Abstract
Emerging evidence indicates that stimulus novelty is affectively potent and reliably engages the amygdala and other portions of the affective workspace in the brain. Using fMRI, we examined whether novel stimuli remain affectively salient across the lifespan, and therefore, whether novelty processing—a potentially survival-relevant function—is preserved with aging. Nineteen young and 22 older healthy adults were scanned during observing novel and familiar affective pictures while estimating their own subjectively experienced aroused levels. We investigated age-related difference of magnitude of activation, hemodynamic time course, and functional connectivity of BOLD responses in the amygdala. Although there were no age-related differences in the peak response of the amygdala to novelty, older individuals showed a narrower, sharper (i.e., “peakier”) hemodynamic time course in response to novel stimuli, as well as decreased connectivity between the left amygdala and the affective areas including orbito-frontal regions. These findings have relevance for understanding age-related differences in memory and affect regulation.
INTRODUCTION
Humans are curious and novelty-seeking creatures. We are wired to prioritize novelty (cf. Mesulam, 2000), and with good reason. Evaluating whether or not a stimulus is novel is one appraisal of an object’s meaning at a particular point in time (Scherer, Schorr, & Johnstone, 2001). Novelty-seeking must have had adaptive advantages because early humans are the only group of hominids to explore the entire world, despite the risk from unknown predators and other enemies (Zuckerman, 2007). The ability to process and respond to novelty translated into an increased change of survival. As humans age, orienting to novel aspects of the outer stimulating environment is thought to prevent mental decline and to sustain cognitive functioning (Scarmeas et al., 2003; Wilson et al., 2002), and to improve mortality across the lifespan (Swan & Carmelli, 1996). Even rats more engaged by novel gustatory/olfactory stimuli tend to exhibit better cognitive functioning (spatial memory measured by water navigation task) (Rowe, Spreekmeester, Meaney, Quirion, & Rochford, 1998). In the article, we examine age-related changes in novelty processing, with an emphasis on the amygdala.
Prior research has shown that novelty is inherently affective. Novelty and uncertainty produce the same cardiovascular responses associated with valence and arousal (Mendes, Blascovich, Hunter, Lickel, & Jost, 2007). Novel faces and pictures engage the same neural workspace as explicitly pleasant, unpleasant, or highly arousing objects, with a most notably enhanced amygdala response (Wright et al., 2003, 2008; Wright, Wedig, Williams, Rauch, & Albert, 2006; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Breiter et al., 1996; for a review, see Strange & Dolan, 2006). Further, novelty enhances amygdala response to valenced and arousing stimuli in an independent and interactive manner, such that greater amygdala activation has been observed to novel negative versus novel positive pictures, but not for familiar pictures (Weierich, Wright, Negreira, Dickerson, & Barrett, 2010). Stimulus novelty also enhances ERPs to affectively hedonic pictures (Yuan, Yang, Meng, Yu, & Li, 2008) as well as skin conductance responses to affectively arousing pictures (Glascher & Adolphs, 2003). Although novelty is affectively potent and interacts with other affective properties, it is dissociable from valence and arousal, in both its peak magnitude and duration of activation in the amygdala, and in its engagement of other parts of the affective workspace, including orbito-frontal cortex (OFC), ventral anterior cingulate, and dorsal anterior cingulate (Weierich et al., 2010).
Our primary question in the current article was whether novelty responses in the brain change across lifespan. If the brain responds similarly to novelty in both young and elderly adults, then it would indicate that novelty processing—a potentially survival-relevant function—is preserved with aging. At present, there are conflicting findings on the issue of age-related changes in novelty processing. ERP studies have failed to find age-related changes to novelty (Polich, 2007; Goldstein, Spencer, & Donchin, 2002; Bin, Jie, Kevin, Joseph, & Emanuel, 2001). Furthermore, two fMRI studies found that amygdala responsivity was preserved with aging to novel fearful (vs. familiar neutral) faces (Wright et al., 2006), and to novel (vs. familiar) neutral faces (Wright et al., 2008). Yet, some studies have documented age-related changes in affective processing (e.g., see Williams et al., 2006; Tessitore et al., 2005; Wedig, Rauch, Albert, & Wright, 2005; Mather et al., 2004; Gunning-Dixon et al., 2003; Iidaka et al., 2002; see St. Jacques, Bessette-Symons, & Cabeza, 2009 for a review), and given the fact that novelty engages the same workspace as valenced and arousing stimuli, it is possible that we might observe changes in responses to novelty with age.
In the present study, we used fMRI to examine age-related differences in the overall magnitude and (for the first time) time course of amygdala response to the visual presentation of novel and familiar images that varied in both valence and arousal. We were particularly interested in examining age-related differences in amygdala time course because there is substantial individual variability of responses across different individuals (Aguirre, Zarahn, & D’Esposito, 1998) and some studies documented age-related changes of hemodynamic response curve (e.g., Madden, Whiting, & Huettel, 2005; Aizenstein et al., 2004; Huettel, Singerman, & McCarthy, 2001; Buckner, Snyder, Sanders, Raichle, & Morris, 2000; D’Esposito, Zarahn, Aguirre, & Rypma, 1999). As there are currently no published reports about details of hemodynamic time course in the amygdala in response to affectively potent stimuli, let alone the age-related changes in the hemodynamic time course, this focus on time course is a unique feature of the current article.
We presented both younger and older participants with images that varied in their valence, arousal, and novelty. We examined whether the novelty responses in the amygdala were moderated by valence and arousal, and whether these responses were related to subjective experiences of arousal in response to the pictures. We also examined age-related differences in functional connectivity within the affective workspace during novelty processing, as measured by temporal correlations between the hemodynamic response to novelty within the amygdala and other brain structures. Such functional connectivity provides clues to potential causes and consequences of changes in amygdala time course. In prior studies, older individuals showed enhanced functional connectivity between the amygdala and ventral anterior cingulate cortex during exposure to negative images (St. Jacques, Dolcos, & Cabeza, 2010). Changes in functional connectivity as a consequence of stimulus novelty are unknown to date.
METHODS
Participants
Nineteen healthy young adults (14 women, 5 men; age: M = 24.5, SD = 3.68, range = 19–32 years) and 22 healthy older adults (14 women, 8 men; age: M = 70.6, SD = 7.09, range = 62–86 years) were included in the final sample for the analyses in this study. Our sample size (n = 41) provided sufficient power to test our hypotheses. Assuming an effect size of (Mather et al., 2004), our sample provided a power = .998.
To obtain our final sample, we had screened larger sample and excluded 16 people. All participants underwent a Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) to confirm the absence of DSM-IV Axis I diagnoses. All were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971), and were free of psychoactive medications. All participants completed the American National Reading Test (AMNART; Grober & Sliwinski, 1991; the American modification of the NART, Nelson, 1982) and the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) to assess that general cognitive ability was equivalent in both groups. One older participant with more than 30 errors in the NART (corresponds to verbal IQ 97, performance IQ 98 and full-scale IQ 98) was excluded. No one was excluded based on the cutoff score (<26) for the MMSE. Eleven participants were excluded before the scanning due to neuropsychiatric problem-like phobia, schizophrenic, ADHD, bulimia, or medication use. Functional data were first visualized over the averaged 3-D image for each individual to ensure that the fMRI signal in the amygdala was not obscured by susceptibility artifact. Data from one participant were excluded on this basis. Further, one young participant and two older participants were also excluded for excessive head motion during scanning (total motion vector >3 mm).
Behavioral Measures
All participants also completed standard cognitive and personality measures because memory and personality processes could be third variables of interest that could explain the age differences that emerged in this study. Participants completed the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 2000) to assess verbal memory abilities. To assess the big five personality dimensions, participants completed the 100-item International Personality Item Pool (IPIP; Goldberg et al., 2006). Each IPIP item is a 5-point, Likert-type scale ranging from 1 (very inaccurate) to 5 (very accurate). Additional measures were also completed, but are beyond the scope of this article.
Affective Pictures
One hundred thirty-two full-color images were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1997) for each of six combinations of arousal and valence (i.e., high arousal negative, high arousal positive, mid arousal negative, mid arousal positive, mid arousal neutral, and low arousal neutral images). It was not possible to parse out the effect of neutral valence in the context of high arousal, or the effects of negative or positive valence (vs. neutral) in the context of low arousal, because these combinations were not available within the standard IAPS stimulus set; nor does the IAPS stimulus set include high arousal neutral images. As a consequence, neutral valence and low arousal were necessarily confounded in this study. Twelve pictures were used for the familiar condition, and the remaining 120 pictures were used for the novel condition. Positive and negative pictures were equated for level of arousal [positive: M = 5.50 SD = 0.74; negative: M = 5.69, SD = 0.79; t(86) = 1.18, p = .24], as were the novel and familiar pictures [novel: M = 5.04, SD = 1.15; familiar: M = 4.95, SD = 1.21; t(130) = .251, p = .80].
Procedure
Prior to scanning, each participant completed a brief practice run outside the scanner to become familiar with the experimental task; practice images were not used in the experimental runs. The task was run using E-Prime experimental software (Psychology Software Tools, Pittsburgh, PA) on a PC, from which images were projected onto a screen in the magnet bore. Participants viewed images via a mirror mounted on the head coil.
The imaging paradigm consisted of five event-related fMRI runs. The first run was a familiarization run. Participants were familiarized to two images in each stimulus category (12 pictures total). The 12 IAPS images were each shown 10 times. Throughout four test runs, participants viewed each familiarized image a total of 10 times and each of the 120 novel images only once. During scanning, participants rated each image for how aroused it made them feel using a 3-point scale (1 = low, 2 = mid, 3 = high) and answered with a button response box. Each run was 340 sec in length and each image was presented for 3.5 sec, with a stimulus onset asynchrony that varied from 4 to 16 sec.
Image Acquisition
We used a Siemens Magnetom Trio Tim 3-T whole-body high-speed imaging device equipped for echo-planar imaging (EPI) (Siemens Medical Systems, Iselin NJ) with a 12-channel gradient head coil. Expandable foam cushions restricted head movement. After an automated scout image was acquired and shimming procedures were performed to optimize field homogeneity, high-resolution 3-D MP-RAGE sequences (TR/TE/flip angle = 2.53 sec/ 3.39 msec/7°) with an in-plane resolution of 1.0 × 1.0 mm, and 1.0 mm slice thickness were collected for spatial normalization and for positioning the slice prescription of the subsequent sequences. fMRI images with blood oxygenation level dependent (BOLD; Ogawa, Lee, Kay, & Tank, 1990; Ogawa, Lee, Nayak, & Glynn, 1990) were acquired using a gradient-echo T2*-weighted sequence (TR/ TE/flip angle = 2.0 sec/30 msec/90°). Prior to each scan, four scans were acquired and discarded to allow longitudinal magnetization to reach equilibrium. The gradient-echo functional images were collected in the same plane (33 coronal slices angled perpendicular to the AC/PC line) with the same slice thickness (5 mm; voxel size 3.12 × 3.12 × 5 mm), excitation order (interleaved), and phase encoding (foot-to-head). We used these parameters based on earlier work that suggested that the parameters helped minimize susceptibility in medial temporal lobe regions (Wright et al., 2001).
Magnitude of Amygdala Response: Anatomical ROI Analyses
Based on our a priori hypothesis that the amygdala plays a central role in the brain’s affective workspace, we first conducted analyses focusing the magnitude of amygdala activation along the time course for each stimulus category. We used an anatomically based approach to conduct ROI analyses of functional data from the amygdala, using FSFAST (http://surfer.nmr.mgh.harvard.edu). We applied automated subcortical segmentation methods to the native 3-D MP-RAGE structural images for each subject to create anatomically defined amygdala ROIs (Fischl et al., 2002), and individual amygdala volumes were also calculated. We manually verified these amygdala ROIs according to our previously published protocols (Wright, Dickerson, Feczko, Negeira, & Williams, 2007; Wedig et al., 2005). The anatomically defined amygdala ROIs were registered to fMRI data, and BOLD signal was extracted for each participant. To explore the details of the time course at the amygdala in both groups, functional data for each condition were modeled using a finite impulse response (FIR) model beginning at 4 sec before stimulus onset, and utilizing 2-sec bins. We estimated the duration of the hemodynamic response to be 16 sec. Percent signal change for combinations of valence, arousal, and novelty versus baseline (fixation) was calculated. Because individuals of the older group have smaller amygdala volumes [right: young, M = 1798.1 (mm3), SD = 197.1; older, M = 1568.2, SD = 282.4; t = 2.98, p = .005; left: young, M = 1670.6, SD = 282.4; older, M = 1406, SD = 244.4; t = 3.69, p = .001], and this directly influences amygdala signal, we adjusted the functional data using individual amygdala volume as a covariate in all analyses.
To examine age-related differences in the magnitude of the BOLD response within the amygdala at different points along the time course, we analyzed our repeated measures design using a multivariate analysis of variance (MANOVA) with multivariate effect estimation (Wilk’s, Pillai’s, etc.). We chose this multivariate approach (where responses were modeled as individual dependent measures) because the sphericity varied enough in at least three time points within the amygdala time course that the statistical assumption of sphericity was violated (making a standard repeated measures ANOVA not advisable; Misangyi, LePine, Algina, & Goeddeke, 2006; Tabachnick & Fidell, 2006, for examples of using this method, see Nitschke et al., 2006; Tilman, Hill, & Lehman, 2006; Tilman, Reich, & Knops, 2006; Koekkoek et al., 2003). We conducted four different repeated measures MANOVAs each for the left and right amygdala to investigate all important effects of interest given that we could not fully cross (balance) valence and arousal due to stimulus limitations.
Curve Fitting Analysis
We conducted additional curve fitting analyses on the amygdala time course data to determine group differences in time course shape. This curve fitting analysis provided additional information about “how” the hemodynamic curve differed for younger and older participants by estimating and comparing parameters obtained by fitting a hemodynamic function to actual time course data. The time course data were fitted with the simplified gamma probability density function that is commonly used as canonical hemodynamic function in neuroimaging studies, given by
where Γ is the gamma function, c is the magnitude parameter (i.e., equivalent to beta coefficient in GLM analysis), d is delay from the onset of the event, a is the “shape” parameter (similar to kurtosis; the larger the a is the broader distribution the function has), b is another scale parameter that affects the magnitude. In our analyses, b was fixed at 1.25 (the value used in FSFAST as a default setting), and a best-fit gamma probability density function was fit to the actual FIR time course data. Parameters a, c, and d were estimated with 95% confidence intervals. In this analysis, we used Curve Fitting Toolbox in Matlab (MathWorks, Natick, MA).
Functional Connectivity Analyses
We conducted functional connectivity analyses to explore how the group difference of time course activation in the amygdala was correlated with activation in other brain areas that belong to the neural reference space for affect [e.g., both sides of the amygdala (AMG), anterior insula (AI), medial posterior OFC at Brodmann’s area 11 to 13 (OFC), thalamus (Thal), hippocampus (Hc), fusiform gyrus (FG), inferior frontal gyri; Brodmann’s area 45 to pars triangularis (IFGtri), and Brodmann’s area 47 to pars orbitalis (IFGorb), ventromedial prefrontal cortex (vmPFC), and ventral ACC (vACC) (Kober et al., 2008; Barrett, Mesquita, Ochsner, & Gross, 2007)]. First, for the purpose of merely extracting the affect-related ROIs, all events versus fixation contrast were estimated by GLM with a canonical hemodynamic response using SPM5, in each group, independently across whole brain (available from the first author on request). Then, using a conjunction analysis, we localized commonly activated areas of two event-related activation maps (all vs. fixation, p < .05 with correction of false discovery rate) of both young and older groups. These common activation areas were further restricted by the structure data of the amygdala and other emotion-related regions adopted from the Automated Anatomical Labeling (AAL) dataset (Tzourio-Mazoyer et al., 2002) using PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, 2003). The regional mean % signal changes across all voxels in an ROI were calculated. Using FIR estimation, all the time course data in each ROI were extracted for each stimulus type separately. Correlation analyses of stimulus-specific time course data were conducted between the right or left amygdala and other ipsilateral ROIs if there was activation or deactivation in these areas, and correlation coefficients were compared between two groups. Using this method, correlation coefficients reflect the similarity of both the magnitude of the peak response as well as the overall pattern of event-related hemodynamic response in two regions.
RESULTS
Behavioral Measures
Memory and Personality Data
Older individuals had decreased CVLT scores compared to younger participants, indicating decreased memory function (see Table 1). The scores in older participants were very close to those in other normative aged samples, however, indicating that they were experiencing normal decrements in memory with age (e.g., Delis et al., 2000; Hu et al., 1999). Young and elderly participants did not differ on the affectively relevant personality dimensions of emotional stability (neuroticism) and extraversion, although younger individuals scored significantly higher on intellect/ imagination (openness to experience).
Table 1.
Comparison of Prescanning Tests between the Young and the Older Group
| YNG
|
OLD
|
t | Significance (two-tailed) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| California Verbal Learning Test | ||||||
| List A Total Recall | 62.8 | 11.5 | 49.6 | 10.1 | 3.67 | .001* |
| List A Total Recall Intrusion | 0.3 | 0.6 | 0.7 | 1.3 | −1.32 | .196 |
| List B Total Recall | 8.8 | 2.7 | 5.8 | 1.9 | 4.03 | <.001* |
| List B Recall Intrusion | 0.1 | 0.3 | 0.3 | 0.6 | −1.26 | .216 |
| Short Delay Free Recall | 13.9 | 2.8 | 9.8 | 3.9 | 3.54 | .001* |
| Short Delay Free Recall intrusion | 0.0 | 0.0 | 0.3 | 0.4 | −2.24 | .031* |
| Short Delay Cued Recall | 14.0 | 2.4 | 10.7 | 3.3 | 3.37 | .002* |
| Short Delay Cued Recall intrusion | 0.3 | 0.5 | 0.9 | 1.1 | −1.83 | .076 |
| Long Delay Free Recall | 13.9 | 2.4 | 9.6 | 3.7 | 3.91 | <.001* |
| Long Delay Free Recall intrusion | 0.0 | 0.0 | 0.6 | 0.8 | −2.82 | .008* |
| Long Delay Cued Recall | 13.9 | 2.6 | 10.2 | 3.4 | 3.55 | .001* |
| Long Delay Cued Recall intrusion | 0.1 | 0.4 | 1.0 | 1.2 | −2.67 | .012* |
| Recognition Performance | 22.7 | 12.6 | 31.4 | 14.9 | −1.83 | .077 |
| International Personality Item Pool | ||||||
| Surgency or Extraversion | 63.4 | 3.9 | 61.8 | 5.5 | 1.04 | .304 |
| Agreeableness | 67.3 | 8.6 | 61.4 | 13.4 | 1.67 | .103 |
| Conscientiousness | 60.3 | 4.5 | 59.9 | 6.8 | 0.24 | .814 |
| Emotional Stability | 49.8 | 7.1 | 50.6 | 6.4 | −0.37 | .712 |
| Intellect or Imagination | 64.2 | 5.2 | 60.6 | 6.1 | 2.07 | .044* |
YNG = younger group; OLD = older group.
Arousal Ratings of IAPS Pictures
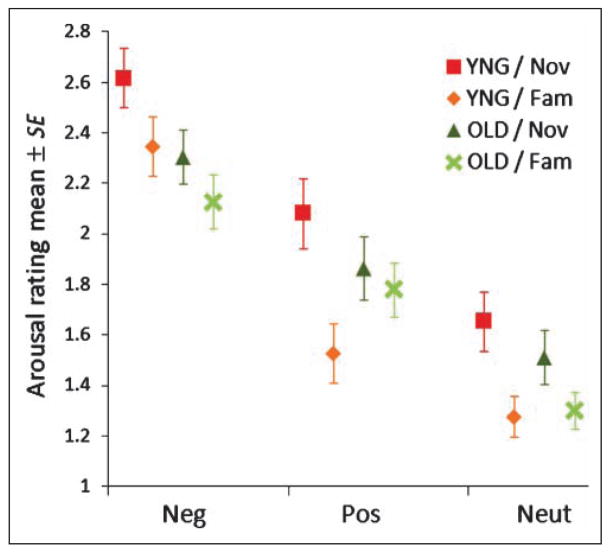
We conducted Novelty (novel, familiar) × Valence (negative, positive, neutral) × Age (young, older) repeated measures ANOVA on subjective arousal rating of IAPS pictures. All participants rated negative pictures as significantly more arousing than positive images, which in turn were more arousing than neutral images (see Figure 1) [F(1.71, 68.28) = 124.77, p < .0001, Greenhouse–Geisser correction]. Older individuals found negative pictures significantly less arousing than did young individuals (see Figure 1) [repeated ANOVA, Valence × Age, F(2, 80) = 3.18, p = .047].
Figure 1.
Rating of arousal level of each valence of IAPS pictures. Mean ± SE of arousal ratings of IAPS pictures during scanning are plotted in each valence of the stimuli (Neg = negative; Pos = positive; Neut = neutral). YNG = younger group; OLD = older group; Nov = novel condition; Fam = familiar condition.
Despite being equated for arousal at the outset, all participants rated novel pictures as more arousing than familiar [novelty effect: F(1, 40) = 31.27, p < .0001]. Older individuals found novel pictures significantly less arousing than did young individuals, however (see Figure 1) [Novelty × Age: F(1, 40) = 5.99, p = .019]. This was particularly true for valenced images as old and young participants found novel, neutral pictures equally arousing [F(1, 40) = 2.133, p = .152].
Subjective arousal ratings also showed a significant three-way Novelty (novel, familiar) × Valence (negative, positive, neutral) × Age (young, older) interaction [F(2, 40) = 5.71, p = .005]. To clarify the three-way interaction, we used a Novelty × Age stratified ANOVA for positive, negative, and neutral pictures separately. We found that there was a significant Novelty × Age interaction for positive picture condition [F = 17.73, p < .001], but this effect did not hold for negative and neutral picture conditions [F = .507, p = .481 for negative, F = 2.133, p = .152 for neutral]. The analyses suggested that, taken together with Figure 1, younger individuals found novel images more arousing than did older individuals, and older individuals found positive familiar images more arousing than did young individuals.
To confirm the effect of stimulus arousal level on subjective arousal ratings, we performed Age × Arousal ANOVA for subjective arousal ratings. There was a main effect of image arousal on subjective arousal ratings, such that all participants experienced high arousal pictures as significantly more arousing than mid, which were more arousing than low [F(1, 40) = 86.65, p < 001 for valenced images; F(1, 40) = 69.20, p < 001 for neutral images]. There were marginally significant age-related difference in the stimulus arousal effects for valenced images (Table 2) [Age × Arousal interaction: F(1, 40) = 3.51, p = .068], suggesting that older individuals found high arousal valenced images less arousing than did young individuals. There was no significant age-related difference of stimulus arousal effect for neutral images, however [F(1, 40) = 0.459, p = .502].
Table 2.
Mean (SE) of the Subjective Arousal Ratings of Different Arousal Levels of IAPS Images in Each Age Group
| Images | Age Group
|
|
|---|---|---|
| YNG | OLD | |
| Valenced | ||
| High arousal | 2.31 (0.11) | 2.13 (0.10) |
| Mid arousal | 1.98 (0.10) | 1.91 (0.09) |
| Neutral | ||
| Mid arousal | 1.64 (0.11) | 1.61 (0.10) |
| Low arousal | 1.29 (0.09) | 1.20 (0.08) |
YNG = young group; OLD = older group.
Magnitude of Amygdala Response
Because of stimulus constraints (it was not possible to fully cross-valence and arousal), two different repeated measures MANOVAs were necessary to examine age-related differences in amygdala’s response to novelty and valence. First, we conducted Novelty (novel, familiar) × Valence (positive, negative, neutral) × Time point (1–8) × Age (young, older) repeated measures MANOVA to examine age-related novelty and valence effects on the amygdala activation. A second analysis was Novelty (familiar, novel) × Time point (1–8) × Age (young, elderly) repeated measures MANOVA for neutral pictures to clarify age-related differences in amygdala responses to novel versus familiar images that were neutral in hedonic valence. To examine age-related differences in amygdala response to novelty and picture arousal level, we conducted Novelty (novel, familiar) × Arousal (high, mid) × Time point (1–8) × Age (young, older) repeated measures MANOVA for amygdala response to valenced images, and Novelty (novel, familiar) × Arousal (mid, low) × Time point (1–8) × Age (young, older) repeated measures MANOVA for amygdala response to neutral images.
FIR Analyses of Age-related Novelty and Valence Effects on the Amygdala Activation
When examining the overall amygdala response, there were no age-related differences in amygdala responses to novelty or valence; there was no Novelty × Age interaction for right amygdala responses [F(1, 38) = 1.06, p = .311], nor for left amygdala responses [F(1, 38) = 1.39, p = .245]. There was no Valence × Age interaction for right amygdala responses [F(2, 37) = 1.79, p = .182], nor for left amygdala responses [F(2, 37) = 0.86, p = .430]. For all participants, both valence [F(2, 37) = 8.32, p = .001] and novelty [F(1, 38) = 5.46, p = .025] significantly engaged the right amygdala. In addition, both valence [F(2, 37) = 4.12, p = .024] and novelty [F(1, 38) = 13.97, p = .0006] engaged the left amygdala.1
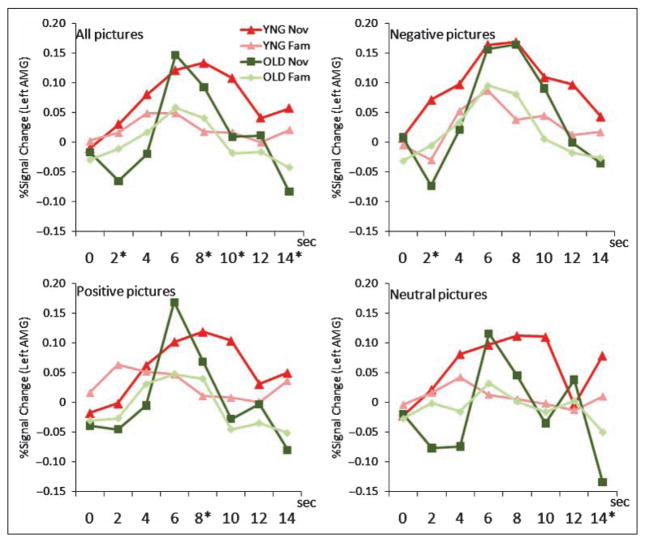
To examine age-related differences in the magnitude of the amygdala along its time course, we conducted a Novelty (familiar, novel) × Valence (positive, negative, neutral) × Time point (1–8) × Age (young, elderly) repeated measures MANOVA on the BOLD response within the right and left amygdala ROIs. Time courses are illustrated in Figure 2. The time course patterns in both left and right amygdala were similar; only the data in the left amygdala are shown. There was an age-related difference in the right and left amygdala time course for novelty [Novelty × Time point × Age: right, F(7.32) = 4.01, p = .003; left, F(7, 32) = 2.46, p = .039], such that younger and older individuals showed a different amygdala time course when viewing novel images. In particular, older (vs. younger) individuals have weaker amygdala responses before and after the peak, leading to a narrower and sharper time course (also see Curve Fit Analysis). The overall Valence × Time point × Age interaction was not significant in the right amygdala, F(14, 25) = 1.65, p = .133, nor in the left amygdala, F(14, 25) = 1.45, p = .200, such that there was no age-related significant difference in the amygdala time course when viewing positive or negative images, although older individuals did appear to show a similar “peakier” response in their amygdala response to negative and positive images when compared to younger individuals.
Figure 2.
Age-related difference of hemodynamic time course in FIR analysis in the left amygdala. Event-related time course of BOLD response (% signal change) in each condition (Valence × Novelty) in two age groups. The right amygdala also showed a similar pattern so only the time course in the left amygdala was illustrated. Red = young group (YNG); green = older group (OLD). The four lines colored in red and green show response to novel (Nov) and familiar (Fam) images in the young and the older groups (Nov/ YNG, Nov/OLD, Fam/ YNG, Fam/OLD), which are displayed on each all, negative, positive, and neutral picture condition panels. Asterisk (*) shows significant Novelty × Age interaction ( p < .05) in each time bin (TR = 2 sec).
The four way Novelty × Valence × Time point × Age interaction was not statistically significant in the right amygdala [F(14, 25) = 0.70, p = .75, ], but was marginally significant in the left amygdala [F(14, 25) = 2.02, p = .061, ]. From Figure 2, this interaction in the left amygdala appeared to be driven by “peakier” amygdala response in the older group than in the young group, particularly in response to novel positive and neutral images rather than to novel negative images. To check this finding, we added a Valence × Time point × Age stratified repeated MANOVA for left amygdala BOLD response only for the novel pictures; we confirmed this marginally significant three-way interaction [F(14, 25) = 2.04, p = .058, ], suggesting that the hemodynamic curves were different for young and older participants, particularly in response to novel positive and neutral images.
Further, we did stratified ANOVAs Novelty (novel, familiar) × Age (young, older) in each time point separately, and found significant Novelty × Age interactions at the time points of 2–4 sec [F(1, 38) = 5.97, p = .02, ], 8–10 sec [F(1, 38) = 4.41, p = .04, ], 10–12 sec [F(1, 38) = 3.88, p = .06, ], and 14–16 sec [F(1, 38) = 4.67, p = .04, ] in response to all three-valence images in the left amygdala; 2–4 sec in response to negative images in the right amygdala [F(1, 38) = 5.16, p = .029, ]; 2–4 sec in response to negative images in the left amygdala [F(1, 38) = 10.1, p = .003, ]; 8–10 sec to positive images in the left amygdala [F(1, 38) = 4.27, p = .046, ]; and 14–16 sec to neutral images in the left amygdala [F(1, 38) = 6.72, p = .013, ]. Taken together with Figure 2, the analyses appeared to show that the group differences of response to novel pictures occurred in early and late phases in the time course.
Age-related Differences in Amygdala Response to Novel vs. Familiar Neutral Images
To further investigate age-related differences within the amygdala time course in response to novelty, we conducted a Novelty (familiar, novel) × Time point (1–8) × Age (young, elderly) repeated measures MANOVA on the BOLD response to the neutral images, within the right and left amygdala ROIs. There was an age-related difference in the left amygdala time course for novelty [Novelty × Time point × Age: F(7.32) = 2.65, p = .028]. This indicates that, even upon observing only neutral images, older individuals had a narrow and sharper amygdala time course to novelty when compared to younger individuals. In the right amygdala, there was no Novelty × Time point × Age interaction [F(7.32) = 1.23, p = .32].
FIR Analyses of Age-related Novelty and Picture Arousal Effects on the Amygdala Activation
To address the question of whether novelty and picture arousal level interact to produce the neural response in the amygdala, we conducted Novelty (novel, familiar) × Arousal (high, mid) × Time point (1–8) × Age (young, older) repeated measures MANOVA for the right and the left amygdala response to valenced images, and Novelty (novel, familiar) × Arousal (mid, low) × Time point (1–8) × Age (young, older) repeated measures MANOVA for the right and the left amygdala response to neutral images. We found significant Novelty × Arousal × Time point × Age interactions for the left amygdala response to valenced images [F(7, 32) = 2.43, p = .041, ], and to neutral images [F(7, 32) = 2.82, p = .021, ], but not for the right amygdala response to valenced images [F(7, 32) = 1.73, p = .13, ], nor to neutral images [F(7, 32) = 0.42, p = .88, ]. Overall, the results were the same as the analyses with novelty and valence; older individuals showed a peakier amygdala response to novel pictures of higher levels of arousal when compared to younger individuals who showed a more sustained response across ~10 sec. The figures are not shown here, and the details of the findings and figures are available from the first author by request.
Curve Fit Analysis
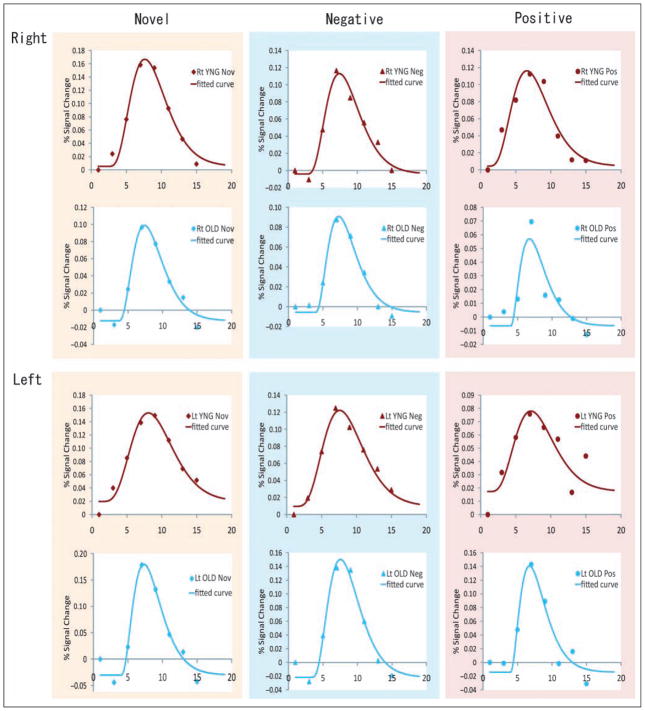
On inspecting the hemodynamic curves from the FIR analysis, older individuals appeared to have a “peakier” amygdala time course when compared to younger individuals, particularly in response to novel stimuli. This was confirmed by an additional curve fitting analysis, showing that older individuals showed a different amygdala time course in response to novel pictures when compared to young individuals (Figures 3 and 4).
Figure 3.
The time course of BOLD response to novel, negative, and positive pictures and fitted curves in curve fitting analyses. Rt = right; Lt = left; red dots and curve = young group; blue dots and curve = older group. The left column = novel condition; middle column = negative condition; right column = positive condition.
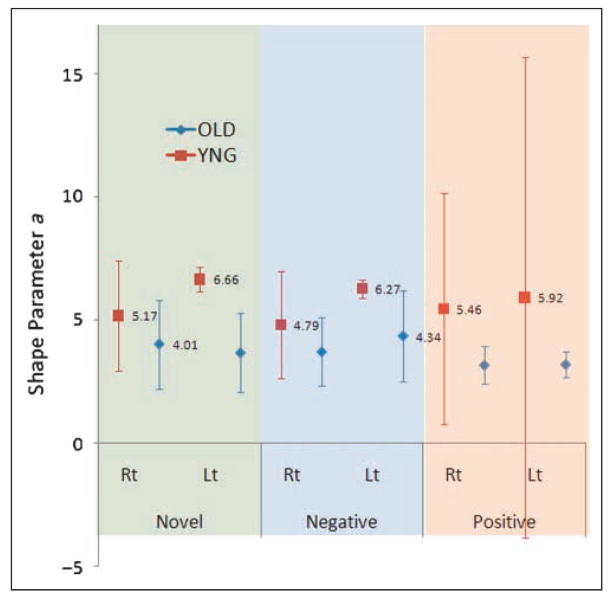
Figure 4.
Shape parameter a in the curve fitting analyses in the novel, negative, and positive condition in the right/left amygdala. BOLD responses produced by FIR analyses in the right and left amygdala were fitted by gamma probability density function with three variable parameters of delay from time 0, height, and shape (broadness). The graph shows shape parameter a in each younger (YNG) and older (OLD) group in novel, negative, and positive condition. Upper = FIR data and fitting line; Lower = estimated value and 95% confidence interval (95% CI) of shape parameter a in both groups.
The simplified gamma probability density function hypothesized in the Methods section fits the observed FIR time course data quite well; all adjusted R2 > .9 and all root-mean-squared-error (RMSE) < .05 (suggested by Browne & Cudeck, 1992). Table 3 shows the mean and 95% confidential interval of estimated shape parameter a for the BOLD time course in novel, negative, and positive conditions in the right and left amygdala. In these analyses, we found that older individuals had lower estimated a than that of the younger group, indicating that the older group had a peakier hemodynamic response to novel and negative pictures in the left amygdala. There was no group difference of parameter a in response to positive images in the left amygdala, or to any images in the right amygdala. Also, we did not observe any statistical age-related difference of delay (d) and height parameter (c).
Table 3.
Estimated Mean and Upper/Lower Bound of 95% Confidence Interval of Shape Parameter a in Curve Fitting Analysis
| YNG
|
OLD
|
|||||
|---|---|---|---|---|---|---|
| Mean | LoCI95 | UpCI95 | Mean | LoCI95 | UpCI95 | |
| Right AMG | ||||||
| Nov | 5.17 | 2.94 | 7.41 | 4.01 | 2.21 | 5.81 |
| Neg | 4.79 | 2.62 | 6.96 | 3.70 | 2.30 | 5.11 |
| Pos | 5.46 | 0.76 | 10.17 | 3.17 | 2.39 | 3.95 |
| Left AMG | ||||||
| Nov | 6.66 | 6.17 | 7.14 | 3.66 | 2.04 | 5.27 |
| Neg | 6.27 | 5.90 | 6.63 | 4.34 | 2.49 | 6.19 |
| Pos | 5.92 | −3.85 | 15.70 | 3.20 | 2.67 | 3.73 |
YNG = younger group; OLD = older group; AMG = amygdala; Nov = novel condition; Neg = negative condition; Pos = positive condition; LoCI95 = lower bound of 95% confidence interval; UpCI95 = upper bound of 95% confidence interval.
Bold type: YNG mean > OLD UpCl95 and OLD mean < YNG LoCl95.
For novel conditions, mean a = 3.66 in older < LoCI95 = 6.17 in younger, mean a = 6.66 in younger > UpCl95 = 5.27 in older; for negative conditions, mean a = 4.34 in older < LoCI95 = 5.90 in younger, mean a = 6.27 in younger > UpCl95 = 6.19 in older.
Additionally, to check if this age-related difference of shape of hemodynamic time course was specific for the amygdala, we compared the hemodynamic time courses for the younger and older groups in other brain areas such as left medial posterior OFC, thalamus, hippocampus, fusiform gyrus, inferior frontal gyri pars triangularis, and inferior frontal gyri pars orbitalis. We also did curve fitting analyses in each ROI on BOLD response to novel stimuli. We did not find any age-related differences of parameter a similar to what were observed in the amygdala (Table 4). This suggests that not all hemodynamic responses across whole-brain areas show an age-related difference in time course shape difference, indicating that hemodynamic time course difference in the amygdala was not due to a general change in vasculature with aging.
Table 4.
Mean and Upper/Lower Bound of Confidential Interval of Shape Parameter a in Curve Fitting Analysis in Affect-related ROIs in the Left Hemisphere
| YNG
|
OLD
|
|||||
|---|---|---|---|---|---|---|
| Mean | LoCI95 | UpCI95 | Mean | LoCI95 | UpCI95 | |
| OFC | 5.26 | 3.02 | 7.51 | 4.60 | 1.89 | 7.31 |
| Thal | 5.16 | 3.43 | 6.90 | 4.51 | 3.15 | 5.86 |
| Hc | 4.17 | 2.55 | 5.80 | 2.97 | 1.57 | 4.37 |
| FG | 2.73 | 2.43 | 3.03 | 4.12 | 2.89 | 5.36 |
| IFG tri | 4.67 | 3.00 | 6.34 | 5.18 | 3.38 | 6.98 |
| IFG orb | 4.90 | 3.21 | 6.59 | 3.36 | 1.57 | 5.15 |
OFC = posterior orbito-frontal cortex at Brodmann’s area 11 to 13; Thal = thalamus; Hc = hippocampus; FG = fusiform gyrus; IFGtri = inferior frontal gyrus (Brodmann’s area 45 to pars triangularis); IFGorb = inferior frontal gyrus (Brodmann’s area 47 to pars orbitalis); LoCI95 = lower bound of 95% confidence interval; UpCI95 = upper bound of 95% confidence interval.
Functional Connectivity Analysis
Functional connectivity analyses indicated that the amygdala of older individuals had a somewhat different pattern of correlated activity than the amygdala of younger individuals when responding to novelty. Correlation coefficients in novel versus familiar picture conditions were compared between the two groups (Figure 5), and those reported were significant according to a Novelty (novel, familiar) × Age (young, older) interaction at p < .05. Only ipsilateral connections (i.e., right amygdala–right ROIs, and left amygdala–left ROIs) are presented because the patterns of contralateral connections were similar.
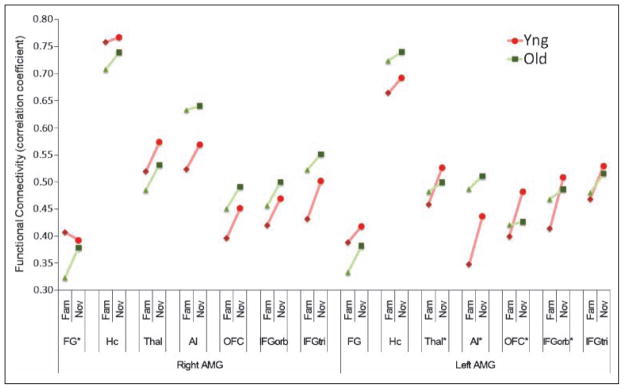
Figure 5.
Functional connectivity between amygdala and other ROI. Correlation coefficients of event-related BOLD response between the amygdala and other emotion-related ipsilateral ROIs in novel (Nov) and familiar (Fam) conditions. Contralateral connectivity showed similar pattern so only ipsilateral connectivity was shown. FG = fusiform gyrus; Hc = hippocampus; Thal = thalamus; AI = anterior insula; OFC = orbito-frontal cortex; IFGorb = inferior frontal gyrus (pars orbitalis); IFGtri = inferior frontal gyrus (pars triangularis); AMG = amygdala. Red circle/ diamond and line = young group (YNG); green triangle/ square and line = older group (OLD). The flesh-colored ROI and asterisk (*) show a connectivity with significant Novelty × Age interaction ( p < .05).
To test the interactive effect of valence and age on the functional connectivity in response to novel (vs. familiar) pictures, estimates of functional connectivity in response to novel/negative, novel/positive, novel/neutral, familiar/ negative, familiar/positive, and familiar/neutral images were first calculated. Next, these estimates of connectivity (correlation coefficients) were entered into Novelty (novel, familiar) × Valence (negative, positive, neutral) × Age (young, older) repeated ANOVA. The results are presented in the Figure 5. Novelty increased the functional connectivity between the amygdala and almost every ipsilateral ROI; for novel pictures, the right amygdala showed greater functional connectivity with the right hippocampus [F(1, 39) = 5.56, p = .024], the right thalamus [F(1, 39) = 10.95, p = .002], the right anterior insula [F(1, 39) = 4.54, p = .039], right medial/posterior OFC [F(1, 39) = 8.68, p = .005], the right inferior frontal gyrus (pars orbitalis) [F(1, 39) = 8.95, p = .005], and the right inferior frontal gyrus (pars triangularis) [F(1, 39) = 15.42, p < .001]. Similarly, the left amygdala showed greater functional connectivity with the left fusiform gyrus [F(1, 39) = 4.86, p = .033], the left hippocampus [F(1, 39) = 8.59, p = .006], the left thalamus [F(1, 39) = 12.76, p = .001], the left anterior insula [F(1, 39) = 12.24, p = .001], left medial/posterior OFC [F(1, 39) = 7.09, p = .011], the left inferior frontal gyrus (pars orbitalis) [F(1, 39) = 11.12, p = .002], and the left inferior frontal gyrus (pars triangularis) [F(1, 39) = 10.84, p = .002]. Functional connectivity did not vary by the valence of the pictures, and the Novelty × Valence interaction did not reach statistical significance.
Furthermore, we found a significant Novelty × Age interaction for the connectivity between the left amygdala and the left thalamus [F(1, 39) = 4.31, p = .045, ], the left anterior insula [F(1, 39) = 4.20, p = .047, ], left medial/posterior OFC [F(1, 39) = 5.63, p = .023, ], and the left inferior frontal gyrus (pars orbitalis) [F(1, 39) = 5.04, p = .031, ]. In response to novel (vs. familiar) pictures, younger individuals showed greater functional connectivity than did older individuals between the left amygdala and the left thalamus, anterior insula, medial/ posterior OFC, and inferior frontal gyrus (pars orbitalis). In contrast, older individuals showed enhanced connectivity between the right amygdala and the right fusiform gyrus; a significant Novelty × Age interaction [F(1, 39) = 4.91, p = .033, ]. This pattern of functional connectivity suggests that the frontal/orbital areas might be involved in sustaining amygdala response in younger individuals.
Both ventromedial prefrontal cortex and ventral anterior cingulate cortex (bilaterally) showed a decrease in activation from fixation baseline in response to positive images (replicating Leclerc & Kensinger, 2008), but we also observed deactivations in response to negative and novel images. Furthermore, the hemodynamic time courses in these two regions were weakly correlated with the amygdala time course (r = 0.0–0.2; data not shown). In functional connectivity analysis, correlations between activation and deactivation hemodynamics are difficult to meaningfully interpret from a methodological standpoint, and so the results of this functional connectivity analysis are not shown here but are available upon request.
DISCUSSION
Our findings clearly indicate that novel stimuli are affectively significant and engage the amygdala in a robust way. This novelty effect was not accounted for by the arousing or valenced nature of the stimuli, as was exhibited even with neutral images. The idea of novelty as a stimulus property with affective salience is consistent with studies in which the amygdala habituates even to very evocative stimuli (e.g., Wright et al., 2001; Fischer, Furmark, Wik, & Fredrikson, 2000), and by animal studies showing that amygdala lesions disrupt normal responses to novelty in primates (e.g., Mason, Capitanio, Machado, Mendoza, & Amaral, 2006; Prather et al., 2001; Burns, Annett, Kelley, Everitt, & Robbins, 1996; for reviews, see Petrides, 2007; Knight & Grabowecky, 1999). Together, these findings shape an emerging view that the amygdala’s function is not to represent negativity or valence per se, but rather to mark the salience of a stimulus and modulate other brain areas to increase the processing of that stimulus to gain information for future use (e.g., Ewbank, Barnard, Croucher, Ramponi, & Calder, 2009; Wedig et al., 2005; Anderson & Phelps, 2001; for a discussion, see Barrett & Bliss-Moreau, 2009; Duncan & Barrett, 2007a, 2007b). This view is also consistent with the view that the amygdala is a key brain structure that is involved in evaluating an object for its goal relevance (Sander, Grafman, & Zalla, 2003).
We did not find age differences of the peak magnitude of the hemodynamic response in amygdala to any evocative images, indicating that, at least in one sense, affective processing within the amygdala, including responsiveness to novelty, is preserved in older people. These results are consistent with prior research showing no age-related changes in novelty processing (Wright et al., 2006, 2008), suggesting that salience (Carstensen & Turk-Charles, 1994) or vigilance (Whalen, 2007) is maintained across the lifespan. These findings are in line with the observation that the amygdala is one of the regions which is relatively structurally preserved with aging when compared to many other brain regions (e.g., West, 1996; Moscovitch & Winocur, 1995; Daigneault & Braun, 1993). Our findings differ from those previously published studies that reported reduced amygdala activation to negative images in older individuals, however, for a number of reasons. One of the possible reasons is that we used FIR analyses to examine our event-related BOLD data, whereas prior studies have used an SPM canonical hemodynamic function (e.g., Mather et al., 2004). The remarkable difference of the shape of the hemodynamic time course in older (vs. younger) individuals that we discovered suggests that a canonical hemodynamic function might provide a worse fit to the actual hemodynamic pattern in older individuals, resulting in a lower estimate of activation (i.e., a lower correlation between actual amygdala response and hypothetical gamma curve). This valence effect in aging remains to be tested with future studies.
Importantly, our results demonstrated age-related difference in the shape of the hemodynamic time course of the amygdala, particularly in response to the novel stimuli that have not previously been reported; older people showed “peakier” hemodynamic response when compared to younger individuals. In previous methodological papers, age-related changes of hemodynamic response were inconclusive (e.g., the rise time of the fMRI signal in motor cortex increased with age during a 10-sec hand-squeezing task, Taoka et al., 1998; spatial extent of activation in older people did not differ from that of young people for a photic stimulation task, Ross et al., 1997; no highly consistent age difference exists in the shape of hemodynamic responses in primary sensorimotor cortex, D’Esposito et al., 1999; and sustained event-related BOLD effect even after the peak in the older group, Aizenstein et al., 2004). These methodological studies indicate the age-related time course difference of fMRI hemodynamic responses may depend on the situations and experimental paradigms, is probably brain region specific, and might not be a general property of the aging brain.
There are three possible ways to explain the origins of age-related amygdala time course differences found in the present study. The first is vascular effects of aging, including stiffening of the arterial wall, decreased blood flow, and so on. Considering the blood flow directly influences the BOLD signal, the present data might reflect vascular issues in aged people. The data showed a negative BOLD change in the initial part of the event-related time course, which might be the “initial dip” (Heeger & Ress, 2002; Vanzetta & Grinvald, 1999; Malonek et al., 1997) caused by an increase in deoxyhemoglobin attributable to a brief uncoupling between blood flow and oxygen utilization; this has been reported in patients with arterial stenoses who exhibited larger initial dip in left primary motor cortex (Roc et al., 2006). Therefore, it might be possible that blood flow in the amygdala in aged people increased slowly, and did not catch up the oxygen consumption, which caused an early large negative BOLD signal. And if the increase of the blood flow ended earlier, the BOLD signal would drop earlier, resulting in their sharpened hemodynamic pattern. Nevertheless, considering that we found such a time course difference between age groups only in the amygdala, and not in other affective brain regions, the observed age-related changes in time course difference cannot be due solely to this vascular change with aging. Nonetheless, future studies should consider measuring participants’ vascular stiffness and other systemic hemodynamic measurements (arterial pressure, pulse wave, etc.) and relating these to the functional data.
The second explanation for age-related changes in the hemodynamic time course of the amygdala is alteration of neurovascular coupling with age. Neurovascular coupling refers to the processes by which neural activity influences the hemodynamic properties of the surrounding vasculature (cf. D’Esposito, Deouell, & Gazzaley, 2003). It is still unclear whether neurovascular coupling is altered with aging (see Fabiani & Gratton, 2004; Rosengarten, Aldinger, Spiller, & Kaps, 2003; Buckner et al., 2000). The fact that we did not find age-related differences in the shape of the time course other brain regions, however, suggests that changes in neurovascular coupling might not be the main source of the age-related differences observed in the current study. This issue should be addressed by future studies.
A third possible explanation for age-related changes in the hemodynamics of the amygdala time course is that other brain areas, such as medial posterior OFC and adjacent inferior frontal gyrus (IFGorb), are up-regulating or sustaining the neural response to novel images in younger individuals, such that brains of younger people appear to hold on to novel information longer than brains of older people. This regulatory hypothesis is plausible given that OFC–IFGorb areas are reciprocally connected with the amygdala (Milad & Rauch, 2007; Petrides, 2007; Rempel-Clower, 2007; Bachevalier & Loveland, 2006). A caudal sector of lateral OFC (Brodmann’s areas 12 and 13) is mainly interconnected with the amygdala (Carmichael & Price, 1995; Barbas & De Olmos, 1990; Aggleton, Burton, & Passingham, 1980), midline thalamus, and temporal pole (Bachevalier & Loveland, 2006). This connection is very unique because the lateral OFC area receives projections from both the amygdala and the temporo-polar area, whereas the rest of prefrontal cortex appear to have fewer connections with the amygdala and temporal pole (Ghashghaei & Barbas, 2002). Posterior OFC has been known to be involved in novelty processing (Petrides, 2007), along with the prefrontal cortices (Mesulam, 1998). Taken together with the results of the present study, this system is altered in older people.
Whether changes in the amygdala time course are due to the vascular effects of aging, alterations of neurovascular coupling, or reduced amygdala regulation by other brain regions in the affective workspace, these findings are consistent with the “aging brain hypothesis” that improved affective stability in later adulthood is a by-product of biological decline including structural and functional degradation of the amygdala and other affect-sensitive brain areas (Scheibe & Carstensen, 2010; Cacioppo, Berntson, Bechara, Tranel, & Hawkley, 2008). This does not mean that older people lose their capacity to respond to affective salient (including novel) environmental conditions, but rather, that older brains do not show sustained processing in this regard.
Furthermore, our results suggest that a consideration of novelty might play a key role in understanding the affective changes that occur with age. Without the moderating influence of stimulus novelty, there were no age-related differences in amygdala activation for positive versus negative stimuli. By including novelty, however, we were able to observe that positive stimuli were perceived as more familiar (and therefore perhaps not as evocative) in older individuals. This is consistent with the recent observation that younger adults exhibited novelty memory bias for the positive items, whereas older adults did not, such that older adults experienced greater overall familiarity for positive items (Spaniol, Voss, & Grady, 2008). On the surface, this might appear inconsistent with earlier published report, but in fact, previous studies of age-related differences in amygdala responsivity have been inconsistent. Older individuals were observed to show increased amygdala responses to positive IAPS images (Mather et al., 2004), but other studies have shown the opposite (Addis, Leclerc, Muscatell, & Kensinger, 2010). Furthermore, positive facial expressions did not activate the amygdala in older individuals more than in young individuals (Gunning-Dixon et al., 2003; Iidaka et al., 2002).
Finally, our findings on the subjective experience of arousal point to potentially important age-related changes in the subjective salience of visual images. Novel pictures were more subjectively arousing for everyone, reflecting their increased salience, but older individuals found them less arousing than did younger individuals. In addition, older individuals found high arousal pictures less arousing when compared to younger individuals. These differences in subjective arousal very likely reflect age-related reductions in interoceptive information from the body. Older individuals are less interoceptively sensitive (e.g., Khalsa, Rudrauf, & Tranel, 2009), and have blunted physiological reactivity (Levenson, Carstensen, Friesen, & Ekman, 1991). In addition, they are less likely to use information from the body to make decisions under uncertainty (Denburg, Tranel, & Bechara, 2005). According to the concept of “maturational dualism” (Mendes, 2010), these age-related changes in sensory feedback from the body has consequences for age-related changes in subjective experience of affect. Given the amygdala’s role in regulating autonomic response, the peakier time course of the amygdala activation in older individuals might be related to these autonomic changes, although this is a point for future research.
Acknowledgments
We thank Mary Foley and Larry White for their technical assistance. This work was supported in part by the National Institutes of Health Director’s Pioneer Award (DP1OD003312) and a National Institute on Aging grant (AG030311) to Lisa Feldman Barrett.
Footnotes
To clarify whether the effect of stimulus novelty on the BOLD response in the amygdala was mediated by subjective arousal, we conducted mediation analyses in the left and right amygdala with stimulus novelty as an independent variable, amygdala BOLD response estimated by FIR analysis as a dependent variable, and subjective arousal rating in every event as a mediator. For the right and left amygdala, subjective arousal only partially mediated amygdala response (indirect effects were significant; z = 7.000, p < .0001 for the right, z = 6.064, p < .0001 for the left). Nonetheless, stimulus novelty continued to directly predict amygdala response (z = 1.89, p = .058 for the right, z = 3.31, p = .0009 for the left). These findings replicate those reported in Weierich et al. (2010), indicating that amygdala responses to novelty were not solely related to the arousing nature of the novel pictures. In addition, we computed a set of correlational analyses to examine whether differences in subjective arousal ratings (novel –familiar) were related to the differences in amygdala BOLD activity in novel (vs. familiar) contrasts. These findings indicated that the difference between subjective arousal in novelty (vs. familiar) and in the amygdala contrasts for novelty (vs. familiar) were related for positive pictures only. The young group showed a larger positive correlation between subjective rating difference scores and right amygdala contrast in response to positive pictures, and a larger negative correlation for neutral pictures, but the older group did not show that pattern. Specifics of the analyses are available from the first author upon request.
References
- Addis DR, Leclerc CM, Muscatell K, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2009;46:425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, et al. The BOLD hemodynamic response in healthy aging. Journal of Cognitive Neuroscience. 2004;16:786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a psychological primitive. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 41. Burlington, VT: Academic Press; 2009. pp. 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin H, Jie L, Kevin MS, Joseph D, Emanuel D. A cortical potential imaging analysis of the P300 and novelty P3 components. Human Brain Mapping. 2001;12:120–130. doi: 10.1002/1097-0193(200102)12:2<120::AID-HBM1009>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociological Methods Research. 1992;21:230–258. [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. Journal of Cognitive Neuroscience. 2000;12(Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: Implication for limbic–striatal interactions. Behavioral Neuroscience. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Bechara A, Tranel D, Hawkley LC. Could an aging brain contribute to subjective well being? The value added by a social neuroscience perspective. In: Tadorov A, Fiske ST, Prentice D, editors. Social neuroscience: Toward understanding the underpinnings of the social mind. New York: Oxford University Press; 2008. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Turk-Charles S. The salience of emotion across the adult life span. Psychology and Aging. 1994;9:259–264. [PubMed] [Google Scholar]
- Daigneault S, Braun CM. Working memory and the self-ordered pointing task: Further evidence of early prefrontal decline in normal aging. Journal of Clinical and Experimental Neuropsychology. 1993;15:881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. Affect is a form of cognition: A neurobiological analysis. Cognition & Emotion. 2007a;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. The role of the amygdala in visual awareness. Trends in Cognitive Sciences. 2007b;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive & Affective Neuroscience. 2009;4:127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G. Electrophysiological and optical measures of cognitive aging. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2004. pp. 85–106. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Arlington, VA: American Psychiatric Publishing; 1996. [Google Scholar]
- Fischer H, Furmark T, Wik G, Fredrikson M. Brain representation of habituation to repeated complex visual stimulation studied with PET. NeuroReport. 2000;11:123–126. doi: 10.1097/00001756-200001170-00024. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, et al. The international personality item pool and the future of public-domain personality measures. Journal of Research in Personality. 2006;40:84–96. [Google Scholar]
- Goldstein A, Spencer KM, Donchin E. The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology. 2002;39:781–790. [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nature Reviews Neuroscience. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- Hu MTM, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Morris RG, Clough C, et al. Evidence for cortical dysfunction in clinically non-demented patients with Parkinson’s disease: A proton MR spectroscopy study. Journal of Neurology, Neurosurgery and Psychiatry. 1999;67:20–26. doi: 10.1136/jnnp.67.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46:1130–1136. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Grabowecky M. Prefrontal cortex, time, and consciousness. In: Gazzaniga M, editor. The new cognitive neurosciences. 2. Cambridge, MA: MIT Press; 1999. pp. 1319–1337. [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SKE, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJH, et al. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: Lang PJ, Balaban RF, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Erlbaum; 1997. pp. 97–134. [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective & Behavioral Neuroscience. 2008;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA. Age-related changes in neural activity during visual perception and attention. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging. New York: Oxford University Press; 2005. p. 171. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: Relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proceedings of the National Academy of Sciences, USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mendes WB. Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review. 2010 doi: 10.1177/ 1754073910364149. [DOI] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Mesulam M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Brain, mind, and the evolution of connectivity. Brain and Cognition. 2000;42:4–6. doi: 10.1006/brcg.1999.1145. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Misangyi VF, LePine JA, Algina J, Goeddeke F., Jr The adequacy of repeated-measures regression for multilevel research: Comparisons with repeated-measures ANOVA, multivariate repeated-measures ANOVA, and multilevel modeling across various multilevel research designs. Organizational Research Methods. 2006;9:5–28. [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature Neuroscience. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences, USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magnetic Resonance in Medicine. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. The orbitofrontal cortex: Novelty, deviation from expectation, and memory. Annals of the New York Academy of Sciences. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Annals of the New York Academy of Sciences. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Roc AC, Wang J, Ances BM, Liebeskind DS, Kasner SE, Detre JA. Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenoses. Stroke. 2006;37:382–387. doi: 10.1161/01.STR.0000198807.31299.43. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Aldinger C, Spiller A, Kaps M. Neurovascular coupling remains unaffected during normal aging. Journal of Neuroimaging. 2003;13:43–47. [PubMed] [Google Scholar]
- Ross MH, Yurgelun-Todd DA, Renshaw PF, Maas LC, Mendelson JH, Mello NK, et al. Age-related reduction in functional MRI response to photic stimulation. Neurology. 1997;48:173–176. doi: 10.1212/wnl.48.1.173. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Spreekmeester E, Meaney MJ, Quirion R, Rochford J. Reactivity to novelty in cognitively-impaired and cognitively-unimpaired aged rats and young rats. Neuroscience. 1998;83:669–680. doi: 10.1016/s0306-4522(97)00464-8. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Review of Neuroscience. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: Implications for the cognitive reserve hypothesis. Archives of Neurology. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. Journal of Gerontology: Series B, Psychological Sciences and Social Sciences. 2010;65:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer KR, Schorr A, Johnstone T. Appraisal processes in emotion: Theory, methods, research. Canary, NC: Oxford University Press; 2001. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Voss A, Grady CL. Aging and emotional memory: Cognitive mechanisms underlying the positivity effect. Psychology and Aging. 2008;23:859–872. doi: 10.1037/a0014218. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: Fronto-amygdalar differences during emotional perception and episodic memory. Journal of the International Neuropsychological Society. 2009;15:819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Anterior medial temporal lobe in human cognition: Memory for fear and the unexpected. Cognitive Neuropsychiatry. 2006;11:198–218. doi: 10.1080/13546800500305096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Curiosity and mortality in aging adults: A 5-year follow-up of the Western Collaborative Group Study. Psychology and Aging. 1996;11:449–453. doi: 10.1037//0882-7974.11.3.449. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Boston, MA: Allyn & Bacon; 2006. [Google Scholar]
- Taoka T, Iwasaki S, Uchida H, Fukusumi A, Nakagawa H, Kichikawa K, et al. Age correlation of the time lag in signal change on EPI-fMRI. Journal of Computer Assisted Tomography. 1998;22:514–517. doi: 10.1097/00004728-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–1600. doi: 10.1126/science.1133306. [DOI] [PubMed] [Google Scholar]
- Tilman D, Reich PB, Knops JM. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: Implications for functional brain imaging. Science. 1999;286:1555–1558. doi: 10.1126/science.286.5444.1555. [DOI] [PubMed] [Google Scholar]
- Wedig MM, Rauch SL, Albert MS, Wright CI. Differential amygdala habituation to neutral faces in young and elderly adults. Neuroscience Letters. 2005;385:114–119. doi: 10.1016/j.neulet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biological Psychiatry. 2007;62:1388–1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Yuan JJ, Yang JM, Meng XX, Yu FQ, Li H. The valence strength of negative stimuli modulates visual novelty processing: Electrophysiological evidence from an event-related potential study. Neuroscience. 2008;157:524–531. doi: 10.1016/j.neuroscience.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking and risky behavior. Washington, DC: American Psychological Association; 2007. [Google Scholar]