Abstract
Posttraumatic stress disorder (PTSD) is a complex psychiatric disorder that involves symptoms from various domains that appear to be produced by the combination of several mechanisms. The authors contend that existing neural accounts fail to provide a viable model that explains the emergence and maintenance of PTSD and the associated heterogeneity in the expression of this disorder (cf. Garfinkel & Liberzon, 2009). They introduce a psychological construction approach as a novel framework to probe the brain basis of PTSD, where distributed networks within the human brain are thought to correspond to the basic psychological ingredients of the mind. The authors posit that it is the combination of these ingredients that produces the heterogeneous symptom clusters in PTSD. Their goal is show that a constructionist approach has significant heuristic value in understanding the emergence and maintenance of PTSD symptoms, and leads to different and perhaps more useful conjectures about the origins and maintenance of the syndrome than the traditional hyperreactive fear account.
The codification of posttraumatic stress disorder (PTSD) as an official diagnosis in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III; American Psychiatric Association [APA], 1980) led to 3 productive decades of theory development and empirical research. Although this work confirmed the legitimacy of PTSD as an important diagnosis and has produced critical insights into the workings of this disorder, many controversies remain (cf. Rosen, 2004). Perhaps the most important unresolved issue is clarity regarding the core features of the disorder (Brewin, Lanius, Novac, Schnyder, & Galea, 2009). The DSM currently classifies PTSD as an anxiety disorder, with fear or anxiety as a necessary component to acquire this disorder: Criterion A2 specifies that an individual must react to a traumatic event with “intense fear, helplessness, or horror.” Many of the other criteria can be interpreted as physiological, behavioral, or psychological correlates or sequelae of fear and anxiety. Yet PTSD is exceptionally heterogeneous in its presentation. First, the experience of fear and anxiety is not highly predictive of PTSD status (e.g., Adler, Wright, Bliese, Eckford, & Hoge, 2008; Bedard-Gilligan & Zoellner, 2008; Breslau & Kessler, 2001; Brewin et al., 2009; Karam et al., 2010; O’Donnell, Creamer, McFarlane, Silove, & Bryant, 2010; Roemer, Orsillo, Borkovec, & Litz, 1998; Schnurr, Spiro, Vielhauer, Findler, & Hamblen, 2002). Clearly, the objective presence of a fear-inducing threat does not uniformly lead to PTSD, in that most individuals who are exposed to threat do not develop the disorder (e.g., Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995), and some individuals appear to develop PTSD without exposure to threat (e.g., Bodkin, Pope, Detke, & Hudson, 2007; Gold, Marx, Soler-Baillo, & Sloan, 2005; Mol et al., 2005; Olde, van der Hart, Kleber, & van Son, 2006). Second, even when present, the experience of fear and anxiety are not specifically diagnostic of PTSD in that they represent one specific manifestation within a range of pathological responses resulting in exposure to a traumatic event (Resick & Miller, 2009). For example, individuals with PTSD also report experiencing anger, sadness, guilt, and disgust (e.g., Brewin, Andrews, & Rose, 2000; Brewin et al., 2009; Kilpartrick et al., 1998; McNally, 2003; Ozer, Best, Lipsey, & Weiss, 2003; Resick & Miller, 2009; for a review, see Bovin & Marx, 2011). In addition, many of the key symptoms appear to implicate dysfunction in more basic mechanisms related to salience and attention, hyperarousal, working memory, and long-term memory (e.g., Brewin, Gregory, Lipton, & Burgess, 2010; Bryant, Creamer, O’Donnell, McFarlane, & Silove; 2008; Bryant & Harvey, 1997; Constans, 2005; Dalgleish, 2004; Marshall, Schell, Glynn, & Shetty, 2006; Moore & Zoellner, 2007; Pineles, Shipherd, Mostoufi, Abramovitz, & Yovel, 2009; Rubin, Bern-sten, & Bohni, 2008; Schell, Marshall, & Jaycox, 2004; Shaw et al., 2009; Shipherd & Salters-Pedneault, 2008), although none of these problems are themselves specifically predictive of PTSD status either (see Bovin & Marx, 2011 for a review). In these regards, PTSD is not unique: Most, if not all, DSM diagnostic categories face these challenges (lack of consistency and specificity; Krueger, Watson, & Barlow, 2005; Sanislow, et al., 2010).
One possibility, of course, is that even if the behavioral, physiological, and experiential aspects of PTSD can be quite varied, the disorder nonetheless results from key alterations in fear circuitry. Animal models describe PTSD in these terms (e.g., Dbiec, J., & LeDoux, 2009; Etkin & Wager, 2007; Jovanovic & Ressler, 2010; Jovanovic, et al., 2009; Neumeister, Henry, & Krystal, 2007; Rauch, Shin, & Phelps, 2006; Shin & Handwerger, 2009; Shin & Liberzon, 2010; Shin, Rauch, & Pitman, 2005, 2006; Weiss, 2007). A common hypothesis in both animal and human neuroscience models of PTSD is that the amygdala, as a key structure in fear circuitry, is hyperreactive to incoming stimuli. This hyperactivity is thought to cause fearful responses associated with a constant disruption in homeostasis, in part because the amygdala is thought to be insufficiently inhibited by areas of prefrontal cortex. This reduced inhibition is to hypoactivation in the anterior cingulate cortex and the medial sector of orbitofrontal cortex (also called the ventromedial prefrontal cortex). Furthermore, deficits in hippocampal processing result in a failure to contextualize responses (e.g., Maren & Holt, 2000; Rudy & O’Reilly 1999—for a review, see Bouton, Westbrook, Corcoran, & Maren, 2006). These brain findings, stemming from animal models of fear-conditioning, are widely interpreted as evidence that PTSD involves enhanced fear learning, a failure to calibrate affective responses to stimuli that no longer represent threat, as well as inadequate top-down regulation of that reactivity. Supportive evidence for this view comes from a recent meta-analysis that empirically compared neuroimaging studies of PTSD, other anxiety disorders, and studies of aversive learning using classical conditioning (Etkin & Wager, 2007). Using the most sophisticated meta-analytic approach available (Wager, Lindquist, & Kaplan, 2007), this meta-analysis showed that individuals diagnosed with PTSD consistently have altered activation in predicted brain structures (depicted in Figure 1), including hyperactivation in the amygdala and the anterior insula, reduced activation in the ventromedial prefrontal cortex extending back to the subgenual (or ventral) anterior cingulate cortex, reduced activation in the more dorsal portion of the anterior cingulate cortex (also called the middle cingulate cortex; Vogt, 2005), and reduced activity in the hippocampus. Relative to matched-comparison subjects (i.e., healthy controls), hyperactivation in the amygdala and insula was less consistent across studies in individuals diagnosed with PTSD than in individuals diagnosed with social anxiety disorder and specific phobias. On the other hand, in relation to matched-comparison subjects, hypoactivation in the ventromedial prefrontal cortex, rostral and dorsal anterior cingulate cortex, and the thalamus was only observed in individuals diagnosed with PTSD (and not individuals diagnosed with social anxiety disorder or specific phobias).
Figure 1.
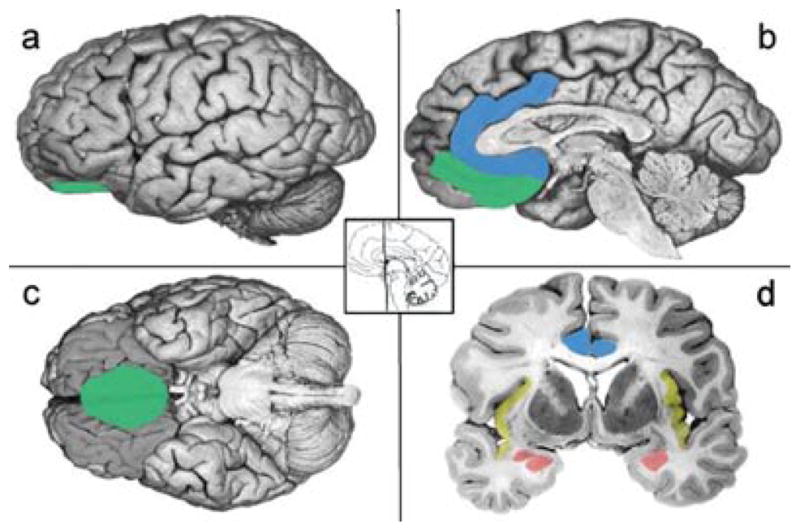
Schematics based on findings from Etkin and Wager (2007). The amygdala is depicted in coral. The insula is depicted in yellow. The ACC is depicted in blue. The medial OFC is depicted in green, Adapted from “The experience of emotion,” by L. F. Barrett, B. Mesquita, K. N. Ochsner, and J. J. Gross, 2007, Annual Review of Psychology, 58, 373–403. Copyright 2007 by Annual Reviews.
There is growing concern that the “hyperreactive, undercontrolled fear” model of PTSD is limited in several important ways. First, there is the continuing matter that the model is limited in its ability to capture the heterogeneity (cf. Shin & Hardwerger, 2009) and variety (cf. Garfinkel & Liberzon, 2009) of prominent symptoms in PTSD. Although there have been attempts to use neuroimaging findings to motivate subtypes of PTSD (one characterized predominantly by hyperarousal and intrusions and another characterized by dissociation; Lanius, Bluhm, Lanius, & Pain, 2006; Lanius, Vermetten, et al., 2010), such a strategy does not solve the problem. Second, even in the context of strong meta-analytic findings (Etkin & Wager, 2007), there is heterogeneity in the neural responses associated with PTSD. For example, in a recent review of neuroimaging findings, Garfinkel and Liberzon (2009) note that several studies do not show enhanced amygdala response in PTSD. They also note that reduced hippocampal, anterior cingulate cortex, amygdala, and insular volumes are common in PTSD, and may represent a preexisting vulnerability to PTSD, but not all individuals who develop the illness actually show these structural changes. Even Etkin and Wager note that fear may be a more defining feature of social anxiety disorder and specific phobias, with PTSD characterized by a broader range of emotional regulation dysfunction. Furthermore, functional connectivity studies (using correlation or causal modeling techniques to identity brain regions whose blood oxygenation level dependent (BOLD) time courses are correlated across time) are either inconsistent with the underregulation hypothesis (Lanius, Vermetten, et al., 2010) or they suggest the opposite interpretation (that the amygdala is influencing medial frontal regions rather than vice versa; Gilboa et al., 2004). Third, several scientists have noted the need for a broader conceptualization of the processes involved in the disorder (Bovin & Marx, 2011; Garfinkel & Liberzon, 2009). In a recent review, Bovin and Marx advocated for a “dimensional approach” to PTSD, with a focus on the underlying processes or properties that are common to all emotions, such as the affective dimensions of valence and arousal (e.g., Ellsworth & Scherer, 2003; Lang, Bradley, & Cuthbert, 1990; Russell & Barrett, 1999).
In this article, we build on these observations and take them one step further to evaluate the adequacy of the dysregulated fear circuitry model of PTSD, and suggest an alternative theoretical framework for guiding scientific inquiry about PTSD. Our goal is not to review the literature for each and every neuroscience study to determine whether PTSD is associated with a hyperresponsive amygdala and a hyporesponsive prefrontal cortex. Many comprehensive review articles (Garfinkel & Liberzon, 2009; Jovanovic & Ressler, 2010; Neumeister, Henry, & Krystal, 2007; Shin & Hardweger, 2009; Shin, Rauch, & Pitman, 2005, 2006), including the Etkin and Wager (2007) meta-analysis confirm this observation. Instead, our goal is simply to ask whether dysregulated fear is the most advantageous and productive approach to interpreting the existing brain evidence in psychological terms.
In asking this question, we do not criticize the painstaking and careful animal studies that have mapped the circuitry for behavioral adaptations such as fight, flight, freezing, or enhanced startle responses (e.g., Davis, 1986, 1992, 2000; Fanselow, 1994; Kapp, Frysinger, Gallagher, & Haselton, 1979; LeDoux, 1990) that occur with the presentation or learning of aversive stimuli. We agree with the generally held view that any valuable theoretical framework for understanding PTSD must incorporate rigorous behavioral neuroscience evidence to understand basic mechanisms and how they go awry or lead to dysfunction elsewhere. We do not plan to argue with the idea that the combination of increased amygdala response combined with reduced prefrontal cortex response results in hyperarousal or enhanced affective reactivity. We do ask, however, whether the interpretation of these results as the underregulation of fear circuitry reveals the mechanisms that contribute to the development and maintenance of PTSD. We examine if another interpretation of the same evidence makes the heterogeneity in PTSD and its similarity to other disorders more predictable and easier to understand.
In this article, we first address whether neuroscientific findings give evidence of a fear network that is largely inhibited by the prefrontal cortex and reconsider the central hypothesis that PTSD should be classified primarily as a fear-based disorder. Next, instead of taking a locationist approach and asking if there are specific brain regions (or interactions between discrete regions) that cause core symptoms in PTSD, we introduce a relatively new psychological construction approach to ask how basic ingredients of the mind (which are represented as distributed networks within the human brain) interact so that PTSD emerges in all its variety and complexity (following Barrett, 2009a). It is becoming increasingly clear that mental states and behavior at any particular time are determined by a number of large-scale distributed networks (e.g., Poldrack, Halchenko, & Hanson, 2009; Smith et al., 2009) that interact in a complex, dynamic fashion constantly influencing and constraining one another in real time. We postulate that these large-scale networks can be thought of as the basic psychological operations or ingredients of the mind that contribute to the construction of normal mental states and behaviors. From this perspective, psychopathology can be understood as a problem in these basic operations or in their influence on one another, so that they provide a vocabulary for describing the endophenotypes that describe in psychological terms the varieties in any diagnostic category (Meyer-Lindenberg, 2008). In a final section, then, we also explore how key insights afforded by a psychological construction approach inform theoretical models of the neural circuitry underlying PTSD.
IS PTSD A FEAR-BASED DISORDER?
Is the Amygdala Specific to Fear in PTSD?
It is generally widely accepted that the amgydala is a crucial brain structure in fear. In animal research, fear is defined as “the behavioural adapation that allows organisms to detect and respond to threats” (LeDoux, 2008, p. 70). Behavioral adaptations are highly heritable, species-general actions that a creature performs to survive (or reproduce). Years of careful study have confirmed that the amygdala plays a crucial role in several behavioral adaptations involved in responding to threat, such as freezing in response to a tone that was previously paired with an electric shock or an enhanced startle response as a function of a threatening or negative stimulus (e.g., Davis, 1992; Fanselow & Poulous, 2005, Fendt & Fanselow, 1999; LeDoux, 2007). In humans, mild electric shock, commonly feared objects (e.g., spiders, snakes), or startled faces depicting fear consistently produce increased activation in the amygdala relative to neutral stimuli. (For a review, see Adolphs, 2010; for a meta-analytic summary, see Etkin & Wager, 2007; Mechias, Etkin, & Kalisch, 2010). It is a great scientific advance to know that animals freeze and startle in the face of a threat, and that the amygdala is a key structure in the circuitry that produces these behavioral adaptations. But is there any scientific reason to understand them as “fear,” over and above the obvious value of making animal research more accessible and relevant to humans? This question is centrally implicated in whether PTSD results from a dysregulated brain circuit for fear or whether there are other ways to understand the psychological relevance of an overreactive amygdala in combination with hypoactivation in prefrontal cortex.
The first important observation here is that there are many behavioral adaptations that an animal can show in response to a threat, and not all of them involve freezing or potentiated startle. Rats avoid the location of uncertain threat when they are free to move around, such as in a testing chamber with several arms (e.g., Kopchia, Altman, & Commissaris,1992; Vazdarjanova & McGaugh, 1998). Rats will also attack a threat if it is known (e.g., Reynolds & Berridge, 2008). Each of these actions (freezing, potentiated startle, defensive aggression, and avoidance) involves different circuitry, and not all of the circuits involve the amygdala; even rats with amygdala ablations show place avoidance following aversive learning. Given this heterogeneity, which is the real fear circuit? If there are many fear circuits, then what makes them all instances of the same category fear—the fact that there is a threat? If this is so, then the perception of threat becomes the key feature in fear, not a specific circuit for a behavior. Is the circuit for avoidance (that does not require an intact amygdala) also a fear circuit? Some scientists suggest that behavioral adaptations occur in stages (i.e., the defense cascade model; Bradley & Lang, 2000; Lang, 1995; Lang, Bradley, & Cuthbert, 1997), but there are others who instead assume that these different adaptations occur in different contexts (depending on the proximity and certainty of a threat; Fanselow, 1994; Fanselow & Lester, 1988). Context is even important as to whether or not stereotyped increases in heart rate or blood pressure occur with freezing behavior (Iwata & LeDoux, 2010), indicating that the autonomic consequences of a putative fear circuit do not always involve hyperarousal. This heterogeneity in the circuits that underlie behavioral responses to threat in animal models makes a direct connection to PTSD less straightforward.
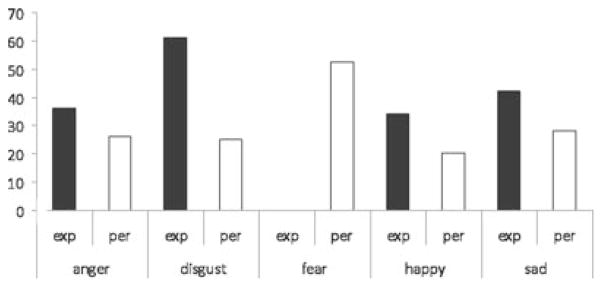
The second observation is that, just as the amygdala is not necessary for withdrawing in the face of a threat, neither is it necessary for the experience or perception of fear. In our most recent meta-analysis of the neuroimaging literature on emotion that summarizes published articles from 1993 to 2007 (Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, in press) using the Wager et al. (2007) method, we found that the amygdala is not consistently activated during fearful experiences at a level greater than what would be expected by chance. Although an increase in amygdala activation was consistently observed for other emotional experiences such as disgust, sadness, and anger (see Figure 2; see also Kober et al., 2008), studies of fear experience did not consistently report an increase in amygdala response. The perception of fear was consistently associated with an increase in amygdala activation, but this is not evidence that the amygdala is necessary for the perception of fear. Even though they signal more imminent danger, the amygdala does not show an increase in activation to startled, fearful faces with averted eye gazes (e.g., Adams, Gordon, Baird, Ambady, & Kleck, 2003; Ewbank, Fox, & Calder, 2010; Straube, Dietrich, Mothes-Lasch, Mentzel, & Miltner, 2010) or fearful faces that are masked with visual noise rather than a neutral face (Kim et al., 2010). Even individuals with amygdala damage can recognize fearful faces (Adolphs et al., 2005; Tsuchiya, Moradi, Felsen, Yamazaki, & Adolphs, 2009) and bodies (Atkinson, Heberlein, & Adolphs, 2007). The lack of consistency in the link between the amygdala and instances of fear experience and fear perception attenuates the straightforward view that PTSD is rooted in an abnormal fear response.
Figure 2.
Amygdala activation in the experience and perception of emotion. The proportion of studies in Lindquist et al. (in press) that report increased activation in the amygdala. The Y-axis reports the proportion of studies. Exp = Experience; Per = perception.
A third observation leading us to question the view that PTSD is predominantly a fear-based disorder is that the amygdala is itself not a brain region that is specific to the experience of fear or even threat. As we noted in our meta-analyses (Lindquist et al., 2010; Wager et al., 2008), increased amygdala response has been implicated in most negative emotions such as disgust, sadness, and anger. As well, activation in the amygdala is best predicted in logistic regressions by high arousal conditions. Increased amygdala response has been observed in high arousal states of surprise and excitement (see meta-analytic summaries in Costafreda, Brammer, David, & Fu, 2008; Sergerie, Chochol, & Armony, 2008), and more generally in response to both positive and negative images (e.g., responses to positive and negative faces, Zald, 2003; positively and negatively valenced pictures, Anders, Eippert, Weiskopg, & Veit, 2008; positively and negatively valenced words, Posner et al., 2009; meta-analytic summaries in Barrett & Wager, 2006; Costafreda et al., 2008; Sergerie et al., 2008; Wager et al., 2008). The amygdala is also implicated in reward and appetitive learning (reviewed in Ball et al., 2009; Balleine & Killcross, 2006; Baxter & Murray, 2002; Seymour & Dolan, 2008). Amygdala response is heightened for novel material (Breiter et al., 1996; Moriguchi et al., in press; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Weierich et al., 2010; Wright et al., 2003; Wright, Wedig, Williams, Rauch, & Albert, 2006; for a review, see Strange & Dolan, 2006) even when it is not explicitly valenced (e.g., Wright et al., 2008), and amygdala lesions disrupt normal responses to novelty in nonhuman primates (e.g., Burns, Annett, Kelley, Everitt, & Robbins, 1996; Mason, Capitanio, Machado, Mendoza, & Amaral, 2006; Prather et al., 2001; for reviews, see Knight & Grabowecky, 1999; Petrides, 2007). The amygdala, in interaction with the orbitofrontal cortex, has also been implicated more generally in reversal-learning-based deficits, with lesion studies showing that lesions to the orbitofrontal cortex or amygdala differentially influence an animal’s ability to adjust its behavior to changing reinforcement contingencies (e.g., Burke, Franz, Miller, & Schoenbaum, 2007; Schoenbaum, Roesch, Stalnaker, & Takahashi, 2009; reviewed in Schoenbaum, Saddoris, & Stalnaker, 2007).
Together, these findings shape an emerging view that the amygdala’s function is not to represent a fear state, or even anything negative per se. An alternative hypothesis that is gaining strength is that the amygdala’s function is related to computing the salience of an uncertain stimulus that is homeostatically relevant, so that it modulates other brain systems to increase the processing of that stimulus to gain information for future use (e.g., Ewbank, Barnard, Croucher, Ramponi, & Calder, 2009; for a discussion, see Adolphs, 2010; Barrett & Bliss-Moreau, 2009; Dayan & Balleine, 2002; Duncan & Barrett, 2007a,b; O’Doherty & Bossaerts, 2008; Seymour & Dolan, 2008). This interpretation is also consistent with the view that the amygdala is a key brain structure that is involved in evaluating an object for its goal relevance (Sander, Grafman, & Zalla, 2003), as well as findings that the amygdala is most active during ambiguity or uncertainty (e.g., Herry, et al., 2007; Rosen & Donley, 2006; Whalen, 1998, 2007). Instead of instantiating a fear state then, the amygdala might help to induce a more general vigilant state (associated with decreased voluntary muscle movement, increased skin conductance associated with sympathetic nervous system activity, and increased blood pressure) to allow a creature to acquire more information to reduce uncertainty and render an adaptive response. There is evidence to show that ambiguity and uncertainty produce the autonomic markers of threat (Mendes, Blascovich, Hunter, Lickel, & Jost, 2005), which create the body context for changes in affect (Barrett & Bliss-Moreau, 2009) that can then be categorized and experienced as a physical symptom, or as a discrete emotion such as fear or anger, or even as the perception that a person is threatening or mean (Barrett, 2006a,b).
In the end, a circuit that produces a behavior is just that—it is not a circuit that produces a broad and complex psychological category like fear. Rats that freeze when they hear a tone paired with a foot shock might be in a state of fear, but they could also be in a state of surprise, anger, a general state of alarm, or merely a state that is conducive to reducing uncertainty (for a similar discussion of this point, see Kagan, 2009). Here is what we know for certain: Freezing (or startle) is an innate action pattern in mammals that has been preserved in some form through natural selection, and freezing is part of the Western script for fear. However, this does not necessarily translate into the assumption that the circuitry producing freezing behavior constitutes evidence for a fear circuit unless one is willing, a priori, to ontologically reduce fear to freezing behavior; a similar line of reasoning can be applied to the startle response.
Clearly, hyperresponsiveness in the amygdala is an important feature of PTSD, but this does not mean that PTSD has an abnormality in fear. If we abandon the necessary connection between fear and the amygdala response, then the brain data become more consistent with the behavioral and experiential data, which also fail to find that fear (whether defined behaviorally or experientially) is a central feature of the disorder. Furthermore, recognition that the amygdala is active in many kinds of unpleasant emotional experiences helps to explain the observation that PTSD routinely involves the experience of other negative emotions. Of course, this would also mean that the psychological meaning of amygdala hyperactivity is still an open scientific question.
Does the Prefrontal Cortex Inhibit a Subcortical Fear Circuit?
One element in the hyperreactive, undercontrolled fear hypothesis is that PTSD symptoms arise from reduced regulation capacity associated with hyporesponsivity of paralimbic cortical regions such as the ventromedial prefrontal cortex in the medial orbitofrontal cortex, and immediately posteriorly, the subgenual anterior cingulate cortex. The fact that the cortex inhibits subcortical regions is often taken as evidence that more evolutionarily recent, cognitive parts of the brain control the more ancient, emotional parts, and any disruption of this control leads to psychopathology such as is observed in PTSD. This somewhat Cartesian understanding of the human mind (the more human parts of cognition inhibit the more animalistic instincts and urges) is rooted in the triune brain concept (MacLean, 1949, 1990). The triune brain concept, however, represents a somewhat outdated understanding of brain evolution (Striedter, 2005). As it turns out, the cortex does more than just inhibit subcortical regions. The true picture of cortical regulation is more complex.
All mammalian nervous systems share the same basic architecture where the cortex modulates subcortical target regions by a complex set of cascading projections, some of which excite and others which subdue subcortical activity (Swanson, 2005). These multiple descending pathways from cortical areas to subcortical autonomic regions in the hypothalamus, periaqueductal gray, and brainstem (schematically depicted in Figure 3) produce a complex pattern of autonomic regulation (again, see Swanson, 2005) that leads to the counter-intuitive hypothesis that in PTSD the cortex might be selectively enhancing (as opposed to failing to control) automatic reactivity. Cortical areas do not merely put the brakes on autonomic reactivity, so that hypoactivation in these areas translates into enhanced affective reactivity. Instead, the cortex exerts a nuanced and complex influence over autonomic nuclei in the brainstem and even in the spinal cord. The direct connections from cortical regions to autonomic centers in the brainstem are actually excitatory (glutamatergic) and would work to enhance autonomic reactivity. Connections from cortical regions also have an inhibitory (GABAergic) effect on these autonomic regions via striatal parts of subcortex, putting the break on autonomic reactivity. In yet another set of connections, the cortical regions have another opportunity to enhance autonomic reactivity—as these regions excite neurons in the striatum, striatal neurons inhibit neurons in the pallidum, which has the effect of releasing these autonomic centers from inhibitory control. From this perspective then, hypoactivation in the ventromedial prefrontal cortex and ventral anterior cingulate cortex does not simply produce an increase in autonomic reactivity in PTSD, but instead it reflects a situation where there is reduced cortical oversight of bodily responses. This interpretation also explains why Etkin and Wager (2007) in their meta-analysis found individuals with PTSD routinely showed hypoactivation in the dorsal amygdala (which is where the central nucleus of the amygdala is located, the central nucleus being part of the striatal system; Swanson & Petrovich, 1998). Furthermore, because some research has shown that PTSD involves reduced GABA-receptor binding (Geuze et al., 2008), it is even possible that at times, cortical regions are enhancing autonomic reactivity, as opposed to failing to control it, because part of their inhibitory action might be selectively impaired. These observations could help explain why arousal dysregulation has been found in PTSD (e.g., Frewen & Lanius, 2006), but the nature and dynamics of the dysregulation requires closer attention.
Figure 3.
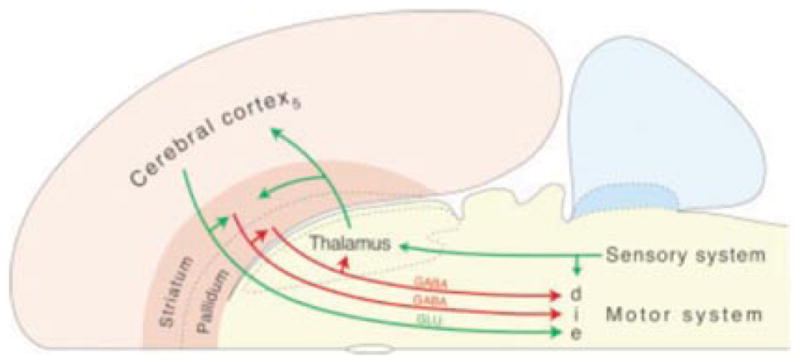
The triple descending pathways of Swanson’s “basic plan” of the nervous system. Taken from Swanson (2005). This “basic plan” of the central nervous system, as well as work by Barbas et al. (2003) and Price and colleagues (Ongur, Ferry, & Price, 2003), helps us to understand the complex cortical oversight of autonomic nervous system reactivity.
The cortical oversight of subcortical autonomic nuclei is also enhanced in humans relative to other mammals like rats and mice. In evolutionary terms, the primate’s brain—particularly the great ape’s—is distinguished by enhanced connectivity; this can be most easily seen in the cortical regulation of body states associated with behavioral adaptations and affective feelings. In primates, including humans, subcortical regions responsible for autonomic reactivity, including the periaqueductal gray and hypothalamus, receive inputs from areas of the prefrontal cortex (An, Bandler, & Price, 1998; Ongur & Price, 2000). These inputs originate from the ventromedial prefrontal cortex (the medial surface of the most posterior parts of Brodmann area 10) along with the ventral anterior cingulate cortex both directly (Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003; Ongur & Price, 2000) and indirectly via the central nucleus of the amygdala and other parts of the striatum (Amaral, Price, Pitkanen, & Carmichael, 1992; Carmichael & Price, 1995; Ghashghaei & Barbas, 2002; McDonald, 1998; Ongur & Price, 2000; Stefanacci & Amaral, 2002). As a result, humans and other great apes have greater direct and indirect cortical control over the subcortex and spinal cord. This allows greater autonomic and behavioral diversity and flexibility and results in a decreased chance of fixed action patterns when compared to rats and other mammals. The direct cortical-brainstem/spinal cord connections require only one synapse in humans. So although it makes sense to say that cortical regions regulate circuits for behavioral adaptations like freezing, fleeing, or fighting, this does not necessarily translate into the understanding that the cortex stands apart from—and separately regulates—fear, or that the reduction of such cortical oversight results in an underregulation of fear.
Summary
When considered as a whole, the evidence suggests that symptom presentations of PTSD are heterogeneous and have a reliable set of brain correlates (enhanced amygdala and insula activity in the context of hypoactive prefrontal and hippocampal regions) that appear important to, but are neither necessary nor specific for fear. Although the existing data do not clearly disconfirm the hyperreactive, undercontrolled fear model of PTSD, they do not clearly support it either. We suggest that the time is ripe to consider other ways of interpreting the amgydala, insula, and prefrontal cortex data, as well as to expand the scope of the brain findings that might be relevant to understanding PTSD.
In the next section, we take a descriptive, conventionally defined diagnostic category like PTSD and attempt to frame its brain correlates in terms of more basic psychological mechanisms or operations. We suggest that such a strategy can open up new vistas for understanding the heterogeneity of symptom presentations of those with PTSD, and also how the processes important in PTSD might be important in the psychopathology of a number of other diagnostic categories.
A PSYCHOLOGICAL CONSTRUCTION APPROACH TO PTSD
The Psychological Construction Approach
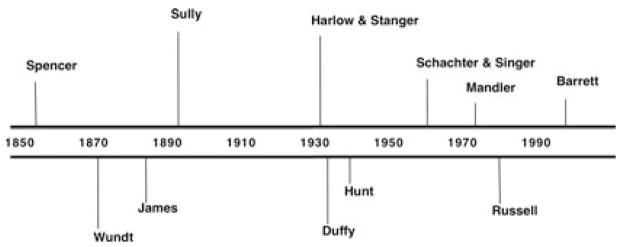
The hyperreactive, undercontrolled fear approach to PTSD belongs to the traditional “faculty” approach to psychology, where the mind is made up of different processes, each corresponding to a different kind of state. There are “cognitive” processes that produce cognitions (e.g., a memory system that produces memories), “emotional” processes that produce emotions (e.g., a fear system that produces fear), and “perceptual” processes” (e.g., a visual system that produces vision). A psychological construction approach to understanding the human mind, in contrast, assumes that the psychological events called emotions, cognitions, and perceptions are not basic, elemental faculties or “atoms” of the mind, but instead are the names people give to mental events that result from the interplay of a more basic, common set of psychological ingredients. Although these models do stretch back to the beginning of psychology (e.g., see Figure 4; for a review, see Gendron & Barrett, 2009), they are largely unintuitive and therefore relatively rare.
Figure 4.
Timeline of psychological construction models in psychology.
Psychological construction models differ in terms of whether the standard faculty categories have any scientific value (Gross & Barrett, in press). Elemental psychological construction models ontologically reduce mental categories to their more basic psychological ingredients, so that categories like fear, memory, and perception have no scientific value (e.g., Russell, 2003). Emergent models view such categories as having meaning, not as explanatory mechanisms, but at other levels of analysis (e.g., as ontologically subjective categories they have functional distinctions for human perceivers in making mental state inferences that allow communicating about and predicting human action; e.g., Clore & Ortony, 2008; for a discussion see Barrett, 2009b). Inspired by the scope of the earliest psychological models, our lab introduced the first psychological construction approach to mind–brain correspondence in 2005 and published several key articles articulating the basic assumptions and hypotheses of the model (Barrett, 2005, 2006b, 2009a; Barrett & Bar, 2009; Barrett & Bliss-Moreau, 2009; Barrett & Lindquist, 2008; Barrett, Lindquist, & Gendron, 2007; Barrett, Lindquist, Bliss-Moreau, et al., 2007; Barrett, Mesquita, Ochsner, & Gross, 2007; Barrett, Ochsner, & Gross, 2007; Duncan & Barrett, 2007b; Gendron & Barrett, 2009; Lindquist & Barrett, 2008a, 2008b). Our working hypothesis is that every human brain contains a number of basic ingredients that are used for making emotions and other mental states (like thoughts, memories, beliefs, and perceptions).
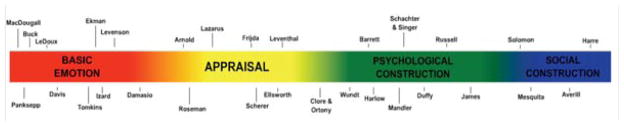
The original impetus for our model, called the conceptual act model, was to try to craft a set of hypotheses to explain (a) the considerable heterogeneity that was observed within any discrete emotion category (i.e., not all instances of fear look alike, feel alike, or appear to be caused in the same way Barrett, 2009a; Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000; Mauss & Robinson, 2009), and (b) the lack of firm boundaries between categories (e.g., the amygdala is implicated in almost every category of emotion at one time or another, as well in nonemotional states like novelty and even in memory and vision; Barrett, 2006b; Barrett, Lindquist, Bliss-Moreau, et al., 2007), while respecting the animal studies that show evidence of basic behavioral adaptations. We hypothesized that emotions are not elemental features of the mind, but they are emergent states that are constructed from a more basic set of psychological operations that are not themselves specific to emotion per se (for a comparison of discrete emotion vs. construction approaches, see Figure 5). We went beyond the emotion domain, however, extending our model to try to understand the degree of neural overlap in other faculties, like in cognition and emotion (Duncan & Barrett, 2007b; for a similar view, see Pessoa, 2008), and in emotion and perception (Barrett & Bar, 2009). In 2009, we developed our psychological construction approach even further to propose a broader set of hypotheses of correspondence between mind and brain (Barrett, 2009b; see Table 1). Taking inspiration from connectionist and network approaches to the brain (e.g., Fuster, 2006; Mesulam, 1998; O’Reilly & Munakata, 2000; Poldrack et al., 2009; Raichle & Snyder, 2007; Seeley et al., 2007; Smith et al., 2009), we hypothesized that basic psychological ingredients correspond to distributed functional networks of brain regions. Like ingredients in a recipe, the weighting and contribution of each network is predicted to vary across instances of each psychological category, or even across instances within the same category. One possibility is that these brain networks have intrinsic connectivity (i.e., show correlated activity during mental activity that is not triggered by an external stimulus). Another possibility is that these networks have dynamic functional connectivity (i.e., producing neural assemblies that routinely emerge in response to an external stimulus). The central idea, however, is to distinguish between the scientific question for psychology of identifying and understanding these basic psychological functions, and investigations and questions in neuroscience that can reveal the underlying brain basis of these psychological ingredients.
Figure 5.
Perspectives on emotion can be loosely arranged along a continuum. We have populated this continuum with representative theorists/researchers drawn from the field of psychology. We distinguish four “zones”: (a) basic emotion, in red (there exists a limited number of biologically basic states that are unique in form, function, and cause from other states such as cognition and perception); (b) appraisal, in yellow (emotion words still name privileged mental states that are unique in form, function, and cause from other mental states, but “anger,” “sadness,” “fear,” and other emotion words do not name distinct, dedicated mental mechanisms per se); (c) psychological construction, in green (emotions are not special mental states, unique in form, function, and cause from other mental states such as cognition and perception; emotions are not “caused” by dedicated mechanisms and emerge from an ongoing, continually modified constructive process that involves more basic ingredients that are not specific to emotion); and (d) social construction (emotions are viewed as social artifacts or culturally prescribed performances that are constituted by sociocultural factors). Given space constraints, as well as the goals of this article, we have limited ourselves to a subset of the many theorists/researchers who might have been included on this continuum (e.g., those who only study one aspect of emotion were not included in this figure). Adapted from “Emotion Generation and Emotion Regulation: One or Two Depends on Your Point of View,” by J. J. Gross and L. F. Barrett, L. F., in press. Emotion Review.
Table 1.
Mind–Brain Ontology
| Psychology | Example | Brain |
|---|---|---|
| Complex psychological category | Emotion (e.g., anger, sadness, fear, etc.), cognition (i.e., thoughts, memories, and beliefs), perception, posttraumatic stress disorder | Neural reference space |
| Psychological ingredient | Core affect, conceptualization/mentalizing, executive attention | Distributed network |
| Basic mechanism | Representing autonomic sensory cues, coding uncertainty, etc. | Circuit |
| Momentary mental state | Specific instance of “anger” | Neural assembly/brain state |
Because a psychological approach to correspondence of mind and brain is relatively new, we do not yet claim to know what the basic psychological ingredients are. Our proposals for basic psychological ingredients thus far are really more like basic domains of psychological functions (e.g., affect, conceptualization, executive function) that are a first approximation in the trajectory of a longer research program; we anticipate that they will be refined as research proceeds. Our psychological ingredients, as they currently stand, probably reflect a class of processes that are associated with assemblies of neurons within a distributed network, rather than a one-to-one mapping of ingredient to network. Ideally, with more research, it will be possible to identify distributed brain networks that are associated with psychological primitives—the most basic psychological descriptions that cannot be further reduced to anything else psychological. This is ambitious and daunting, but the search has to begin somewhere.
If psychological states are constructed, emergent phenomena will not reveal their more primitive elements any more than a loaf of bread reveals all its constituent ingredients. Therefore, neuroimaging evidence is particularly useful for examining the validity of a psychological construction approach. Preliminary support for a psychological construction approach to the mind comes from a growing appreciation in the neuroimaging literature that the same brain regions and networks are implicated across a variety of different task domains. In addition to the amygdala being broadly involved in both positive and negative affect, as well as in novelty, memory, and even in vision, there are other brain regions that show this pattern of general activation as well. The anterior insula (involved in representing visceral cues in subjective awareness; Craig, 2002, 2009) is one brain region that shows increased activation across a range of tasks, including working memory, task switching, emotion, language, and sensory processing (Nelson et al., 2010). Ventromedial prefrontal cortex and dorsomedial prefrontal cortex are active during emotion, person perception, object perception, and long-term memory (Barrett, 2009b). The left inferior frontal junction is involved in working memory, long-term memory, inhibition, and task switching (Van Snellenberg & Wager, 2009). Taking these examples as a set, though one might claim that a brain region is performing multiple tasks, a more parsimonious hypothesis is that each is performing a more basic process that is required across task domains.
Another important source of relevant evidence comes from a meta-analytic project summarizing the neuroimaging literature on emotion (Barrett, Mesquita, et al., 2007; Kober et al., 2008; Lindquist et al., 2010; Wager et al., 2008). Meta-analyses of the neuroimaging literature are useful for evaluating the success of psychological construction models for at least three reasons. First, a meta-analysis summarizes hundreds of empirical studies by statistical means; this is particularly beneficial given the high rate of false-positives and the considerable variability of experimental and statistical methods used (see Wager et al., 2007). Second, not only are meta-analytic results more reliable than the findings from any given study, but they also make it possible to mathematically model the influence of between-study methodological and statistical differences. Third, most individual experiments contrast only one emotion with another or with a neutral state, suggesting that activity is only different, but not necessarily specific, to that emotion. Meta-analytic studies can help overcome this limitation by directly comparing the activation patterns of several different discrete emotions to each other to assess the hypothesis that different emotions correspond to distinct locales (or networks) of brain activation.
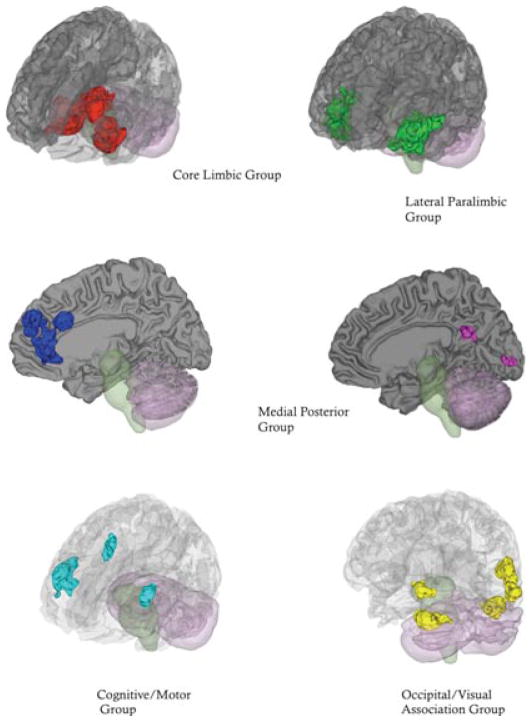
Based on an inductive analysis (using cluster analysis and multiple dimensional scaling), we have identified six functional groupings consistently co-activated across published neuroimaging studies of emotional experience and emotion perception; these groups support the most simple aspects of our psychological construction approach to the mind (Kober et al., 2008; see Table 2 and Figure 6). These functional groupings in Kober et al. (2008) bear a family resemblance to dynamic networks that exist within the intrinsic connectivity of the human brain.1 Intrinsic connectivity reveals many topographically distinct networks that appear to have distinct mechanistic functions, some of which appear similar to the psychological ingredients with the conceptual act model (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002; Dosenbach et al., 2007; Seeley et al., 2007; Smith et al., 2009; Sridharan, Levitin, & Menon, 2008).
Table 2.
Functional Groupings Identified by Kober et al., 2008
| Grouping | Key brain areas | Psychological ingredient |
|---|---|---|
| Core limbic | Amygdala/left hippocampus, thalamus extending into the periaqueductal gray, ventral striatum, and lateral hypothalamus | Core affect |
| Lateral paralimbic | Ventral striatum, ventral-posterior insula, dorsal-anterior insula, ventral interior insula/posterior orbital gyrus, and temporal pole | Core affect |
| Medial posterior | Posterior cingulated cortex/primary visual cortex (occipital lobe) | Conceptualizing/visual processing |
| Medial prefrontal cortex group | Dorsal and pregenual subsection of the anterior cingulate cortex, dorsal medial prefrontal cortex | Conceptualizing |
| Cognitive/motor | Frontal operculum, bilateral inferior frontal gyrus, and the presensory motor area/left middle frontal gyrus | Executive attention/language/motor |
| Lateral occipital/visual association | Right and left lateral occipital gyrus, right occipital/temporal cortex, cerebellum | Visual processing |
Figure 6.
Functional groupings with the Neural Reference Space for emotion. See Table 2 for brain regions within each functional grouping. Adapted from “Functional Networks and Cortical-Subcortical Interactions in Emotion: A Meta-Analysis of Neuroimaging Studies,” by H. Kober, L. F. Barrett, J. Joseph, E. Bliss-Moreau, K. A. Lindquist and T. D. Wager, 2008. Neuroimage, 42, 998–1031. Copyright 2008 by Elsevier.
In the Kober et al. (2008) analysis, we identified two functional groups in emotion (core limbic and paralimbic) that involve regions that are most relevant to PTSD. These are the brain regions that have been traditionally considered intrinsically emotional in nature because these groupings include brain regions that are involved in representing and regulating a person’s autonomic state and changes in homeostasis. These include areas related to processing salience and uncertainty (the amgydala; Whalen, 1998; Weierich et al., 2010), subcortical control of autonomic and hormonal responses (the periaqueductal gray and lateral hypothalamus), cortical control of autonomic and hormonal responses (ventromedial prefrontal cortex and ventral anterior cingulate cortex; Ongur, Ferry, & Price, 2003), representing internal sensations from the body (anterior insula; Craig, 2002), and areas that integrate somatovisceral information into higher-order multimodal representations (lateral orbitofrontal cortex; Ongur et al., 2003). In psychological terms, these two groupings correspond to the domain of affect. One ingredient in all psychological construction models is some form of information from the body such as raw somatic, visceral, vascular, or motor cues (James, 1884), affect (Harlow & Stagner, 1932; Hunt, 1941; Wundt, 1897/1998), arousal (Duffy, 1957; Mandler, 1975, 1990; Schachter & Singer, 1962) or what we refer to as core affect (Barrett, 2006c; Barrett & Bliss-Moreau, 2009; Russell, 2003; Russell & Barrett, 1999). The network producing core affect connects information within the body to information outside of the body and in doing so helps determine the personal salience of external stimuli and helps coordinate a behavioral response. In most cases the response is adaptive; nonadaptive behavioral responses may well contribute to psychopathology. Core affect is present in every waking moment of life (as the brain is always processing and presenting sensory input from the body), and is often represented consciously as a feeling of pleasure or displeasure with some degree of arousal (Barrett & Bliss-Moreau, 2009). Because changes in core affect are rooted in representations of sensory input from the body, they are not only influenced by psychological events; affect can change because of hormonal fluctuations, insulin levels, sleep, or many other processes that influence homeostasis. Nonetheless, humans typically experience core affect in psychological terms, as changes in a barometer that help them recognize and respond to salient cues in the environment. One of the abnormalities associated with PTSD involves disruptions in affect (Bovin & Marx, 2011) in both positive (e.g., emotional numbing may represent problems with appetitive responding) and negative (e.g., hypervigilance, intrusive symptoms) states and associated behaviors (Litz & Gray, 2002).
Sensory input from the body might be represented very precisely, but it is experienced in very general terms (as combinations of valence and arousal) because people tend not to be interoceptively sensitive (for reviews, see Barrett, Quigley, Bliss-Moreau, & Aronson, 2004; Cacioppo, Berntson, & Klein, 1992). Just like with any sensory input, representations of bodily sensations must be made meaningful. Two of the functional groupings in the Kober et al. analysis might accomplish this meaning-making process. These functional groups make up the so-called default network or long-term memory network consisting of areas of the ventromedial prefrontal cortex, posterior cingulate/retrosplenial cortex, inferior parietal lobule, lateral temporal cortex, dorsal medial prefrontal cortex, and the hippocampal formation. This network is active whenever people engage in spontaneous, highly associative mental activity (Raichle et al., 2001), construct an imagining of the future or a memory of the past (Buckner & Carroll, 2007), or construct a perception of the present (Bar, 2007). This grouping of brain regions has been called the episodic memory network (e.g., Vincent et al., 2006), the prospective brain (Schacter, Addis, & Buckner, 2007) and the network involved in self-referential processing (see Mitchell, 2009). It is active during context-sensitive object perception (Bar, 2009), and theory of mind (Saxe & Kanwisher, 2003). It is also implicated in first impressions, fictitious imaginings, emotion regulation, and moral decision making, as well as in emotion experience and perception (for reviews see Adolphs, 2001; Bar, 2007; Blakemore, Winston, & Frith, 2004; Buckner, Andrews-Hanna, & Schacter, 2008; Lane & McRae, 2004; Ochsner et al., 2004; Wager et al., 2008). We believe this network plays a role in retrieving information about the past to conceptualize incoming information to construct the present moment. This conceptualization involves reactivation of prior experience to make the present moment meaningful in a way that involves episodic projection or simulation, forming what Edelman (1989) refers to as “the remembered present.” It is not surprising, then, that this network is critical to the phenomena that psychologists refer to as categorization, memory, and conceptual knowledge. In psychological terms, this psychological process makes the current sensory array meaningful in an ongoing, automatic, and effortless manner. We have elsewhere referred to this process as categorization or situated conceptualization (Barrett, 2006b; Wilson-Mendenhall, Barrett, Simmons, & Barsalou, in press).
The Kober et al. analysis produced another functional group containing key nodes in executive attention and language networks involving the inferior frontal gyrus and frontal operculum, and extending to the ventral lateral prefrontal cortex, as well as the anterior temporal lobe that is associated with language. The inferior frontal gyrus and frontal operculum are thought to be involved in tasks requiring cognitive control: These include task switching, working memory, and response inhibition (e.g., Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Aron, Shohamy, Clark, Myers, Cluck, & Poldrack, 2004; Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Martin & Chao, 2001; Poldrack et al., 1999; Wager, Jonides, Smith, & Nichols, 2005; Wagner, Maril, Bjork, & Schacter, 2001). The ventral lateral prefrontal cortex has been implicated in the retrieval, maintenance, and manipulation of conceptual knowledge stored elsewhere in the brain (Gabrieli, Poldrack, & Desmond, 1998; Martin & Chao, 2001; Poldrack, et al., 1999; Wagner et al., 2001). Recent findings link the anterior temporal lobe to the representation of abstract social categories (e.g., Ross & Olson, 2010; Zahn et al., 2009). In the conceptual act model, we had hypothesized that both executive function (Barrett, 2009a; Barrett, Tugade, & Engle, 2004) and language (especially mental state words like the emotion words that anchor emotion categories) are an important ingredient in emotion (Barrett, 2006b; Barrett, Lindquist, & Gendron, 2007; Barrett, Mesquita, & Gendron, in press; Lindquist & Barrett, 2008b).
From a psychological construction perspective, then, instances of emotion (as well as other mental states like memories and perceptions) involve constant streams of affect and sensory cues from the world that are made meaningful. Sensory cues are shaped into meaningful percepts by prior knowledge about the world that over time the brain has organized into categories. This categorization process is managed by an attentional matrix in the brain that involves (among other things) executive function. So, for example, when your heart is racing and you see a snake in the woods, that instance falls into the category of fear. But if your heart was racing and you saw a snake in a pet shop, it might fall into the category of excitement, especially if you are an 11-year-old girl hoping for a pet. Or it might belong to the category of irritation if you are the parent who does not want yet another pet in the house. This type of process may appear completely unintuitive and frankly difficult to believe or accept. But it is not that different from what happens in speech perception or in vision. Top-down knowledge from the perceiver is necessary to make the incoming sensory input meaningful.
A Psychological Construction Approach to PTSD
From a psychological construction approach, disorders of mental life result from dysregulation of basic psychological ingredients or how those processes influence and constrain each other. Particular configurations of symptoms in PTSD might result from enhanced affective reactivity resulting from an overactive nervous system. Or symptoms could derive from a contextually impoverished conceptual system that is used to make meaning of the affective reactivity and transform it into fear, anger, or sadness, or even perceptions of the world as threatening. They could stem from reducing executive function that manages conceptual activations or a focus on internal versus external sources of information. Finally, they could be produced by any combination of the above. In normal life, some changes in homeostasis are experienced as physical symptoms (e.g., heart racing from too much coffee), some as affect (e.g., feeling wound up or tired), as emotions (e.g., as anger or fear), and some as perceptions of the world (e.g., a person is mean or food is delicious). In PTSD, perhaps a higher base rate of changes in home-ostasis (hyperarousal) provide a greater opportunity for physical events to be experienced and acted on as psychologically meaningful. Given the relative novelty of the conceptual act model, and the early stage of theory building within this theoretical framework, the value of a psychological construction approach can be gauged by the extent to which it is generative and opens up new avenues for understanding the heterogeneity within the disorder as well as the commonalities between disorders such as panic disorder and PTSD.
Core affect in PTSD
An amygdala-salience hypothesis provides another way to think about the theoretical importance of amygdala hyperresponsivity in PTSD. Instead of understanding PTSD as an exaggerated activation of fear circuitry, it is possible that information from the world has stronger affective value—it is more motivationally salient and homeostatically relevant, even when it ought not to be. This interpretation is consistent with recent findings that the amygdala is part of an intrinsic brain network that helps to determine the personal or motivational salience of an object or event (Menon & Uddin, 2010; Seeley et al., 2007; Sridharan et al., 2008), a process that is often, but not always, associated with fear. Neutral stimuli might not acquire fear-eliciting properties in PTSD (Bush, Schafe, & LeDoux, 2009; Keane, Zimering, & Caddell, 1985; Mineka & Zinbarg, 2006), but might instead be coded as inherently uncertain and of unrelenting personal relevance. The result could be an abnormally autonomic reactivity (associated with hyperarousal), which then the perceiver must make meaningful in some way (perhaps via mentalizing with the reactivation of prior experience). Some have suggested that hyperarousal is an important but often neglected aspect of PTSD (Kemp et al., 2009). Longitudinal research demonstrates that levels of hyperarousal shortly after experiencing a traumatic event are more predictive of later PTSD symptoms than are the other symptom clusters (e.g., Marshall et al., 2006; Schell, Marshall, & Jaycox, 2004). Such an interpretation might also help explain why amygdala hyperreactivity is also observed in depression and schizophrenia, and in a host of stress-related responses. This constant state of hyperarousal could well translate into the conscious experience of feeling on edge and unpleasantly aroused. The Etkin and Wager (2007) meta-analysis showed that individuals with PTSD have hyperreactivity in the anterior insula. This portion of the insula is evolutionarily recent (and perhaps unique in humans) and is involved in the conscious representation of affective feelings that arise from interoceptive sensory cues from the body (see Craig, 2002, 2009).
The link between hyperarousal and reexperiencing/intrusive symptoms
Speculating even further, it might be the case that the heightened amygdala response, combined with a reduced cortical oversight of autonomic responses, produces a situation where core affect is more linked to internal representations (related to reactivation of prior experience) versus more fully elaborated representations of the external world with all the contextual information that brings the present moment to life in a vivid and real way. This idea, that PTSD can be characterized by the salience of internal thoughts and feelings (as opposed to engagement by events and objects in the external world that are salient) is somewhat consistent with Brewin and colleagues’ dual representation theory of intrusive symptoms (Brewin, Dalgleish, & Joseph, 1996; Brewin et al., 2010) where individuals with PTSD are thought to represent uncontextualized perceptual images and details from the past that lead to hyperarousal and intrusive symptoms, but they fail to construct more contextually nuanced aspects of memory. This hypothesis is consistent with the hypoactivation in the anterior hippocampus and parahippocampal gyrus observed in PTSD in the Etkin and Wager neuroimaging meta-analysis. It is also consistent with recent evidence of enhanced “default network” connectivity observed in PTSD during a functional working memory task when typically individuals should be less internally focused and more externally focused (Daniels et al., 2010).
In addition, it is well documented that the amygdala interacts with important memory structures or systems such as the hippocampus that help facilitate the encoding of consciously accessible episodic memories (for a recent review, see Dere, Pause, & Pietrowsky, 2010). In addition, high levels of affective arousal often enhance the vividness with which affective details of a memory are experienced, often to the exclusion of other less evocative details (Kensinger & Schacter, 2008), potentially leading to symptoms related to reexperiencing. Although there are several existing neuroimaging studies that examine activations in brain regions associated with attention to internal mental activity, in these studies termed the default network (Bluhm et al., 2009; Lanius, Bluhm, et al., 2010; Lui et al., 2009), methodological limitations and analytic choices preclude us from drawing conclusions about the exact nature of internal versus external focus in posttraumatic symptomatalogy. Nonetheless, the Etkin and Wager meta-analysis did find PTSD to be selectively associated with greater reactivity in the precuneus area of the parietal cortex, and this brain region is part of the intrinsic network that is associated with self-relevant, mentalizing, and a focus on internally generated thoughts and experiences. We believe that these concepts represent an important avenue for future research to pursue.
The idea that enhanced arousal might lead to an internal focus could also be explored in terms of the most basic aspects of cortical arousal. There is neuroanatomical evidence that the paralimbic cortex along with the amygdala projects to the ascending reticular activation system that regulates the degree of cortical arousal and processing of sensory information from the world through the thalamus. As detailed by Parvizi and Damasio (2001), the ascending reticular activation system originates in a variety of reticular nuclei in the brainstem and can activate widespread regions of the cortex through multiple routes. Not only do these brainstem nuclei project directly to multiple cortical areas, but they also indirectly activate cortical regions via several different brain areas such as the intralaminar and reticular nuclei of the thalamus and basal fore-brain nuclei. The vast majority of pathways of the ascending reticular activation system are ascending, projecting to higher cortical areas. These widespread projections terminate in many networks that contribute to memory and attentional processes associated with PTSD. There is only one primary descending pathway that projects to the ascending reticular activation system and areas in the basal forebrain that regulate cortical activity, and this descending pathway originates from the amygdala and other areas of the paralimbic ventromedial prefrontal cortex (Mesulam, 2000). It is in this way that core affective circuitry can entrain the rest of the cortex to influence levels of cortical arousal and help to regulate the neural assemblies that form the core of consciousness (Edelman & Tononi, 2000). This interpretation, at least on the surface, is consistent with findings that parts of the core affect network (e.g., anterior insula) are part of an attentional switching network (Corbetta et al., 2008) that helps to regulate between attention to the world and attention to internal mentation. To the extent that core affect is not well yoked to what is going on in the external world, and therefore requires processing to be made meaningful, it might cue the brain to select internal representations for increased attention over external sensory input.
The notion that internal sensations such as physiological arousal can trigger intrusive symptoms is consistent with many theoretical models of PTSD. For instance, according to Foa’s affective processing theory (e.g., Foa, Huppert, & Cahill, 2006), which was heavily influenced by Lang’s (1979) bioinformational model of emotion, traumatic memories are networks made up of meaning, memory, context, stimulus, and response elements. So, a physiological response that is part of the response element of this network could prime the activation of other parts of the network and trigger intrusive symptoms. Although empirical investigations of physiological activity as triggers for intrusive symptoms are sparse, an investigation of acute stress disorder demonstrated that a hyperventilation challenge task produced a greater increase in trauma memories in participants with acute stress disorder compared to those without. This is consistent with the notion that elevated arousal can trigger intrusive symptoms (Nixon & Bryant, 2005). Thus, one important factor that could account for the heterogeneity of intrusive symptoms is the nature of the triggering event—mental state or external stimulus.
Nonetheless, for a psychological construction account of PTSD to be a profitable approach to understanding the basic ingredients that underlie the variety in reexperiencing symptoms, a more detailed and comprehensive accounting of the heterogeneity in intrusive symptoms is also necessary. Current models (e.g., Brewin et al., 2010; Ehlers & Clark, 2000; Rubin et al., 2008) offer several candidates: (a) the controllability of the memory (i.e., automatically activated vs. more voluntarily retrieved); (b) the degree to which a memory is represented in conscious experience; (c) the degree to which the memory involves raw sensory experiences (e.g., imagery) versus other more abstract representations tied to language; (d) the degree to which the memory is fragmentary or organized into a coherent narrative, which might not be independent of (c) above; (e) the involvement of physiological activity or representations of physiological activity; (f) whether the memory is experienced from a first- or third-person perspective; and (g) the temporal–spatial qualities of the memory (i.e., was it experienced as if it were happening in the current moment or in the distant past).
In addition, how a traumatic memory is triggered is likely to be an important factor in the processes involved in the memory. A memory could be initiated exogenously by external stimuli, or endogenously by other memories or internal sensations. Experimental research in PTSD has almost exclusively focused on exogenously triggered memories, and a more thorough accounting of endogenously triggered memories would contribute to a more comprehensive understanding of intrusive symptoms. Given that one of the primary functions of the default network (discussed above) is to activate associations stored in memory to generate predictions about the future (Bar, 2009), research elucidating the role of the default network in PTSD will likely involve understanding how memory systems can endogenously trigger intrusive symptoms.
Changes in executive attention in PTSD
A psychological construction approach suggests several novel hypotheses regarding the ways in which another psychological ingredient—executive attention—might be disrupted or altered in PTSD. Attentional processes play a key role in PTSD. Multiple DSM-IV (APA, 1994) criteria specify disrupted attentional processes including dissociative processes that can occur during reliving experiences (Criterion B3), the distractibility associated difficulties in concentrating (Criterion D3), and the pathological alertness associated with hypervigilance (Criterion D4). Several experimental investigations have also documented abnormalities across multiple domains of attention including enhanced detection of threatening information (i.e., attentional facilitation; e.g., Bryant & Harvey, 1997), difficulties disengaging from threatening information (i.e., attentional interference; e.g., Pineles et al., 2009), and problems with more effortful top-down types of attention such as working memory (e.g., Shaw et al., 2009; for relatively recent reviews of the PTSD-attention literature see Constans, 2005; Shipherd & Salters-Pedneault, 2008).
To understand these symptoms, it might be helpful to consider the concept of an “attentional matrix” (Mesulam, 2000). At any given moment, numerous internal and external sources of stimulation compete for our limited processing resources. Mesulam (2000) defines attention as a generic term that describes a variety of processes involved in deciding “which of many suitable mental or external events will have preferential access to the narrow portals of consciousness and action… At the psychological level, attention implies a preferential allocation of processing resources and response channels to events that have become behaviorally relevant. At the neural level, attention refers to alterations in the selectivity, intensity, and duration of neuronal responses to such events” (p. 174). The processes making up this attentional matrix range from automatic processes that are exogenously stimulus-driven, bottom-up, reflexive (i.e., exogenous attention) to controlled processes that are top-down and goal-directed (i.e., endogenous attention; Barrett, Tugade, et al., 2004). More recently, we have distinguished between goal-based attention, where attention is applied because the person has a goal to perform a task, and affective attention, where attention is applied to certain representations because they are of affective value. These are not competing forms of attention, but instead they work together in complex ways to determine what is salient to a person at a given moment in time. Over the past decade, neuroimaging studies have identified multiple intrinsic connectivity networks that correspond to these different forms of attention, and these networks interact to guide the control and expression of attention (e.g., LaBerge, 2002; Menon & Uddin, 2010; Sridharan et al., 2008). It is possible that some of the symptomatic presentations in PTSD involve disruption in one or more of these attentional networks. For example, a recent neuroimaging study of working memory involving nontrauma-related stimuli in participants with PTSD (vs. without) found a relative inefficiency in allocating resources to different processes contributing to working memory (i.e., updating vs. maintaining information) in those with PTSD, perhaps due to an increased reliance on these resources to manage the associated hyperarousal (Shaw et al., 2009). Although such findings are tantalizing, a more direct test of this hypothesis awaits future research. Take, for example, the Etkin and Wager (2007) meta-analysis where individuals with PTSD showed hyperactivation in inferior parietal cortex; this brain region is part of the attention switching network reported by Corbetta and colleagues (2002, 2008), is often associated with visuospatial processing of information in the immediate surroundings, and it is also part of the mentalizing network described by Buckner and colleagues (Buckner, & Carroll, 2007, Buckner et al., 2008). These findings illustrate that a closer examination of this parietal area might be instructive in PTSD because adjacent yet distinct regions of the parietal lobe can be activated in a push–pull manner by top-down attention to memory versus the external environment (Sestieri, Shulman, & Corbetta, 2010, p. 8453). For the moment, it is difficult to test this hypothesis with existing neuroimaging studies of attention in PTSD because they do not report their findings in a way that allows parsing apart these intrinsic networks.
Nonetheless, interesting and testable hypotheses regarding attentional processes contributing to PTSD arise when adopting a psychological construction perspective. For instance, one viable hypothesis is that dissociative processes often experienced by individuals with PTSD are the result of an overactive mentalizing long-term memory network or the failure of core affect to activate attentional networks to attend to the external environment to more fully encode and represent context in a nuanced and detailed way, or both. The hypervigilance exhibited over and over again by individuals with PTSD could well be associated with a breakdown in communication among networks. Likewise, the distractibility associated with PTSD could stem from a combination of the networks that interact to determine the expression of attention, for example, the interaction of inefficient executive attention (Shaw et al., 2009), core affect characterized by high arousal, an overactive mentalizing network, and the processes described above related to dissociation and vigilance.
CONCLUDING REMARKS
In this article, our goal was to introduce the psychological construction approach as a way of understanding the brain basis of PTSD, while offering new interpretations and insights for future research. This kind of psychological construction approach, though uncommon in psychology, is consistent with many recent transdiagnostic or unified approaches that attempt to identify psychological and biological processes that are common to many types of psychopathology (e.g., Fairholme, Boisseau, Ellard, Ehrenreich, & Barlow, 2010; Harvey, Watkins, Mansell, & Shafran, 2004; Kendler, 2008; Kring, 2008). It is consistent with the research domain criteria approach recently proposed (Sanislow et al., 2010). Underscoring the wide applicability of this approach, the psychological construction approach is also consistent with current neurotransmitter models of anxiety, which recognize that many neurotransmitters and receptors serve multiple, and often contrasting, roles in the modulation of anxious states depending on the precise cerebral circuits with which they interact (e.g., Millan, 2003). Taken together with these transdiagnostic approaches to mental illness, a psychological construction perspective helps to recognize that current psychiatric diagnostic categories, like other complex psychological categories (e.g., emotions), are at once heterogeneous and also the products of more general causes that might also go awry in other mental disorders. Furthermore, a psychological construction approach can help explain the increasing realization that psychiatric categories (like any other complex psychological category) are not natural kinds. As Haslam (2002) recently wrote, “the naïve-realist or objectivist position that mental disorders are essence-based, classically definable, objectively grounded, and discovered by carving psychiatric nature at its joints has generally taken a beating” (p. 203). Hopefully, this realization can facilitate efforts to overcome the divide that exists between integrative neuroscience and clinical research (Sanislow et al., 2010) and inform efforts to refine the measurement of PTSD symptoms and elucidate underlying processes that can be targeted by interventions.
In our psychological construction approach to PTSD, we have suggested the dysregulation in one basic psychological ingredient—core affect—represents a key feature of the disorder, although the way that affect manifests itself (as changes in homeostasis, felt experience, or even cortical arousal) remains an important avenue for future research. We should be careful to point out that we do not propose core affect is specific to this disorder, in that disruptions in core affect are common to almost all mental illness categories (Kring, 2008), nor do we imply that it is the core feature of the disorder, in that it is just one ingredient in the family of recipes that create PTSD experiences.
Furthermore, we considered other ingredients that might constitute the recipes of PTSD, including a focus on internal mentalizing potentially at the expense of context-sensitive perceptions of the external world. Such anchoring in the head, instead of in the world, if it were identified in PTSD, might be supported by executive attention and/or the kind of affective attention that results from hyperarousal. These ingredients (e.g., core affect characterized by high arousal and goal-based attention hypersensitive to threat) likely contribute to both the emergence and the qualities of the intrusive symptomatology that is central to PTSD. We hope that our ideas, while just a sketch, provide a context that might help to develop models (perhaps several are needed depending upon the nature/level of the inquiry) that can account for the complexity of PTSD and the brain in the most parsimonious manner to meaningfully inform clinical assessment, conceptualizations, and interventions.
For the present, a psychological construction approach to PTSD can concretely contribute to clinical assessment and treatment by refining the precision of assessment measures. A psychological construction approach could help guide the development of a psychometrically sound and valid measure that captures the heterogeneity of the intrusive and reexperiencing symptoms experienced by individuals diagnosed with PTSD. As already discussed, a variety of trauma researchers (e.g., Brewin et al., 2010; Dalgleish, 2004) have recognized the importance of better understanding the heterogeneity of intrusive symptoms associated with PTSD. However, the lack of a comprehensive model accounting for this heterogeneity and a psychometrically sound measure to assess this heterogeneity has limited research efforts in this area. For instance, Items B-1 and B-2 of the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995), considered the gold standard for assessing PTSD, ask interviewees if they have had “unwanted memories” or if they have ever gotten “emotionally upset when something reminded you of (Event).” The corresponding items of the Posttraumatic Checklist (PCL; Weathers, Litz, Herman, Huska, & Keane, 1993) assess the occurrence of “repeated, disturbing memories, thoughts or images” (one single item) and “feeling very upset when something reminded you of a stressful experience…). The vagueness of this language is unlikely to lead to a comprehensive understanding of intrusive symptoms, though it must be acknowledged that this is not the goal of either measure. The vast cognitive and affective neuroscience literature that details the nature of multiple memory mechanisms could greatly contribute to the development of a comprehensive theoretical account and psychometrically sound measure capturing the heterogeneity of PTSD.
Finally, psychological construction not only offers a somewhat different take on the causes and descriptions of PTSD, but it also suggests important methodological implications for neuroimaging studies of PTSD. Traditional approaches to understanding the brain basis of psychopathology have typically focused on identifying the neural basis of a particular process by using a specific task to isolate that process. Over the past few years there has been an increased recognition that fully understanding the neural networks and interactions among these networks that produce a mental state or behavior also requires examining data across multiple tasks (e.g., Kober et al., 2008; Poldrack et al., 2009). The vast majority of studies examining the neural circuitry of PTSD have adopted the former, more traditional specific task approach; we suggest that a multiple task approach is more likely to bear fruit. Furthermore, there should be a focus on studies that attempt to isolate and model underlying psychological primitives, in combination with studies that attempt to understand symptoms as the dynamic interplay (under specific conditions) between the brain networks that realize different psychological ingredients. Finally, an explicit comparison of PTSD to other disorders (not just anxiety disorders, but also to depression and other classes of mental illness) will protect researchers from making claims about the specificity of brain regions or networks in PTSD that are manifest in other disorders as well and are very likely to be better understood as general processes that contribute to both regulated and dysregulated mental life.
Acknowledgments
Many thanks to Paul Gade and Danny Kaloupek for commenting on earlier drafts of this manuscript. Preparation of this manuscript was supported by the National Institutes of Health Director’s Pioneer Award (DP1OD003312), a grant from the National Institute of Aging (AG030311), and a contract with the U.S. Army Research Institute for the Behavioral and Social Sciences (W91WAW-08-C-0018) to Lisa Feldman Barrett. The views, opinions, and/or findings contained in this article are solely those of the author(s) and should not be construed as an official Department of the Army or DOD position, policy, or decision, unless so designated by other documentation.
Footnotes
Intrinsic connectivity networks are identified by examining correlations in low-frequency signals in functional magnetic resonance imaging (fMRI)data recorded when there is no external stimulus or task (hence this misnomer “resting state” or “default” activity; Beckmann, DeLuca, Devlin, & Smith, 2005; Biswal, Yetkin, Haughton, & Hyde, 1995; Buckner & Vincent, 2007; Fox et al., 2005; Greicius, Krasnow, Reiss, & Menon, 2003). The temporal dynamics of these low-frequency signals reveals networks of regions that increase and decrease in their activity together in a correlated fashion.
Contributor Information
Michael K. Suvak, Women’s Health Sciences Division, VA National Center for PTSD, VA Boston Healthcare System
Lisa Feldman Barrett, Department of Psychology, Northeastern University, and Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School.
References
- Adams RB, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adler AB, Wright KM, Bliese PD, Eckford R, Hoge CW. A2 Diagnostic criterion for combat-related posttraumatic stress disorder. Journal of Traumatic Stress. 2008;21:301–308. doi: 10.1002/jts.20336. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinions in Neurobiology. 2001;11:231–239. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggelton J, editor. The amygdala. New York, NY: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in Macaque monkeys. Journal of Comparative Neurology. 1998;401:455–479. doi: 10.1002/(SICI)1096-9861(19981130). [DOI] [PubMed] [Google Scholar]
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: An fMRI study. Social Cognitive and Affective Neuroscience. 2008;3:233–243. doi: 10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. Journal of Neurophysiology. 2004;92:1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- Atkinson AP, Heberlein AS, Adolphs R. Spared ability to recognise fear from static and moving whole-body cues following bilateral amygdala damage. Neuropsychologia. 2007;45:2772–2782. doi: 10.1016/j.neuropsychologia.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler R, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Ball T, Derix J, Wentlandt J, Wieckhorst B, Speck O, Schulze-Bonhage A, Mutschler I. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. Journal of Neuroscience Methods. 2009;180:57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: An integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends in Cognitive Sciences. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;10:4–25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Feeling is perceiving: Core affect and conceptualization in the experience of emotion. In: Barrett LF, Niedenthal PM, Winkielman P, editors. Emotions: Conscious and unconscious. New York, NY: Guilford Press; 2005. pp. 255–284. [Google Scholar]
- Barrett LF. Emotions as natural kinds? Perspectives on Psychological Science. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006b;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Valence as a basic building block of emotional life. Journal of Research in Personality. 2006c;40:35–55. [Google Scholar]
- Barrett LF. The future of psychology: Connecting mind to brain. Perspectives on Psychological Science. 2009a;4:326–339. doi: 10.1111/j.1745-6924.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Variety is the spice of life: A psychological construction approach to understanding variability in emotion. Cognition & Emotion. 2009b;23:1284–1306. doi: 10.1080/02699930902985894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bar M. See it with feeling: Affective predictions in the human brain. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364:1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a psychological primitive. Advances in Experimental Social Psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Lindquist K. The embodiment of emotion. In: Semin G, Smith E, editors. Embodied grounding: Social, cognitive, affective, and neuroscience approaches. New York, NY: Cambridge University Press; 2008. pp. 237–262. [Google Scholar]
- Barrett LF, Lindquist K, Bliss-Moreau E, Duncan S, Gendron M, Mize J, Brennan L. Of mice and men: Natural kinds of emotion in the mammalian brain? Perspectives on Psychological Science. 2007;2:297–312. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Lindquist K, Gendron M. Language as a context for emotion perception. Trends in Cognitive Sciences. 2007;11:327–332. doi: 10.1016/j.tics.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Gendron M. Emotion perception in context. Current Directions in Psychological Science (in press) [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Ochsner KN, Gross JJ. On the automaticity of emotion. In: Bargh J, editor. Social psychology and the unconscious: The automaticity of higher mental processes. New York, NY: Psychology Press; 2007. [Google Scholar]
- Barrett LF, Quigley K, Bliss-Moreau E, Aronson KR. Arousal focus and interoceptive sensitivity. Journal of Personality and Social Psychology. 2004;87:684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130:553–573. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Wager T. The structure of emotion: Evidence from the neuroimaging of emotion. Current Directions in Psychological Science. 2006;15:79–85. doi: 10.1111/j.0963-7214.2006.00411.x. [DOI] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard-Gilligan M, Zoellner LA. The utility of the A1 and A2 criteria in the diagnosis of PTSD. Behaviour Research and Therapy. 2008;46:1062–1069. doi: 10.1016/j.brat.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuro-science: where are we heading? Trends in Cognitive Science. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Osuch E, Frewen P, Stevens T, Boksman K, Lanius RA. Alterations in default network connectivity in post-traumatic stress disorder related to early-life trauma. Journal of Psychiatry & Neuroscience. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Bodkin JA, Pope HG, Detke MJ, Hudson JI. Is PTSD caused by traumatic stress? Journal of Anxiety Disorders. 2007;21:176–182. doi: 10.1016/j.janxdis.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP. The importance of the peritraumatic experience in defining traumatic stress. Psychological Bulletin. 2011;137:47–67. doi: 10.1037/a0021353. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane R, Nadel L, editors. Cognitive neuroscience of emotion. New York, NY: Oxford University Press; 2000. pp. 242–276. [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expressions. Neuron. 1996;17:875–877. doi: 10.1016/S0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC. The stressor criterion in DSM-IV post-traumatic stress disorder: An empirical investigation. Biological Psychiatry. 2001;50:699–704. doi: 10.1016/S0006-3223(01)01167-2. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Rose S. Fear, helplessness, and horror in posttraumatic stress disorder: Investigating DSM-IV Criterion A2 in victims of violent crime. Journal of Traumatic Stress. 2000;13:499–509. doi: 10.1023/A:1007741526169. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Dalgleish T, Joseph S. A dual representation theory of post-traumatic stress disorder. Psychological Review. 1996;103:670–686. doi: 10.1037/0033-295X.103.4.670. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychological Review. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Lanius RA, Novac A, Schnyder U, Galea S. Reformulating PTSD for DSM-V: Life after Criterion A. Journal of Traumatic Stress. 2009;22:366–373. doi: 10.1002/jts.20443. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, McFarlane AC, Silove D. A multisite study of initial respiration rate and heart rate as predictors of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2008;69:1694–1701. doi: 10.4088/JCP.v69n1104. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Attentional bias in posttraumatic stress disorder. Journal of Traumatic Stress. 1997;10:635–644. doi: 10.1002/jts.2490100409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: Default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G. Conditioned reinforcement can be mediated by either outcome-specific or general affective representations. Frontiers in Integrative Neuroscience. 2007;1:1–11. doi: 10.3389/neuro.07/011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: Implication for limbic-striatal interactions. Behavioral Neuroscience. 1996;110:60–73. doi: 10.1037/0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Bush DEA, Schafe GE, LeDoux JE. Neural basis of fear conditioning. In: Berntson Gary G, Cacioppo John T., editors. Handbook of neuroscience for the behavioral sciences. Vol. 2. Hoboken, NJ: Wiley; 2009. pp. 762–764. [Google Scholar]
- Cacioppo JT, Berntson GG, Klein DJ. What is emotion? The role of somatovisceral afference, with special emphasis on somatovisceral “illusions. Review of Personality and Social Psychology. 1992;14:63–98. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, editors. The handbook of emotion. New York, NY: Guilford Press; 2000. [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of Comparative Neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Clore GL, Ortony A. Appraisal theories: How cognition shapes affect into emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3. New York, NY: Guilford Press; 2008. pp. 628–642. [Google Scholar]
- Constans JI. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. New York, NY: Guilford Press; 2005. Information-processing biases in PTSD; pp. 105–130. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Cognitive approaches to posttraumatic stress disorder: The evolution of multirepresentational theorizing. Psychological Bulletin. 2004;130:228–260. doi: 10.1037/0033-2909.130.2.228. [DOI] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark RC, Shaw ME, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. Journal of Psychiatry & Neuroscience. 2010;35:258–266. doi: 10.1503/jpn.090010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behavioral Neuroscience. 1986;100:814–824. doi: 10.1037/0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala. Vol. 2. Oxford, England: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/S0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dȩbiec J, LeDoux J. The amygdala and the neural pathways of fear. In: Shiromani PJ, Keane TM, LeDoux JE, editors. Post-traumatic stress disorder: Basic science and clinical practice. Totowa, NJ: Humana Press; 2009. pp. 23–38. [DOI] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and eposodic memory in neuropsychiatric disorders. Behavioral Brain Research. 215:162–171. doi: 10.1016/j.bbr.2010.03.017. (in press) [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KL, Dosenbach RAT, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy E. The psychological significance of the concept of “arousal” or “activation. Psychological Review. 1957;64:265–275. doi: 10.1037/h0048837. [DOI] [PubMed] [Google Scholar]
- Duncan SL, Barrett LF. The amygdala in visual awareness. Trends in Cognitive Sciences. 2007a;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. Affect as a form of cognition: A neurobiological analysis. Cognition and Emotion. 2007b;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. The remembered present: A biological theory of consciousness. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Edelman GM, Tononi G. A universe of consciousness: How matter becomes imagination. New York, NY: Basic Books; 2000. [Google Scholar]
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy. 2000;38:319–345. doi: 10.1016/S0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Ellsworth PC, Scherer K. Appraisal processes in emotion. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York, NY: Oxford University Press; 2003. pp. 572–595. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive and Affective Neuroscience. 2009;4:127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Fox E, Calder AJ. The interaction between gaze and facial expression in the amygdala and extended amygdala is modulated by anxiety. Frontiers in Human Neuroscience. 2010;4:1–11. doi: 10.3389/fnhum.2010.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairholme CP, Boisseau CL, Ellard KK, Ehrenreich JT, Barlow DH. Emotions, emotion regulation, and psychological treatment: A unified perspective. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology. New York, NY: Guilford Press; 2010. pp. 283–309. [Google Scholar]
- Fanselow M. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110:73–81. doi: 10.1016/S0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Hillsdale, NJ: Erlbaum; 1988. pp. 185–212. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/S0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Cahill SP. Emotional processing theory: An update. In: Rothbaum BO, editor. Pathological anxiety: Emotional processing in etiology and treatment. New York, NY: Guilford Press; 2006. pp. 3–24. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anti-correlated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The cognit: A network model of cortical representation. International Journal of Psychophysiology. 2006;60:125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Liberzon I. Neurobiology of PTSD: A review of neuroimaging findings. Psychiatric Annals. 2009;39:370–372. 376–381. doi: 10.3928/00485713-20090527-01. [DOI] [Google Scholar]
- Gendron M, Barrett LF. Reconstructing the past: A century of ideas about emotion in psychology. Emotion Review. 2009;1:1–24. doi: 10.1177/1754073909338877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, van Berckel B, Lammertsma A, Boellaard R, de Kloet C, Vermetten E, Westenberg HGM. Reduced GABA[sub]A[/sub] benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Molecular Psychiatry. 2008;13:74–83. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/S0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gold SD, Marx BP, Soler-Baillo JM, Sloan DM. Is life stress more traumatic than traumatic stress? Journal of Anxiety Disorders. 2005;19:687–698. doi: 10.1016/j.janxdis.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Barrett LF. Emotion generation and emotion regulation: One or two depends on your point of view. Emotion Review. doi: 10.1177/1754073910380974. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Stagner R. Psychology of feelings and emotions. I. Theory of feelings. Psychological Review. 1932;39:570–589. doi: 10.1037/h0072961. [DOI] [Google Scholar]
- Harvey A, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Haslam N. Kinds of kinds: A conceptual taxonomy of psychiatric categories. Philosophy, Psychiatry, & Psychology. 2002;9:203–217. doi: 10.1353/ppp.2003.0043. [DOI] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA. Recent developments in the field of emotion. Psychological Bulletin. 1941;38:249–276. doi: 10.1037/h0054615. [DOI] [Google Scholar]
- Iwata J, LeDoux JE. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behavioral Neuroscience. 1988;102:66–76. doi: 10.1037/0735-7044.102.1.66. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. The American Journal of Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. What is an emotion? History, measures, meaning. New Haven CT: Yale University Press; 2009. [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: Effect on heart rate conditioning in the rabbit. Physiology Behavior. 1979;6:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Karam EG, Andrews G, Bromet E, Petukhova M, Ruscio AM, Salamoun M, Kessler RC. The role of criterion A2 in the DSM-IV diagnosis of posttraumatic stress disorder. Biological Psychiatry. 2010;68:465–473. doi: 10.1016/j.biopsych.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Zimering RT, Caddell JM. A behavioral formulation of posttraumatic stress disorder in Vietnam veterans. The Behavior Therapist. 1985;8:9–z12. [Google Scholar]
- Kemp AH, Felmingham KL, Falconer E, Liddell BJ, Bryant RA, Williams LM. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: An fMRI study. Psychiatry Research: Neuroimaging. 2009;174:158–161. doi: 10.1016/j.pscychresns.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Explanatory models for psychiatric illness. The American Journal of Psychiatry. 2008;165:695–702. doi: 10.1176/appi.ajp.2008.07071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Memory and emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. Vol. 3. New York, NY: Guilford Press; 2008. pp. 601–617. [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Freedy JR, Pelcovitz D, Resick P, Roth S, van der Kolk B. In: Posttraumatic stress disorder field trial: Evaluation of the PTSD construct—Criteria A through EDSM–IV sourcebook. Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis W, Kline M, editors. Vol. 4. Washington, DC: American Psychiatric Association; 1998. pp. 803–844. [Google Scholar]
- Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, Whalen PJ. Behind the mask: The influence of mask-type on amygdala response to fearful faces. Social Cognitive and Affective Neuroscience. 2010;5:363–368. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist KA, Wager TD. Functional networks and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Grabowecky M. Prefrontal cortex, time, and consciousness. In: Gazzaniga M, editor. The new cognitive neurosciences. 2. Cambridge, MA: MIT Press; 1999. pp. 1319–1337. [Google Scholar]
- Kopchia KL, Altman HJ, Commissaris RL. Effects of lesions of the central nucleus of the amygdala on anxiety-like behaviors in the rat. Pharmacology, Biochemistry, and Behavior. 1992;43:453–461. doi: 10.1016/0091-3057(92)90176-G. [DOI] [PubMed] [Google Scholar]
- Kring A. Emotion disturbances as transdiagnostic processes in psychopathology. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York, NY: Guilford Press; 2008. pp. 691–705. [Google Scholar]
- Krueger RF, Watson D, Barlow DH. Introduction to the special section: Toward a dimensionally based taxonomy of psychopathology. Journal of Abnormal Psychology. 2005;114:491–493. doi: 10.1037/0021-843X.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D. Attentional control: Brief and prolonged. Psychological Research/Psychologische Forschung. 2002;66:220–233. doi: 10.1007/s00426-002-0097-2. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K. Neural substrates of conscious emotional experience: A cognitive–neuroscientific perspective. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdam: John Benjamins; 2004. pp. 87–122. [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lanius R, Bluhm R, Coupland N, Hegadoren K, Rowe B, Théberge J, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. Journal of Psychiatric Research. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J. The amygdala. Current Biology. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ledoux J. Emotional colouration of consciousness: How feelings come about. In: Weiskrantz L, Davies M, editors. Frontiers of consciousness: The Chichele Lectures. New York, NY: Oxford University Press; 2008. pp. 69–130. [Google Scholar]
- LeDoux JE. Information flow from sensation to emotion plasticity in the neural computation of stimulus values. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: Bradford Books/MIT Press; 1990. pp. 3–52. [Google Scholar]
- Lindquist K, Barrett LF. Emotional complexity. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York, NY: Guilford Press; 2008a. pp. 513–530. [Google Scholar]
- Lindquist K, Barrett LF. Constructing emotion: The experience of fear as a conceptual act. Psychological Science. 2008b;19:898–903. doi: 10.1111/j.1467-9280.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. 2010. Manuscript submitted for publication. [Google Scholar]
- Litz BT, Gray MJ. Emotional numbing in posttraumatic stress disorder: Current and future research directions. Australian and New Zealand Journal of Psychiatry. 2002;36:198–204. doi: 10.1046/j.1440-1614.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. Psychosomatic disease and the visceral brain: Recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: Role in paleocerebral functions. New York, NY: Plenum; 1990. [DOI] [PubMed] [Google Scholar]
- Mandler G. Mind and emotion. New York, NY: Wiley; 1975. [Google Scholar]
- Mandler G. William James and the construction of emotion. Psychological Science. 1990;1:179–180. doi: 10.1111/j.1467-9280.1990.tb00193.x. [DOI] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural Brain Research. 2000;110:97–108. doi: 10.1016/S0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Schell TL, Glynn SM, Shetty V. The role of hy-perarousal in the manifestation of posttraumatic psychological distress following injury. Journal of Abnormal Psychology. 2006;115:624–628. doi: 10.1037/0021-843X.115.3.624. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/S0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: A review. Cognition and Emotion. 2009;23:209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/S0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Progress and controversy in the study of post-traumatic stress disorder. Annual Review of Psychology. 2003;54:229–252. doi: 10.1146/annurev.psych.54.101601.145112. [DOI] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Attentional networks, confusional states, and neglect syndromes. In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. 2. New York, NY: Oxford University Press; 2000. pp. 174–256. [Google Scholar]
- Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: Brain networks under genetic control. Human Brain Mapping. 2008;30:1938–1946. doi: 10.1002/hbm.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Progress in Neurobiology. 2003;70:83–244. doi: 10.1016/S0301-0082(03)00087-X. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philosophical Transactions B. 2009;364:1309. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol SSL, Arntz A, Metsemakers JFM, Dinant G, Vilters-Van Montfort PAP, Knottnerus JA. Symptoms of post-traumatic stress disorder after non-traumatic events: Evidence from an open population study. British Journal of Psychiatry. 2005;186:494–499. doi: 10.1192/bjp.186.6.494. [DOI] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA. Overgeneral autobiographical memory and traumatic events: An evaluative review. Psychological Bulletin. 2007;133:419–437. doi: 10.1037/0033-2909.133.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich MR, Dautoff R, Dickerson BC, Wright CI, Barrett LF. Differential hemodynamic response in affective circuitry with aging: An fMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21527. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Structure & Function. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Henry S, Krystal JH. Neural circuitry and neuroplasticity in PTSD. In: Friedman MJ, Keane TM, Resick PA, editors. Handbook of PTSD: Science and practice. New York, NY: Guilford Press; 2007. pp. 151–165. [Google Scholar]
- Nixon RDV, Bryant RA. Induced arousal and reexperiencing in acute stress disorder. Journal of Anxiety Disorders. 2005;19:587–594. doi: 10.1016/j.janxdis.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Bossaerts P. Toward a mechanistic understanding of human decision making. Current Directions in Psychological Science. 2008;17:119–123. doi: 10.1111/j.1467-8721.2008.00560.x. [DOI] [Google Scholar]
- O’Donnell ML, Creamer M, McFarlane AC, Silove D, Bryant RA. Should A2 be a diagnostic requirement for posttraumatic stress disorder in DSM-V? Psychiatry Research. 2010;176:257–260. doi: 10.1016/j.psychres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Olde E, van der Hart O, Kleber R, van Son M. Posttraumatic stress following childbirth: A review. Clinical Psychology Review. 2006;26:1–16. doi: 10.1016/j.cpr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Munakata JA. Computational explorations in cognitive neuroscience: Understanding the mind by simulating the brain. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DW. Predictors of post-traumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Damasio AR. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/S0010-0277(00)00127-X. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews: Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Petrides M. The orbitofrontal cortex: Novelty, deviation from expectation, and memory. Annals of the New York Academy of Sciences. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Mostoufi SM, Abramovitz SM, Yovel I. Attentional biases in PTSD: More evidence for interference. Behaviour Research and Therapy. 2009;47:1050–1057. doi: 10.1016/j.brat.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Halchenko YO, Hanson SJ. Decoding the large-scale structure of brain function by classifying mental states across individuals. Psychological Science. 2009;20:1364–1372. doi: 10.1111/j.1467-9280.2009.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull M, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuro Image. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Posner J, Russell JA, Gerber A, Gorman D, Colibazzi T, Yu S, Peterson BS. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30:883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather M, Lavenex P, Mauldin-Jourdain M, Mason W, Capitanio J, Men-doza S, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/S0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Resick PA, Miller MW. Posttraumatic stress disorder: Anxiety or traumatic stress disorder? Journal of Traumatic Stress. 2009;22:384–390. doi: 10.1002/jts.20437. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments return the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer L, Orsillo SM, Borkovec TD, Litz BT. Emotional response at the time of a potentially traumatizing event and PTSD symptomatology: A preliminary retrospective analysis of the DSM-IV criterion A-2. Journal of Behavior Therapy and Experimental Psychiatry. 1998;29:123–130. doi: 10.1016/S0005-7916(98)00007-X. [DOI] [PubMed] [Google Scholar]
- Rosen G. Posttraumatic stress disorder: Issues and controversies. New York, NY: Wiley; 2004. [DOI] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: Relevance to human research. Biological Psychology. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49:3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. A memory-based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review. 2008;115:985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113:867– 880. doi: 10.1037/0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–172. doi: 10.1037/0033-295X.110.1.145. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology. 1999;76:805–819. doi: 10.1037/0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Review of Neuroscience. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind. Neuroimage. 2003;19:1835–1842. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of an emotional state. Psychological Review. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schell TL, Marshall GN, Jaycox LH. All symptoms are not created equal: The prominent role of hyperarousal in the natural course of posttraumatic psychological distress. Journal of Abnormal Psychology. 2004;113:189–197. doi: 10.1037/0021-843X.113.2.189. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Spiro A, Vielhauer MJ, Findler MN, Hamblen JL. Trauma in the lives of older men: Findings from the normative aging study. Journal of Clinical Geropsychology. 2002;8:175–187. doi: 10.1023/A:1015992110544. [DOI] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Annals of the New York Academy of Sciences. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: Functional specialization and dynamic competition in human posterior parietal cortex. The Journal of Neuroscience. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Dolan RJ. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Moores KA, Clark RC, McFarlane AC, Strother SC, Bryant RA, Taylor JD. Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2009;172:235–241. doi: 10.1016/j.pscychresns.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? Journal of Traumatic Stress. 2009;22:409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety. Neuropsychopharmacology Reviews. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Structural and functional anatomy of PTSD: Findings from neuroimaging research. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. New York, NY: Guilford Press; 2005. pp. 59–82. [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal functioning in PTSD. Annuals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shipherd JC, Salters-Pedneault K. Attention, memory, intrusive thoughts, and acceptance in PTSD: An update on the empirical literature for clinicians. Cognitive and Behavioral Practice. 2008;15:349–363. doi: 10.1016/j.cbpra.2008.01.003. [DOI] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. Journal of Comparative Neurology. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Anterior medial temporal lobe in human cognition: Memory for fear and the unexpected. Cognitive Neuropsychiatry. 2006;11:198–218. doi: 10.1080/13546800500305096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Dietrich C, Mothes-Lasch M, Mentzel H, Miltner WHR. The volatility of the amygdala response to masked fearful eyes. Human Brain Mapping. 2010;31:1601–1608. doi: 10.1002/hbm.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. Principles of brain evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: Neural circuits underlying voluntary control of basic motivated behaviors. The Journal of Comparative Neurology. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neuroscience. 1998;21:323–331. doi: 10.1016/S0166-2236(98)01265-X. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nature Neuroscience. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Wager TD. Cognitive and motivational functions of the human prefrontal cortex. In: Christensen A-L, Bougakov D, Goldberg E, editors. Luria’s legacy in the 21st century. New York, NY: Oxford University Press; 2009. pp. 30–61. [DOI] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proceedings of the National Academy of Sciences. 1998;95:15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, … Mize J. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York, NY: Guilford Press; 2008. pp. 249–271. [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE. Towards a taxonomy of attention-shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:127–143. doi: 10.3758/CABN.5.2.127. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Pre-frontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the annual meeting of the International Society of Traumatic Stress Studies; San Antonio, TX. 1993. Oct, [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Neurobiological alterations associated with traumatic stress. Perspectives in Psychiatric Care. 2007;43:114–122. doi: 10.1111/j.1744-6163.2007.00120.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. doi: 10.1111/1467-8721.ep10836912. [DOI] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, Simmons WK, Barsalou LW. Grounding emotion in situated conceptualization. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2010.12.032. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. NeuroImage. 2003;18:660–669. doi: 10.1016/S1053-8119(02)00037-X. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuro Image. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging. 2005.01.014. [DOI] [PubMed] [Google Scholar]
- Wundt W. In: Outlines of psychology. Judd CH, translator. Bristol, England: Thoemmes Press; 1998. (Original work published 1897) [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J. The neural basis of human social values: evidence from functional MRI. Cerebral Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/S0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]