Abstract
Glucocorticoids (GCs) are the most commonly used antiinflammatory and immunosuppressive drugs. Their outstanding therapeutic effects, however, are often accompanied by severe and sometimes irreversible side effects. For this reason, one goal of research in the GC field is the development of new drugs, which show a reduced side-effect profile while maintaining the antiinflammatory and immunosuppressive properties of classical GCs. GCs affect gene expression by both transactivation and transrepression mechanisms. The antiinflammatory effects are mediated to a major extent via transrepression, while many side effects are due to transactivation. Our aim has been to identify ligands of the GC receptor (GR), which preferentially induce transrepression with little or no transactivating activity. Here we describe a nonsteroidal selective GR-agonist, ZK 216348, which shows a significant dissociation between transrepression and transactivation both in vitro and in vivo. In a murine model of skin inflammation, ZK 216348 showed antiinflammatory activity comparable to prednisolone for both systemic and topical application. A markedly superior side-effect profile was found with regard to increases in blood glucose, spleen involution, and, to a lesser extent, skin atrophy; however, adrenocorticotropic hormone suppression was similar for both compounds. Based on these findings, ZK 216348 should have a lower risk, e.g., for induction of diabetes mellitus. The selective GR agonists therefore represent a promising previously undescribed class of drug candidates with an improved therapeutic index compared to classical GCs. Moreover, they are useful tool compounds for further investigating the mechanisms of GR-mediated effects.
Keywords: inflammation, nuclear receptor, dissociated glucocorticoid receptor ligand
Glucocorticoids (GCs) have been widely and successfully used in the treatment of acute and chronic inflammatory diseases for >50 yr. They exert their effects by different mechanisms interfering with most inflammatory pathways. Unfortunately, the desired antiinflammatory and immunosuppressant effects are often accompanied by severe and/or partially non-reversible side effects (e.g., diabetes mellitus, peptic ulcer, Cushing's syndrome, osteoporosis, skin atrophy, psychosis, glaucoma, and many others). The use of GCs is limited by these side effects, and there is a major need for the development of compounds with the antiinflammatory potency of standard GCs but with reduced side effects (1-3).
New insights into the molecular mechanisms of GC-mediated actions have provided opportunities for identification of substances with a better therapeutic index (4-9). After entry into target cells, GCs bind to the GC receptor (GR), which then translocates into the nucleus to regulate gene transcription directly or indirectly. The ligand-activated GR binds as a homodimer to consensus sequences, termed GC response elements, in the promoter region of GC-sensitive genes to induce transcription (transactivation) of genes such as tyrosine aminotransferase (TAT) (10, 11). An indirect negative regulation of gene expression (transrepression) is achieved by GR-protein interaction. The ligand activated receptor binds as a monomer to transcription factors, such as NF-κB, activator protein-1, and others (1) to inhibit the activity of many proinflammatory transcription factors. This transrepression is considered the key mechanism for the antiinflammatory activity of GCs. Indeed, this assumption has been confirmed by investigations with transgenic mice expressing a dimerization-deficient GR (GRdim/dim). Although transactivation is suppressed in these mice, the transrepression function is intact and GCs inhibit edema in a croton oil-induced ear inflammation test as effectively as in wild-type animals (12).
In contrast, several side effects are thought to be predominantly mediated via transactivation. Thus, ligands that preferentially induce the transrepression and not transactivation function of the GR should be as effective as standard GCs but with fewer undesirable effects (1). Although the molecular mechanisms of GC-induced side effects are complex and often not yet well understood, it appears justified to assume that some of these undesired effects, such as steroid diabetes, require GR-DNA interaction and transactivation. For example, the two most important enzymes of gluconeogenesis, an essential pathway in the development of diabetes, phosphoenolpyruvate carboxykinase (13, 14) and glucose-6-phosphatase (15), are both induced by GCs. In contrast, a key mechanism for suppression of the hypothalamo-pituitary-adrenal axis, the decreased release of adrenocorticotropic hormone (ACTH) by corticotrophin-releasing hormone, is mediated by the GR via a transrepression mechanism (8). Other GC-mediated side effects (e.g., osteoporosis, skin atrophy, growth retardation, Cushing's syndrome) are subject to complex regulation involving a variety of mechanisms. However, a transactivation mechanism seems to be at least partially involved in the regulation also of these side effects (16).
To prove the concept that ligand-activated GRs predominantly mediate their antiinflammatory effects via transrepression and side effects predominantly via transactivation, we aimed to identify GR agonists with a dissociated profile. To identify such compounds, we have assessed several hundred compounds with regard to their binding to the GR and to other nuclear receptors [progesterone (PR) and mineralocorticoid receptors (MR)] and their activity in transactivation (induction of TAT) and transrepression (inhibition of IL-8 production) assays. Here we describe a nonsteroidal selective GR agonist (SEGRA). This compound, ZK 216348, has antiinflammatory activity similar to prednisolone (PRED) in rodents and induces less transactivation-mediated side effects. However, one side effect induced mainly by transrepression, the suppression of the hypothalamo-pituitary-adrenal axis, was also seen with ZK 216348 after systemic treatment of rats. ZK 216348 represents a previously undescribed class of nonsteroidal GR ligands that preferentially induce transrepression, resulting in an improved therapeutic index in vivo.
Materials and Methods
Receptor-Binding Assays. Cytosol preparations of Sf9 cells, infected with recombinant baculovirus coding for the human GR, MR, or PR, were used for the binding assays. After centrifugation (15 min, 600 × g) Sf9 pellets were resuspended in 1/20 volume of 20 mM Tris·HCl, pH 7.5/0.5 mM EDTA/2 mM DTT/20% glycerol/400 mM KCl/20 mM sodium molybdate/0.3 μM aprotinin/1 μM pepstatin/10 μM leupeptin and shock frozen in liquid nitrogen. After three freeze-thaw cycles, the homogenate was centrifuged for 1 h at 100,000 × g. Protein concentration of the resulting supernatant was between 10 and 15 mg/ml. Aliquots were stored at -40°C.
For the binding assays for GR, MR, and PR, [3H]dexamethasone (DEX) (≈20 nM), [3H]aldosterone or [3H]progesterone, SF9 cytosol (100-500 μg protein), test compound, and binding buffer (10 mM Tris·HCL, pH 7.4/1.5 mM EDTA/10% glycerol) were mixed in a total volume of 50 μl and incubated for 1 h at room temperature. Specific binding was defined as the difference between binding of [3H]DEX, [3H]aldosterone, and [3H]progesterone in the absence and presence of 10 μM unlabeled DEX, aldosterone, and progesterone. After incubation, 50 μl of cold charcoal suspension was added for 5 min, and the mixtures were transferred to microtiter filtration plates. The mixtures were filtered into Picoplates (Canberra Packard, Dreieich, Germany) and mixed with 200 μl of Microszint-40 (Canberra Packard). The bound radioactivity was determined with a Packard Top Count plate reader. The concentration of test compound giving 50% inhibition of specific binding (IC50) was determined from Hill analysis of the binding curves.
Induction of TAT. Induction of TAT by test compounds was determined in vitro in the rat liver hepatoma cell line H4-II-EC3. Cells were grown in MEM (Life Technologies, Karlsruhe, Germany) supplemented with 10% FBS (Life Technologies), 2 mML-glutamine (Life Technologies), and 1% NEAA (nonessential amino acids) (Life Technologies). For compound testing, cells were seeded in 96-well plates (2 × 104 cells per well) and incubated with test compounds or DEX for 20 h. Cells were then lysed and TAT activity assayed as described below for hepatic TAT induction in vivo.
Inhibition of IL-8 Production. IL-8 synthesis was induced by stimulation of the human promyeloic cell line THP-1 with lipopolysaccharide (LPS, Escherichia coli serotype 0127:B8; Sigma). Cells (2.5 × 104 cells per well) were treated with 10 μg/ml LPS in the absence or presence of test compounds or DEX for 18 h. IL-8 concentration was determined in the supernatant by an IL-8 specific ELISA (Beckman Coulter).
Inhibition of Monokine Secretion. Monocytic secretion of tumor necrosis factor α (TNF-α) and IL-12 p70 was determined after stimulation of peripheral blood mononuclear cells from healthy donors with 1 μg/ml LPS (E. coli serotype 0127:B8; Sigma) and 10 ng/ml IFN-γ 1b (Imukin, Boehringer Ingelheim).
After 24-h stimulation at 37°C, 5% CO2, in the absence or presence of different concentrations of the compounds, concentrations of IL-12 p70 and TNF-α in culture supernatants were determined by using commercial ELISA kits from R & D Systems (IL-12 HS Immunoassays, Nivelles, Belgium) and Bio-Source International TNF-α EASIA, Wiesbaden, Germany]. The calculated IC50 value represents the concentration of compound giving 50% inhibition of the maximal TNF-α and IL-12 p70 production.
Animal Models. Animals. NMRI mice (26-28 g) and Shoe:Wistar rats (140-160 g) were housed according to institutional guidelines with access to food and water ad libitum. Eight to 11 animals were randomly allocated to the different treatment groups.
Croton oil-induced ear inflammation. Topical application of the nonspecific contact irritant croton oil, a mixture of several phorbol esters, leads to acute inflammation characterized by edema and a mainly granulocytic cell infiltration into the skin (17). Experiments were performed as recently described in detail (18). Briefly, for topical application, compounds were dissolved in the same vehicle as used for croton oil and were coapplied. Systemic application of compounds (s.c.) was performed 2 h before croton oil application. At the maximum of the inflammatory reaction, animals were killed, and ears (mice, area ≈1 cm2) or a punch biopsy (rats, 10-mm diameter) were weighed as an indicator of edema formation, then snap-frozen in liquid nitrogen in polypropylene tubes and kept at -20°C for up to 24 h. Peroxidase activity assay. Peroxidase activity, as a measure for total granulocyte infiltration, was assayed by using a modification of a previously described method (19), as described by Schottelius et al. (18).
Induction of TAT in vivo. TAT induction was evaluated 6 h after compound administration (s.c.) to juvenile rats by determination of TAT activity in liver homogenates. Animals were killed, and biopsies (10-mm diameter) were taken from the liver and snap frozen. Biopsies were homogenized in 2 ml of homogenizing buffer (140 mM KCl in 20 mM KPO4 buffer, pH 7.6) and centrifuged at 24,000 × g for 20 min at 4°C. Supernatants were assayed for protein content by the BCA (bicinchoninic acid) Protein Assay Kit from Pierce (20). Twenty microliters of supernatant diluted 1:50 in PBS was incubated for 30 min at 37°C with 200 μl of TAT-reaction buffer (tyrosine 1.2 mg/ml or 6.6 mM/45 mM KH2PO4/0.06 mM pyridoxal-5′-phosphate/12 mM oxoglutaric acid, adjusted to pH 7.6 with KOH). After stopping the reaction with 10 M KOH and further incubation for 30 min, extinction was measured at 340 nm, and TAT activity was calculated in relation to 500 μg/ml total protein content.
TAT induction was defined as x-fold increase in TAT activity measured as OD at 340 nm in comparison to the TAT activity in vehicle-treated animals.
ACTH suppression in rats. Animals were isolated before the study to avoid stress. Six hours after application of compounds, animals were killed, and EDTA-anticoagulated blood was collected from abdominal aorta. Plasma content of ACTH was determined by using the ACTH 125I-assay system (ImmuChem Double Antibody hACTH) following the instructions of the vendor (ICN). Increased blood glucose in rats. Animals were isolated before the study to avoid stress and fasted for 16 h. Six hours after application of compounds, animals were killed, and EDTA blood was collected from abdominal aorta.
Glucose in plasma was measured by colorimetric serum glucose determination by using hexokinase and glucose-6-phosphate dehydrogenase (21) with a Hitachi 904 automatic analyzer (Roche Diagnostics).
Skin atrophy. To assess the atrophogenic potential, ZK 216348 or the reference compound PRED (75 μl on a marked area of 9 cm2 in 95% ethanol/5% isopropyl myristate) was applied daily for 19 days to the dorsal skin of nude rats (strain hr-hr, 120-140 g, Iffa-Credo). Animals were killed on day 20. Skin thickness was determined by using a specifically designed dial thickness gauge (Schering). Mean values were derived by measuring two adjacent treated skin areas. To determine skin-breaking strength of treated skin, a dorsal skin patch (5 × 5 cm) was removed. The skin patch was placed on filter paper, and two double-T-piece skin strips (50 mm long, 4 mm wide at the narrowest point) were punched in a caudal-cranial direction out of the patch. The skin strips were covered with moistened filter paper to avoid drying and were fixed with the wider ends into an apparatus developed in-house to measure skin-breaking strength. At a constant rate of stretch (200 mm/min), the force necessary to tear the skin strip was determined with a pressure sensor and was expressed as the skin-breaking strength (in N).
Thymus, spleen, adrenal, and body weight. To determine effects on body weight, animals were weighed before and after completion of treatment. Thymus, spleen, and adrenal glands were removed from animals killed after systemical or topical treatment and weighed. Organ weights were expressed as absolute values or in percent inhibition when compared with untreated animals.
Statistical analysis. For all animal models, statistical analysis was performed with the “modified Hemm” (inhibition) test developed by Schering based on the program SAS SYSTEM FOR WINDOWS 6.12 (SAS Institute, Cary, NC) (18). Statistical significance in TAT, glucose induction, and ACTH suppression was assessed by using Dunnet's test. Differences between ZK 216348 and the corresponding PRED group were assessed by using the Mann-Whitney test.
Results
In Vitro Investigations. Receptor binding, IL-8 suppression, and TAT induction in cell lines. In the GR-binding screen, we first identified the racemic compound ZK 209614 with a high binding affinity to the GR. Although the (-)-enantiomer (ZK 216347) did not show any binding to the GR, the (+)-enantiomer (ZK 216348) (Fig. 1) bound to the GR with a similar affinity as DEX and with an ≈3-fold higher affinity than PRED (Table 1). ZK 216348 also binds to the PR and MR.
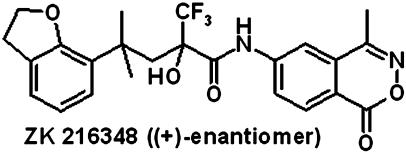
Fig. 1.
Structure of ZK 216348. ZK 216348 [(+)-enantiomer] was synthesized by standard methods starting with the commercially available dihydrobenzofurane.
Table 1. Binding affinities to nuclear receptors.
| Binding IC50, nM
|
|||
|---|---|---|---|
| GR | PR | MR | |
| Dexamethasone | 14 ± 6 | >1,000 | >1,000 |
| PRED | 68 ± 30 | >1,000 | >1,000 |
| ZK 216347 (—) | >1,000 | >1,000 | >1,000 |
| ZK 216348 (+) | 20.3 ± 2.6 | 20.4 ± 1.3 | 79.9 ± 1.3 |
Binding to the recombinant human GR, PR, and MR was determined in comparison to dexamethasone, progesterone, and aldosterone, respectively. IC50 values represent the concentration of compounds that inhibited 50% of specific binding of [3H]dexamethasone, [3H]progesterone, and [3H]aldosterone. Mean ± SD of three to five separate experiments are shown.
To determine whether this ligand exhibits dissociation between transactivation and transrepression on binding to the GR, the inhibition of IL-8 synthesis in THP-1 cells (transrepression) and the induction of TAT in liver hepatoma cells (transactivation) were investigated. Compared with PRED, ZK 216348 inhibited IL-8 secretion with only a 2-fold lower potency and a lower efficacy (Table 2). Importantly, this compound is ≈60-fold less potent than PRED with regard to TAT induction. In comparison to DEX, ZK 216348 was ≈14-fold less potent in the IL-8 assay, whereas DEX was >300-fold more potent in induction of TAT. Consequently, ZK 216348 induces significantly less transactivation compared to standard GCs in this assay and shows a clear selectivity for transrepression. Therefore, this compound is considered as a SEGRA.
Table 2. Effect on IL-8 secretion and TAT activity in cell lines.
| IL-8 inhibition IC50, nM | IL-8 inhibition efficacy, % | TAT induction EC50, nM | TAT induction efficacy, % | |
|---|---|---|---|---|
| DEX. | 2.6 ± 1 | 100 | 0.3 | 100 |
| PRED | 18 ± 12 | 92 ± 8 | 1.5 | 100 |
| ZK 216348 | 35 ± 10 | 52 ± 15 | 95 | 88 |
Inhibition of IL-8 secretion in monocytic THP-1 cells as a parameter for transrepression and induction of TAT in hepatic H4-II-E-C3 cells as a transactivation parameter were determined for ZK 216348 in comparison to standard GCs. Efficacy is expressed relative to the maximal response to DEX. For IL-8 inhibition, mean ± SD values of three to five separate experiments are shown, whereas single values are given for TAT induction.
Antiinflammatory effects in human peripheral blood mononuclear cells (PBMCs). Additionally, we investigated whether the compound is active in inhibiting TNF-α and IL-12 production in LPS-stimulated PBMCs. Similar to the results obtained in THP-1 cells, DEX and PRED were more potent than ZK 216348. However, the compound inhibited TNF-α and IL-12 with an efficacy similar to the standards (Table 3).
Table 3. Effect on monokine production in human PBMC.
| IL-12 p70 IC50, nM | IL-12 p70 efficacy, % | TNF-α IC50, nM | TNF-α efficacy, % | |
|---|---|---|---|---|
| DEX | 3.2 | 89 | 3.1 | 97 |
| PRED | 7.9 | 84 | 11 | 93 |
| ZK 216348 | 52 | 89 | 89 | 63 |
The effect of ZK 216348 on secretion of IL-12 p70 and TNF-α 24 h after LPS stimulation of human peripheral blood mononuclear cells was determined in comparison to the effect of standard GCs. Efficacy is expressed as absolute maximal inhibition of LPS-induced increase in cytokine release. Single values are shown.
In Vivo Activity. In the in vivo studies, the SEGRA compound was compared with the classical GC PRED.
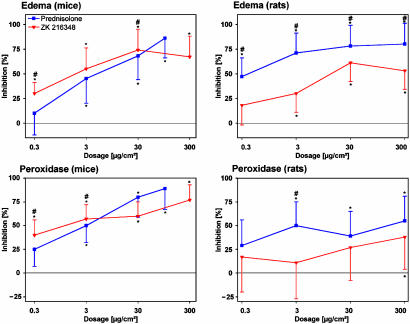
Antiinflammatory activity. The SEGRA compound ZK 216348 was tested in the croton oil model of ear inflammation in rodents. After topical application, the antiinflammatory effect of ZK 216348 in mice was similar to that of PRED (Fig. 2). ZK 216348 had comparable potency regarding the inhibition of ear edema (ED50: 0.02 μg/cm2 SEGRA, 0.03 μg/cm2 PRED) and granulocyte infiltration as assessed by peroxidase activity (ED50: 0.03 μg/cm2 SEGRA, 0.04 μg/cm2 PRED). In this experiment, PRED was tested only up to 100 μg/cm2, although the highest dose of ZK 216348 was 300 μg/cm2. We know from previous experiments that the concentration used here gives the maximal antiinflammatory effect of PRED.
Fig. 2.
Antiinflammatory activity in the croton oil-induced ear inflammation model after topical application in mice and rats. Compounds were topically administered at doses from 0.3 to 300 μg/cm2. In mice, 1 cm2 and in rats, 2 cm2 were treated. After 24 h, the ear weight, as a measure for edema, and peroxidase activity, as a parameter for granulocyte infiltration, were determined. Results are shown as percent inhibition (mean ± SD, n = 10) in comparison to solvent control group. *, P < 0.05 vs. solvent group in modified the Hemm test. #, P < 0.05 vs. PRED in the Mann-Whitney test.
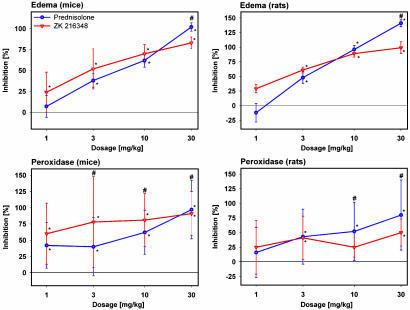
In rats, the compound is significantly less active than PRED after topical application (Fig. 2). Given s.c., ZK 216348 inhibited ear edema similarly to PRED in both mice (ED50: 2 mg/kg SEGRA, 9 mg/kg PRED) and rats (ED50: 2 mg/kg SEGRA, 3.5 mg/kg PRED) (Fig. 3).
Fig. 3.
Antiinflammatory activity in croton oil-induced ear inflammation model after s.c. application in mice and rats. Compounds were administered s.c. at doses from 1 to 30 mg/kg. After 24 h, the ear weight, as a measure for edema, and peroxidase activity, as a parameter for granulocyte infiltration, were determined. Results are shown as percent inhibition (mean ± SD, n = 10) in comparison to vehicle group. *, P < 0.05 vs. vehicle group in the modified Hemm test. #, P < 0.05 vs. PRED in the Mann-Whitney test.
Overall, in this model of acute inflammation, the antiinflammatory activity of ZK 216348 is similar to that of PRED, with the exception of the lower activity seen after topical application and s.c. administration on granulocyte infiltration in rats.
Side-effect induction. Several side-effect parameters were determined in parallel to the antiinflammatory effects described above.
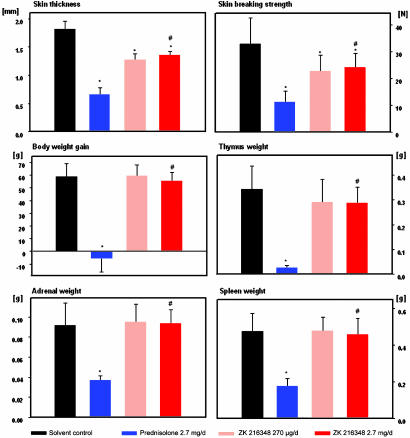
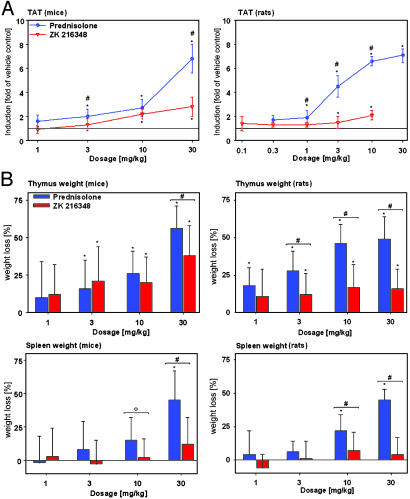
Skin atrophy represents one of the most common side effects of GCs after long-term topical treatment. In nude rats treated with PRED or ZK 216348 topically once daily for 3 wk at doses showing comparable antiinflammatory effects, the SEGRA compound reduced skin thickness and skin-breaking strength significantly less than did PRED (Fig. 4). A similar advantage of ZK 216348 over PRED was seen with regard to systemic effects after long-term topical treatment (reduction of body-weight gain and reduction of adrenal weight) (Fig. 4). In addition, the compound induced significantly less deterioration related to immune cell apoptosis (reduction in thymus and spleen weight) (Fig. 4).
Fig. 4.
Topical and systemic side effects after long-term topical treatment in nude rats. Compounds were applied on 9 cm2 of dorsal skin of rats for 19 consecutive days. ZK 216348 was administered at 30 μg/cm2 per day (270 μg/d) and 300 μg/cm2 per day (2.7 mg/d) and PRED at 300 μg/cm2 per day (2.7 mg/d). On day 20, a skin patch was removed to measure skin thickness and breaking strength. Body-weight gain was determined, and thymus, spleen, and adrenal glands were weighed. Data are given as mean ± SD of 10 animals per group. *, P < 0.05 vs. solvent control group in the modified Hemm test. #, P < 0.05 vs. PRED at a dose of 2.7 mg/d in the Mann-Whitney test.
The quantification of TAT induction in rodents was used to determine whether the dissociation demonstrated in vitro is also seen in vivo after systemic administration with the emphasis on effects mediated by transactivation.
In mice, the induction of TAT by ZK 216348 was significantly less than that seen with PRED (Fig. 5A). Neither PRED nor ZK 216348 reached a plateau effect at the doses tested, but it was not possible to test higher doses due to the low solubility of the SEGRA compound. In rats, the dissociation was even more marked in favor of ZK 216348. Even at a dose of 3 mg/kg, ZK 216348 induced TAT significantly less than PRED. In a separate experiment, ZK 216348 was also tested at a dose of 30 mg/kg in rats and did not show a significant increase in TAT activity (data not shown).
Fig. 5.
Effect on hepatic TAT activity and thymus and spleen weights after s.c. application in mice and rats. (A) Induction of TAT 6 h after s.c. treatment was determined in mice and rats and is shown as x-fold induction (mean ± SD; mice n = 10, rats n = 5) compared to vehicle control. *, P < 0.05 vs. vehicle group in Dunnet's test. #, P < 0.05 vs. PRED in the Mann-Whitney test. (B) Thymus and spleen weights 24 h after s.c. treatment were determined in mice and rats. The reduction in organ weight is shown as percent weight loss (mean ± SD, n = 10) compared to vehicle control. *, P < 0.05 vs. vehicle group in the modified Hemm test. #, P < 0.05; °, P = 0.052 vs. PRED in the Mann-Whitney test.
Effects on thymus and spleen weight were tested after systemic administration (Fig. 5B). In mice, ZK 216348 reduced thymus weight significantly less than PRED only at the highest dose, whereas in rats, ZK 216348 caused significantly less thymus weight reduction than PRED at doses of 3 mg/kg and more. With regard to spleen weight, the SEGRA compound had no significant effect in either mice or rats, whereas PRED clearly reduced spleen weight at doses of 10 and 30 mg/kg.
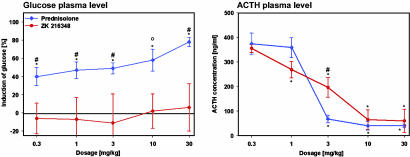
The transactivation-mediated increase in blood glucose concentration by GCs reflects the risk for induction of diabetes mellitus. After s.c. treatment in rats, PRED triggered a dose-dependent increase in blood glucose. In contrast, the SEGRA compound ZK 216348 did not lead to a significant increase in blood glucose even at the highest dose of 30 mg/kg (Fig. 6).
Fig. 6.
Effect on blood glucose and ACTH concentrations after s.c. application in rats. Blood glucose concentration was determined 6 h after s.c. treatment of rats that were fasted 16 h before treatment. ACTH plasma concentrations were determined 6 h after s.c. treatment of rats that had been isolated to avoid stress. Data are given as mean ± SD of five animals per group. *, P < 0.05 vs. vehicle group in Dunnet's test. #, P < 0.05; °, P = 0.056 vs. PRED in the Mann-Whitney test.
Regarding ACTH suppression, an advantage of SEGRAs over classical GCs was not expected, because transrepression seems to be the key mechanism underlying this effect. Indeed, ZK 216348 suppressed ACTH plasma concentrations in rats as effectively as PRED (Fig. 6). Although there is a significant difference between PRED and ZK 216348 at a dose of 3 mg/kg, the effects of both compounds are similar at higher doses.
Taken together, the separation of transactivation from transrepression demonstrated in vitro is reflected in vivo by a similar antiinflammatory activity but reduced transactivation-mediated side effects.
Discussion
Recent studies have provided new insights into the molecular mechanisms of GR-mediated effects and have led to the hypothesis that therapeutic effects are mediated to a major extent via a transrepression process, whereas side effects are predominantly mediated by transactivation (12). Using a screen discriminating between these mechanisms, we have identified the nonsteroidal SEGRA compound ZK 216348 that binds to the GR with high affinity and preferentially induces the transrepression function of the GR. Binding of ZK 216348 to the PR and MR leads to a predominantly antagonistic activity at the PR (50% efficacy) and a fully antagonistic activity at the MR (100% efficacy) (data not shown). Although ZK 216348 binds to the GR with an affinity similar to DEX, it is less active in all cellular in vitro assays. Possible explanations for this discrepancy might be that the chemical structure of ZK 216348 results in different physicochemical properties and cellular uptake behavior and/or the ligand does not induce a receptor conformation required for full repression effects.
We demonstrated, however, that the antiinflammatory activity of the SEGRA compound ZK 216348 is comparable to that of PRED. A somewhat lower activity of ZK 216348 in rats in comparison to mice might be caused by a different metabolism. Taken together, our data strongly suggest that selective transrepression is sufficient for antiinflammatory activity in vivo and are in line with the observations by Schütz and coworkers in GRdim/dim mice (12).
Moreover, we have shown that the dissociation between transrepression and transactivation observed with ZK 216348 in vitro is reflected by an improved therapeutic index in vivo after both topical and systemic administration. The compound induces significantly fewer transactivation-mediated side effects, such as increased blood glucose and induction of hepatic TAT in rats. Therefore, we believe that the risk of diabetes induction with ZK 216348 should be clearly lower than that for PRED. The reduction of lymphoid organ weights was also diminished after systemic and long-term topical administration of ZK 216348 in comparison to PRED. In contrast, no clear differences were observed regarding the suppression of the hypothalamo-pituitary-adrenal axis. Because the secretion of ACTH to corticotrophin-releasing hormone (CRH) stimulation is affected by a GR-protein interaction via the transrepression mechanism of CRH itself (22, 23), we do not expect an advantage of this compound class in comparison to standard GCs here. Although species-specific differences in activity have been observed for ZK 216348, the compound shows a better therapeutic index in comparison to PRED in both mice and rats. It has to be determined in clinical studies whether this effect translates into a similar dissociation in humans.
Our results regarding a reduced activity of the SEGRA compound with regard to induction of TAT and increased blood glucose but a sustained ACTH suppression correspond to the current knowledge of the underlying mechanisms of GC-mediated side effects. The precise mechanisms of many other GC effects, however, are not yet well understood. Moreover, many of the side effects (e.g., skin atrophy, osteoporosis, Cushing's syndrome) seem to use more than one mechanism (24-26). Thus, it is difficult to predict the behavior of SEGRA compounds with regard to these effects. Investigations using ZK 216348 as a tool compound or GC treatment in GRdim/dim mice will help to determine the molecular mechanisms involved in these GC side effects. For example, the less-pronounced loss of skin thickness and skin-breaking strength observed after long-term topical treatment with ZK 216348 in comparison to PRED might suggest that transactivation is involved in skin atrophy.
In the past, optimization of pharmacokinetic properties was used to improve the therapeutic index of GR ligands. Deflazacort, a prodrug derivative of PRED, has been shown clinically to induce fewer side effects than PRED while having only slightly less antiinflammatory activity (27).
Currently the development of steroidal and nonsteroidal GC ligands that induce an altered GR activation profile has moved into the focus of several groups. Representatives of this new generation of GR ligands are the RU compounds 40066, 24782, and 24858 (3). These compounds show a high affinity to the GR and repress activator protein-1 very efficiently. Regarding transactivation, they reach only 8-35% of DEX efficacy. Unfortunately, this dissociated in vitro profile was not reflected in vivo; therefore, RU 24858 had antiinflammatory activity in vivo comparable to PRED but failed to show any reduction in systemic side effects (osteoporosis or reduction of body and thymus weights) (28). Another group described that RU 24858 showed stronger transactivation activity in vivo than in vitro (29). We agree with the conclusion drawn by the authors that these results, rather than disproving the concept, represent a problem of this particular compound.
This assumption is supported by other new GR ligands such as ZK 216348 and AL-438 (30). AL-438 was shown to be more efficacious in transrepression than in transactivation in vitro. It was antiinflammatory active in vivo and showed less increase in blood glucose than PRED. Additionally, Coghlan et al. (31) reported a lesser osteoporotic potential for AL-438 in rats compared to PRED. Therefore, although the antiinflammatory activity of AL-438 was weaker than PRED in the reported models, the compound showed a dissociated profile not only in vitro but also in vivo.
In this respect, AL-438 behaves similarly to the SEGRA compound ZK 216348, which we found to be equipotent in antiinflammation to PRED in our murine model. ZK 216348 demonstrates that the principle of an improved therapeutic index by the separation of the molecular mechanisms of the GR regarding therapeutic effect and side effects is possible.
In summary, ZK 216348 is a previously undescribed SEGRA, representing a compound class with an improved therapeutic index compared to classical GCs. Because these compounds have a reduced risk of developing certain side effects, such as diabetes mellitus, they are representatives of a generation of GR ligands with a high clinical value. Moreover, the compound will serve as a tool to further investigate the molecular basis of GC side effects.
Acknowledgments
We thank Andrew Cato (Forschungszentrum Karlsruhe, Karlsruhe, Germany) for helpful discussions, Steven Katz (National Institutes of Health, Bethesda), and Fiona McDonald (Schering AG, Berlin) for the critical reading of the manuscript. We specifically thank M. Backhus, D. Schröder, H.-U. Klein, R. Schmidt, D. Gerhard, E. Matzke, D. Opitz, B. Schulz, and S. Schoepe (Schering AG, Berlin) for excellent technical assistance.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACTH, adrenocorticotropic hormone; DEX, dexamethasone; GC, glucocorticoid; GR, GC receptor; MR, mineralocorticoid receptor; PR, progesterone receptor; PRED, prednisolone; SEGRA, selective GR agonist; TAT, tyrosine aminotransferase; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α.
References
- 1.Barnes, P. J. (1998) Clin. Sci. 94, 557-572. [DOI] [PubMed] [Google Scholar]
- 2.Resche-Rigon, M. & Gronemeyer, H. (1998) Curr. Opin. Chem. Biol. 4, 501-507. [DOI] [PubMed] [Google Scholar]
- 3.Vayssiere, B. M., Dupont, S., Choquart, A., Petit, F., Garcia, T., Marchandeau, C., Gronemeyer, C. & Resche-Rigon, M. (1997) Mol. Endocrinol. 11, 1245-1255. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto, K. R. (1985) Annu. Rev. Genet. 19, 209-252. [DOI] [PubMed] [Google Scholar]
- 5.Heck, S., Kullmann, M., Gast, A., Ponta, H., Rahmsdorf, H. J., Herrlich, P. & Cato, A. C. (1994) EMBO J. 13, 4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beato, M., Herrlich, P. & Schütz, G. (1994) Cell 83, 851-857. [DOI] [PubMed] [Google Scholar]
- 7.Cole, T. J., Blendy, J. A., Monaghan, A. P., Krieglstein, K., Schmidt, W., Aguzzi, A., Fantuzzi, G., Hummler, E., Unsicker, K. & Schütz, G. (1995) Genes Dev. 9, 1608-1621. [DOI] [PubMed] [Google Scholar]
- 8.Reichardt, H. M., Kaestner, K. H., Tuckermann, J., Kretz, O., Wessely, O., Bock, R., Gass, P., Schmidt, W., Herrlich, P., Angel, P. & Schütz, G. (1998) Cell 93, 531-541. [DOI] [PubMed] [Google Scholar]
- 9.Kassel, O., Sancono, A., Krätzschmar, J., Kreft, B., Stassen, M. & Cato, A. C. (2001) EMBO J. 20, 7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jantzen, H.-M., Strähle, U., Gloss, B., Stewart, F., Schmidt, W., Boshart, M., Miksicek, R. & Schütz, G. (1987) Cell 49, 29-38. [DOI] [PubMed] [Google Scholar]
- 11.Rigaud, G., Roux, J., Pictet, R. & Grange, T. (1991) Cell 67, 977-986. [DOI] [PubMed] [Google Scholar]
- 12.Reichardt, H. M., Tuckermann, J. P., Göttlicher, M., Vujic, M., Weih, F., Angel, P., Herrlich, P. & Schütz, G. (2001) EMBO J. 20, 7168-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien, R. M., Noisin, E. L., Suwanichkul, A., Yamasaki, T., Lucas, P. C., Wang, J.-C., Powell, D. R. & Granner, D. K. (1995) Mol. Cell. Biol. 15, 1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosson, S. M. & Roesler, W. J. (2000) J. Biol. Chem. 275, 5804-5809. [DOI] [PubMed] [Google Scholar]
- 15.Yoshinuchi, I., Shingu, R., Nakajima, H., Hamaguchi, T., Horikawa, Y., Yamasaki, T., Oue, T., Ono, A., Miyagawa, J. I., Namba, M., et al. (1998) J. Clin. Metab. 83, 1016-1019. [DOI] [PubMed] [Google Scholar]
- 16.Schäcke, H., Döcke, W.-D. & Asadullah, K. (2002) Pharmacol. Ther. 96, 23-43. [DOI] [PubMed] [Google Scholar]
- 17.Berg, D. J., Leach, M. W., Kuhn, R., Rajewski, K., Muller, W., Davidson, N. J. & Rennick, D. (1995) J. Exp. Med. 182, 99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schottelius, A. J., Giesen, C., Asadullah, K., Fierro, I. M., Colgan, S. P., Bauman, J., Guilford, W., Perez, H. D. & Parkinson, J. F. (2002) J. Immunol. 169, 7063-7070. [DOI] [PubMed] [Google Scholar]
- 19.Goka, J. & Farthing, M. J. G. (1987) J. Immunoassay 8, 29-41. [DOI] [PubMed] [Google Scholar]
- 20.Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J. & Klenk, D. C. (1985) Anal. Biochem. 150, 76-85. [DOI] [PubMed] [Google Scholar]
- 21.Carroll, J. J., Smith, N. & Babson, A. L. (1971) Biochem. Med. 2, 171-180. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt, H. M. & Schütz, G. (1998) Mol. Cell. Endocrinol. 146, 1-6. [DOI] [PubMed] [Google Scholar]
- 23.Reichardt, H. M., Tronche F., Bauer, A. & Schütz, G. (2000) Biol. Chem. 381, 961-964. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler, R. & Kasperk, C. (1998) Steroids 63, 344-348. [DOI] [PubMed] [Google Scholar]
- 25.Manelli, F. & Giustina, A. (2000) Trends Endocrinol. Metab. 11, 79-85. [DOI] [PubMed] [Google Scholar]
- 26.Patschan, D, Loddenkemper, K. & Buttgereit, F. (2001) Bone 6, 498-505. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous (1999) Drug Ther. Bull. 3, 57-58. [Google Scholar]
- 28.Belvisi, M. G., Wicks, S. L., Battram, C. H., Bottoms, S. E. W., Redford, J. E., Woodman, P., Brown, T. J., Webber, S. E. & Foster, M. L. (2001) J. Immunol. 166, 1975-1982. [DOI] [PubMed] [Google Scholar]
- 29.Tanigawa, K., Tanaka, K., Nagase, H., Miyake, H., Kiniwa, M. & Ikizawa, K. (2002) Biol. Pharm. Bull. 25, 1619-1622. [DOI] [PubMed] [Google Scholar]
- 30.Miner, J. N. (2002) Biochem. Pharmacol. 64, 355-361. [DOI] [PubMed] [Google Scholar]
- 31.Coghlan, M. J., Jacobson, P. B., Lane, B., Nakane, M, Lin, C. W., Elmore, S. W., Kym, P. R., Luly, J. R., Carter, G. W., Turner, R., et al. (2003) Mol. Endocrinol. 17, 860-869. [DOI] [PubMed] [Google Scholar]