Abstract
Previous studies examined the serum immunoglobulin levels in relation to coronary artery disease (CAD). We hypothesized that the salivary immunoglobulins might better estimate oral infections in this relationship. Multivariate logistic regression analyses utilizing the data from 256 angiographically confirmed CAD patients and 250 non-CAD individuals that controlled for age, sex, smoking, diabetes, total/HDL cholesterol ratio, hypertension, and education revealed the trends that salivary IgA was positively and salivary IgG was inversely associated with CAD. The odds ratios (OR) of each increasing quartile of salivary IgA were 1.00 (first and second quartiles combined), 1.97, and 1.37 (p-value for trend = 0.06), while those for salivary IgG were 1.00, 0.77, 0.60, and 0.51 (p-value for trend = 0.02). Additionally, salivary IgA correlated positively with C-reactive protein and Asymptotic Dental Score (dental infection score), while IgG was inversely associated with these inflammation markers. Salivary IgA warrants further studies to confirm its role in the risk assessment of CAD.
Keywords: coronary artery disease, inflammation, infection, salivary immunoglobulins, mucosal antigenicity
Introduction
Inflammatory changes that occur during atherogenesis are known to influence plaque vulnerability (Birnie et al., 2005), and the synergy of extravascular infections, autoimmunity, and inflammation may play a role in the development of atherosclerosis (Huittinen et al., 2003). Oral infection is postulated to initiate such changes by activating the innate and adaptive immune system to express cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) (Yamamoto et al., 2006; Roth et al., 2007). At the same time, treatment of periodontitis, a major oral infection, has been associated with a corresponding reduction in systemic inflammatory (D’Aiuto et al., 2004) and endothelial dysfunction markers (Tonetti et al., 2007). Increased serum IgG levels against periodontal pathogens were associated with increased intima-media thickness (IMT) (Beck et al., 2005), and increased serum IgA levels specific to periodontopathogens were predictive of future myocardial infarction (Pussinen et al., 2005) and stroke (Pussinen et al., 2004).
Most of these studies examined the immune response to the infection in the serum, by measuring IgG as the marker of interest, as opposed to the actual site of the infectious insult, namely, the mucosa, more appropriately measured by local IgA. This is important, since higher levels of serum IgA against Chlamydia pneumoniae were found to confer a modest increase in risk of coronary heart disease (CHD) (Danesh et al., 2002, 2003), while an alternate study found that serum IgG did not (Ridker et al., 1999b). Although antibiotic treatments against Chlamydia pneumoniae have yielded no improvement in CVD outcomes, the trial might have been flawed, because the intervention was targeted without considering the participants’ seropositivity or infection status (Wong and Gnarpe, 2005).
In contrast to previous studies, we investigated the relationship between coronary artery disease (CAD) and salivary immunoglobulins (Igs) at the site of infection, the oral mucosa. The aim of this study was to identify answers to the following questions:
(i) Which salivary immunoglobulin would best estimate the strength of local infection in the oral cavity? (ii) Which immunoglobulin supports the current inflammation paradigm better? To support the query further, we explored the correlation of these immunoglobulins to the markers of systemic and oral inflammation as assessed, respectively, by C-reactive protein (CRP) and the Asymptotic Dental Score (ADS) (Janket et al., 2004).
Materials & Methods
Ethical and Protection Consideration for Human Participants
This is a secondary analysis of existing case-control data from the Kuopio Oral Health and Heart (KOHH) Study. The Joint Ethical Committee (Institutional Review Board) of the Kuopio University Hospital and the University of Kuopio, Finland, approved the study protocol. Written informed consent was obtained from all participants according to the Declaration of Helsinki and the Belmont Accord.
Participants
For cases, we recruited 256 consecutive cardiac patients at Kuopio University Hospital and confirmed them as having CAD. We also recruited, as controls, 250 age- and sex-matched non-CAD individuals from the departments of general surgery and otorhinolaryngology. We excluded: individuals who had been on antibiotics during the previous 30 days; those with chronic infection other than dental disease; those needing emergency coronary by-pass surgery or valvular replacement surgery; those whose disease status was so grave that dental examination or x-ray could not be taken safely; and those needing antibiotic prophylaxis prior to dental examination.
Ascertainment of Outcome
CAD was confirmed by angiography at the cardiothoracic examination, and a positive diagnosis was made if at least one major coronary vessel had 50% occlusion of the lumen. To estimate the burden of oral infection, we derived the Asymptotic Dental Score (ADS), an oral infection score generated by the stochastic summation of the weighted likelihood ratio for 5 common oral infections as reported previously (Janket et al., 2004). Serum CRP levels were used as an index of systemic inflammation.
Measurement of Predictors
(i) Saliva Collection
The predictors of interest were salivary IgA and IgG levels. To avoid diurnal fluctuation, we collected saliva samples from the participants between 7 and 9 a.m. They had been advised not to eat or smoke 1 hr before the collection. With the free-flow method, whole saliva was collected into a 10-mL test tube for 5 min after initial swallowing. Whenever possible, saliva was centrifuged (10 min, 12,000 g) and analyzed immediately. If immediate processing was not possible, the centrifuged supernatants were frozen and kept in -80°C until the time for analyses within the ensuing 6 mos.
(ii) Immunoglobulin Analyses
After being thawed, salivary immunoglobulins were analyzed by a modified Enzyme-linked ImmunoSorbent Assay (ELISA) by previously described methods involving microtiter plates (Lehtonen et al., 1984). We used rabbit anti-human IgA and IgG (Dako A/S, Glostrup, Denmark) for the primary adhesion layer, and the respective peroxidase-conjugated rabbit anti-human immunoglobulins (Dako A/S) as the secondary antibodies. Immunoglobulin concentrations were derived from standard curves based on known amounts of human serum standards of IgA and IgG (Behringwerke AG, Marburg, Germany). We carried out all assays in duplicate and included cases and controls in all analyses for even distribution of potential environmental or measurement errors.
Other Confounding Risk Factors
Age was recorded in yrs; sex was coded 0 for males, 1 for females; and smoking was categorized as never smoked, past smokers, and current smokers. We calculated body mass index (BMI) by dividing weight in kilograms by the squared height in meters. Participants’ diabetes mellitus (DM) status was coded as a categorical variable, with 0 for absence of disease and 1 if they had been diagnosed or were receiving treatment for DM. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL) were measured by an automated enzymatic technique, and the total-to-HDL cholesterol ratio was calculated by a statistical function. CRP was measured by a high-sensitivity immunoturbidimetry assay utilizing the Hitachi 717 analyzer (Boehringer Mannheim, Mannheim, Germany).
Statistical Methods
We used Statistical Analysis System (SAS) version 9.1 (SAS Institute, Cary, NC, USA), to compare the general characteristics of the cases and controls, using t tests for variables with a normal distribution and Chi-square tests or the Wilcoxon rank sum test for variables with non-normal distribution. For the purposes of this study, we expressed levels of salivary IgA and salivary IgG as quartiles. Cut-off values for each quartile of salivary IgG levels were < 5.75, 5.75-11.50, 11.50-20.78, and ≥ 20.78 µg/mL, and for each quartile of salivary IgA, they were < 43.5, 43.5-61.5, 61.5-95.4, and ≥ 95.4 µg/mL. Using multivariable logistic regression methods, we calculated odds ratios (OR) of CAD for each quartile of salivary immunoglobulins, salivary IgG, and salivary IgA, compared with the reference (lowest) quartile, adjusting for other established risk factors. Since the first and second quartiles of salivary IgA were not statistically different, we combined them as a reference category. We also calculated the non-parametric correlation coefficient of salivary immunoglobulins with ADS and CRP to assess the association between salivary immunoglobulins and the extent of local and systemic inflammation. All p-values were two-tailed, and all confidence intervals (CI) were computed at the 95% level.
Results
The general characteristics of this cohort have been described previously (Janket et al., 2004), and the distribution of CAD risk factors according to quartiles of salivary IgA and salivary IgG are presented in Table 1. In multivariate analyses, we found an increased likelihood of CAD for those in the third (odds ratios [OR] = 1.97) or fourth (OR = 1.37) quartile of salivary IgA, compared with those in the combined two lowest quartiles (p for trend = 0.06). We also found a decreased likelihood of CAD for those in the second (OR = 0.77), third (OR = 0.60), and fourth (OR = 0.51) highest quartiles of salivary IgG (p for trend = 0.02). Thus, salivary IgA level appeared to be positively (p = 0.06) and salivary IgG appeared to be inversely associated with CAD (p < 0.02). These results are presented in Table 2.
Table 1.
Distribution of CHD Risk Factors According to the Quartiles of Immunoglobulin G (IgG) and Immunoglobulin A (IgA)
| Immunoglobulin A (µg/mL) |
Immunoglobulin G (µg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Age (mean ± SD) | 58.3 (10.1) | 59.6 (9.7) | 59.7 (9.37) | 61.5 (8.8) | 61.6 (9.6) | 58.7 (9.3) | 58.9 (9.7) | 59.9 (9.4) |
| BMI (mean ± SD ) Kg/M2 | 24.7 ( 3.6) | 24.8 (2.82) | 25.2 (4.11) | 25.4 (3.99) | 24.5 (3.7) | 25.0 (3.2) | 25.2 (3.6) | 25.4 (4.1) |
| Number of teeth(mean ± SD) | 15.1 (11.3) | 13.9 (10.3) | 11.9 (9.8) | 10.8 (10.4) | 7.01 (9.7) | 12.7 (10.1) | 14.6 (10.2) | 17.0 (9.7) |
| Asymptotic Dental Score(mean ± SD) | 6.3 ( 7.3 ) | 7.32 (8.72) | 8.95 (8.6) | 10.3 (8.8) | 11.6 (8.4) | 7.2 (7.8) | 7.5 (8.8) | 6.6 (8.1) |
| Diabetes, N (%) | 6 (4.96) | 9 (7.38) | 19 (15.7) | 16 (13.6) | 12 (10.3) | 12 (9.9) | 14 (11) | 12 (10.1) |
| Smoking N (%) | ||||||||
| Never smokers | 85 (69.11) | 76 (61.79) | 80 (64.5) | 90 (75.0) | 70 (59.3) | 81 (65.3) | 85 (66.9) | 95 (78.5) |
| Past smokers | 25 (20.3) | 34 (27.6) | 28 (22.6) | 22 (18.3) | 24 (20.3) | 31 (25.0) | 35 (27.6) | 19 (15.7) |
| Current smokers | 13 (10.6) | 13 (10.6) | 16 (12.9) | 8 (6.7) | 24 (20.3) | 12 ( 9.7) | 7 (5.5) | 7 (5.8) |
| Total/HDL cholesterol ratio (mean ± SD) | 5.0 (1.52) | 4.9 (1.42) | 5.1 (1.31) | 5.2 (1.29) | 5.0 (1.5) | 4.9 (1.3) | 5.1 (1.4) | 5.1 (1.4) |
| LDL cholesterol* (mmol/L) mean (± SD) | 3.7 (0.95) | 3.7 (0.88) | 3.6 (0.89) | 3.5 (0.88) | 3.5 (0.9) | 3.6 (0.9) | 3.7 (0.9) | 3.6 (0.9) |
| Triglyceride (mmol/L) mean (± SD) | 1.9 (1.17) | 1.7 (0.77) | 2.1 (1.22) | 2.0 (0.98) | 2.0 (1.1) | 1.9 (0.9) | 2.0 (1.2) | 2.0 (0.9) |
| HDL cholesterol (mmol/L) mean (± SD) | 1.2 (0.3) | 1.2 (0.33) | 1.2 (0.30) | 1.13 (0.31) | 1.2 (0.4) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| C-reactive protein mg/L (Log-transformed mean ± SD) | 1.9 ± 0.9 | 2.0 ± 1.0 | 2.3 ± 1.0 | 2.1 ± 1.0 | 2.3 ± 1.1 | 2.2 ± 0.93 | 1.9 ± 0.9 | 2.09 ± 1.04 |
LDL cholesterol was calculated by the Friedewald equation.
Due to missing values, for some variables of N < 506.
Medians were compared by non-parametric methods.
Asymptotic Dental Score: mathematically compiled oral infection score.
Table 2.
Multivariate Models to Predict the Probability of CAD
| Immunoglobulin A |
Immunoglobulin G |
|||||
|---|---|---|---|---|---|---|
| Main Predictor | Odds Ratio | 95% CI | p-value for trend | Odds Ratio | 95% CI | p-value for trend |
| Quartile 1 | 1.00 | (Q1. Q2 combined as a reference) | 1.00 | |||
| Quartile 2 | 0.77 | (0.42 - 1.40) | ||||
| Quartile 3 | 1.97 | (1.17 - 3.32) | 0.06 | 0.60 | (0.33 - 1.11) | 0.02** |
| Quartile 4 | 1.37 | (0.81 - 2.31) | 0.51 | (0.27 - 0.94) | ||
| Covariates | ||||||
| Age | ||||||
| ≤ 50 yrs | 1.00 | 1.00 | ||||
| Each year increase | 0.98 | 0.96 - 1.01 | 0.20 | 0.98 | 0.96 - 1.01 | 0.17 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 2.04 | 1.25 - 3.34 | 0.004** | 1.94 | 1.19 - 3.16 | 0.008** |
| Smoking | ||||||
| Never | 1.00 | 1.00 | ||||
| Past | 11.4 | 6.08 - 21.4 | < 0.0001** | 10.6 | 5.63 - 19.8 | < 0.0001** |
| Current | 2.35 | 1.16 - 4.76 | 0.02** | 1.98 | 0.97 - 4.03 | 0.06 |
| Total/HDL cholesterol ratio | ||||||
| Reference (≤ 3.5) | 1.00 | 1.00 | ||||
| > 3.5 | 2.19 | 1.10 - 4.37 | 0.03** | 2.42 | 1.22 - 4.79 | 0.01** |
| Diabetes | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.72 | 0.82 - 3.61 | 0.15 | 1.89 | 0.91 - 3.93 | 0.09 |
| Hypertension | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 3.87 | 2.42 - 6.17 | < 0.0001** | 4.10 | 2.56 - 6.56 | < 0.0001** |
| Education | ||||||
| Up to high school | 1.00 | 1.00 | ||||
| College and above | 0.87 | 0.82 - 0.95 | 0.0006** | 0.88 | 0.82 - 0.95 | 0.0007** |
Statistically significant.
CAD: coronary artery disease.
All covariates were controlled simultaneously with IgA and IgG quartiles.
Additionally, we found a positive correlation between salivary IgA levels and serum CRP (r = 0.09, p < 0.05) and ADS (r = 0.18, p < 0.0001), while salivary IgG levels were inversely associated with both CRP (r = -0.11, p = 0.01) and ADS (r = −0.21, p < 0.0001). Together, these suggest that oral infection may contribute to systemic inflammation, and that salivary IgA appeared to assess mucosal antigenicity better than did salivary IgG. These results are presented in Table 3.
Table 3.
Spearman Correlation Matrix: Correlation of IgA and IgG to Local and Systemic Inflammation
| IgA (µg/mL) | IgG (µg/mL ) | Asymptotic Dental Infection Score (ADS) | C-reactive Protein (CRP) | |
|---|---|---|---|---|
| IgA (µg/mL) | 1.00 | 0.39 | 0.18 | 0.09 |
| p < 0.0001 | p < 0.0001 | p = 0.05 | ||
| IgG (µg/mL) | 0.39 | 1.00 | − 0.21 | − 0.11 |
| p < 0.0001 | p < 0.0001 | p = 0.01 | ||
| Asymptotic Dental Infection score | 0.18 | − 0.21 | 1.00 | 0.26 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | ||
| C-reactive protein (CRP) | 0.09 | − 0.11 | 0.26 | 1.00 |
| p = 0.05 | p = 0.01 | p < 0.0001 |
The non-parametric correlation matrix demonstrates unadjusted correlation coefficients between the two variables.
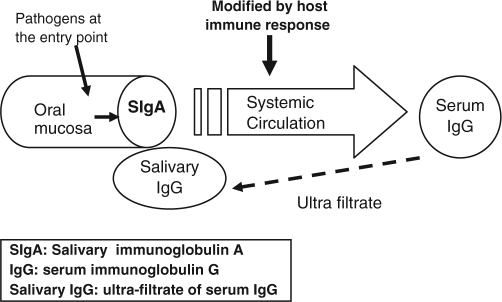
We conceptualized that salivary IgA on the oral mucosa would best approximate the strength of pathogenic insult (Fig.). In contrast, salivary IgG is an ultrafiltrate of serum IgG that is already modulated by the individual immune response.
Figure.
Schematic diagram for conceptual mechanism
Discussion
This is the first multivariate study that investigated the relationship of immunoglobulins assessed at the site of infection, the oral cavity, and CAD. According to previous suggestions (Ridker et al., 1999a), we assessed the total infection burden rather than one or a group of pathogen-specific immunoglobulins. We improved on the methodology further by investigating IgA; since most extravascular infections associated with CAD originate on the mucosa, it is plausible that local IgA would provide a more precise estimate of infections than corresponding serum antibodies. This point has been advocated by Ridker and colleagues by acknowledging the deficiencies of their own study (Ridker et al., 1999a).
Salivary IgG is an ultrafiltrate of serum IgG (Söderling, 1989), which is modified by the host’s general immune response and may not accurately reflect the strength of infection. This may explain the inverse association between salivary IgG and CAD that we report, as well as the paradoxical relationship of serum IgG to periodontal pathogens reported previously (Kinane et al., 1993). We postulated that the most proximal assessment of infection near the entry point of pathogens would approximate the strength of infection more accurately than a distal measure such as serum IgG levels.
Further support for this concept comes from our calculation of the specificity of salivary IgA and salivary IgG. The specificity for the highest quartile of salivary IgA was 0.76, and that of the lowest quartile of salivary IgG was 0.80. Thus, salivary immunoglobulins generated much better specificity than the CRP level at 3 mg/L, which yielded 0.34. Unfortunately, we cannot provide the specificities of serum immunoglobulin, but we are confident that salivary immunoglobulins will provide a much better test due to their proximity to the entry point of infectious stimuli. Some studies have reported specificity figures of 90% or above, but often these figures are a reflection of low prevalence rather than evidence of a superior test performance (Zweig and Campbell, 1993). For example, at a prevalence of 1%, if everyone is classified as not having the disease without any test, then having no test at all will generate a specificity of 99%.
The significant positive correlations of salivary IgA with ADS and CRP further support the view that salivary IgA may be a better measure of local inflammation. This was in agreement with the report that IgA titers against C. pneumoniae were better markers for the risk of ischemic stroke than were IgG titers (Elkind et al., 2006). Furthermore, the fact that oral infection score (ADS) was significantly (r = 0.26, p < 0.0001) correlated with CRP levels suggests an important contribution of oral infection to systemic inflammation. Change in ADS could reasonably explain 26% of the changes in CRP measurement in a global sense (Kleinbaum et al., 1998).
Neither of the immunoglobulins demonstrated any clear trend with lipid profile. This is in agreement with a previous report that 50% of cardiac events occur among those without elevated cholesterol levels, and that inflammation may be the core etiological factor in these cases (Blake and Ridker, 2001).
Our results on IgA are in agreement with our previous report (Janket et al., 2003) and with those of others who used serum IgA (Danesh et al., 2000, 2002; Karvonen et al., 2003; Johansson et al., 2005; Liu et al., 2005). An interesting parallel exists between our results and results by others using IgA and IgG against human cytomegalovirus. Danesh and associates observed increased risk of CHD with increased levels of serum IgA against cytomegalovirus (OR = 1.40; 95% CI = 0.96-2.05), while Ridker and associates reported an inverse association of IgG against the same cytomegalovirus (OR = 0.72; 95% CI = 0.6-0.9) (Ridker et al., 1998). The ratio of relative risks assessed by IgA and IgG (1.40:0.70) is very similar to our results (1.20:0.80), namely, showing a direct association of salivary IgA with CHD (OR = 1.2; CI = 0.99-1.40) and an inverse association of salivary IgG with CHD (OR = 0.80, CI = 0.70-1.00). These ratios of relative risk underscore the changes in the direction of association depending on the immunoglobulin used, and are summarized in Appendix Table 1.
Some caveats concerning our results are worth mentioning. The immunological associations (Muhlestein and Anderson, 2003) may reflect individual variation in immune response rather than the strength of causative infection or inflammation. Currently, there is no published evidence that seropositivity and infection has a 1:1 or even linear relationship (Muhlestein and Anderson, 2003). Additionally, the current study was a cross-sectional investigation and did not allow us to interpret our results in a causal context.
Although the p-value for the relationship between salivary IgA and CAD did not reach the traditional significance level of α = 0.05, we should note that since a significant p-value is a function of sample size and effect size (George, 1984), the point estimate and the confidence intervals are more clinically meaningful (Gardner and Altman, 1986).
Periodontitis assessed by pocket depth may not be a precise assessment measure of oral infection/inflammation (Vettore et al., 2008), because it includes the evidence of past periodontitis. Thus, a precise marker of periodontitis or oral infection is needed. Although modest, our results provide some evidence that salivary IgA might be used as a biomarker in the assessment of oral infection. However, as with any scientific truth, our findings must be iterated by future studies.
In conclusion, salivary immunoglobulins may be associated with prevalent CAD, and salivary IgA appeared to approximate the infectious insult on the oral mucosa.
Supplementary Material
Footnotes
This study was supported by a grant from the American Heart Association (#0635351N) to S. Janket. J.H. Meurman is supported by grant TYH 3245 from the Helsinki University Central Hospital, Helsinki, Finland. T.E. Van Dyke is supported by NIH grant DE13191 and USPHS grant DE15566. The abstract of this study was presented at the 76th Congress of the European Atherosclerosis Society in Helsinki, Finland, in June, 2007.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Beck JD, Eke P, Lin D, Madianos P, Couper D, Moss K, et al. (2005). Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis 183:342-348 [DOI] [PubMed] [Google Scholar]
- Birnie DH, Vickers LE, Hillis WS, Norrie J, Cobbe SM. (2005). Increased titres of anti-human heat shock protein 60 predict an adverse one year prognosis in patients with acute cardiac chest pain [see comment]. Heart 91:1148-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. (2001). High sensitivity C-reactive protein for predicting cardiovascular disease: an inflammatory hypothesis. Eur Heart J 22:349-352 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Ready D, Tonetti MS. (2004). Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res 39:236-241 [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. (2000). Chlamydia pneumoniae IgG titres and coronary heart disease: prospective study and meta-analysis [see comment]. BMJ 321:208-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Lewington S, Walker M, Lennon L, Thomson A, et al. (2002). Chlamydia pneumoniae IgA titres and coronary heart disease; prospective study and meta-analysis [see comment]. Eur Heart J 23:371-375 [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M. (2003). Chlamydia pneumoniae IgA titres and coronary heart disease: prospective study and meta-analysis [comment]. Eur Heart J 24:881. [DOI] [PubMed] [Google Scholar]
- Elkind MSV, Tondella ML, Feikin DR, Fields BS, Homma S, Di Tullio MR. (2006). Seropositivity to Chlamydia pneumoniae is associated with risk of first ischemic stroke [see comment]. Stroke 37:790-795 [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Altman DG. (1986). Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J Clin Res Ed 292:746-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SL. (1984). The required size and length of a phase III trial. In: Cancer clinical trials: methods and practice. Buyse ME, Staquet MI, Sylvester RJ, editors. New York, NY: Oxford University Press, pp. 296-301 [Google Scholar]
- Huittinen T, Leinonen M, Tenkanen L, Virkkunen H, Manttari M, Palosuo T, et al. (2003). Synergistic effect of persistent Chlamydia pneumoniae infection, autoimmunity, and inflammation on coronary risk. Circulation 107:2566-2570 [DOI] [PubMed] [Google Scholar]
- Janket SJ, Baird AE, Chuang SK, Jones JA. (2003). Meta-analysis of periodontal disease and risk of coronary heart disease and stroke [see comment]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:559-569 [DOI] [PubMed] [Google Scholar]
- Janket SJ, Qvarnström M, Meurman JH, Baird AE, Nuutinen P, Jones JA. (2004). Asymptotic dental score and prevalent coronary heart disease [see comment]. Circulation 109:1095-1100 [DOI] [PubMed] [Google Scholar]
- Johansson A, Johansson I, Eriksson M, Ahrén AM, Hallmans G, Stegmayr B. (2005). Systemic antibodies to the leukotoxin of the oral pathogen Actinobacillus actinomycetemcomitans correlate negatively with stroke in women. Cerebrovasc Dis 20:226-232 [DOI] [PubMed] [Google Scholar]
- Karvonen J, Päivänsalo M, Kesäniemi YA, Hörkkö S. (2003). Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108:2107-2112 [DOI] [PubMed] [Google Scholar]
- Kinane DF, Mooney J, MacFarlane TW, McDonald M. (1993). Local and systemic antibody response to putative periodontopathogens in patients with chronic periodontitis: correlation with clinical indices. Oral Microbiol Immunol 8:65-68 [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, editors (1998). Applied regression analysis and other multivariable methods. 3rd ed. Pacific Grove, CA: Duxbury Press [Google Scholar]
- Lehtonen OP, Grahn EM, Stahlberg TH, Laitinen LA. (1984). Amount and avidity of salivary and serum antibodies against Streptococcus mutans in two groups of human subjects with different dental caries susceptibility. Infect Immun 43:308-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yamamoto M, Moroi M, Kubota T, Ono T, Funatsu A, et al. (2005). Chlamydia pneumoniae immunoreactivity in coronary artery plaques of patients with acute coronary syndromes and its relation with serology. Am Heart J 150:681-688 [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Anderson JL. (2003). Infectious serology and atherosclerosis: how burdensome is the risk? Circulation 107:220-222 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. (2004). Antibodies to periodontal pathogens and stroke risk. Stroke 35:2020-2023 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Nyyssönen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. (2005). Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol 25:833-838 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Kundsin R, Shih J. (1999a). Baseline IgG antibody titers to Chlamydia pneumoniae, Helicobacter pylori, herpes simplex virus, and cytomegalovirus and the risk for cardiovascular disease in women. Ann Intern Med 131:573-577 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Kundsin RB, Stampfer MJ, Poulin S, Hennekens CH. (1999b). Prospective study of Chlamydia pneumoniae IgG seropositivity and risks of future myocardial infarction. Circulation 99:1161-1164 [DOI] [PubMed] [Google Scholar]
- Roth GA, Moser B, Roth-Walter F, Giacona MB, Harja E, Papapanou PN, et al. (2007). Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 190:271-281 [DOI] [PubMed] [Google Scholar]
- Söderling A. (1989). Practical aspects of salivary analyses: proteins. In: Human saliva: clinical chemistry and microbiology. Tenovuo JO, editor. Boca Raton, FL: CRC Press, pp. 9-17 [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. (2007). Treatment of periodontitis and endothelial function. N Engl J Med 356: 911-920 [DOI] [PubMed] [Google Scholar]
- Vettore MV, Leal M, Leão AT, da Silva AM, Lamarca GA, Sheiham A. (2008). The relationship between periodontitis and preterm low birthweight. J Dent Res 87:73-78 [DOI] [PubMed] [Google Scholar]
- Wong BYL, Gnarpe J. (2005). Chlamydia pneumoniae and acute coronary syndrome. N Engl J Med 353:525-528 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. (2006). Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res 41:554-559 [DOI] [PubMed] [Google Scholar]
- Zweig MH, Campbell G. (1993). Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39:561-77 [Erratum appears in Clin Chem 1993 Aug;39(8):1589] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.