Abstract
Two recent hurricanes passed directly over the northern Bahamas 2 years apart, allowing a comparison of their effects on lizard populations inhabiting exactly the same islands. The hurricanes differed in two ways: one struck during the reproductive season and was relatively severe; the other struck after most reproduction had taken place and was milder. The late-season hurricane produced a significant relation between population reduction and lowness of the island that lasted at least through two seasons; the earlier hurricane produced no such relationship. The late-season hurricane wiped out populations of lizards on two islands (two of the three lowest) that the earlier hurricane failed to exterminate even though it was stronger. We relate these effects to the fact that the study lizards regenerated from the earlier hurricane only via the egg stage, whereas eggs were unavailable when the later storm struck and regeneration was via hatched lizards. We discriminate and illustrate four kinds of hurricanes, cross-classified by two contrasts: earlier vs. later and stronger vs. weaker. A later, stronger hurricane completely exterminated lizard populations at a second Bahamian site, whereas an earlier, weaker hurricane had no detectable effect at a third Bahamian site. We suggest that, in addition to severity, the timing of a hurricane as it coincides with reproductive scheduling or other phenological aspects may determine the magnitude of its effect on a variety of organisms.
Why should hurricanes sometimes have devastating effects on natural populations and sometimes not? One factor is severity: the more extreme the storm surge or wind speed, the greater the destruction. A second factor, more subtle yet potentially as significant, is timing: a hurricane will have greater impact when scheduling of reproduction is unfavorable for survival and/or recovery. Which factor predominates depends on the mechanism by which populations survive hurricanes, how they weather the storm. Recently, we discovered that lizards of the species Anolis sagrei on small islands are especially vulnerable to inundation from the storm surge of a hurricane (1), but they are sometimes able to survive this destructive force as eggs (2, 3).
On October 19, 1996, Hurricane Lili, generating a 5-m storm surge, wiped out all A. sagrei populations on the 11 “exposed” islands off Great Exuma, Bahamas, that were under continuous observation (1). On September 14, 1999, Hurricane Floyd, generating a 3-m storm surge, failed to exterminate eight continuously observed island populations of A. sagrei off Great Abaco, Bahamas (4). In the second case, although all lizards perished, their eggs apparently survived and subsequently hatched, allowing populations to recover. Laboratory experiments with relatively young eggs confirmed that those submerged in sea water for periods comparable to the maximum duration of the storm surge hatched equally well as eggs not so treated (5).
The fact that Lili was more devastating than Floyd is consistent with both the factors suggested above. First, because Lili was more destructive, eggs were more likely to be dislodged and washed away. Second, because Lili struck later in the year and Anolis in the Bahamas (6) and the adjacent Florida mainland (7) shows seasonal reproduction that winds down by October, no or too few eggs were available to perpetuate the population. Further progress on this problem awaited the perfect storm, a hurricane less powerful than the first yet later in the year than the second.
Obligingly, such a hurricane has now happened. On November 5, 2001, Hurricane Michelle passed over the same islands as did Floyd but generated a storm surge only somewhat >1 m. Was Michelle unable to affect A. sagrei populations much because of its relatively low power, or was Michelle very devastating, just as was Lili, because it was so late in the season despite its lesser power?
Materials and Methods
The Subject Species. A. sagrei is a widespread species distributed throughout the Caribbean (8) and occurring on islands both large and small (9), down to areas <100 m2 as at the present site (10). It is a “trunk-ground” species (11), inhabiting low vegetation from the understory of forest to barren scrub. Among anoles, it is small to medium and highly sexually dimorphic in size. On the Little Bahama Bank, where the study islands are located, mean sizes (snout-vent-length means of the largest third of all specimens examined) ranged from 48.6 to 55.6 mm for males and from 38.5 to 42.5 mm for females (unpublished data). On just the study islands alone, the maximum male snout-vent length we recorded was 57 mm, and the maximum female snout-vent length was 44 mm (12). Food of the species is primarily medium-to-large insects, supplemented with a few fruits (13, 14). A. sagrei is highly territorial, with adults of both sexes and subadult males having overdispersed home ranges (15, 16). Hence, certain individuals of this species, those along the shore and/or in low-lying sites, might be especially vulnerable to the inundating effect of hurricanes, particularly as their changing location would likely be resisted strongly by other lizards.
Study Site. The main area of comparison in the present study is located adjacent to the very large island of Great Abaco and consists of several “creek” waterways fanning out from Buckaroon Bay and Snake Cay. This area, ≈7 × 2 km in extent, contains numerous small islands, many of which have A. sagrei populations. The eight islands on which we focused range from 137.2 to 270.3 m2 in vegetated area. Typically, islands were covered with fairly densely spaced shrubs and trees, rarely exceeding 2 m in height. The islands were quite low in altitude, ranging from 0.65 to 2.44 m. During the 3 years preceding the first hurricane, mean numbers of A. sagrei on these islands (nonexperimental; see below) ranged from 31 to 75 individuals.
Islands at this site were the subjects of a field experiment prior to Hurricane Floyd [the passage of which in fact abruptly terminated that experiment (4, 12)]. Five islands having A. sagrei were selected randomly for invasion by the larger lizard Leiocephalus carinatus, and six others served as controls. In addition, we monitored an island naturally invaded by L. carinatus shortly before the manipulation. Populations of A. sagrei showed an immediate effect from L. carinatus, and by the time the hurricane struck, ≈2 years after the introduction, experimental populations had been reduced to approximately half those on control islands. The hurricane led to the extinction of A. sagrei on four of the six L. carinatus islands but on none of the six controls. The hurricane wiped out the L. carinatus on the two islands where it had not exterminated A. sagrei, and the latter then recovered fairly quickly. These two islands, plus the six controls, constitute the eight islands that are the focus of the present study. L. carinatus, incidentally, did not become extinct everywhere, but survived on two of the six islands on which it was established before the hurricane; both were relatively high, a point to which we will return below.
Population-Size Estimates. Lizard numbers were estimated by using the multiple-recapture method following Heckel and Roughgarden (17) and Fienberg (18); in three censuses on each of three different days lizards are marked with a census-specific color of water-soluble latex paint administered long-distance with Idico spraying devices (Forestry Suppliers Incorporated, Jackson, MS). No long-term ill effects on the lizards have been detected in the ≈20 years that we have been using this method.
Raw lizard counts were converted into estimates as follows: The multivariate contingency-table method of Fienberg (18) fits the data to a variety of models, distinguished by which interactions (associations between the three censuses) or combinations of interactions, if any, are included. The simplest of the models fitting the data adequately, in the sense that the χ2 value of the fit was less than the α = 0.05 χ2 value for the particular model, was selected, and the estimate that model provided used in the analysis. A pascal program doing the fits was kindly provided by Joan Roughgarden (Stanford University, Stanford, CA). In the great majority of cases, the simplest model, that of complete independence, was adequate. Occasionally no model could be run on the data (e.g., when a number of categories equaled zero); then we took the average of the possible pairwise Lincoln estimates as the estimates to be used in the analysis. Finally, if the numbers of different individuals seen exceeded the Lincoln computational estimate, we used the former in the analysis. Note that we could not always verify that we were able to spray a particular lizard; in those cases, we counted a lizard as probably marked (0.75 individual) or possibly marked (0.25 individual).
Data and Analysis. Estimates of population counts themselves are not the variable of most interest in the present study. Instead, we converted those estimated counts to a percentage change as follows. First, we determined the best estimate of the “standing” or chronically typical population size for each island. For data after Floyd, we used the mean of estimates from the immediately preceding 3 years (April 1997–1999) as the standing population size for the six control islands, and we used April 1997 only as the standing population size for the two experimental islands (the introduction of L. carinatus was done just after the April 1997 census, so that the succeeding 2 years showed the diminishing effect of the introduction and could not be used). For data after Michelle and for the six controls, we used the same 3 years as for Floyd plus data from April 2001 to compute the mean standing population size. For the two experimental islands, we used only April 1997 and April 2001 to compute this mean.
The percent change then was calculated by the following formula if the posthurricane population was smaller than the standing population: 100 (A – B)/B, where A is April population size after the hurricane and B is the standing population size computed at the mean of appropriate previous years as given above. If the posthurricane population was larger than the standing population, we calculated the symmetrical measure as percent change: 100(A – B)/A. The logarithm of altitude gave a more linear relation to percent change than did arithmetic values, and therefore we used that transformation in all analyses.
We used Pearson correlation coefficients to describe the relation between percent change and altitude, evaluating significance in the usual way; one-tailed P values were used because we had the a priori expectation that the relation would be positive. Differences between the two hurricanes in the percent change 1 and 2 years into recovery were evaluated with two-tailed t tests.
Results
A substantial number of “hatched” individuals were apparently able to survive Michelle, in contrast to Floyd. Such individuals would be medium to large (>34-mm snout-vent length) by the end of the year. Nearly all individuals seen then after Michelle [12 of 13 (92.7%)] were that size. In complete contrast, none of the 65 different individuals seen during the three censuses performed 2 months after Floyd struck exceeded 34 mm (and only two exceeded 31 mm), implying that all those individuals had hatched from eggs that survived the inundation of the hurricane.
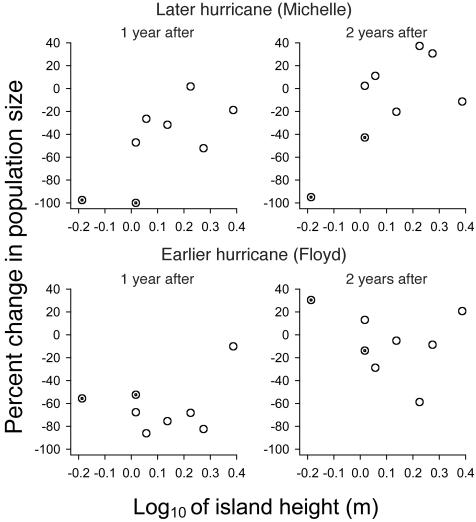
Estimates from the census session the April after Michelle (2002) determined that, among eight islands (those on which A. sagrei survived Floyd), the degree of population reduction from Michelle significantly decreased with increasing island height (r = 0.70; P = 0.027; Fig. 1 Upper). Indeed, two islands lost viable populations altogether (one had no lizards, and the other had one male). In contrast, no such significant correlation existed for estimates from three censuses the April after Floyd (r = 0.30; P = 0.238; Fig. 1 Lower). Moreover, the two islands undergoing lizard extinction from Michelle had, if anything, relatively small population reductions after Floyd (Fig. 1, marked circles). Thus, populations recovering via the egg stage showed no clear relation of numbers to island height, in contrast to those recovering via surviving hatched individuals.
Fig. 1.
Percent change in A. sagrei population size as a function of log island height after two hurricanes: the weaker but later Michelle (Upper) and the earlier but stronger Floyd (Lower). Plots are shown for 1 (Left) and 2 (Right) years after the hurricane. The same islands are used in all four plots; they are situated near Great Abaco, Bahamas.
Censuses during the second April after the hurricane generated the same pattern (Fig. 1): a strong relation for Michelle (r = 0.70; P = 0.028) and again no relation for Floyd (r = 0.27; P = 0.260). Thus the effect of Michelle has persisted at least into the second year. One of the islands losing A. sagrei altogether during Michelle was recolonized sometime between April and December 2002, at which date a few individuals were seen; in April 2003, censuses gave an estimate of 17.3, well below the standing long-term average of 30.3, but still quite firmly established. The other such island had only two males in April 2003 and therefore could not be considered colonized.
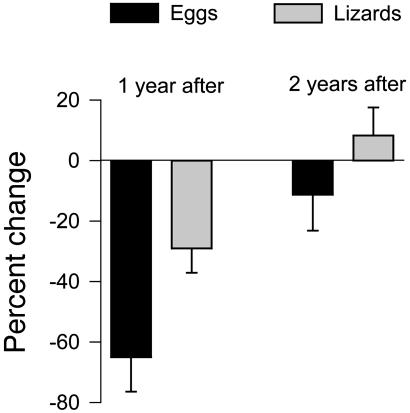
The six islands on which A. sagrei were never completely wiped out can be used to ascertain whether recovery solely from eggs (Floyd) or mainly (if not solely) from hatched individuals (Michelle) is more pronounced. The more negative the percent change after the hurricane, the farther the population value from its standing mean, and the less recovered the population is. Using the April census immediately after the hurricane, i.e., within 1 year later, percent change is far more negative after Floyd than after Michelle (Fig. 2 Left; t = 2.58; P < 0.027). Thus recovery at this time is more behind when it is entirely via the egg stage than when mostly (or entirely) hatched individuals are the progenitors. By the second April after the hurricane, the difference between Floyd and Michelle had mostly disappeared (Fig. 2 Right; t = 1.30; P = 0.222), and indeed populations by then in both cases bracketed standing means before the hurricane.
Fig. 2.
Values of percent change in A. sagrei population size for islands recovering via eggs (Floyd) vs. via hatched lizards (Michelle). (Error bars, 1 SE.)
Discussion
Why is there no relation of population reduction to island altitude for populations recovering via the egg stage (Floyd) but a strong relation for populations recovering via surviving hatched individuals (Michelle)? Note that islands with populations that recover entirely from eggs are covered completely by the storm surge no matter what the altitude. Although submergence time may increase the lower the island, we showed experimentally that A. sagrei eggs immersed in sea water for the maximum duration of the storm surge hatched no less successfully than eggs immersed half as long (5). In contrast, islands having surviving hatched individuals probably had some portion always above water, that increasing with island height.
It also follows that islands with no emergent portion should lose populations if eggs are not present, as indeed happened during Michelle: one exterminated population was on the lowest (0.65 m) island, and the other was on an island tied for second lowest (1.04 m), whereas all lizard populations of both species on higher (1.14–2.54 m) islands (five of A. sagrei; two of L. carinatus) persisted. Thus, islands with a maximum altitude that exceeded the maximum height of the storm surge of Michelle, even by a little, were able to retain their lizard populations.
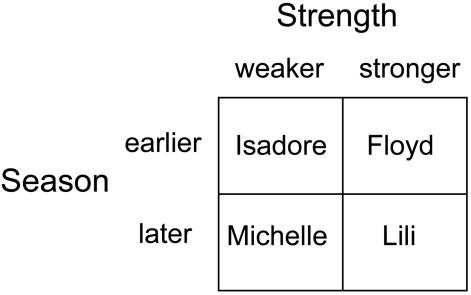
The preceding discussion has compared two levels of hurricanes: a later-season, relatively weak storm, and an earlier-season, relatively strong storm. The two axes of this comparison suggest that two other kinds of hurricanes might be of interest: strong, late-season storms and weak, early-season storms. The former are expected to be especially devastating because of both the immediate destructive power and the lack of a relatively immune stage that can reseed the population. In complete contrast, the latter are expected to show few substantial effects. The four possibilities are diagrammed in Fig. 3. Observations we made in the general area of the present study (the Bahamanian archipelago) of other hurricanes allow us to fill in examples of the two categories of hurricanes not analyzed in the present article. As mentioned in the introduction, Hurricane Lili struck late in the season and was very devastating to islands exposed to the full force of its storm surge (1): the exposed islands off Great Exuma lost all individual lizards and were not reconstituted from eggs, because they presumably were no longer available that late in the season. An example of an earlier-season, relatively weaker hurricane, Isadore, struck the islands around Staniel Cay in the central Exumas in late September of 1984. At that time, it was only a tropical storm (winds of 40 mph). Such a storm would be expected to have little effect on A. sagrei, in particular (i) giving values of percent change near zero and (ii) producing no relation of percent change to island altitude.
Fig. 3.
Four kinds of hurricanes, with examples, resulting from the two-way classification of season (earlier or later) and strength (stronger or weaker).
Although collected for another purpose, we are fortunate in having precise lizard population estimates (done as for the Abaco studies reported above) for eight islands near Staniel Cay from data collected the springs immediately preceding and immediately after Isadore. Using the same kind of plot of percent change vs. altitude as shown above (Fig. 1) for Michelle and Floyd, no significant correlation exists (P = 0.37), and it is negative (r = –0.372) rather than positive, as for Michelle. Furthermore, percent change is both positive (five values) and negative (three values), and the mean is not statistically different from zero (t = 1.53; P = 0.164).
We have just seen that, given enough data, one can begin to distinguish kinds of hurricanes, classifying these relatively rare events with respect to their likely ecological effects. Thus we extend the growing literature on the biological effects of hurricanes in particular (e.g., refs. 19–23) and of physical disturbance in general (e.g., refs. 24–28). In the present study, a late-season hurricane wiped out two populations of lizards that an earlier hurricane failed to exterminate even though the earlier hurricane was stronger. Only the late-season hurricane produced a significant ecological pattern: a positive relation between population reduction and lowness of the island. Thus both timing and severity of the storm seem to matter: the weaker storm caused population extinction on some islands because it occurred after the reproductive season. Although details will differ, we expect that how the timing of a hurricane or other physical disturbance coincides with reproductive scheduling or other phenological aspects will determine the magnitude of its effect in a variety of systems.
In a recent commentary, Brooks and Smith (3) point out that the Caribbean is one of the designated world “hot spots” (29) of biodiversity. They imagine “a conservation vision across the region, with the land and seascape of surviving habitat fragments connected by corridors of restored habitat,” “zones of low-impact human activity,” and “interdependent systems of tiny, largely pristine, islands.” One might assume the third component of this system at least to be in potential danger from natural storms; how good in fact would such a system in the hurricane belt be for conservation purposes?
For lizards in a compact archipelago of small islands tightly fringing much larger ones, the answer as suggested above and in previous articles (1, 2, 4) is probably very good indeed. Even though major hurricanes can wipe out lizards completely from certain small islands, lizards can recolonize rapidly when near to a very large “source” island, as, for example, for one of the two islands on which A. sagrei was exterminated by Michelle. When islands are farther from a potential source of colonists such as the exposed islands to the west of Great Exuma (1), recolonization becomes much more unlikely, but for the continuous and numerous islands lying just off Great Abaco that we studied here, immigration is relatively frequent and restoration of the prehurricane situation quite rapid (2).
Nonetheless, once islands become sufficiently small they are no longer inhabited continuously, or even ever inhabited, by lizards. Such “no-lizard islands” are, however, an enormous conservation resource for invertebrates (such as spiders) negatively affected by lizards. We have shown repeatedly (e.g., refs. 10, 12, 30) that lizards have devastating effects on spider numbers, including the frequent extermination of entire populations on small islands; strong negative effects on a variety of other arthropods have also been shown or inferred. Because severe storms can convert a lizard island into a no-lizard island, hurricanes must have a significant role in enhancing the diversity of many kinds of arthropods on small islands, probably also helping to preserve their diversity over the entire region. This is despite their potentially short-term destructive effects on arthropods, effects that can disappear rapidly when dispersal is high, as we have shown elsewhere (1) for spiders. In conclusion, even in areas chronically impacted by hurricanes, the “interdependent systems of tiny, largely pristine islands” that Brooks and Smith characterize are likely to be invaluable in conserving the biota of the Bahamas, an important portion of the Caribbean hotspot.
Acknowledgments
We thank J. M. Diamond for reading a previous draft and the National Science Foundation for support.
References
- 1.Spiller, D. A., Losos, J. B. & Schoener, T. W. (1998) Science 291, 695–697. [DOI] [PubMed] [Google Scholar]
- 2.Schoener, T. W., Spiller, D. A. & Losos, J. B. (2001) Science 294, 1525–1528. [DOI] [PubMed] [Google Scholar]
- 3.Brooks, T. & Smith, M. L. (2001) Science 294, 1469–1471. [DOI] [PubMed] [Google Scholar]
- 4.Schoener, T. W., Spiller, D. A. & Losos, J. B. (2001) Nature 412, 183–186. [DOI] [PubMed] [Google Scholar]
- 5.Losos, J. B., Schoener, T. W. & Spiller, D. A. (2003) Oecologia 137, 360–362. [DOI] [PubMed] [Google Scholar]
- 6.Licht, P. A. & Gorman, G. C. (1970) Univ. Calif. Publ. Zool. 95, 1–52. [Google Scholar]
- 7.Lee, J. C., Clayton, D., Eisenstein, S. & Perez, H. (1989) Copeia 189, 930–937. [Google Scholar]
- 8.Williams, E. E. (1969) Q. Rev. Biol. 44, 345–389. [Google Scholar]
- 9.Schoener, T. W. & Schoener, A. (1983) J. Anim. Ecol. 52, 209–235. [Google Scholar]
- 10.Schoener, T. W. & Spiller, D. A. (1996) Nature 381, 691–694. [Google Scholar]
- 11.Rand, A. S. & Williams, E. E. (1969) Breviora 327, 1–19. [Google Scholar]
- 12.Schoener, T. W., Spiller, D. A. & Losos, J. B. (2003) Ecol. Monogr. 72, 383–407. [Google Scholar]
- 13.Schoener, T. W. (1968) Ecology 49, 704–726. [Google Scholar]
- 14.Spiller, D. A. & Schoener, T. W. (1990) Oecologia 83, 150–161. [DOI] [PubMed] [Google Scholar]
- 15.Schoener, T. W. & Schoener, A. (1980) J. Anim. Ecol. 49, 19–53. [Google Scholar]
- 16.Schoener, T. W. & Schoener, A. (1981) Ecology 63, 809–823. [Google Scholar]
- 17.Heckel, D. G. & Roughgarden, J. (1979) Ecology 60, 966–975. [Google Scholar]
- 18.Fienberg, S. E. (1972) Biometrika 45, 591–603. [Google Scholar]
- 19.Vandermeer, J., de la Cerda, I. G., Boucher, D., Perfecto, I. & Ruiz, J. (2000) Science 290, 788–791. [DOI] [PubMed] [Google Scholar]
- 20.Schowalter, T. W. & Ganio, L. M. (1999) Ecol. Ent. 24, 191–201. [Google Scholar]
- 21.Hunter, M. D. & Forkner, R. E. (1999) Ecology 80, 2676–2682. [Google Scholar]
- 22.Rathcke, B. J. (2000) Ecology 81, 1951–1958. [Google Scholar]
- 23.Wunderle, J. J., Jr. (1995) Condor 97, 879–896. [Google Scholar]
- 24.Sousa, W. F. (1984) Annu. Rev. Ecol. Syst. 15, 353–392. [Google Scholar]
- 25.Pickett, S. T. A. & White, P. S. (1985) The Ecology of Natural Disturbance and Patch Dynamics (Academic, New York). [DOI] [PubMed]
- 26.Dunson, W. A. & Travis, J. (1991) Am. Nat. 101, 97–107. [Google Scholar]
- 27.Power, M. E., Parker, M. S. & Wootton, J. T. (1996) in Food Webs, eds. Polis, G. A. & Winemiller, K. O. (Chapman & Hall, New York), pp. 286–297.
- 28.Turner, M. G., Collins, S. L., Lugo, A. L., Magnuson, J. J., Rupp, T. S. & Swanson, F. J. (2003) BioScience 53, 46–56. [Google Scholar]
- 29.Mittermeier, R. A., Myers, N. & Mittermeier, C. G. (1999) Hotspots (Cemex Conservation International, Mexico City).
- 30.Schoener, T. W. & Spiller, D. A. (1999) Am. Nat. 153, 347–358. [DOI] [PubMed] [Google Scholar]