner. T1-weighted image showed hyperintensity in the left lentiform nucleus [Figure 1a]. No significant signal abnormality was detected on T2-weighted [Figure 1b], fluid attenuation and inversion recovery (FLAIR) [Figure 1c] and diffusion weighted (DW) images [Figure 1d]. On SWI, hyperintense signal was seen in left putamen. Symmetrical hypointensities were seen in bilateral globus palidii suggesting age-related mineralization. Asymmetrical hypointensity was also seen in posterolateral aspect of left putamen indicating mineral deposition. Prominent vessels appearing hypointense on SWI were seen along the left sylvian fissure and left temporal region [Figure 2a–c]. A provisional diagnosis of Hemichorea-hemiballism syndrome was made. Fasting blood glucose was 309 mg/dl. No ketones were detected in urinalysis, which confirmed the diagnosis of hyperglycemic hemichorea-hemiballism. The patient was started on s/c insulin and glycemic control was achieved. He received gabapentin (600 mg/day) and frisium (20 mg/day). His condition improved significantly and was subsequently discharged in a satisfactory condition [Figure 2d].
Figure 1 (a-d).
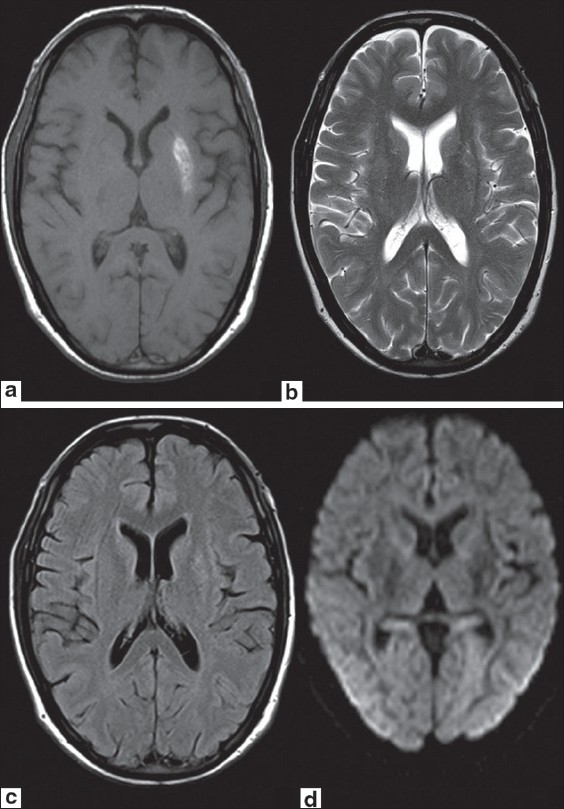
Axial T1W MR image (a) shows hyperintense signal in left lentiform nucleus. No significant signal abnormality is seen on axial T2W (b), FLAIR (c) or DW (d) images
Figure 2 (a-d).
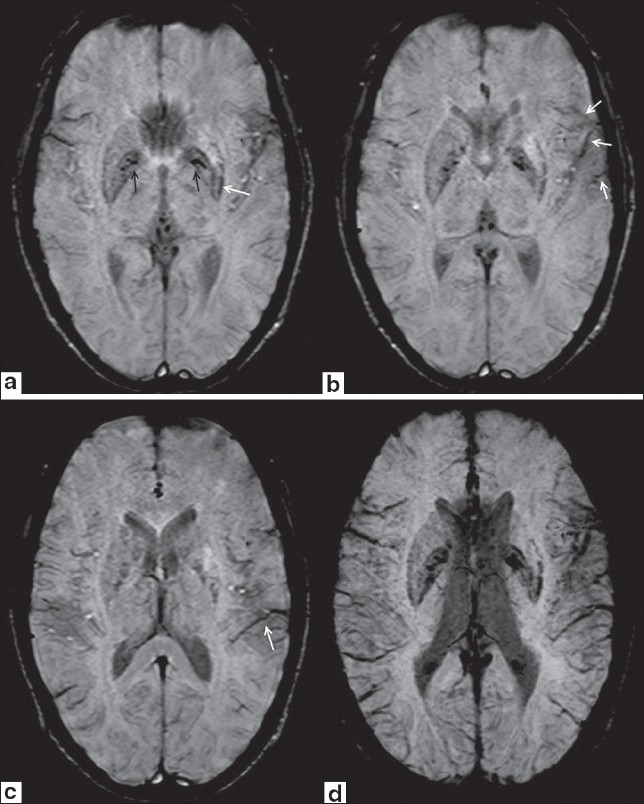
Axial SWI images show hyperintense signal in left lentiform nucleus. Symmetrical hypointensities are seen in bilateral globus palidii suggesting age-related mineralization (black arrows in 2a). Asymmetrical hypointensity is also seen in posterolateral aspect of left putamen indicating mineral deposition (white arrow in 2a). Prominent vessels appearing hypointense of SWI are seen along left sylvian fissure (white arrows in 2b) and in left temporal region (white arrow in 2c). Axial minimum intensity projection (MinIP) SWI image (2d) again shows prominent hypointense vessels along left sylvian fissure and in left temporal region to better advantage
Discussion
The characteristic imaging manifestation of hyperglycemia-induced HCHB syndrome is striatal hyperintensity on T1 W images with no signal abnormality on T2, FLAIR, gradient echo (GRE), or DW images.[2] The cause of T1 shortening is not exactly known. This has been attributed to basal ganglia cerebral blood flow reduction, petechial hemorrhage, depletion of both GABA and acetylcholine, or metabolic acidosis.[3] Others have implicated presence of abundant gemistocytes as the cause of striatal hyperintensity.[2]
SWI is a relatively new technique. It is a 3D sequence that exploits differences in tissue magnetic susceptibilities to provide a unique contrast.[4] It is exquisitely sensitive to the presence of even minute amounts of blood products. Based on same principle, it provides unique contrast that is similar to blood oxygen level-dependent (BOLD) imaging.[5] If any region of brain in hypoperfused due to deficient arterial flow, it promotes local vasodilation in the ischemic tissues, and increases extraction of available amount of oxygen in the blood. This results in increased amount of deoxhemoglobin in venous blood in that region. As deoxyhemoglobin is paramagnetic, it shows prominent hypointense signal on SWI and can be detected as prominent hypointense venous vessels in the affected region.
Petechial hemorrhages have been postulated as a cause of T1 shortening in HCHB syndrome.[6] However, isolated pathological studies have failed to show presence of hemorrhage.[2] Also, follow-up scans have failed to demonstrate any residue of hemorrhage. SWI is the most sensitive technique for detecting hemorrhage. Large area of T1 hyperintensity is expected to be much more pronounced on SWI if it were due to haemorrhage. This refutes petechial hemorrhages as the etiology.
Cerebral ischemia is considered by many as the most appropriate explanation of signal alterations in HCHB syndrome.[7–9] Other studies have documented relative hypometabolism in the involved area on positron emission tomography imaging.[7] In our case, we observed prominent hypointense vessels in the overlying cerebral convexity on the side of putaminal hyperintensity. This could be due to increased amount of deoxyhemoglobin in the veins secondary to ischemia in ipsilateral cerebral hemisphere. Indeed in experimental studies in rats,[8] similar hyperintense basal ganglia lesions have been produced by transient middle cerebral artery (MCA) occlusion followed by reperfusion. Although not in the distribution of basal ganglia venous drainage, the prominent veins on the ipsilateral cerebral convexity could indicate transient MCA ischemia on the same side. This observation supports cerebral ischemia as the cause of HCHB syndrome. Another experimental study in monkeys[10] has also shown than it is the partial striatal damage rather than complete infarction that results in chorea-like features. The cause of selective vulnerability of basal ganglia and precise anatomical localisation to corpus striatum is not clear. Stereotactic biopsy in a previous study[2] has shown presence of abundant gemistocytes (which are basically reactive swollen astrocytes) without any evidence of hemorrhage. Another study of 10 patients[11] has shown presence of mineral deposition in hypertrophied astrocytes in ischemic brain tissues. Based on these observations, ischemia seems to be the cause of HCHB syndrome with reactive gemistocyte proliferation and mineral deposition. Asymmetrical putaminal hypointensity on SWI also supports this hypothesis.
In conclusion, we describe SWI findings in a case of HCHB syndrome. SWI does not show any evidence of hemorrhage, and therefore, refutes petechial hemorrhages as the etiology of HCHB syndrome. Presence of prominent cortical veins on ipsilateral side supports transient vascular insult on the ipsilateral side. Further studies on more number of patients are required to further elucidate the incidence and significance of these findings. Finally, SWI is a new technique that provides unique contrast-based on tissue susceptibility differences, that is different from conventional MR sequences. It provides unique opportunities to show perfusion alterations without using perfusion-weighted imaging. This unique contrast may help explain the pathophysiology of many other conditions, which is obscure at present.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Cherian A, Thomas B, Baheti NN, Chemmanam T, Kesavadas C. Concepts and controversies in nonketotic hyperglycemia-induced hemichorea: Further evidence from susceptibility-weighted MR imaging. J Magn Reson Imaging. 2009;29:699–703. doi: 10.1002/jmri.21672. [DOI] [PubMed] [Google Scholar]
- 2.Shan DE, Ho DM, Chang C, Pan HC, Teng MM. Hemichorea-hemiballism: An explanation for MR signal changes. AJNR Am J Neuroradiol. 1998;19:863–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Felicio AC, Chang CV, Godeiro-Junior C, Okoshi MP, Ferraz HB. Hemichorea-hemiballism as the first presentation of type 2 diabetes mellitus. Arq Neuropsiquiatr. 2008;66:249–50. doi: 10.1590/s0004-282x2008000200022. [DOI] [PubMed] [Google Scholar]
- 4.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232–52. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altafullah I, Pascual-Leone A, Duvall K, Anderson DC, Taylor S. Putaminal hemorrhage accompanied by hemichorea-hemiballism. Stroke. 1990;21:1093–4. doi: 10.1161/01.str.21.7.1093. [DOI] [PubMed] [Google Scholar]
- 7.Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea.A PET study. J Neurol. 2004;251:486–90. doi: 10.1007/s00415-004-0571-4. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka M, Taoka T, Matsuo Y, Hiramatsu KI, Sakaki T. Novel brain ischemic change on MRI: Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999;30:1043–6. doi: 10.1161/01.str.30.5.1043. [DOI] [PubMed] [Google Scholar]
- 9.Shan DE. Hemichorea-hemiballism associated with hyperintense putamen on T1-weighted MR images: An update and a hypothesis. Acta Neurol Taiwan. 2004;13:170–7. [PubMed] [Google Scholar]
- 10.Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- 11.Neal JW, Singhrao SK, Jasani B, Newman GR. Immunocytochemically detectable metallothionein is expressed by astrocytes in the ischaemic human brain. Neuropathol Appl Neurobiol. 1996;22:243–7. [PubMed] [Google Scholar]


