Abstract
Bcl2 functions to suppress apoptosis and retard cell cycle entry. Single-site phosphorylation at serine 70 (S70) is required for Bcl2's antiapoptotic function, and multisite phosphorylation at threonine 69 (T69), S70, and S87 has been reported to inactivate Bcl2. To address this apparent conflict and identify the regulatory role for Bcl2 phosphorylation in cell death and cell cycle control, a series of serine/threonine (S/T) → glutamate/alanine (E/A) mutants including T69E/A, S70E/A, S87E/A, T69E/S70A/S87A (EAA), T69A/S70E/S87A (AEA), T69A/S70A/S87E (AAE), T69E/S70E/S87E (EEE), and T69A/S70A/S87A (AAA) was created to mimic or abrogate, respectively, either single-site or multisite phosphorylation. The survival and cell cycle status of cells expressing the phosphomimetic or nonphosphorylatable Bcl2 mutants were compared. Surprisingly, all of the E but not the A Bcl2 mutants potently enhance cell survival after stress and retard G1/S cell cycle transition. The EEE Bcl2 mutant is the most potent, indicating a possible cumulative advantage for multisite phosphorylation of Bcl2 in survival and retardation of G1/S transition functions. Because the E-containing Bcl2 mutants, but not the A-containing mutants, can more potently block cytochrome c release from mitochondria during apoptotic stress, even at times when steady-state expression levels are similar for all mutants, we conclude that phosphorylation at one or multiple sites within the flexible loop domain of Bcl2 not only stimulates antiapoptotic activity but also can regulate cell cycle entry.
The Bcl-2 family proteins consist of both anti- and proapoptotic members and regulate cell death by controlling mitochondrial membrane permeability. Dysregulation of mitochondrial integrity is crucial for facilitating activation of the caspase cascade that leads to the morphologic consequences of apoptosis (1). Bcl2 functions as the prototypic antagonist of caspase activation by blocking Bax or Bak-induced oligomerization, which leads to loss of mitochondrial membrane integrity with cytosolic leakage of caspase activators including cytochrome c (2–5). Previous reports indicate that the endogenous Bcl2 expressed in various cells can be phosphorylated and that phosphorylation of Bcl2 is closely associated with regulation of apoptosis (6, 7). We and others (8–10) recently discovered that growth factors like IL-3- or erythropoietin-induced phosphorylation of Bcl2 at S70 can positively regulate Bcl2's antiapoptotic function. However, it has also been reported that Bcl2 can be phosphorylated at multiple sites in the flexible loop domain (FLD), including T69, S70, and S87, in association with inhibition of microtubule dynamics (11). A role for multisite Bcl2 phosphorylation at T69, S70, and S87 induced by paclitaxel or other microtubule-damaging agents has been proposed to inactivate Bcl2 specifically at the G2/M phase and facilitate apoptotic cell death from this phase of the cycle (11). Because deletion of the FLD that contains these phosphorylation sites can enhance Bcl2's survival function, it was also suggested that the FLD may negatively regulate Bcl2's antiapoptotic function (12). Therefore, phosphorylation may regulate Bcl2's function by potentially attenuating or enhancing the negative regulatory properties conferred by this domain. However, because WT Bcl2 expression blocks and/or delays paclitaxel-induced apoptosis (13), an alternative explanation is that multisite phosphorylation may also function to enhance survival function but is not sufficient to prevent cell death upon continuous exposure to such toxins.
Mounting evidence indicates that the cell cycle and apoptosis are inextricably linked (14, 15). For example, expression of the oncogene c-myc can initiate proliferation and increase sensitivity to apoptosis under low serum conditions when antiapoptotic mechanisms are not activated (16). By contrast, Bcl2 was reported to suppress both apoptosis and cell cycle entry by a mechanism that is not yet clear but is thought to result from two distinct and genetically separable functions of Bcl2 (14, 17). Surprisingly, results reported here genetically link Bcl2's antiapoptotic function and retardation of cell cycle entry function through phosphorylation.
Materials and Methods
Materials. Bcl2 and cytochrome c antibodies were purchased from Santa Cruz Biotechnology. Paclitaxel was purchased from Bristol-Myers Squibb.
Plasmids, cDNA, Cell Lines, and Stable Transfections. Murine Bcl2 cDNA was cloned in pUC19 plasmid. Nucleotides corresponding to each serine (S) or threonine (T) residue were substituted to create a conservative alteration to alanine (A) or glutamic acid (E) with a site-directed mutagenesis kit (Clontech). Each single mutant was confirmed by sequencing the cDNA and then cloned into the pCIneo mammalian expression vector (Promega). The pCIneo plasmid containing each Bcl2 mutant cDNA was transfected into murine IL-3-dependent NSF/N1.H7 and lung cancer H157 cells by electroporation (10). Clones stably expressing WT or mutant Bcl2 were selected in medium containing G418 (0.6 mg/ml).
Metabolic Labeling and Pulse–Chase Analysis of WT and Mutant Bcl2 Proteins. Cells were metabolically labeled with [35S]methionine for 60 min. The [35S]methionine-labeled cells were washed and incubated in fresh methionine-replete RPMI medium 1640 for various time points up to 48 h. 35S-labeled Bcl2 was immunoprecipitated by using Bcl2 antibody. The samples were subjected to SDS/10–20% PAGE. The t1/2 of Bcl2 was determined by electronic autoradiography.
Assay of Bcl2 Degradation in Vitro. WT Bcl2 and A- or E-mutant Bcl2 were immunoprecipitated from lysates of cells expressing WT or various A- or E-Bcl2 mutants. The natural multisite phosphorylated Bcl2 was immunoprecipitated from lysates of paclitaxel-treated WT Bcl2-expressing cells. These products were then incubated with an apoptotic extract in assay buffer (20 mM Pipes/100 mM NaCl/10 mM DTT/1 mM EDTA/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/10% sucrose, pH 7.2) at 37°C for 2 h. The apoptotic extract used was generated from staurosporine-treated NSF/N1 H7 cells undergoing apoptosis in which the proteases were activated as described (18). Bcl2 was analyzed by Western blotting with a Bcl2 antibody.
Subcellular Fractionation and Cytochrome c Release. Cells were washed with cold PBS, suspended in HIM buffer (0.2% BSA/200 mM mannitol/70 mM sucrose/10 mM Hepes-KOH/1 mM EGTA, pH 7.5), and then homogenized with a Polytron homogenizer (Kinematica, Lucerne, Switzerland) operating for six bursts of 10 seconds each at a setting of 5. The cell lysate was then centrifuged at 200 × g to pellet nuclei. The supernatant was centrifuged at 150,000 × g to pellet heavy and light membranes. The resulting supernatant contains the cytosolic fractions. Cytosolic protein was subjected to SDS/PAGE and analyzed by Western blotting by using a cytochrome c antibody.
Cell Viability Assay. The apoptotic and viable cells were detected by using the ApoAlert Annexin V kit (Clontech) according to the manufacturer's instructions. The percentage of annexin Vlow cells (percentage of viable cells) or annexin Vhigh cells (percentage of apoptotic cells) was determined by using the data obtained by fluoresence-activated cell sorting analysis as described (19). In addition, an APO-BRDU Kit (Phoenix Flow Systems, San Diego) was used to determine apoptotic cells according to the manufacturer's instructions. The APO-BRDU kit is a two-color terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay to analyze apoptotic cells in various phases of the cell cycle by flow cytometry (20).
Transmission Electron Microscopy. Cells were fixed in 2% glutaraldehyde buffer and postfixed in 1% buffered osmium tetroxide. After dehydration, cells were embedded in Epon 812. Ultrathin sections were stained with 2% uranyl acetate and lead citrate and observed under transmission electron microscopy as described (21).
Results
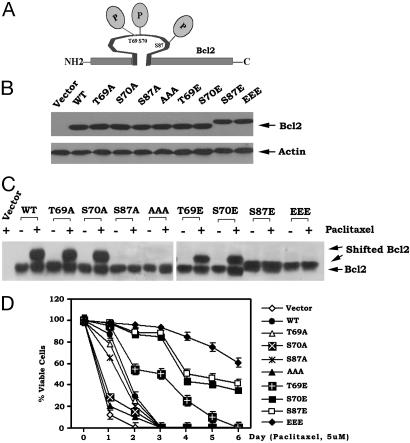
Mono- and Multisite Phosphorylation of Bcl2 in the FLD Inhibits Paclitaxel-Induced Apoptosis. To directly test and compare the function and potency of mono- and multisite phosphorylation, individual E(T69E, S70E, S87E) and EEE (T69E/S70E/S87E) or A(T69A, S70A, S87A) and AAA (T69A/S70A/S87A) Bcl2 mutants were created to mimic or abrogate mono- or multisite phosphorylation. Mutant Bcl2 was stably transfected in IL-3-dependent NSF/N1.H7 cells, and clones expressing quantitatively similar levels of Bcl2 were selected and tested. Western blot analysis indicates that introduction of an E at the S87 site produces an altered mobility pattern of Bcl2 that is observed in growing cells after denaturing electrophoresis because only the single S87E or the multiple EEE mutant, but not the S70E and T69E mutants, demonstrates this mobility pattern (Fig. 1B). These results indicate that the charge conferred by phosphorylation at S87 is required to induce the conformational change responsible for Bcl2's altered mobility pattern under denaturing conditions (Fig. 1 B and C).
Fig. 1.
Multisite phosphorylation of Bcl2 at T69, S70, and S87 in the FLD maximally inhibits paclitaxel-induced apoptosis. (A) Schematic representation of phosphorylation sites in the Bcl2 FLD. (B) WT and various A- or E-Bcl2 mutant cDNAs were stably transfected in NSF.N1/H7 cells. Bcl2 protein expression levels were determined by Western blotting by using a Bcl2 antibody. (C) Cells expressing WT, A-, or E-Bcl2 mutants were treated with 5 μM paclitaxel for 24 h. Bcl2 was analyzed by Western blotting as above. (D) Cells expressing WT or various A- or E-Bcl2 mutants were treated with 5 μM paclitaxel for various time points as indicated. Cell viability was determined by analyzing annexin V binding on fluoresence-activated cell sorting as described (19). Data represent the mean ± SD of three determinations.
To test the functional significance of mono- or multisite Bcl2 phosphorylation on cell survival, cells expressing the E- or A-Bcl2 mutants were initially treated with 5 μM paclitaxel for various times as indicated. Cell viability was determined (19). All phosphomimetic Bcl2 mutants containing one or more E-substitution (i.e., at T69, S70, or S87) demonstrate significantly prolonged survival whereas the nonphosphorylatable A-containing mutants have much reduced antiapoptotic activity when compared with WT Bcl2 (Fig. 1D). The E-substitutions at either S70 or S87 are more potent than that at T69, whereas the EEE mutant is more potent than any single-site E-mutant. This indicates a cumulative survival advantage for multisite phosphorylation (Fig. 1D). Because the E-Bcl2 mutants can more potently inhibit paclitaxel-induced cell death compared with A-mutants (Fig. 1D), this suggests a mechanism by which either mono- or multisite phosphorylation may be sufficient to activate Bcl2's antiapoptotic function and prolong cell survival after stress. Based on these findings, we can conclude that the charge conferred by multisite phosphorylation of Bcl2 in the FLD produces maximal antiapoptotic activity and does not inactivate Bcl2. Enhancement of Bcl2's survival function by multisite phosphorylation potentially represents a stress-acquired intracellular defense mechanism that can be regulated by various stress-activated Bcl2 kinases. This may explain why Bcl2 is the target of multiple such protein kinases, including c-Jun N-terminal kinase 1, protein kinase A, PKC, and mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 (7, 8, 19, 22, 23).
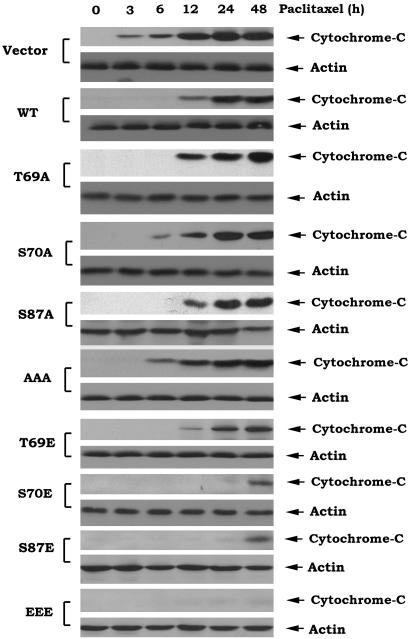
Bcl2 Gain-of-Function Glutamate (E) but Not Phosphorylation-Deficient Alanine (A) Mutants Block Paclitaxel-Induced Cytochrome c Release and Cell Death. The current model for initiating and amplifying the requisite caspase activation cascade that leads to apoptosis holds that Bcl2's antiapoptotic function, at least in part, results in preserving mitochondrial integrity by preventing Bax- or Bak-induced release of cytochrome c into the cytosol (4, 5). To evaluate a functional requirement for a charge conferred by mono- or multisite phosphorylation of Bcl2, the phosphomimetic and nonphosphorylatable Bcl2 mutants were compared for their effect on cytochrome c release from mitochondria after treatment with 5 μM paclitaxel. Time course studies reveal that cytochrome c is released early (i.e., by 3–6 h) from cells expressing the nonphosphorylatable A-Bcl2 mutants or the vector-only control cells but is substantially delayed (i.e., 12 h) in cells expressing WT Bcl2 (Fig. 2). By contrast, cytochrome c release is significantly more delayed in cells expressing the E-Bcl2 mutants (24–48 h; Fig. 2). Because expression levels of the WT and Bcl2 mutants are relatively similar until 24 h after treatment with paclitaxel (Fig. 1C), this indicates that the mono- or multisite phosphorylation may directly activate Bcl2's antiapoptotic function such that reduced levels of Bcl2 are not likely to explain the functional differences observed, at least at early times after stress (Figs. 1 C and D and 2).
Fig. 2.
Bcl2 gain of function glutamate (E) but not phosphorylation deficient alanine (A) mutants block paclitaxel-induced cytochrome c release. Cells expressing WT or A- or E-Bcl2 mutants were treated with 5 μM paclitaxel for various times as indicated. Cytochrome c release was assessed by using subcellular fractionation as described in Materials and Methods.
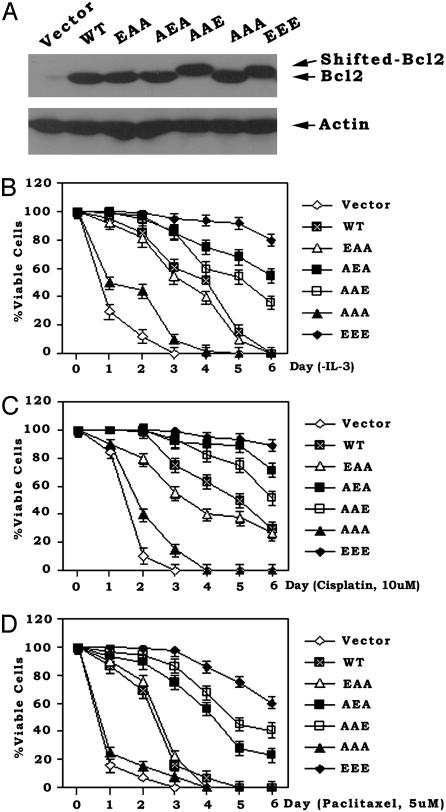
In a further attempt to flesh out the functional contribution of individual phosphorylation sites in the FLD, we tested compound Bcl2 mutants including T69E/S70A/S87A (EAA), T69A/S70E/S87A (AEA), and T69A/S70A/S87E (AAE). H7 cells expressing equivalent amounts of the EAA, AEA, AAE, AAA, or EEE Bcl2 mutants were compared (Fig. 3A). Viability was assessed after either IL-3 withdrawal or treatment of the cells with the clinically useful chemotherapeutic agents paclitaxel and cisplatin. The EEE mutant was found to be the most potent for prolonging cell survival, indicating a maximal antiapoptotic role for multisite Bcl2 phosphorylation. The AEA and AAE Bcl2 phosphomimetic mutants were found to have more antiapoptotic activity than the EAA mutant, suggesting that phosphorylation at S70 or S87 is more important than at T69, at least in supporting survival (Fig. 3). Interestingly, cells expressing the AEA Bcl2 mutant display more antiapoptotic activity than cells expressing the AAE mutant after either IL-3 withdrawal (a physiologic apoptotic stress) or cisplatin treatment, with the ordering of survival potency as follows: EEE→AEA→AAE→EAA (Fig. 3 B and C). These data indicate the importance of the S70 site under these conditions. However, the AAE Bcl2 mutant appears to be slightly more protective than the phosphomimetic AEA or EAA mutants when cells were treated with the microtubule-damaging agent paclitaxel (Fig. 3D). Because single-site phosphorylation at S87 apparently does not occur and S87 phosphorylation has only been reported to occur in the context of multisite Bcl2 phosphorylation after treatment with paclitaxel or related agents (11), we are not sure of the physiologic relevance of S87 single-site phosphorylation. Therefore, because growth factors like IL-3 or erythropoietin can stimulate Bcl2 phosphorylation exclusively at the S70 site under physiologic growth conditions (7, 10), we have concluded that S70 is the major physiologic phosphorylation site for Bcl2's survival function. However, if S87 could be individually phosphorylated under some conditions, results with the phosphomimetic AAE Bcl2 mutant indicate that it would be sufficient to activate Bcl2's antiapoptotic function (Fig. 3). Thus, single-site phosphorylation at S87 may be sufficient but is not required for Bcl2's antiapoptotic function.
Fig. 3.
Mono- or multisite phosphorylation of Bcl2 potently prolongs cell survival after IL-3 withdrawal or treatment with chemotherapeutic agents. (A) WT, EAA, AEA, AAE, AAA, or EEE Bcl2 mutants were stably transfected in NSF.N1/H7 cells. Expression levels of Bcl2 were determined by Western blotting by using Bcl2 antibody. (B–D) Cells expressing WT or various Bcl2 mutants were deprived of IL-3 or treated with cisplatin or paclitaxel for various times as indicated. Cell viability was determined as described for Fig. 1D. Data represent the mean ± SD of three determinations.
To rule out a possible cell type or species-specific effect for E- or A-Bcl2 mutants, similar studies were performed with human H157 epithelial lung cancer cells. As for H7 cells, expression of the multisite phosphomimetic EEE Bcl2 mutant in H157 cells confers the most potent survival phenotype (data not shown).
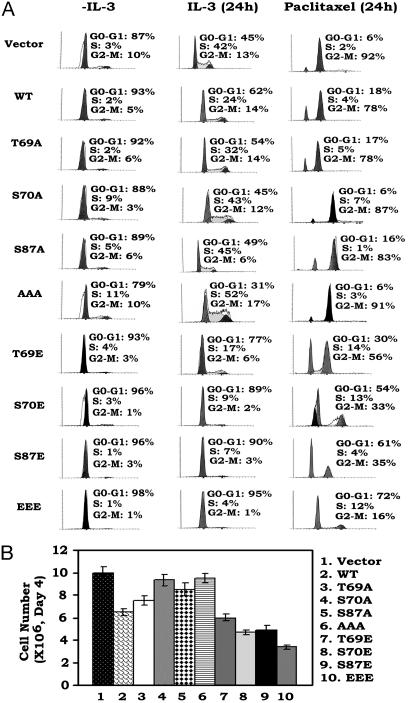
Mono- or Multisite Bcl2 Phosphorylation Retards Cell Cycle Progression After IL-3 Addition or Treatment with a Chemotherapeutic Agent. It was reported that WT Bcl2 not only inhibits apoptosis but also restrains cell cycle entry (17, 24). To test the effect of Bcl2 phosphorylation on cell cycle progression, cells expressing WT or A- or E-Bcl2 mutants were accumulated in G0/G1 by IL-3 withdrawal for 24 h (Fig. 4A, column 1). Cells were then monitored for reentry into the S phase after addition of IL-3 or treatment with 5 μM paclitaxel in the presence of IL-3. Results indicate that cells expressing WT Bcl2 reenter the cell cycle after IL-3 addition with ≈24% of cells in S phase and that paclitaxel arrests the majority of cells (i.e., 78%) in the G2/M phase by 24 h (Fig. 4A). Cells expressing the nonphosphorylatable A-Bcl2 mutants display a slightly higher percentage of cells in S phase (32–52%) and are not significantly more sensitive to paclitaxel-induced G2/Marrest(Fig. 4A). By contrast, expression of the single-E or EEE mutant Bcl2 potently retards but does not prevent cell cycle progression with 4–17% of cells in S phase. However, although paclitaxel treatment can arrest these cells in G2/Mby24h(16–56%), the slower cycling status of these cells results in significantly delayed G2/M arrest (Fig. 4). Cell counts show that survival-hearty cells expressing the E-containing Bcl2 mutants grow more slowly than cells expressing either WT or the A-containing Bcl2 mutants (Fig. 4B). These findings indicate that mono- or multisite phosphorylation of Bcl2 not only positively regulates cell survival but also affects cell proliferation by retarding G1/S transition.
Fig. 4.
Mono- or multisite Bcl2 phosphorylation retards cell cycle progression after IL-3 addition or treatment with paclitaxel. (A) NSF.N1/H7 cells expressing WT or various A- or E-Bcl2 mutants were deprived of IL-3 for 24 h. Cells were monitored for reentry into the S phase after addition of IL-3 or treatment with 5 μM paclitaxel in the presence of IL-3. Cell cycle status was analyzed by flow cytometry after propidium iodide staining. (B) The same number (i.e., 1 × 106) of cells expressing WT, A- or E-mutant Bcl2 were initially plated in individual cell culture flasks. Cell growth was assessed by using a Coulter counter at day 4.
Mono- and Multisite Bcl2 Phosphorylation Prolongs Cell Survival in the G2/M Phase. Because clinically useful chemotherapeutic drugs generally target dividing cells, malignant cells expressing phosphorylatable Bcl2 may conceivably acquire chemoresistance by means of this mechanism. For example, paclitaxel arrests cells in the G2/M phase, where cells may be more sensitive to apoptosis (11, 25). To determine whether this posttranslational modification can affect Bcl2's survival function in the G2/M phase of the cell cycle, an APO-BRDU two-color assay (anti-BrdUrd FITC and propidium iodide) was used to label DNA strand breaks that result from apoptosis and total cellular DNA, respectively (20). After treatment with 5 μM paclitaxel for 48 h, 84–92% of cells expressing WT or A-Bcl2 mutants but only 43–82% of cells expressing the E-containing mutants were arrested in the G2/M phase (Fig. 5A). For EEE Bcl2-expressing cells, the percentage of cells in G2/M at 48 h represents a 2.6-fold increase compared with the 24-h time point (i.e., 43% vs. 16%; compare Figs. 4 and 5A). This finding further indicates that phosphorylated Bcl2 can retard but does not prevent paclitaxel-induced G2/M arrest. More importantly, however, the cells expressing the A-containing Bcl2 mutants display more apoptotic cells in the G2/M phase compared with cells expressing the single E- or EEE mutant Bcl2 (i.e., 63.6–83% vs. 2.9–43%; Fig. 5B). Cells expressing WT or A- or E-Bcl2 under normal culture conditions display similar morphologies with a single nucleus compared with the vector control cells (Fig. 5C and data not shown). After paclitaxel treatment for 48 h, most cells expressing WT or A-containing Bcl2 mutants display the characteristic morphological changes associated with apoptosis including cytoplasmic organelle disruption and increased chromatin condensation with multiple broken nuclei (Fig. 5C). In contrast, the majority of the single E- or EEE-Bcl2-expressing cells examined exhibit normal-appearing cytoplasmic structures and multiple nuclei consistent with G2/M phase arrest, but without chromatin condensation. These data indicate that these cells, while arrested in G2/M phase, remain viable (Fig. 5 B and C). Therefore, both mono- and multisite phosphorylation of Bcl2 can more potently prolong cell survival in all phases of the cell cycle.
Fig. 5.
Mono- or multisite Bcl2 phosphorylation prolongs cell survival in G2/M phase. (A) Cells expressing WT or various A- or E-Bcl2 mutants were treated with 5 μM paclitaxel for 48 h. The percentage of cells in G2/M phase was determined by flow cytometry after propidium iodide staining. (B) Cells were treated as in A, and a two-color terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay using a APO-BRDU kit was used to assess apoptotic cells in G2/M phase. The percentage of apoptotic cells in G2/M was analyzed by flow cytometry. (C) Cells expressing WT or various Bcl2 mutants were treated with 5 μM paclitaxel for 48 h, then observed under transmission electron microscopy. (Scale bar, 2 μm.)
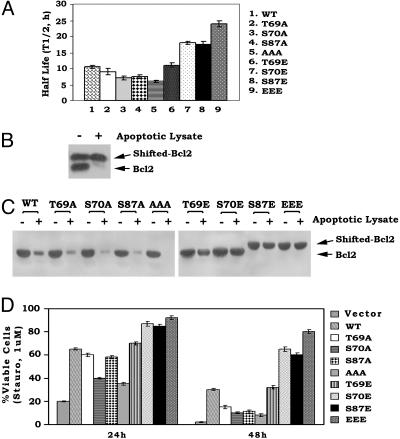
Mono- or Multisite Phosphorylation Enhances Bcl2's Stability to Proteolysis in Vitro and in Vivo. Bcl2 has been reported to be proteolytically cleaved and degraded during apoptosis, and inhibition of degradation was found to prolong Bcl2's survival function (26, 27). To test whether Bcl2 phosphorylation at T69, S70, and S87 may affect Bcl2's stability, t1/2 studies were performed in cells expressing WT or A- or E-containing Bcl2 mutants with the classical [35S]methionine pulse–chase methods. The t1/2 of E-containing Bcl2 mutants was found to be significantly longer (i.e., 12–24 h) than that for either WT (i.e., 10.5 h) or A-Bcl2 mutants (i.e., 6–9h; Fig.6A). These results indicate that the charge conferred by phosphorylation enhances Bcl2's stability to turnover. To directly assess whether mono- or multisite phosphorylation affects Bcl2's proteolysis in vitro, Bcl2 was immunoprecipitated from lysates of cells expressing WT Bcl2 after treatment with paclitaxel to induce multisite Bcl2 phosphorylation and the recognizable mobility shift. Because the potent protein kinase inhibitor staurosporine can induce apoptosis (7, 24), an apoptotic extract containing protease activity capable of degrading Bcl2 was obtained from staurosporine-treated cells undergoing apoptosis (18). The extract was used to incubate with immunoprecipitated, mobility-shifted Bcl2 or the various phosphomimetic Bcl2 proteins. Results indicate that the paclitaxel-induced, multisite phosphorylated WT Bcl2 (Fig. 6B, upper band) is more stable to degradation than the nonphosphorylated Bcl2 (Fig. 6B, lower band). Furthermore, the non-mobility-shifted WT and the nonphosphorylatable A-containing Bcl2 mutants are rapidly degraded by this apoptotic lysate, whereas the E-containing Bcl2 mutants remain relatively more resistant (Fig. 6C). These findings reveal that the WT and A-Bcl2 mutants are significantly more sensitive to degradation by an apoptotic extract derived from cells undergoing apoptosis. Furthermore, the phosphomimetic Bcl2 mutants are less susceptible to proteolysis but are superior to either WT or the A-containing Bcl2 mutants in prolonging cell survival after treatment with staurosporine (Fig. 6D). Because S70 is the site of physiologic Bcl2 phosphorylation during cell growth, whereas the T69 and S87 sites are apparently only phosphorylated simultaneously along with S70 after treatment with microtubule disrupting agents (11), we propose that placing a charge equivalent to a phosphate at one or more of the phosphorylation sites in the FLD will, in addition to directly activating Bcl2's ability to delay cytochrome c release from mitochondria (Fig. 2), also protect Bcl2 from proteolytic degradation. Because staurosporine might be expected to inhibit phosphorylation of WT Bcl2, our results show that WT Bcl2 still has more survival activity than any of the A-Bcl2 mutants in the presence of staurosporine (Fig. 6D). Cells expressing either T69A or S87A Bcl2 have increased viability compared with cells expressing S70A, at least at 24 h, because the S70 site remains intact (Fig. 6D). This finding can explain our previous findings that high concentrations of staurosporine (i.e., 0.1–1 μM) only incompletely inhibit Bcl2 phosphorylation because of the existence of a staurosporine-resistant Bcl2 kinase, extracellular signal-regulated kinase 1/2, that can also phosphorylate Bcl2 at S70 (8).
Fig. 6.
Multisite phosphorylation of Bcl2 induced by either paclitaxel or substitution of glutamate can enhance Bcl2's stability and resistance to proteolysis. (A) Cells expressing WT or various A- or E-Bcl2 mutants were metabolically labeled with [35S]methionine. The t1/2 of WT and mutant Bcl2 was determined by classic pulse–chase analysis. (B and C) Mobility-shifted Bcl2 was immunoprecipitated from lysates of cells expressing WT Bcl2 after treatment with paclitaxel to induce multisite Bcl2 phosphorylation. A- and E-containing Bcl2 mutants were immunoprecipitated from lysates of untreated cells expressing A- or E-mutants. These Bcl2 immune complexes were then incubated with an apoptotic extract for 2 h, and Bcl2 was analyzed by Western blotting by using Bcl2 antibody. (D) NSF.N1/H7 cells expressing equivalent amounts of WT or A- or E-Bcl2 mutants were treated with 1 μM staurosporine for 24 and 48 h. Cell viability was assessed as described for Fig. 1D. Data represent the mean ± SD of three independent determinations.
Discussion
We previously reported that the hematopoietic growth factors IL-3 and erythropoietin induce Bcl2 phosphorylation exclusively at S70 and that S70 phosphorylation may be required for Bcl2's potent survival function (7, 8, 10). Subsequently, other studies have reported that inhibition of microtubule dynamics with paclitaxel and related agents can induce Bcl2 phosphorylation simultaneously at multiple sites including T69, S70, and S87 in association with cell death. From these results, it was also proposed that multisite phosphorylation can inactivate Bcl2 (6, 11). However, there is no report that phosphorylation of Bcl2 can occur at the single T69 or S87 site. Thus, Bcl2 may be phosphorylated either at the S70 site alone or at all three sites simultaneously, depending on the type of intracellular signal(s) generated (i.e., growth vs. microtubule damage). We chose to abrogate Bcl2 phosphorylation by introducing a conserved, nonphosphorylated alanine (A) at the known serine or threonine phosphorylation sites. Similar mutants carrying a glutamate (E) at these same sites were prepared to functionally mimic the charge conferred by the phosphate (i.e., phosphomimetic). We believe that this comparative approach can provide a definitive answer to explain how, and at which site(s), phosphorylation in the FLD affects Bcl2's antiapoptotic and cell cycle regulatory functions. Results clearly indicate that both monosite (S70E, T69E, S87E, EAA, AEA, or AAE) and multisite (EEE) phosphomimetic mutations can activate Bcl2's antiapoptotic function. Importantly, the EEE Bcl2 mutant was found to display the most potent antiapoptotic effect, whereas the AAA-Bcl2 mutant had minimal residual antiapoptotic activity (Figs. 1 and 3). Results from these comparable studies strongly support a role for mono- and multisite phosphorylation in positively regulating Bcl2's survival function and are difficult to reconcile with the conclusion that multisite phosphorylation of Bcl2 can negatively regulate its function (11). Although the reason for the discrepancy is not completely clear, it is possible (but not likely) that it results from a species- or cell-type-specific effect particularly because both murine myeloid and human lung cancer cells behave similarly to apoptosis-inducing stress when expressing the mutant Bcl2 transgenes. Still, we chose not to test these Bcl2 mutants in human Jurkat T or murine WEHI-231 B cells (11) because these cells already express relatively high levels of endogenous Bcl2 and Bcl-XL, which can potentially complicate the interpretation of results. We conclude that phosphorylation of Bcl2 in the FLD positively regulates its antiapoptotic function.
Mechanistically, Bcl2 phosphorylation closely links its potent survival function with the ability to prevent cytochrome c release from mitochondria and resistance to proteolytic degradation. That is, cells expressing phosphomimetic Bcl2 treated with inducers of apoptosis represent delayed cytochrome c release from mitochondria and more potent antiapoptotic activities. Bcl2's stability to proteolytic degradation was also enhanced by mono- or multisite phosphorylation. Prolonged stability could help to increase survival after treatment of cells with staurosporine (Fig. 6). These findings support recent reports that dephosphorylation of Bcl2 can promote its degradation by facilitating ubiquitination (26, 27) and reveal a mechanism by which the A-containing Bcl2 mutants are more sensitive to degradation (Fig. 6).
In addition to its well established role in suppressing apoptosis, Bcl2 also retards cell cycle entry (14, 17). Results here establish that these properties may be genetically linked and regulated through phosphorylation of Bcl2. We suggest that this linkage may provide a cooperative function for Bcl2 in managing cell stress because cells can generally withstand the effects of an apoptotic stress better in G1/G0 than during DNA replication and/or cell mitotic division (14, 25). Results indicate that mono- and multisite phosphomimetic Bcl2 mutants are more potent than WT or A-mutants in retarding G1/S transition (Fig. 4), which closely correlates with their enhanced survival function. Thus, Bcl2 phosphorylation appears to tightly link its antiapoptotic and cell cycle retardation functions. Tyrosine phosphorylation of Bcl2 at Y28 was initially considered as a possible phosphorylation target that might regulate Bcl2's cell cycle retardation properties (24). However, because Bcl2 is not phosphorylated at tyrosine but only on serine or threonine (7, 10, 11), and because we find that phosphorylation of Bcl2 at one or more of such sites in the FLD can retard G1/S transition, we propose that phosphorylation at S70, S87, and T69 can regulate both cell cycle entry and apoptosis.
Collectively, the findings reported here help to resolve a controversy surrounding the role of phosphorylation in regulating Bcl2's survival function by demonstrating genetically that mono- or multisite phosphorylation can enhance, but not inhibit, Bcl2's survival activity. In addition, phosphorylation of Bcl2 may directly link its survival and cell cycle retardation properties in a mechanism that is not yet clear. Thus, either physiologic growth factor-induced or stress-activated Bcl2 phosphorylation may play an activating role in prolonging cell survival and regulating the cell cycle. The functional linkage of these two properties of Bcl2 may be relevant for oncogenesis and drug resistance mechanisms.
Acknowledgments
This work was supported by National Institutes of Health Grant CA44649-11.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: FLD, flexible loop domain.
References
- 1.Shimizu, S., Ide, T., Yanagida, T. & Tsujimoto, Y. (2000) J. Biol. Chem. 275, 12321–12325. [DOI] [PubMed] [Google Scholar]
- 2.Ranger, A. M., Malynn, B. A. & Korsmeyer, S. J. (2001) Nat. Genet. 28, 113–118. [DOI] [PubMed] [Google Scholar]
- 3.Mikhailov, V., Mikhailova, M., Pulkrabek, D. J., Dong, Z., Venkatachalam, M. A. & Saikumar, P. (2001) J. Biol. Chem. 276, 18361–18374. [DOI] [PubMed] [Google Scholar]
- 4.Wei, M., Zong, W-X., Cheng, E. H., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K., MacGregor, G., Thompson, C. & Korsmeyer, S. (2001) Science 292, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang, J., Liu, X., Bhalla, K., Kim, C. N., Ibrado, A. M., Cai, J., Peng, T., Jones, D. & Wang, X. (1997) Science 274, 1129–1132. [DOI] [PubMed] [Google Scholar]
- 6.Haldar, S., Jena, N. & Croce, C. M. (1995) Proc. Natl. Acad. Sci. USA 92, 4507–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May, W. S., Tyler, P. G., Ito, T., Armstrong, D. K., Qatsha, K. A. & Davidson, N. E. (1994) J. Biol. Chem. 269, 26865–26870. [PubMed] [Google Scholar]
- 8.Deng, X., Ruvolo, P., Carr, B. K. & May, W. S. (2000) Proc. Natl. Acad. Sci. USA 97, 1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiuchi, M., Hayashida, W., Kambe, T., Yamada, T. & Dzau, V. J. (1997) J. Biol. Chem. 272, 19022–19026. [DOI] [PubMed] [Google Scholar]
- 10.Ito, T., Deng, X., Carr, B. & May, W. S. (1997) J. Biol. Chem. 272, 11671–11673. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto, K., Ichijo, H. & Korsmeyer, S. (1999) Mol. Cell. Biol. 19, 8469–8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, B. S., Minn, A. J., Muchmore, S. W., Fesik, S. W. & Thompson, C. B. (1997) EMBO J. 16, 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, S., Widmann, C. & Johnson, G. L. (1999) J. Biol. Chem. 274, 10916–10922. [DOI] [PubMed] [Google Scholar]
- 14.Mazel, S., Burtrum, D. & Petrie, H. (1996) J. Exp. Med. 183, 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin, L., Phipott, K. L. & Brooks, S. F. (1993) Curr. Biol. 3, 391–394. [DOI] [PubMed] [Google Scholar]
- 16.Evan, G. I., Wyllie, A. H., Gilbert, C. S., Littlewood, T. D., Land, H., Brooks, M., Waters, C. M., Penn, L. Z. & Hancock, D. C. (1992) Cell 69, 119–128. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly, L. A., Huang, D. & Strasser, A. (1996) EMBO. J. 15, 6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, X., Zelenski, N. G., Yang, J., Sakai, J., Brown, Michael, M. S. & Golgstein, J. L. (1996) EMBO J. 15, 1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 19.Deng, X., Xiao, L., Lang, W., Gao, F., Ruvolo, P. & May, W. S. (2001) J. Biol. Chem. 276, 23681–23688. [DOI] [PubMed] [Google Scholar]
- 20.Li, X. & Darzynkiewicz, Z. (1995) Cell Prolif. 28, 572–579. [DOI] [PubMed] [Google Scholar]
- 21.Tzung, S., Kim, K., Basanez, G., Giedt, C., Simon, J., Zimmerberg, J., Zhang, K. & Hockenbery, D. (2001) Nat. Cell Biol. 3, 183–191. [DOI] [PubMed] [Google Scholar]
- 22.Ruvolo, P. P, Deng, X., Carr, B. K & May, W. S. (1998) J. Biol. Chem. 273, 25436–25442. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava, R. K., Srivastava, A. R., Korsmeyer, S. J., Nesterova, M., Cho-Chung, Y. S. & Longo, D. L. (1998) Mol. Cell. Biol. 18, 3509–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, D., O'Reilly, A., Strasser, A. & Cory, S. (1997) EMBO J. 16, 4628–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazitt, Y. Rothenberg, M. L., Hilsenbeck, S. G., Fey, V., Thomas, C. & Montegomrey, W. (1998) Int. J. Oncol. 13, 839–848. [DOI] [PubMed] [Google Scholar]
- 26.Breitschopf, K., Haendeler, J., Malchow, P., Zeiher, A. M. & Dimmeler, S. (2000) Mol. Cell. Biol. 20, 1886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmeler, S., Breitschopf, K., Haendeler, J. & Zeiher, A. M. (1999) J. Exp. Med. 189, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]