Abstract
p38α, p38β, p38γ, and p38δ are four isoforms of p38 mitogen-activated protein (MAP) kinase (MAPK) involved in multiple cellular functions such as cell proliferation, differentiation, apoptosis, and inflammation response. In the present study, we examined the mRNA expression pattern of each of the four isoforms during erythroid differentiation of primary erythroid progenitors. We show that p38α and p38γ transcripts are expressed in early hematopoietic progenitors as well as in late differentiating erythroblasts, whereas p38δ mRNA is only expressed and active during the terminal phase of erythroid differentiation. On the other hand, p38β is minimally expressed in early CD34+ hematopoietic progenitors but not expressed in lineage-committed erythroid progenitors. We also determined the phosphorylation/activation of p38α, MAPK kinase 3/6, and MAPKAP-2 in response to erythropoietin and stem cell factor. We found that phosphorylation of p38α, MAPK kinase kinase 3/6 and MAPKAP-2 occurs only upon growth factor withdrawal in primary erythroid progenitors. Moreover, our data indicate that activation of p38α does not induce apoptosis or promote proliferation of erythroid progenitors. On the other hand, under steady-state culture conditions, both p38α and p38δ isoforms are increasingly phosphorylated activated in the terminal phase of differentiation. This increased phosphorylation/activity was accompanied by up-regulation of heat shock protein 27 phosphorylation. Finally, we demonstrate that tumor necrosis factor α, an inflammatory cytokine that is modulated by p38α, is expressed by differentiating erythroblasts and inhibition of p38α or tumor necrosis factor α results in reduction in differentiation. Taken together, our data demonstrate that both p38α and δ isoforms function to promote the late-stage differentiation of primary erythroid progenitors and are likely to be involved in functions related to erythrocyte membrane remodeling and enucleation.
The family of p38 mitogen-activated protein (MAP) kinases (MAPKs) include p38α, β, δ, and γ isoforms. The four p38 MAPK isoforms are defined by the common TGY motif and has significant homology with each other at the amino acid level (1, 2). Phosphorylation of serine and threonine residues by MAPK kinase (MKK) 6 and MKK3 (β isoform is not activated by MKK3), activates all four isoforms leading to transcriptional activation of ATF-2 (3–5). However, studies demonstrating that each of the isoforms also targets other substrates in a selective fashion suggest that each of the isoforms may have unique functions depending on the tissue type (3, 6, 7). The tissue distribution of mRNA for each isoform has been examined. The p38α and p38β isoforms are expressed in most tissues, but expression of p38γ is limited to the skeletal muscle (6–11). On the other hand, p38δ is expressed in lung, pancreas, kidney, testis, and small intestine (5). By contrast, expression of these isoforms in hematopoietic cells, especially during erythroid maturation, has not been examined. Studies performed in inflammatory cell lineages such as monocytes, macrophages, neutrophils, and T lymphocytes have shown differential expression patterns for all isoforms (12). However, whether expression of isoforms is differentiation stage-specific has not been explored.
Erythropoietin (Epo) promotes cell proliferation and differentiation in addition to preventing apoptosis of erythroid progenitors. Early committed human erythroid progenitors are dependent on Epo beginning at the burst-forming unit erythroid (BFU-E) stage until they reach late colony-forming unit erythroid (CFU-E) stage of differentiation (13–15). Previous studies have established the key signaling pathways regulating erythroid cell proliferation and cell viability. These studies demonstrated the involvement of Jak/Stat, Shc/Grb2/Ras, Gab1/2, extracellular response kinase (ERK), and phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (PKB) pathways in the regulation of proliferation and cell viability (16–22). The activation of MAPK, p38α in Epo-dependent cell lines has been also examined although its function during differentiation of primary erythroid progenitors is not well understood (23–26). Moreover, expression and activation of the other three-kinase isoforms has not been examined in Epo-dependent cell lines or in primary erythroid progenitors. Our present study demonstrates a distinct pattern of expression and/or activation of the four p38 MAPK isoforms during erythroid differentiation. In addition we demonstrate that Epo and stem cell factor (SCF) suppress activity of p38α, MKK3/6, and MAPKAP-2 during the growth factor-dependent phase of erythroid differentiation and observe increased phosphorylation/activity of p38α and δ during the Epo-independent terminal-phase of differentiation. Furthermore, we show evidence that in primary erythroid progenitors the p38α isoform is involved in induction of differentiation but not apoptosis or cell proliferation. Finally, we investigated the role of tumor necrosis factor α (TNFα) and heat shock protein 27 (Hsp27), downstream targets of p38α and MAPKAP-2 respectively, in the late stages of differentiation. The addition of neutralizing antibodies slowed differentiation of erythroid progenitors in cultures. With respect to Hsp27, we show that Hsp27 is phosphorylated only in the late stages of erythroid maturation.
Methods
Antibodies and Reagents. Polyclonal antibodies against p38α, p38δ, and ATF-2 fusion protein were purchased from Santa Cruz Biotechnology. An antibody against the MAPKAP-2 was obtained from Upstate Biotechnology. Polyclonal antibodies against the phospho-specific p38α, MKK3/6, and Hsp27 were obtained from Cell Signaling Technology (Beverly, MA). Neutralizing antibody against TNFα and growth factors was purchased from R & D Systems. The p38α/β-specific inhibitor SB203580 was obtained from Calbiochem.
Cell Isolation and Culture. Human primary erythroid progenitor cells were derived by in vitro culture of CD34+ cells isolated from growth factor mobilized peripheral blood (purchased from ALL Cells, Berkeley, CA). CD34+ cells were isolated as described (22, 27). The culture media contained 15% FCS, 15% human AB serum, Iscove's modified Dulbecco's medium (IMDM), 10 ng/ml IL-3, 2 units/ml Epo, and 50 ng/ml SCF. Eighty to ninety percent of these cells were erythroid cells as determined by flow cytometry for glycophorin A and CD71.
Flow Cytometry. Flow cytometry analysis was performed on cells undergoing differentiation by using fluorochrome-labeled glycophorin A and CD71 antibodies purchased from BD Biosciences (San Jose, CA). Cells were analyzed for apoptosis by determining the percentage of cells that were positive for annexin V and propidium iodide.
PCR Amplification. Total RNA isolated at various time points during the 14-day culture period was used to synthesize cDNA. PCR amplification was performed with specific forward and reverse primers for p38α (forward, 5′-58GTGCCCGAGCGTTACCAGACC78-3′; reverse, 5′-370CTGTAAGCTTCTGACATTTC351-3′), p38β (forward, 5′-746CACCCAGCCCTGAGGTTCT764-3′; reverse, 5′-1110AATCTCCAGGCTGCCAGG1093-3′), p38γ (forward, 5′-782ACATGAAGGGCCTCCCCG799-3′; reverse, 5′-1093TCTCCTTGGAGACCCTGG1076-3′), p38δ (forward, 5′-64CCCAAGACCTACGTGTCCC82-3′; reverse, 5′-385ATTGGATCTTCTCCTCACTG366-3′) by using the following conditions: 1 cycle at 95°C for 2 min, 30 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and 1 cycle at 72°C for 5 min.
Control PCR amplifications were performed with specific forward and reverse primers for the 18S ribosomal RNA gene (forward, 5′-948CGCCGCTAGAGGTGAAATTCT968-3′; reverse, 5′-1479CAATCTCGGGTGGCTGAAC1461-3′) by using similar conditions. The specificity of the p38 primers was tested and confirmed by using p38α, β, γ, and δ plasmid DNA. Transcripts for TNFα were amplified by using primers and positive controls purchased from R & D Systems.
Immunoblot Analysis. Immunoblotting was performed as described (19).
p38δ MAPK Assay. On days 9, 13, and 16 of culture, cells were harvested, lysed, and subsequently immunoprecipitated by using an antibody against p38δ. The immunocomplexes were washed with phosphorylation lysis buffer containing 0.1% Triton X-100 with kinase buffer (25 mM Hepes/25 mM MgCl2/25 mM β-glycerophosphate/2mMDTT/0.1 mM Na3VO4/20 μM ATP) and resuspended in 30 μl of kinase buffer containing 5 μg of glutathione S-transferase-ATF-2 fusion protein, and 10 μCi of [γ-32P]ATP was added (1 Ci = 37 GBq). The reaction was incubated for 30 min at room temperature and was terminated by the addition of SDS-sample buffer. Proteins were analyzed by SDS/PAGE, and the phosphorylated form of ATF-2 was detected by autoradiography.
MAPKAP-2 Assays. Day-7 cells were growth factor-starved and subsequently stimulated with Epo or SCF. The cells were then lysed and in vitro kinase assays were performed as in previous studies (28).
Cell Proliferation. Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay (Sigma) as described (29).
Results
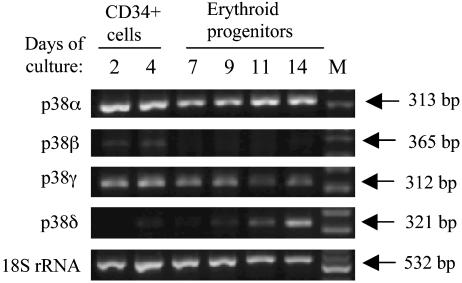
We initially sought to determine the mRNA expression pattern of p38 MAPK isoforms in the 14-day culture period during which early hematopoietic cells (CD34+) proliferate and differentiate to reticulocyte. PCR amplification by using isoform-specific primers indicated that p38 MAPK isoforms α and γ are expressed at every stage of differentiation, whereas p38 MAPK isoform β transcripts are expressed only early in the differentiation program. (Fig. 1). In contrast, expression of the p38δ was observed only at orthochromatic stage of differentiation. These results suggest that p38α and γ are important in early as well as late erythroid differentiation, whereas p38δ is functional only in the terminal phase of erythroid differentiation.
Fig. 1.
Expression pattern of p38 MAPK α, β, δ, and γ mRNA in CD34+ early hematopoietic cells and differentiating primary erythroid progenitors. RT-PCR analysis was performed by using p38 MAPK isoform-specific primers using early-uncommitted CD34+ cells and differentiating erythroid progenitors as indicated. As positive controls, 18S ribosomal RNA gene was also amplified by RT-PCR.
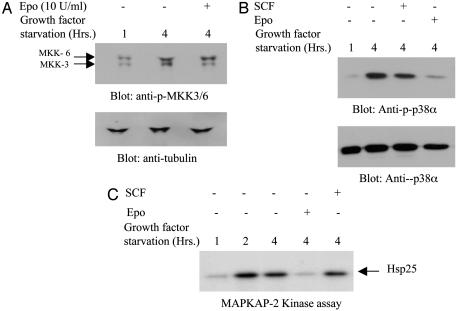
In further experiments, we focused on the p38α isoform, because this isoform is abundantly expressed throughout differentiation and has been widely studied in many cell types. We also examined the phosphorylation of MKK3/6, an upstream kinase and MAPKAP-2, an immediate downstream target of p38α. We examined the phosphorylation of MKK3/6 and p38α proteins in response to Epo or SCF addition or withdrawal in erythroid progenitors that are at the basophilic erythroblast stage of differentiation. Our experiments indicated that MKK3/6 and p38α were phosphorylated only when growth factors were withdrawn from cultures (Fig. 2 A and B). Readdition of Epo after 4 h of starvation down-regulated phosphorylation of MKK3 and p38α (Fig. 2 A and B). In contrast, readdition of SCF into cultures induced very little or no down-regulation of phosphorylation of p38α. We then examined the kinase activity of MAPKAP-2, the immediate downstream target of p38α isoform during growth factor withdrawal and restimulation with Epo or SCF. Our results indicated that, during growth factor starvation, MAPKAP-2 activity increased greatly peaking at 2 h after growth factor withdrawal (Fig. 2C). Readdition of Epo into cultures that were growth factor deprived down-regulated kinase activity. These results suggested that p38α MAPK is not active in cells that are at the basophilic erythroblast stage of differentiation, although the transcripts were present in the cells throughout differentiation. Once again, we did not observe down-regulation of MAPKAP-2 kinase activity in cultures that were treated with SCF.
Fig. 2.
Growth factor withdrawal induces phosphorylation/activation of MKK3/6, p38α, and MAPKAP-2 in primary erythroid progenitors. Primary erythroid progenitors on day 8 of culture were deprived of Epo and SCF for indicated periods or stimulated with Epo or SCF after growth factor starvation. Cell lysates were collected and analyzed by immunoblotting with anti-phospho MKK3/6 antibodies (A) or anti-phospho p38α antibodies (B). Each of the blots were then stripped and reprobed with either anti-tubulin (A) or anti-p38α (B) antibodies to confirm equal protein loading. (C) Day 8 cells were growth factor starved as indicated and stimulated with Epo or SCF. Total cell lysates were immunoprecipitated with antibodies against MAPKAP-2. The immunocomplexes were then used in kinase assays. Hsp25 protein was used as a substrate along with [γ-32P]ATP in the reactions. Subsequently, proteins were analyzed by SDS/PAGE, and the phosphorylated form of Hsp25 was detected by autoradiography.
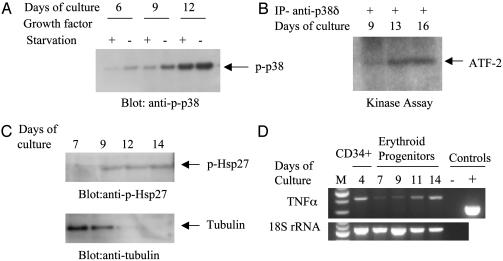
We then sought to determine whether p38 isoforms play a role in terminal differentiation of erythroid progenitors. Because RT-PCR experiments indicated that p38α and p38δ are highly expressed during the terminal phase of erythroid differentiation, we determined whether p38α and p38δ isoforms are phosphorylated and active respectively in the late stages of the erythroid differentiation program. Fig. 3A shows that, under steady-state culture conditions (in the presence of growth factors), the level of p38α phosphorylation gradually increased as the cells differentiated into orthochromatic erythroblasts. In the same experiment, we also included as controls cells that were collected after growth factor deprivation, which exhibited increased level of phosphorylation as expected (Fig. 3A). We then determined whether p38δ isoform shows kinase activity in the terminal phase of differentiation. Fig. 3B shows that very little kinase activity was observed on day 9 of culture, although a high level of kinase activity was observed on days 13 and 16 when these cells are at the orthochromatic stage of differentiation and undergoing enucleation. Finally, because Hsp27 is a target for phosphorylation by MAPKAP-2, we investigated the level of phosphorylation of Hsp27 during the differentiation program. Fig. 3C shows that Hsp27 is only slightly phosphorylated on day 7 and then increased phosphorylation is observed on days 9, 12, and 14 of culture, indicating that the p38α signaling pathway is active in these cells in the Epo-independent phase. Because TNFα is a cytokine that is expressed in response to p38 activation, we determined whether TNFα mRNA is detectable in the late stages of differentiation. Our results showed that TNFα transcripts are expressed in erythroid progenitors in early as well as late stages of differentiation (Fig. 3D).
Fig. 3.
Phosphorylation/activation of p38α, p38δ, and Hsp27 and expression of TNFα during differentiation of erythroid progenitors. (A) During steady-state culture conditions, cells were collected on days 6, 9, and 12 in the presence or absence of Epo and SCF. Total cell lysates were used to perform immunoblot analysis using phospho-specific p38α antibodies to determine the level of phosphorylation on each day of culture. As positive controls, samples were also collected on each day after depriving the cells of Epo and SCF for 4 hours. (B) In vitro kinase assays were performed on samples collected on days as indicated. Proteins were analyzed by SDS/PAGE, and phosphorylated ATF-2 was detected by autoradiography. (C) Cells were collected during differentiation of erythroid progenitors, and 20 μg of total proteins per sample were used for immunoblotting with an anti-phospho Hsp27 antibody. The blot was stripped and immunoblotted with tubulin to demonstrate that cells were undergoing terminal differentiation as Hsp27 was increasingly phosphorylated. (D) RT-PCR analysis using specific primers for TNFα on primary differentiating erythroid progenitors. PCR amplification was performed by using 1/10th of cDNA obtained from the reverse transcriptase reaction. 18S ribosomal RNA gene was also amplified in addition to the positive control sample provided by the manufacturer.
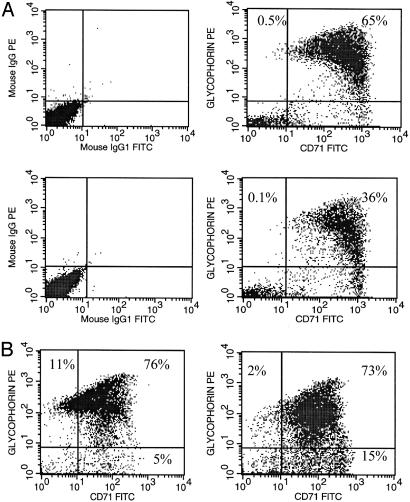
We then sought to determine the consequence of p38α inhibition on differentiation of erythroid progenitors during the culture period. We used glycophorin A, which is an erythroid-specific differentiation marker and CD71 (transferrin receptor) to determine the level of differentiation when kinase activity of p38α was inhibited. Fig. 4A shows cells that were cultured in the presence or absence of the p38α inhibitor SB203580 during the day 10–12 culture period and analyzed for the level of glycophorin A and CD71 on day 12 by flow cytometry. Decreased levels of glycophorin A was observed in cultures that were treated with the p38α inhibitor in comparison to cultures that were not treated with the inhibitor (36% vs. 65%), suggesting that the p38α pathway plays a role in promoting differentiation. We next determined whether TNFα plays a role in promoting differentiation of erythroid progenitors. Our results indicated that addition of TNFα neutralizing antibodies reduced the percentage of cells that were glycophorin Ahigh/CD71low (Fig. 4B), suggesting that TNFα promotes differentiation of primary erythroid cells.
Fig. 4.
p38α MAPK and TNFα promote terminal differentiation of primary erythroid progenitors. (A) Erythroid progenitors were treated with (Lower) or without (Upper) p38α inhibitor SB203580 on day 10 of culture. Cells were collected and analyzed for glycophorin A and CD71 expression by flow cytometry (Right). As controls, cells were also analyzed by using IgG isotype-specific antibodies (Left). (B) Erythroid progenitors (2 × 105 cells) were treated with (Right) or without (Left) neutralizing antibody against TNFα on day 10 and analyzed for glycophorin A and CD71 expression on day 12 by flow cytometry. The percentage of glycophorin A- and CD71-positive cells are indicated in each dot plot.
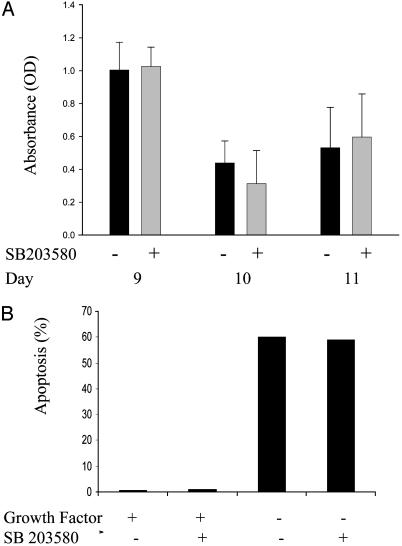
To determine the functional consequence of p38α inhibition on proliferation and apoptosis of primary erythroid progenitors during the differentiation program, we treated cells on days 9, 10, and 11 of culture with the p38 inhibitor and then determined the impact on proliferation at 24-h intervals. Fig. 5A shows the results of such an experiment where we found no difference in the level of proliferation of cells treated or untreated with SB203580. Because our results demonstrated that growth factor withdrawal phosphorylates p38α, we were interested in determining whether induction of apoptosis, which occurs in erythroid progenitors deprived of Epo is due to activation of the p38 MAPK pathway. Our results indicated that cultures deprived of growth factors showed >60% apoptosis regardless of the presence or absence of p38α inhibitor, whereas cultures with growth factor present showed no apoptosis (Fig. 5B). These results indicate that induction of apoptosis that occurs as a result of growth factor withdrawal is not caused by p38α MAPK phosphorylation/activation.
Fig. 5.
Effects of inhibition of p38α on proliferation and apoptosis of primary erythroid progenitors. (A) Erythroid progenitors cultured in serum-containing media with Epo and SCF were treated with SB203580 inhibitor 24 h before harvesting the cells for proliferation assays. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed on days indicated along with control samples that were not treated with the inhibitor. The data are means ± SE of triplicate measurements. (B) Erythroid progenitors on day 8 were washed to eliminate growth factors (Epo and SCF) and were recultured with the SB203580 inhibitor and growth factors or without the inhibitor treatment but with growth factors. Also, two samples were recultured without growth factors as indicated. After 20 h, cells were harvested and the percentage of apoptotic cells in each sample was determined by flow cytometry using annexin V and propidium iodide as apoptotic markers.
Discussion
The p38 MAPK subfamily is generally activated in response to environmental stresses. In most studies activation of p38 MAPKs, especially p38α isoform ultimately leads to an inflammation or an apoptotic response (30, 31). However, recent studies have shown that activation of p38α also can lead to other biological outcomes such as proliferation, cell survival, and differentiation, depending on the context and the cell type (32–39). Several studies have demonstrated that Epo induces a mitogenic response in hematopoietic cell lines through p38 MAPK pathway (23–25). However, such studies were performed using cell lines that do not recapitulate the normal growth and differentiation program. We investigated the expression, activation, and function of four isoforms of p38 MAPK by using primary erythroid progenitors that terminally differentiate into reticulocytes during in vitro culture. Interestingly, our data show that only p38α and p38γ isoforms are continuously expressed throughout differentiation from lineage uncommitted CD34+ early hematopoietic cells to terminally differentiated enucleating erythroblasts, suggesting distinct functions for these two isoforms in hematopoiesis. On the other hand, p38β is not expressed in differentiating erythroid progenitors. This is in contrast to nonhematopoietic tissues where p38β appears almost universally expressed (1). Our results also show expression of the p38δ isoform beginning days 9–10 of culture thus indicating a specific function for this isoform during the terminal phase of differentiation. The timing of expression of p38δ coincides with the time when erythroid progenitors become Epo independent and cease to proliferate suggesting a specific function other than regulation of proliferation or apoptosis (13). This is the first report demonstrating a possible role for p38δ MAPK isoform at any stage of hematopoietic differentiation.
To determine the role of Epo and SCF in the activation of the p38α isoform in primary erythroid cells we performed experiments to determine phosphorylation of p38α in response to Epo or SCF. Initial studies indicated that neither Epo nor SCF induced phosphorylation of p38α in these primary cells (data not shown). However, growth factor withdrawal resulted in phosphorylation of p38α, whereas readdition of Epo down-regulated phosphorylation, indicating that Epo and to a certain degree SCF suppress the activation of p38α in these cells. These results were further reinforced by our observation that the upstream target, MKK3/6 and the immediate downstream target, MAPKAP-2 of p38α followed the same pattern of activation and suppression. Our results differ from data with cell lines showing phosphorylation of p38α in response to Epo (23–25). To further clarify the discrepancy between primary cell cultures and data from cell lines, we used the Epo-responsive cell line HCD57, which has been used in studies to determine whether Epo induces phosphorylation of p38α. These studies confirmed that Epo phosphorylated p38α in HCD57 cells, further indicating that results in cell lines do not always reflect the biology of primary cells.
Because none of the Epo-responsive cell lines can truly duplicate the differentiation process of primary cells, we were interested to determine the role of p38α during the erythroid differentiation program of normal erythroid progenitors. Interestingly we found that, although p38α transcripts were expressed throughout differentiation, their phosphorylation/activation was minimal during basophilic erythroblast stage (under steady-state culture conditions), whereas a gradual increase in phosphorylation/activation was observed during differentiation reaching a maximum by day 12. In fact, by day 12, the amount of phosphorylated p38α under steady-state culture conditions was similar to that in cells deprived of growth factor, suggesting that activation of p38α gradually increases as the cells become independent of Epo and SCF. Examination of kinase activity of p38δ isoform demonstrated that, as erythroid cells differentiate, p38δ MAPK activity is up-regulated, suggesting a specific function related to differentiation for this isoform. A similar observation was made in intestinal epithelial cells, where differentiation-state-selective roles for p38 isoforms were ascribed (40).
Because p38α is phosphorylated during the Epo-independent phase of differentiation, we were interested in determining whether Hsp27, one of the downstream targets of MAPKAP-2, is also phosphorylated in these cells. Our data showed very little phosphorylation of Hsp27 in day-7 cells but increasing phosphorylation between days 9 and 14 of culture. The fact that Hsp27, which is known to stabilize actin filaments (41), is phosphorylated during the end-stages of erythroid differentiation supports the idea that phosphorylated form of Hsp27 may contribute to the enucleation process during erythroid differentiation. Our previous studies have shown that actin is localized in the constriction between the extruding nucleus and the incipient reticulocyte, ultimately suggesting that specific cellular signals regulate the enucleation of mammalian erythroblasts (42).
It is well established that TNFα is one of the cytokines that is expressed as a result of p38α activation (43). In addition, recent studies have shown that TNFα is expressed in both CD34+ hematopoietic cells and in human erythroid progenitors at colony-forming unit erythroid E stages of differentiation (44). Moreover, it has been demonstrated that, in neutrophils, Hsp27 protein expression is directly regulated by TNFα (45). These observations lead us to determine whether TNFα is expressed during late stages of differentiation. Our results indicated that TNFα transcripts could be easily detected on day 14 of culture, suggesting a role for this cytokine during differentiation. To determine whether TNFα regulates differentiation, we determined whether the differentiation program is affected in cultures that contained neutralizing antibodies against TNFα. Our results showed that addition of neutralizing antibodies against TNFα retards differentiation of primary erythroid progenitors as demonstrated by the decreased level of glycophorin Ahigh/CD71low cell population. Similar results were also observed with the p38α inhibitor SB203580 (Fig. 4). Together these results suggest that effect of p38α activation with respect to promoting differentiation of erythroid progenitors is mediated at least in part through TNFα. Interestingly, in a recent study it was demonstrated that transforming growth factor (TGF)-β1 also accelerated terminal differentiation of cord-blood-derived erythroid progenitors and resulted in increased number of enucleated erythroblasts (46), suggesting that both TNFα and TGFβ1 may have similar overall effects on erythroid cells to promote differentiation. However, precise functions of these two cytokines in erythroid differentiation have not been determined.
Numerous studies have used the appearance of β-globin as indicative of erythroid cell differentiation. By contrast, in our experiments using p38 MAPK inhibitor or TNFα neutralizing antibodies we did not observe a significant effect on the amount of β-globin between the samples that were treated or untreated with these reagents. Because globin synthesis begins relatively early (late burst-forming unit erythroid) during erythroid differentiation and reaches a maximum by day 10 of culture (13), these observations are consistent with our results demonstrating that p38α is only phosphorylated/active during late stages of differentiation and any effects of p38α inhibition is only observed by using a late differentiation marker such as glycophorin A. Finally, the data showing that inhibition of p38α does not affect proliferation or apoptosis during the Epo-sensitive phase of erythroid maturation is also consistent with the notion that p38α is not active during this phase.
Although further studies are required to precisely define the roles of TNFα and Hsp27 in promoting differentiation-related events such as enucleation and erythrocyte membrane remodeling, here we demonstrate the effect of various p38 isoforms in hematopoiesis. Furthermore, this study highlights the importance of using primary cells in studies involving signal transduction and events related to cellular differentiation because many of the growth factor-responsive cell lines are either derived from leukemic cells or do not naturally differentiate into reticulocytes.
Acknowledgments
We thank Drs. Koen van Besien and Eugene Chang for critical review of the manuscript. This work was supported in part by the Wendy Will Case Cancer Fund (to A.W. and S.U.).
Abbreviations: MAP, mitogen-activated protein; MAPK, MAP kinase; MKK, MAPK kinase; Epo, erythropoietin; SCF, stem cell factor; Hsp27, heat shock protein 27; TNFα, tumor necrosis factor α.
References
- 1.Ono, K. & Han, J. (2000) Cell. Signaling 12, 1–13. [DOI] [PubMed] [Google Scholar]
- 2.Shi, Y. & Gaestel, M. (2202) Biol. Chem. 383, 1519–1536. [DOI] [PubMed] [Google Scholar]
- 3.Kessler, G. A., Bray, J., Hunt, J., Johnson, D. A., Gleason, T., Yao, Z., Wang, S.-W., Parker, C., Yamone, H., Cole, C. & Lichenstein, H. S. (1998) Express Purif. 14, 221–230. [DOI] [PubMed] [Google Scholar]
- 4.Enslen, H., Raingeaud, J. & Davis, R. J. (1998) J. Biol. Chem. 273, 1741–1748. [DOI] [PubMed] [Google Scholar]
- 5.Kumar, S., McDonnel, P. C., Gum, R. J., Hand, A. J. & Young, P. R. (1997) Biochem. Biophys. Res. Commun. 235, 533–538. [DOI] [PubMed] [Google Scholar]
- 6.Li, Z., Jiang, Y., Ulevitch, J. & Han, J. (1996) Biochem. Biophys. Res. Commun. 228, 334–340. [DOI] [PubMed] [Google Scholar]
- 7.Cuenda, A. & Dorow, D. S. (1998) Biochem J. 333, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner, C., Zahalka, M. A., Giot, J.-F., Moler, N. P. H. & Ulrich, A. (1996) Proc. Natl. Acad. Sci. USA 93, 4355–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, Y., Chen, C., Li, Z., Guo, W., Genger, J., Lin, S. & Hann, J. (1996) J. Biol. Chem. 271, 17920–17926. [DOI] [PubMed] [Google Scholar]
- 10.Stein, B., Yang, M. X., Young, D. B., Janknecht, R., Hunter, T., Murray, B. W. & Barbosa, M. S. (1997) J. Biol. Chem. 272, 19509–19517. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, Y., Gram, H., Zhao, M., New, L., Gu, J., Feng, L., DiPadova, F., Ulevitch, R. & Han, J. (1997) J. Biol. Chem. 272, 30122–30128. [DOI] [PubMed] [Google Scholar]
- 12.Hale, K. K., Trollingor, D., Rihanek, M. & Manthey, C. L. (1999) J. Immumol. 162, 4246–4252. [PubMed] [Google Scholar]
- 13.Wickrema, A., Krantz, S. B., Winkelmann, J. C. & Bondurant, M. C. (1992) Blood 80, 1940–1949. [PubMed] [Google Scholar]
- 14.Muta, K., Krantz, S. B., Bondurant, M. C. & Wickrema, A. (1994) J. Clin. Invest. 94, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krantz, S. B. (1994) Blood 77, 419–443. [Google Scholar]
- 16.Sawyer, S. T. & Penta, K. (1996) J. Biol. Chem. 270, 32430–32437. [DOI] [PubMed] [Google Scholar]
- 17.Damen, J. E., Liu, L., Cutler, R. L. & Krystal, G. (1993) Blood 270, 11055–11061. [PubMed] [Google Scholar]
- 18.Jacobs-Helber, S. M., Penta, K., Sun, Z. H., Lawson, A. & Sawyer, S. T. (1997) J. Biol. Chem. 272, 6850–6853. [DOI] [PubMed] [Google Scholar]
- 19.Wickrema, A., Uddin, S., Chen, F., Alsayed, Y., Ahmad, S., Sawyer, S. T., Krystal, G., Yi., T., Nishada, K., Hibi, M., et al. (1999) J. Biol. Chem. 274, 24469–24474. [DOI] [PubMed] [Google Scholar]
- 20.Damen, J. E., Mui, A. L., Puii, J. L., Pawson, T. & Krystal, G. (1993) Blood 81, 3204–3210. [PubMed] [Google Scholar]
- 21.Uddin, S., Kottegoda, S., Stigger, D., Platanias, L. C. & Wickrema, A. (2000) Biochem. Biophys. Res. Commun. 275, 16–19. [DOI] [PubMed] [Google Scholar]
- 22.Mahmud, D. L., Amlak, M.-G., Deb, D. K., Platanias, L. C., Uddin, S. & Wickrema, A. (2002) Oncogene 21, 15561562. [DOI] [PubMed] [Google Scholar]
- 23.Nagata, Y., Takahoshi, N., Davis, R. J. & Todokoro, K. (1998) Blood 92, 1859–1869. [PubMed] [Google Scholar]
- 24.Nagata, Y. & Todokoro, K. (1999) Blood 94, 853–863. [PubMed] [Google Scholar]
- 25.Jacobs-Helber, S. M., Ryan, J. J. & Sawyer, S. T. (2000) Blood 96, 933–940. [PubMed] [Google Scholar]
- 26.Somervaille, T. C. P., Linch, D. C. & Khwaja, A. (2003) Br. J. Haemotol. 120, 876–886. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, J. F., Wickrema, A., Potapova, O., Milanick, M. & Yingst, D. R. (2002) Proc. Natl. Acad. Sci. USA 99, 14572–14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddin, S., Majchrzak, B., Woodson, J., Arunkumar, P., Alsayed, Y., Pine, R., Young, P. R., Fish, E. N. & Platanias, L. C. (1999) J. Biol. Chem. 274, 30127–30131. [DOI] [PubMed] [Google Scholar]
- 29.Uddin, S., Fish, E. N., Sherr, D. & Platanias, L. C. (1997) Blood 90, 2574–2582. [PubMed] [Google Scholar]
- 30.Kummer, J. L., Rao, P. K. & Heidenreich, K. A. (1997) J. Biol. Chem. 272, 20490–20494. [DOI] [PubMed] [Google Scholar]
- 31.Xia, Z., Dickems, M., Raingeaud, J., Davis, R. J. & Greenberg, M. E. (1995) Science 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- 32.Maher, P. (1999) J. Biol. Chem. 274, 17491–17498. [DOI] [PubMed] [Google Scholar]
- 33.Rausch, O. & Marshall, C. J. (1999) J. Biol. Chem. 274, 4096–4105. [DOI] [PubMed] [Google Scholar]
- 34.Mackoy, K. & Mochlly-Rosen, D. (1999) J. Biol. Chem. 274, 6272–6279. [DOI] [PubMed] [Google Scholar]
- 35.Nemato, S., Sheng, Z. & Lin, A. (1998) Mol. Cell. Biol. 18, 3518–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao, Z., Boni, A., Xia, F., Nadel-Vicens, M. & Grrenberg, M. E. (1999) Science 286, 785–790. [DOI] [PubMed] [Google Scholar]
- 37.Wang, X. Z. & Ron, D. (1996) Science 272, 1347–1349. [DOI] [PubMed] [Google Scholar]
- 38.Ouenda, A. & Cohen, P. (1999) J. Biol. Chem. 274, 4341–4346. [DOI] [PubMed] [Google Scholar]
- 39.Zeter, A., Grdinger, E. & Bengal, E. (1999) J. Biol. Chem. 274, 5193–5200. [DOI] [PubMed] [Google Scholar]
- 40.Vachon, P. H., Harnois, C., Greier, A., Dufour, G., Bouchard, V., Han, J., Landry, J., Beaulieu, J. F., Vezina, A., Dydenborg, A. B., et al. (2002) Gastroenterology 123, 1980–1991. [DOI] [PubMed] [Google Scholar]
- 41.Lavoie, J. N., Gingras-Breton, G., Tanguay, R. M. & Nandry, J. (1993) J. Biol. Chem. 268, 3420–3429. [PubMed] [Google Scholar]
- 42.Wickrema, A., Koury, S. T., Dai, C.-H. & Krantz, S. B. (1994) J. Cell Physiol. 160, 417–426. [DOI] [PubMed] [Google Scholar]
- 43.Garcia, J., Lemercier, B., Roman-Roman, S. & Rawadi, G. (1998) J. Biol. Chem. 273, 34391–34398. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs-Helber, S. M., Roh, K. H., Bailey, D., Dessypris, E. N., Ryan, J. J., Chen, J., Wickrema, A., Barber, D. L., Dent, P. & Sawyer, S. T. (2003) Blood 101, 524–531. [DOI] [PubMed] [Google Scholar]
- 45.Niwa, M., Hotta, K., Kanamori, Y., Hatakeyama, D., Hirade, K., Katayama, M., Hara, A., Hideki, M., Ito, H., Kato, K., et al. (2003) Eur. J. Pharmacol. 466, 245–253. [DOI] [PubMed] [Google Scholar]
- 46.Akel, S., Petrow-Sadowski, C., Laughlin, M. J. & Ruscetti, F. W. (2003) Stem Cells 21, 557–567. [DOI] [PubMed] [Google Scholar]