Abstract
SIGN-R1, a recently discovered C-type lectin expressed at high levels on macrophages within the marginal zone of the spleen, mediates the uptake of dextran polysaccharides by these phagocytes. We now find that encapsulated Streptococcus pneumoniae are rapidly cleared by these macrophages from the bloodstream, and that capture also takes place when different cell lines express SIGN-R1 after transfection. To assess the role of the capsular polysaccharide of S. pneumoniae (CPS) in the interaction of SIGN-R1 with pneumococci, we first studied binding and uptake of serotype 14 CPS in transfected cells. Binding was observed and was of a much higher avidity (3,000-fold) for CPS 14 than dextran. The CPSs from four different serotypes were also cleared by marginal zone macrophages in vivo. To establish a role for SIGN-R1 in this uptake, we selectively down-regulated expression of the lectin by pretreatment of the mice with SIGN-R1 antibodies, including a newly generated hamster monoclonal called 22D1. For several days after this transient knockout, the marginal zone macrophages were unable to take up either CPSs or dextrans. Therefore, marginal zone macrophages in mice have a receptor that interacts with capsular pneumococcal polysaccharides, setting the stage for further studies of the functional consequences of this interaction.
The spleen functions at several points in innate and adaptive immunity. A major innate function is exerted by macrophages that are abundant in vascular regions termed the splenic red pulp, whereas adaptive functions are carried out by B and T lymphocytes, typically located in white pulp nodules. At the junction of each white pulp nodule with the red pulp is a specialized region called the marginal zone, which is composed of several concentric regions (1). Innermost is a ring of macrophages termed marginal metallophils, expressing a hemagglutinin termed sialoadhesin or CD169 (2, 3). Then there is a vascular sinus that receives blood via penetrating small arterial vessels from the white pulp. Surrounding the marginal sinus is a zone composed of large macrophages as well as specialized B lymphocytes (4). Within and surrounding the marginal zone are also dendritic cells (5, 6), possibly in the process of migrating from the blood to the T cell regions of the white pulp.
With respect to host defense, the spleen plays a special role during blood-borne infection with encapsulated microorganisms, particularly Streptococcus pneumoniae bacteria (7–12). A critical role of the spleen is the formation of antibodies by marginal zone B cells (13–15), particularly complement-fixing antibodies (16–20). The role of macrophages in the processes of microbial clearance and resistance and antibody formation to S. pneumoniae needs to be considered (21), particularly given recent data that marginal zone macrophages interact and retain B cells in this region (22). Here we show that marginal zone macrophages express a receptor called SIGN-R1 that is able to bind and internalize the capsular pneumococcal polysaccharide (CPS). SIGN-R1 is a C-type lectin that is a member of a recently identified family related to DC-SIGN (23). It was recently reported that SIGN-R1 is expressed at high levels in marginal zone macrophages of the spleen, as well as other macrophages in the lymph node (24, 25). Furthermore, SIGN-R1 mediates the clearance of the polysaccharide dextran in vivo (24, 25). We therefore asked whether SIGN-R1 also was involved in the uptake of pneumococci and its capsular polysaccharide. We find that this is the case, and that CPS uptake can be eliminated in mice that are selectively depleted of SIGN-R1 by treatment with specific antibody to this lectin.
Methods
Mice and Cell Culture. C57BL/6 mice from The Jackson Laboratory were kept under specific pathogen-free conditions until use at 6–10 weeks of age. All experiments were conducted according to institutional guidelines. Chinese hamster ovary (CHO) and OKT8 cells were cultured in DMEM with 10% FCS/100 units/ml penicillin G/100 μg/ml streptomycin. DCEK, a mouse L cell fibroblast line, was cultured in RPMI medium 1640 with 10% FCS and antibiotics. Stable CHO transfectants expressing cDNAs of mouse SIGN-R1, DC-SIGN, SIGN-R3, and DEC205 were generated as described (25) and cloned under G418 (1.5 mg/ml) selection pressure. Stable OKT8 and DCEK SIGN-R1 transfectants were generated by using a pMX retroviral vector (26) as described (27).
Antibodies and Microscopy. A soluble SIGN-R1 antigen of fusion between the extracellular portion of SIGN-R1 and mouse IgG Fc was produced, affinity purified from transfected mammalian cells, and used as antigen to generate a new hamster monoclonal antibody, 22D1, in the Hybridoma Core Facility at Mt. Sinai School of Medicine. Rabbit polyclonal antibodies against the C-terminal 13-aa peptide of SIGN-R1 (PAb-C13) were described (25). Similarly, rabbit polyclonal antibodies against the 16-aa peptide of mouse DC-SIGN (NH2–FRDDGWNDTKCTNKKF-COOH) and SIGN-R3 (NH2–FSGDGWDLSCDKLLF–COOH) carbohydrate recognition domains were generated by Invitrogen, as described (25). Antibodies to DEC205 (CD205), I-A (MHC II), sialoadhesin (CD169), and F4/80 were purified from the supernatants of the NLDC-145, KL295, SER-4, and F4/80 hybridomas (25). Antibodies to the following targets were purchased: Actin (Abcam, Cambridge, MA), SIGN-R1 [ERTR9 (28), BMA Biomedicals], MARCO [ED31 (29), Serotec], transferrin receptor (C2F2, BD Biosciences PharMingen), and IgM (Southern Biotechnology Associates). Serotype-specific polyclonal rabbit antibodies to pneumococcal polysaccharides were purchased from Statens Serum Institute (Copenhagen). A deconvolution microscope (Olympus, Melville, NY) and one-, two-, or three-color fluorescence labeling were used.
SDS/PAGE and Western Blot Analysis. Spleens were lysed in RIPA buffer (150 mM NaCl/50 mM Tris·HCl, pH 8.0/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) supplemented with protease inhibitor cocktails (Sigma) and stored at -80°C. Each lysed sample was mixed with an equal volume of 2× SDS sample buffer with 2-mercaptoethanol and boiled at 95°C for 5 min. The samples of lysate were separated in 4–15% gradient SDS/PAGE, transferred onto poly(vinylidene difluoride) membranes, followed by incubation with antibodies. Antibody-reactive bands on the blots were visualized with peroxidase-labeled secondary antibodies followed by ECL+plus chemiluminescent substrate (Amersham Pharmacia Biosciences) and exposure in Kodak BioMax Light film (Eastman Kodak).
Polysaccharides. FITC-Ficoll (Biosearch) and CPSs of various serotypes (American Type Culture Collection, Manassas, VA) were purchased. The following materials were purchased from Sigma: FITC-dextran (2,000 kDa), dextran (2,000 kDa), and Ficoll (400 kDa). To study endocytosis of these polysaccharides in vivo, 100 μg per mouse was administered i.v. and uptake studied from 1 to 24 h later; alternatively, the polysaccharides were added in vitro at 1–50 μg/ml for 1–2 h on ice or at 37°C to cell lines transfected with SIGN-R1, and mDC-SIGN or empty vector as negative control. To test for inhibition of uptake, we used 100 μg of antibody per animal given i.v. before injecting 100 μg of FITC-dextran or CPSs.
S. pneumoniae Strains and Fluorescent Labeling. S. pneumoniae capsular type 3 (DCC1714) and 14 (DCC1490) were grown in Brain Heart Infusion broth (Difco) to midlogarithm phase and inactivated with 50 μg/ml mitomycin-C (Sigma) for 1 h or heat killed by incubation at 95°C for 10 min, which removes the capsule. These bacteria were labeled with the PKH2 (green) or PKH26 (red) fluorescent cell linker kits (Sigma). Suspensions of 109 bacteria per milliliter were incubated at 25°C for 10 min in a diluent containing 2 μM PKH dyes. After incubation, the excess of fluorescent dye was removed by extensive washing in PBS. The fluorescent bacteria (1 × 108) were given i.v., and mouse spleen sections were examined by deconvolution fluorescence microscopy.
FACS Analysis and Inhibition Experiments. Transfected CHO cells were incubated with antibodies for 30 min, washed, and incubated with secondary antibodies, followed by analysis with the FACSCalibur Flow Cytometry System (BD Biosciences). For FACS analysis of SIGN-R3-transfected CHO cells, we used incubation with FITC-dextran to verify SIGN-R3 expression, because the rabbit anti-SIGN-R3 peptide polyclonal antibody could detect SIGN-R3 molecules only in Western blots and not FACS. To stain for CPSs, transfectants were permeabilized by using Cytofix/Cytoperm kit (BD Biosciences PharMingen) before staining. For inhibition experiments, cells were first incubated for 10 min with varying concentrations of polysaccharides at 4°C, followed by incubation with 1 μg/ml FITC-dextran at 37°C for 1 h. The percentage of inhibition of FITC-dextran uptake in the presence of inhibitors was: % Inhibition = {(MFI of cells stained without inhibitors)-(MFI of cells stained with inhibitors)} × 100 ÷ (MFI of cells stained without inhibitors) (MFI, mean fluorescence index).
Results
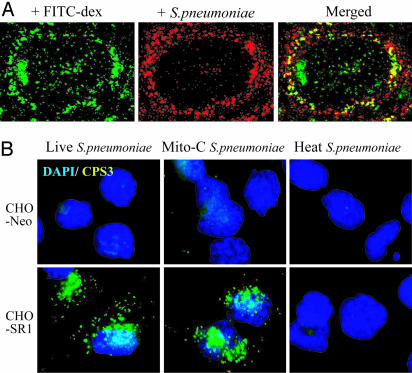
The Lectin SIGN-R1 Mediates Capture of Encapsulated S. pneumoniae. We began our studies by following the uptake of encapsulated fluorescent-labeled S. pneumoniae into the spleen. Thirty minutes after injecting 108 mitomycin-treated organisms, the spleens were sectioned and examined by deconvolution fluorescence microscopy. The S. pneumoniae were primarily found in the marginal zone (Fig. 1A). The red fluorescent organisms were within macrophages, as evidenced by two new criteria (24, 25), i.e., uptake of the polysaccharide FITC-dextran (Fig. 1 A) or staining with an antibody to SIGN-R1 (not shown). To test whether SIGN-R1 was itself involved in microbial uptake, we exposed SIGN-R1 transfectants to S. pneumoniae in culture at a multiplicity of 100:1. For both CHO cells (Fig. 1B) and DCEK cells (not shown), SIGN-R1 transfectants bound encapsulated S. pneumoniae but not capsule-depleted heated organisms. In contrast, SIGN-R1 transfectants did not bind Alexa488-labeled Staphylococcus aureus. To verify the role of SIGN-R1 in the binding of S. pneumoniae by transfectants, we showed that the ER-TR9 antibody to SIGN-R1 blocked binding, whereas control antibody or antibody to the transferrin receptor did not (not shown). These initial findings indicate that encapsulated S. pneumoniae are rapidly taken up by SIGN-R1+ splenic macrophages in situ and captured by SIGN-R1 transfectants in culture.
Fig. 1.
The lectin SIGN-R1 mediates the uptake of encapsulated S. pneumoniae. (A) Mice were injected i.v. with 108 fluorescent (red, PKH26) organisms after 100 μg of FITC-dextran i.v. injection for 1 h, the latter to label SIGN-R1+ marginal zone macrophages as described (24, 25). (B) CHO cells transfected with control (neomycin) DNA or with SIGN-R1 cDNA were challenged for 1 h with live, mitomycin-treated, or heat-treated S. pneumoniae serotype 3 (1,000,000 green organisms per 10,000 cells), and uptake was assessed alongside a 4′,6-diamidino-2-phenylindole (blue) nuclear label.
SIGN-R1 Mediates Uptake of the Capsular Polysaccharide of S. pneumoniae. The uptake of encapsulated but not heated organisms in Fig. 1B suggested that the CPS was being recognized by SIGN-R1. We therefore studied the uptake by DCEK cells of CPS from three serotypes, 14, 23, 26. Cells transfected with SIGN-R1 took up these polysaccharides (Fig. 2A). No uptake was seen in wild-type cells or cells transfected with three other putative lectins (DC-SIGN in Fig. 2 A; SIGN-R3 and DEC-205, not shown). SIGN-R1 also mediated uptake of FITC-dextran (Fig. 2 A) as described (24, 25), whereas cells exposed to both dextran and CPS showed considerable double labeling of the intracellular endocytic compartments (Fig. 2 A Inset). In addition to these microscopic assays, uptake of CPS14 by SIGN-R1 was evident with FACS analyses of either DCEK or RAW transfectants (not shown). To directly demonstrate binding to the cell surface of the transfectants, binding of CPS14 and FITC-dextran at 4°C was additionally found (Fig. 2B). Interestingly, when we tried to block uptake of FITC-dextran by using FACS assays, CPS14 was a much more effective blocker than cold dextran (Fig. 2C). We therefore used this FACS assay to quantify the capacity of different CPSs (30, 31) to block uptake of FITC-dextran via SIGN-R1. CPS14 was ≈3,000 times better in blocking the uptake of FITC-dextran than dextran itself (Fig. 2D), whereas CPS3 was ≈50 times better. IC50 values for CPS14, CPS3, and cold dextran were 0.009, 0.5, and 31 mg/ml, respectively. These findings indicate that SIGN-R1 mediates the uptake of several different CPSs, and among the serotypes we studied, CPS14 was most effectively recognized.
Fig. 2.
SIGN-R1 mediates the uptake of the capsular polysaccharide of S. pneumoniae. (A) Both FITC-dextran (green) and CPS 14 (red) are taken up by SIGN-R1-transfected DCEK cells but not by mouse DC-SIGN transfectants. The white arrow (Lower Right) illustrates extensive double labeling of CPS14 and FITC-dextran in endocytic vacuoles. (B)Asin A, but the polysaccharides were applied at 4°C to assess binding to the cell surface. (C) CPS14 (pretreatment at 100 μg/ml for 30 min at 37°C) but not cold dextran or NP-ficoll (both at 500 μg/ml) blocked uptake of FITC-dextran, given for 1 h at 1 μg/ml. (D) Graded doses of each of the indicated polysaccharides were added for 30 min before and 1 h during exposure to 1 μg/ml FITC-dextran.
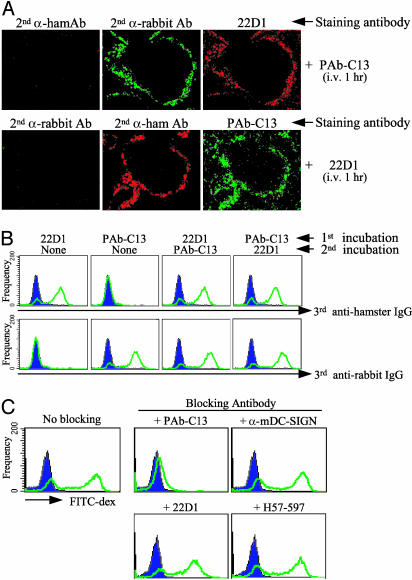
Isolation of the 22D1 Hamster Monoclonal Antibody to Mouse SIGN-R1. Two antibodies to SIGN-R1 had been used in prior work to block the interaction of FITC-dextran with this lectin. One was a rabbit polyclonal (here termed PAb-C13) to a COOH terminal 13-aa peptide (SIGN-R1 is a type II transmembrane protein), whereas the other was a commercial rat IgM monoclonal termed ERTR9 (28). After immunization of hamsters with SIGN-R1 fusion protein, we retrieved a stable monoclonal, named 22D1, that selectively bound to SIGN-R1 but not to cells transfected with other lectins (data not shown). The 22D1 antibody also immunoblotted SIGN-R1 (see below). However, the hamster antibody recognized a site distinct from the peptide recognized by rabbit PAb-C13 antibody. For example, if mice were given 100 μg of either rabbit or hamster antibody i.v., and 1 h later the spleens were sectioned and stained for both the injected antibody or the alternative anti-SIGN-R1, there was clear double labeling, i.e., the binding of one anti-SIGN-R1 antibody did not block staining with the other (Fig. 3A). Similar results were obtained by FACS analysis, i.e., staining with 22D1 did not block staining with PAb-C13, and staining with PAb-C13 did not block reactivity with 22D1 (Fig. 3B). Polyclonal PAb-C13 was able to block the uptake of FITC-dextran by SIGN-R1 transfectants, but neither 22D1 nor antibodies to other lectins was inhibitory (Fig. 3C). Therefore, 22D1 is a distinct monoclonal capable of recognizing mouse SIGN-R1 but unable to block uptake of the polysaccharide FITC-dextran by SIGN-R1 transfectants.
Fig. 3.
Isolation of the 22D1 hamster monoclonal antibody to mouse SIGN-R1. (A) One hundred micrograms of rabbit PAb-C13 to SIGN-R1 or hamster 22D1 monoclonal was injected i.v., and 1 h later the spleen sections were stained as indicated above each micrograph. (B) Same as A, but SIGN-R1-transfected OKT8 cells were exposed successively for 30 min to one species (hamster monoclonal, rabbit polyclonal) of antibody, followed by a second incubation with the alternative antibody, and then the cells were stained for binding of hamster Ig (Upper) or rabbit Ig (Lower) to demonstrate that each anti-SIGN-R1 antibody does not block the binding of the other. (C) Blocking of FITC-dextran uptake (1 μg/ml for 1 h at 37°C) in OKT8 cells transfected with SIGN-R1 (blue tracing is the FITC signal from nontransfected cells exposed to FITC-dextran). The blocking antibodies to SIGN-R1 were rabbit PAb-C13 (and α-mDC-SIGN as a nonreactive control) and hamster 22D1 (and H57–597 to the T cell receptor as a nonreactive control).
Selective and Prolonged Down-Regulation of SIGN-R1 After Injection of Antibody. We then discovered that the antibodies could be injected i.v. and used to induce a selective, prolonged, but transient (5–15 days) down-regulation of SIGN-R1 protein as assessed by staining of tissue sections and immunoblotting. We refer to the antibody-treated mice as transient knockouts (TKOs). As shown in Fig. 4A, we injected mice with 100 μg of control hamster Ig or 22D1 monoclonal antibody, and then 1 day later, we gave FITC-dextran for 1 h. The 22D1-treated mice could no longer take up dextran into the marginal zone macrophages (Fig. 1 A), although liver uptake was ostensibly unperturbed. When the sections were stained for the injected hamster antibody, or with rabbit PAb-C13 antibody to a second SIGN-R1 epitope (above), no staining was found, indicating that both SIGN-R1 and the antibody had been down-regulated (Fig. 4A, second and third rows). The down-regulation of SIGN-R1 was specific, because other macrophage receptors, Marco and SER4, were still expressed on marginal zone macrophages and metallophils, respectively (Fig. 4A, fourth and fifth rows). This selective loss was confirmed by immunoblotting; treatment with SIGN-R1 antibody for 24 h led to the disappearance of the lectin but not several other molecules in macrophages and other cells of the spleen (Fig. 4A Right).
Fig. 4.
Selective down-regulation of SIGN-R1 after injection of antibody. (A) Mice were given 100 μg of control hamster Ig or 22D1 anti-SIGN-R1 antibody (similar results were obtained with rabbit PAb-C13 antibody). One day later, mice were given 100 μg of FITC-dextran i.v., and 1 h later, the spleens were sectioned to assess FITC-dextran uptake (Top) or for staining for hamster Ig, SIGN-R1 (with PAb-C13), and the macrophage receptors MARCO and SER4 (CD169, sialoadhesin). In parallel (Right), samples of spleen tissue were Western-blotted with antibodies to document the selective down-regulation of SIGN-R1. (B) In contrast to A, mice were first given 100 μg of FITC-dextran i.v. and 1 h later 100 μg of control hamster Ig or 22D1 anti-SIGN-R1. One day later, the spleens were examined for the persistence of FITC-dextran in the marginal zone macrophages (green) and the expression of SIGN-R1 (red) by staining with PAb-C13. (C) TKO of SIGN-R1 (green) but not MARCO (red) in macrophages given control Ig or 22D1 hamster monoclonal anti-SIGN-R1 1 day earlier. (Left) The antibody was given i.v. to target macrophages in spleen. (Right) The hamster antibody was given i.p. to target macrophages in mesenteric lymph nodes.
To further verify that antibody was depleting SIGN-R1 but not the respective macrophages, we first allowed the macrophages to take up FITC-dextran for 1 h, and then we injected control hamster Ig or 22D1 antibody (Fig. 4B). One day later, we looked for FITC-dextran and SIGN-R1 by using the rabbit PAb-C13. Treatment with 22D1 did not eliminate the dextran-laden macrophages but did eliminate the SIGN-R1 (Fig. 4B).
To determine whether antibody could transiently knock out SIGN-R1 in both spleen and lymph node, we gave mice 100 μg of 22D1 antibody i.v. or 200 μg i.p. to access the spleen and mesenteric lymph nodes, respectively. i.v. injection of antibody down-regulated SIGN-R1 expression in spleen, not lymph node, whereas i.p. injection primarily eliminated the lectin from lymph nodes (Fig. 4C). The elimination of SIGN-R1 persisted for ≈1 wk. Therefore, antibodies to SIGN-R1 are able to selectively knock out the lectin, permitting analysis of its function in vivo.
Uptake of Several Serotypes of Capsular Pneumococcal Polysaccharide Is Blocked in Mice Selectively Depleted of SIGN-R1. We first injected CPS from several different pneumococcal serotypes (types 3, 14, 23, and 26) i.v., and 30 min later we looked for the injected CPS in spleen sections. In each case, the CPSs were noted in abundance in the marginal zone macrophages, double labeled with PAb-C13 (Fig. 6, which is published as supporting information on the PNAS web site). At higher power, the CPS14 was located in internal granules within SIGN-R1+ macrophages (Fig. 5A). However, if we injected the 22D1 antibody i.v. 24 h earlier to create a SIGN-R1 TKO, and then we injected CPS i.v., there was no uptake of either FITC-dextran or CPS into the marginal zone macrophages, but uptake into the liver was retained (Fig. 5B). To establish the specific role for SIGN-R1 in CPS uptake in vivo, we injected mice with ED31, a commercial monoclonal to MARCO, a scavenger receptor also expressed on SIGN-R1-positive marginal zone macrophages (32). CPS uptake was blocked only in mice given 22D1 anti-SIGN-R1 but not in mice injected with ED31 anti-MARCO antibody (Fig. 5C). We conclude that antibody to SIGN-R1 selectively down-regulates this macrophage endocytic receptor for days in vivo, and that this method reveals SIGN-R1 to be a major receptor for the uptake of CPS in vivo.
Fig. 5.
Uptake of several serotypes of pneumococcal CPS is blocked in mice selectively depleted of SIGN-R1. (A) Mice were injected i.v. with 100 μg of CPS from different serotypes, and 30 min later, the spleens were taken for sectioning and staining for the injected CPS (by using serotype-specific rabbit polyclonal antibody, ATCC, red) and for SIGN-R1 (rabbit PAb-C13, green) to observe double labeling of the marginal zone macrophages. Here, CPS14 lies within SIGN-R1+ macrophages. (B) TKO of SIGN-R1 was induced by injecting 100 μg of 22D1 hamster Ig (or nonreactive hamster Ig control), and 24 h later, 100 μg of FITC-dextran (green) or CPS 14 (red) was given i.v. Thirty minutes later, spleen and livers were taken for sectioning and visualization of the injected SIGN-R1 ligands. (C) Additional mice were injected with a commercial rat IgG1 monoclonal to MARCO (named ED31) or a control polyclonal antibody to the cytosolic domain of mouse DC-SIGN. Each mouse was given 100 μg of CPS 14. Thirty minutes later, we visualized SIGN-R1 (green PAb-C13, Top), MARCO (green mAb, Middle), or the injected CPS 14 (red polyclonal to CPS14, Bottom).
Discussion
Despite the importance of the spleen in defense against encapsulated bacteria (7–12), particularly S. pneumoniae, there have been relatively few studies of the fate of these organisms or the CPS in vivo. In pioneering studies of the uptake of polysaccharides, primarily dextran, in mouse spleen (33, 34), Humphrey and Grennan (33) also reported the uptake of CPS in the macrophages of the marginal zone and red pulp. Recent investigations have shown that the marginal zone macrophages express a C-type lectin, SIGN-R1, that mediates dextran uptake (24, 25). Here we find that this lectin also contributes to the uptake of all four different CPS serotypes that we tested. Our work applies to the early clearance of CPS, 30–60 min after injection. At later time points, when complement begins to be fixed, the CPS is found on B cells and the follicular dendritic cells of the B cell areas, as reported (17, 35), because both cell types express the CD21 receptors for C3, the third component of complement.
At this time, the consequences of SIGN-R1 function after uptake of CPS in vivo remain to be determined. Interestingly, a related C-type lectin, human DC-SIGN, has multiple functions in vitro, including (i) uptake into processing compartments (36), (ii) sequestration within nonlysosomal nonendosomal vacuoles (37, 38), and (iii) binding to intercellular adhesion molecules on other cells like ICAM-3 on T cells (39) and ICAM-2 on endothelial cells (40). In an analogous manner, it will be important to assess whether the CPS is (i) digested by marginal zone macrophages, (ii) retained in an intact form available for subsequent presentation to B cells, and/or (iii) involved in interaction with other cells, such as marginal zone B cells, which are the lymphocytes involved in antibody formation to polysaccharides (15).
Likewise, the role of SIGN-R1 in the host response to the intact pneumococcus remains to be determined. Although SIGN-R1 mediates the uptake of encapsulated organisms (Fig. 1B), we suspect that there are additional recognition systems. First, when we transiently knock out SIGN-R1 with antibody, uptake of pneumococci by marginal zone macrophages is still observed. In addition, uptake of CPS in the TKO mice is extensive in liver macrophages (Fig. 5B). Conceivably there are additional receptors that recognize CPS and other pneumococcal cell wall components, such as the peptidoglycan.
An important method devised for our studies was the use of antibodies to transiently knock out a surface receptor for as long as 1 wk. When we administered a high dose of either monoclonal hamster antibody or polyclonal rabbit antibody, SIGN-R1 disappeared by using staining and immunoblotting methods (Fig. 4A). The loss of SIGN-R1 was specific, because an antibody to a distinct epitope on the molecule (hamster antibody 22D1 in the case of rabbit anti-SR1 TKO and vice versa) was no longer reactive with SIGN-R1 in vivo, even though the marginal zone macrophage was still intact, as evidenced by expression of the MARCO endocytic receptor and retention of preadministered dextran (Fig. 4 A and B). We do not know whether this method will apply more broadly to other molecules. SIGN-R1 may be a particularly good candidate for this TKO approach, e.g., because the receptor when ligated may be able to traffic digestive lysosomal compartments and because the turnover of the receptor and of the marginal zone macrophages may be slow in vivo. These features remain to be determined, but we anticipate that the TKO method will help to assess the need for SIGN-R1 in the host response to CPS.
Supplementary Material
Acknowledgments
We acknowledge support from National Institutes of Health Grants AI 13013 and AI 057158.
Abbreviations: CPS, capsular polysaccharide of S. pneumoniae; TKO, transient knockout; CHO, Chinese hamster ovary.
References
- 1.Kraal, G. (1992) Int. Rev. Cytol. 132, 31-74. [DOI] [PubMed] [Google Scholar]
- 2.Crocker, P. & Gordon, S. (1989) J. Exp. Med. 169, 1333-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Berg, T. K., Breve, J. J. P., Damoisequx, J. G. M. C., Dopp, E. A., Kelm, S., Crocker, P. R., Dijkstra, C. D. & Kraal, G. (1992) J. Exp. Med. 176, 647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin, F. & Kearney, J. F. (2002) Nat. Rev. Immunol. 2, 323-335. [DOI] [PubMed] [Google Scholar]
- 5.Witmer-Pack, M. D., Crowley, M. T., Inaba, K. & Steinman, R. M. (1993) J. Cell Sci. 105, 965-973. [DOI] [PubMed] [Google Scholar]
- 6.Balazs, M., Martin, F., Zhou, T. & Kearney, J. F. (2002) Immunity 17, 341-352. [DOI] [PubMed] [Google Scholar]
- 7.Cowan, M. J., Ammann, A. J., Wara, D. W., Howie, V. M., Schultz, L., Doyle, N. & Kaplan, M. (1978) Pediatrics 62, 721-727. [PubMed] [Google Scholar]
- 8.Mitchell, A. & Morris, P. J. (1983) Clin. Haematol. 12, 565-590. [PubMed] [Google Scholar]
- 9.Evans, D. I. (1985) J. Clin. Pathol. 38, 309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amlot, P. L. & Hayes, A. E. (1985) Lancet 1, 1008-1011. [DOI] [PubMed] [Google Scholar]
- 11.Di Padova, F., Dürig, M., Harder, F., Di Padova, C. & Zanussi, C. (1985) Br. Med. J. 290, 14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zandvoort, A. & Timens, W. (2002) Clin. Exp. Immunol. 130, 4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mond, J. J., Lees, A. & Snapper, C. M. (1995) Annu. Rev. Immunol. 13, 655-692. [DOI] [PubMed] [Google Scholar]
- 14.Snapper, C. M. & Mond, J. J. (1996) J. Immunol. 157, 2229-2233. [PubMed] [Google Scholar]
- 15.Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. V. (2000) Nat. Immunol. 1, 31-36. [DOI] [PubMed] [Google Scholar]
- 16.Winkelstein, J. A. & Tomasz, A. (1977) J. Immunol. 118, 451-454. [PubMed] [Google Scholar]
- 17.Peset Llopis, M. J., Harms, G., Hardonk, M. J. & Timens, W. (1996) J. Allergy Clin. Immunol. 97, 1015-1024. [DOI] [PubMed] [Google Scholar]
- 18.Pozdnyakova, O., Guttormsen, H. K., Lalani, F. N., Carroll, M. C. & Kasper, D. L. (2003) J. Immunol. 170, 84-90. [DOI] [PubMed] [Google Scholar]
- 19.Haas, K. M., Hasegawa, M., Steeber, D. A., Poe, J. C., Zabel, M. D., Bock, C. B., Karp, D. R., Briles, D. E., Weis, J. H. & Tedder, T. F. (2002) Immunity 17, 713-723. [DOI] [PubMed] [Google Scholar]
- 20.Saeland, E., Vidarsson, G., Leusen, J. H., Van Garderen, E., Nahm, M. H., Vile-Weekhout, H., Walraven, V., Stemerding, A. M., Verbeek, J. S., Rijkers, G. T., et al. (2003) J. Immunol. 170, 6158-6164. [DOI] [PubMed] [Google Scholar]
- 21.Kraal, G., Terhart, H., Meelhuizen, C., Venneker, G. & Claassen, E. (1989) Eur. J. Immunol. 19, 675-680. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson, M. C., Guinamard, R., Bolland, S., Sankala, M., Steinman, R. M. & Ravetch, J. V. (2003) J. Exp. Med. 198, 333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, C. G., Takahara, K., Umemoto, E., Yashima, Y., Matsubara, K., Matsuda, Y., Clausen, B. E., Inaba, K. & Steinman, R. M. (2001) Int. Immunol. 13, 1283-1290. [DOI] [PubMed] [Google Scholar]
- 24.Geijtenbeek, T. B. H., Groot, P. C., Nolte, M. A., van Vliet, S. J., Gangaram-Panday, S. T., van Duijnhoven, G. C. F., Kraal, G., van Oosterhout, A. J. M. & van Kooyk, Y. (2002) Blood 100, 2908-2916. [DOI] [PubMed] [Google Scholar]
- 25.Kang, Y.-S., Yamazaki, S., Iyoda, T., Pack, M., Bruening, S., Kim, J. Y., Takahara, K., Inaba, K., Steinman, R. M. & Park, C. G. (2003) Int. Immunol. 15, 177-186. [DOI] [PubMed] [Google Scholar]
- 26.Onishi, M., Kinoshita, S., Morikawa, Y., Shibuya, A., Phillips, J., Lanier, L. L., Gorman, D. M., Nolan, G. P., Miyajima, A. & Kitamura, T. (1996) Exp. Hematol. 24, 324-329. [PubMed] [Google Scholar]
- 27.Park, C. G., Lee, S. Y., Kandala, G. & Choi, Y. (1996) Immunity 4, 583-591. [DOI] [PubMed] [Google Scholar]
- 28.Dijkstra, C. D., Van Vliet, E., Dopp, E. A., van der Lelij, A. A. & Kraal, G. (1985) Immunology 55, 23-30. [PMC free article] [PubMed] [Google Scholar]
- 29.van der Laan, L. J., Dopp, E. A., Haworth, R., Pikkarainen, T., Kangas, M., Elomaa, O., Dijkstra, C. D., Gordon, S., Tryggvason, K. & Kraal, G. (1999) J. Immunol. 162, 939-947. [PubMed] [Google Scholar]
- 30.Lindberg, B., Lonngren, J. & Powell, D. A. (1977) Carbohydr. Res. 58, 177-186. [DOI] [PubMed] [Google Scholar]
- 31.Kamerling, J. P. (2000) Pneumococcal Polysaccharides: A Chemical View (Liebert, New York).
- 32.Elomaa, O., Kangas, M., Sahiberg, C., Tuukkanen, J., Sormunen, R., Liakka, A., Thesleff, I., Kraal, G. & Tryggvason, K. (1995) Cell 80, 603-609. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey, J. H. & Grennan, D. (1981) Eur. J. Immunol. 11, 221-228. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey, J. H. (1981) Eur. J. Immunol. 11, 212-220. [DOI] [PubMed] [Google Scholar]
- 35.Harms, G., Hardonk, M. J. & Timens, W. (1996) Infect. Immun. 64, 4220-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engering, A., Geijtenbeek, T. B., van Vliet, S. J., Wijers, M., van Liempt, E., Demaurex, N., Lanzavecchia, A., Fransen, J., Figdor, C. G., Piguet, V., et al. (2002) J. Immunol. 168, 2118-2126. [DOI] [PubMed] [Google Scholar]
- 37.Kwon, D. S., Gregario, G., Bitton, N., Hendrickson, W. A. & Littman, D. R. (2002) Immunity 16, 135-144. [DOI] [PubMed] [Google Scholar]
- 38.Trumpfheller, C., Park, C. G., Finke, J., Steinman, R. M. & Granelli-Piperno, A. (2003) Int. Immunol. 15, 289-298. [DOI] [PubMed] [Google Scholar]
- 39.Geijtenbeek, T. B. H., Torensma, R., van Vliet, S. J., van Duijnhoven, G. C. F., Adema, G. J., van Kooyk, Y. & Figdor, C. G. (2000) Cell 100, 575-585. [DOI] [PubMed] [Google Scholar]
- 40.Geijtenbeek, T. B. H., Krooshoop, D. J. E. B., Bleijs, D., van Vliet, S. J., van Duijnhoven, G. C. F., Grabovsky, V., Alon, R., Figdor, C. G. & van Kooyk, Y. (2000) Nat. Immunol. 1, 353-357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.