Abstract
Human settlements are expanding in species-rich regions and pose a serious threat to biodiversity conservation. We quantify the degree to which this threat manifests itself in two contrasting continents, Australia and North America, and suggest how it can be substantially alleviated. Human population density has a strong positive correlation with species richness in Australia for birds, mammals, amphibians, and butterflies (but not reptiles) and in North America for all five taxa. Nevertheless, conservation investments could secure locations that harbor almost all species while greatly reducing overlap with densely populated regions. We compared two conservation-planning scenarios that each aimed to represent all species at least once in a minimum set of sampling sites. The first scenario assigned equal cost to each site (ignoring differences in human population density); the second assigned a cost proportional to the site's human population density. Under the equal-cost scenario, 13–40% of selected sites occurred where population density values were highest (in the top decile). However, this overlap was reduced to as low as 0%, and in almost all cases to <10%, under the population-cost scenario, when sites of high population density were avoided where possible. Moreover, this reduction of overlap was achieved with only small increases in the total amount of area requiring protection. As densely populated regions continue to expand rapidly and drive up land values, the strategic conservation investments of the kind highlighted in our analysis are best made now.
The size, growth rate, and consumption patterns of the global human population are significant threats to biodiversity (1–5), yet little attention has been given to the conservation implications of where people live. Human settlement patterns impact biodiversity directly (e.g., habitat alteration) and indirectly by influencing land prices and other costs of achieving conservation (6–9). Human population density is positively correlated with deforestation in tropical forests (10, 11), abundance of invasive species (12, 13), and extinction rates and the proportion of threatened species in important taxa (14–18). These studies suggest that human population density may be a useful surrogate measure of the impact on biodiversity of a range of activities associated with human settlements (e.g., habitat clearance, waste disposal, recreation, and hunting). There is an urgent need, therefore, to characterize the level of spatial overlap between densely populated and biodiverse areas and to evaluate options for alleviating potential conflict (19–21).
Methods
Population Density and Taxon Correlations. We investigated the spatial congruence between human population density and species distributions of birds, mammals, reptiles, amphibians, and butterflies across Australia (including Tasmania and Kangaroo, Bathurst, and Melville islands) and North America (the continental United States and Canada). We examined broad correlative relationships between human population density and (i) species richness, (ii) the percentage of threatened species, and (iii) the percentage of species with restricted geographic distributions (the latter two groups are of particular conservation concern) (22, 23).
For Australia, data were collected at a spatial resolution of 1°-grid cells, from The Atlas of Australian Birds (www.birdsaustralia.com.au/atlas/index.html) and published sources (24–26). Threatened species were those listed nationally as endangered or vulnerable by the Australian Federal Government under the Environment Protection and Biodiversity Conservation Act 1999 (www.erin.gov.au/biodiversity/threatened/species/_index.html). Species with restricted geographic distributions were defined as those that occurred in four or fewer grid cells (mostly contiguous). For North America, data were collected across 110 ecoregions (see ref. 27 for details of data acquisition). Threatened species were those listed as “critically imperiled,” “imperiled,” and “vulnerable” by NatureServe (2001) (www.natureserve.org/explorer). Restricted species in North America were defined as those occupying only one ecoregion or those with a total range of <50,000 km2. We divided the number of threatened or restricted species in each grid cell or ecoregion by the total number of species therein to obtain a percentage of threatened or restricted species (i.e., a normalized index). Only native terrestrial or freshwater species were included. Estimates of human population density for 1995 were obtained from the Gridded Population of the World, Version 2 (http://sedac.ciesin.columbia.edu/plue/gpw/index.html).
We used the following index (modified from ref. 6), which combines all five taxa, weighted equally, into a single measure of overall species richness:
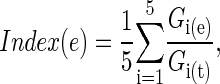 |
where Gi(e) is the number of species of group i in the grid cell or ecoregion, and Gi(t) is the total number of species of group i in the Australian or North American databases. This index reports the average proportion of the continental species pool that is found in a given ecoregion or grid cell and weights each taxon equally to diminish the influence of species-rich taxa.
As grid cells and ecoregions are contiguous, data are likely to be spatially autocorrelated, thereby reducing P values by overestimating degrees of freedom (28). Therefore, we do not include P values in our analyses, instead using correlation coefficients (which are unaffected by spatial autocorrelation) to represent the strength of association between variables. Non-parametric Spearman rank correlations were used because the distributions of most data were heavily skewed.
Complementarity Analysis. We examined the degree to which careful location of conservation efforts could alleviate spatial conflict between human settlements and biodiversity by using a complementarity analysis. Even with substantial congruence between human population density and species richness, certain conservation goals still may be achieved if target areas are carefully selected. For example, if species occur in multiple grid cells or ecoregions, it may be possible to select a set of cells or ecoregions that avoid densely populated areas yet still contain most species. We investigated this issue in Australia (where data were most amenable to this approach) for each taxon and all taxa combined. We used the simulated annealing algorithm in the sites v. 1.0 software program (which is based on spexan reserve selection software; see refs. 29 and 30 for details) with 1,000,000 iterations and 10 repeat runs and selected the best run.
Our conservation goal was to represent each species at least once in a minimum set of grid cells under two scenarios: (i) ignoring human population density by assuming that the cost of acquiring each grid cell was equal and (ii) assigning each grid cell an acquisition cost proportional to its human population density (i.e., avoiding cells of high population density where possible). For both scenarios, we recorded the percentage of selected grid cells in the optimum set that also ranked in the top 10% for human population density and the number of cells required to meet the conservation goal (i.e., representation of each species at least once).
Results and Discussion
Population Density and Taxon Correlations. Human population density was strongly correlated with the overall species richness of all taxa combined in both continents (Fig. 1), and with the richness of each taxon except reptiles in Australia (Fig. 2 A). The negative correlation with Australian reptiles probably occurred because many of these species occupy the sparsely populated arid and semiarid regions in the center of the continent. Our results emphasize two crucial points. First, the most biodiverse regions of each continent are also the most threatened by high human population densities. Second, the level of spatial congruence differs among taxa, so relying on popular indicator taxa (e.g., birds) to set conservation policy may lead to failure (31).
Fig. 1.
Correlations between [logarithm10 (Log10)] human population density and weighted species richness for all taxa in Australia (Upper) and North America (Lower).
Fig. 2.
Correlations between human population density and the richness of each taxon (A), the percentage of threatened species in each taxon (B), and the percentage of restricted species in each taxon (C) across Australia (n = 764) and North America (n = 109). Error bars represent one standard error.
Australia and North America differed markedly in the relationship between human population density and percent of threatened species. In Australia, population density was positively correlated only with threatened reptiles and birds, whereas all threatened taxa in North America had relatively strong positive correlations with population density (Fig. 2B). In contrast, correlation coefficients between human population density and the percentage of species with restricted geographic ranges were positive for all taxa in Australia, and for each taxon except birds in North America (Fig. 2C). Correlations between human population density and threatened species or those with restricted distributions are of particular concern because these species are likely to be especially susceptible to the impacts associated with human settlements (22, 23, 32).
It is possible that biogeographical relationships involving species richness may be dominated by wide-ranging species that contribute the majority of distribution records (33). If these wide-ranging species are largely responsible for the patterns we find above, the congruence between human populations and biodiversity may be less of a concern, because these species could potentially avoid conflict in less impacted areas of their broad range.
To test this suggestion, we repeated the above correlations with each taxon split into range-size quartiles (based on the methods of ref. 33) and found remarkably consistent patterns. For almost all taxa, there were strong positive correlations between human population density and species richness in each range-size quartile (Fig. 3), contrary to the findings of ref. 33 for birds in sub-Saharan Africa. The exceptions were mammals (Australia and North America) and reptiles (Australia). Only for reptiles in Australia did wide-ranging species have a substantial impact on the overall result. Positive correlations were recorded for the first (smallest) to third range-size quartiles (Fig. 3A), but the strong negative correlation with wide-ranging reptile species in Australia led to a slightly negative correlation overall (Fig. 2 A). Clearly, the influence of wide-ranging species does not dominate our principal results; human population distribution is a threat to the majority of species regardless of range size.
Fig. 3.
Correlations between human population density and range-size quartiles for each taxon in Australia (A) and North America (B). The first quartile represents the smallest range size. Error bars represent one standard error.
Sampling bias also may inflate observed relationships between species richness and human population density, because more surveys are likely to be conducted close to population centers. For birds in Australia (the only taxon for which suitable data were available), we found that the number of surveys was indeed highest in densely populated areas (rs = 0.652, n = 772). However, after controlling for sampling intensity with partial correlations (34), there was still a relatively strong positive correlation between human population density and bird species richness (rs = 0.272). In Australia, sampling intensity for all taxa is probably lowest in the arid inland regions, but these generally species-poor regions likely would not yield many more additional species with further sampling. Sampling bias is unlikely to be a problem in the North American data set, because the size of ecoregions and the relatively high level of sampling suggest that most species have been identified and reasonably well mapped (31).
Complementarity Analysis. Under the first conservation planning scenario, which assumed equal acquisition cost for each grid cell (i.e., ignoring human population density), 13–40% of the cells in the selected set were in the highest decile of population density values. However, when human population density was explicitly factored into the selection algorithm, the overlap between population density and species distribution was reduced to as low as 0%, and in almost all cases to <10% (Fig. 4A). Moreover, this remarkable reduction in overlap was achieved with only small increases in the amount of area requiring protection (Fig. 4B). Therefore, it may be possible to reduce conflicts between biodiversity conservation and human settlements, with only a small increase in the total conservation area required, if factors such as population density are explicitly considered when assessing conservation options.
Fig. 4.
(A) Percent of overlap between grid cells in selected sets and those occurring in the top decile of human population density under two scenarios: (i) equal cost (differences in human population density ignored) and (ii) population cost (where cost is proportional to the population density of each cell). (B) The number of grid cells representing all species at least once under the two cost scenarios.
The threats that human populations pose to biodiversity are well recognized, and the positive correlation between human population density and species richness occurs in both developing (20) and developed (21) regions. Surprisingly, this realization is not commonly translated explicitly into the conservation planning process (but see refs. 20 and 35–37). The results of our study indicate that there may be substantial opportunities for protecting biodiversity, despite the overlap between human settlements and species-rich regions.
Three caveats deserve mention here. First, completely avoiding this conflict is unlikely because numerous range-restricted species occur in densely populated areas. Therefore, it remains an imperative that conservation also occurs where most people live. Second, in some places site selection may be further restricted by substantially modified regions with low population density (e.g., agricultural landscapes where most of the native habitat has been cleared), and this effect also needs to be considered when developing conservation strategies. Finally, consideration should be given to biodiverse areas with low current population densities but rapid population growth rates, because they represent areas where biodiversity is likely to experience substantial threats in the future (9).
Conclusion
The results of our study present a clear message to conservation planners. In addition to the standard biological data that are used to guide planning decisions (38), human settlement patterns must be explicitly considered as factors from the very beginning of planning processes. This consideration is crucial to reduce the conflict between population density and biodiversity and to minimize the cost of conservation (9). As human population density increases, land prices inevitably rise, making conservation an expensive exercise near human settlements. Therefore, considering both biological and demographic data can help to ensure effective conservation at the least cost.
Ultimately, conserving a single representative sample of each species is a poor substitute for the protection of ecosystem processes, viable species populations, and other elements of biodiversity (39–42) that are often included in many systematic conservation plans (43). Our study represents a broad-brush approach designed to illustrate the threat posed to biodiversity conservation by human settlement patterns. Predicted population growth in Australia, North America, and other regions underscores the immediate need to develop effective conservation strategies that alleviate the spatial conflict between people and biodiversity.
Acknowledgments
We thank P. and H. Bing for financial support; G. Barret, M. Braby, G. Ceballos, H. Cogger, and R. Poulter for facilitating access to distribution data; S. Bailey, J. Fay, and A. McMillan for assistance with geographical information systems application and production of sampling maps; M. Jemente and R. Phelps for assistance with data collection; and T. Allnutt, A. Balmford, C. Boggs, N. Burgess, P. Ehrlich, W. Jetz, C. Loucks, H. Possingham, T. Sisk, and an anonymous reviewer for helpful comments on the manuscript. This work was supported by the Moore Family Foundation, the Koret Foundation, and the Winslow Foundation.
References
- 1.Myers, N. (1979) The Sinking Ark: A New Look at the Problem of Disappearing Species (Pergamon, New York).
- 2.Pimm, S. L., Russell, G. J., Gittleman, J. L. & Brooks, T. M. (1995) Science 269, 347–350. [DOI] [PubMed] [Google Scholar]
- 3.Sala, O. E., Chapin, F. S., III, Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., et al. (2000) Science 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- 4.Wilson, E. O. (2002) The Future of Life (Alfred A. Knopf, New York).
- 5.Wackernagel, M., Schulz, N. B., Deumling, D., Linares, A. C., Jenkins, M., Kapos, V., Monfreda, C., Loh, J., Myers, N., Norgaard, R., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 9266–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sisk, T. D., Launer, A. E., Switky, K. R. & Ehrlich, P. R. (1994) Bioscience 44, 592–604. [Google Scholar]
- 7.Ando, A., Camm, J., Polasky, S. & Solow, A. (1998) Science 279, 2126–2128. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor, C., Marvier, M. & Kareiva, P. (2003) Ecol. Lett. 6, 706–711. [Google Scholar]
- 9.Balmford, A., Gaston, K. J., Blyth, S., James, A. & Kapos, V. (2003) Proc. Natl. Acad. Sci. USA 100, 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurance, W. F., Albernaz, A. K. M., Schroth, G., Fearnside, P. M., Bergen, S., Venticinque, E. M. & Da Costa, C. (2002) J. Biogeogr. 29, 737–748. [Google Scholar]
- 11.Steininger, M. K., Tucker, C. J., Townshend, J. R. G., Killeen, T. J., Desch, A., Bell, V. & Ersts, P. (2001) Environ. Conserv. 28, 127–134. [Google Scholar]
- 12.McKinney, M. L. (2001) Biol. Conserv. 100, 243–252. [Google Scholar]
- 13.Pyšek, P., Jarošík, V. & Kuèera, T. (2002) Biol. Conserv. 104, 13–24. [Google Scholar]
- 14.Thompson, K. & Jones, A. (1999) Conserv. Biol. 13, 185–189. [Google Scholar]
- 15.McKinney, M. L. (2002) Biodivers. Conserv. 11, 1317–1325. [Google Scholar]
- 16.Parks, S. A. & Harcourt, A. H. (2002) Conserv. Biol. 16, 800–808. [Google Scholar]
- 17.Dobson, A. P., Rodríguez, J. P. & Roberts, W. M. (2001) Ecol. Appl. 11, 1019–1026. [Google Scholar]
- 18.Brashares, J. S., Arcese, P. & Sam, M. K. (2001) Proc. R. Soc. London Ser. B 268, 2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cincotta, R. P., Wisnewski, J. & Engelman, R. (2000) Nature 404, 990–992. [DOI] [PubMed] [Google Scholar]
- 20.Balmford, A., Moore, J. L., Brooks, T., Burgess, N., Hansen, L. A., Williams, P. & Rahbek, C. (2001) Science 291, 2616–2619. [DOI] [PubMed] [Google Scholar]
- 21.Araújo, M. B. (2003) Global Ecol. Biogeogr. 12, 5–12. [Google Scholar]
- 22.Manne, L. L., Brooks, T. M. & Pimm, S. L. (1999) Nature 399, 258–261. [Google Scholar]
- 23.Purvis, A., Gittleman, J. L., Cowlishaw, G. & Mace, G. M. (2000) Proc. R. Soc. London Ser. B 267, 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braby, M. F. (2000) Butterflies of Australia: Their Identification, Biology and Distribution (CSIRO, Collingwood, Australia).
- 25.Strahan, R. (1995) Mammals of Australia (Reed Books, Chatswood, Australia).
- 26.Cogger, H. G. (2000) Reptiles and Amphibians of Australia (Ralph Curtis Books, Sanibel Island, FL).
- 27.Ricketts, T. H., Dinerstein, E., Olson, D. M., Loucks, C. J., Eichbaum, W., Kavanagh, K., Hedao, P., Hurley, P., Carney, K. M., Abell, R., et al. (1999) Terrestrial Ecoregions of North America: A Conservation Assessment (Island, Washington, DC).
- 28.Burrough, P. A. (1995) in Data Analysis in Community and Landscape Ecology, eds. Jongman, R. H. G., Ter Braak, C. J. F. & Van Tongeren, O. F. R. (Cambridge Univ. Press, New York), pp. 213–251.
- 29.Ball, I. R. (2000) Ph.D. thesis (Univ. of Adelaide, Adelaide, Australia).
- 30.Possingham, H. P., Ball, I. R. & Andelman, S. (2000) in Quantitative Methods for Conservation Biology, eds. Ferson, S. & Burgman, M. (Springer, New York), pp. 291–306.
- 31.Ricketts, T. H., Dinerstein, E., Olson, D. M. & Loucks, C. (1999) Bioscience 49, 369–381. [Google Scholar]
- 32.Gaston, K. J. (1998) Nature 394, 229–230. [Google Scholar]
- 33.Jetz, W. & Rahbek, C. (2002) Science 297, 1548–1551. [DOI] [PubMed] [Google Scholar]
- 34.Serlin, R. C. & Harwell, M. R. (1993) Commun. Stat. Simul. Comput. 22, 545–567. [Google Scholar]
- 35.Abbitt, R. J. F., Scott, J. M. & Wilcove, D. S. (2000) Biol. Conserv. 96, 169–175. [Google Scholar]
- 36.Rouget, M., Richardson, D. M., Cowling, R. M., Lloyd, J. W. & Lombard, A. T. (2003) Biol. Conserv. 112, 63–85. [Google Scholar]
- 37.Cowling, R. M., Pressey, R. L., Rouget, M. & Lombard, A. T. (2003) Biol. Conserv. 112, 191–216. [Google Scholar]
- 38.Groves, C. R. (2003) Drafting a Conservation Blueprint (Island, Washington, DC).
- 39.Ehrlich, P. R. & Daily, G. C. (1993) Ambio 22(2/3), 64–68. [Google Scholar]
- 40.Hughes, J. B., Daily, G. C. & Ehrlich, P. R. (1997) Science 278, 689–692. [DOI] [PubMed] [Google Scholar]
- 41.Kareiva, P. & Marvier, M. (2003) Am. Sci. 91, 344–351. [Google Scholar]
- 42.Luck, G. W., Daily, G. C. & Ehrlich, P. R. (2003) Trends Ecol. Evol. 18, 331–336. [Google Scholar]
- 43.Margules, C. R. & Pressey, R. L. (2000) Nature 405, 243–253. [DOI] [PubMed] [Google Scholar]






