Abstract
Production of monoclonal antibodies requires immortalization of splenocytes by somatic fusion to a myeloma cell line partner (hybridomas). Although hybridomas can be immortal, they may depend on a feeder cell layer and may be genetically unstable. Since the inception of hybridoma technology, efforts to improve efficiency and stability of monoclonal antibody-producing cell lines have not brought about substantial progress. Moreover, suitable human multiple myeloma-derived cell lines for the production of human antibodies have been very difficult to develop. Here we report a strategy that greatly simplifies the generation of antibodies and eliminates the need for hybridomas. We show that splenocytes derived from transgenic mice harboring a mutant temperature-sensitive simian virus 40 large tumor antigen under the control of a mouse major histocompatibility promoter are conditionally immortal at permissive temperatures and produce monoclonal antibodies. This simple approach may become a method of choice for generation and production of both polyclonal and monoclonal antibodies with advantages in high-throughput discovery and antibody-based immunotherapy.
Monoclonal antibodies are proteins with such exquisite specificity and sensitivity in their reactions with specific sites on target molecules that they have become reagents of central importance in modern biological research, including the analysis and treatment of human disease. However, more than a quarter century after their introduction, monoclonal antibodies are still produced only by somatic cell hybrid clones of splenocytes fused to multiple myeloma-derived cells (1). These “hybridomas” can produce monoclonal antibodies for years, but production involves a labor-intensive multistep process limited by the constant risk of contamination, often requires feeder cells, and may be genetically unstable (2). Despite later developments, such as phage-display technology for in vitro generation of monoclonal antibodies (3, 4), chimerization or humanization strategies (5), and human myeloma cell lines suitable for hybridoma formation (6), a simple and effective strategy for generating monoclonal antibody-producing immortal cells would represent a major advance.
Here we show that splenocytes derived from transgenic mice harboring a mutant temperature-sensitive (ts) simian virus 40 large tumor antigen under the control of a mouse major histocompatibility promoter (H-2Kb-tsA58 mouse or ImmortoMouse; ref. 7) are conditionally immortal at permissive temperatures and produce monoclonal antibodies.
Materials and Methods
Immunization. H-2Kb-tsA58 mice (Charles River Breeding Laboratories) were immunized with filamentous fd-tet phage (8) every other week for 12 weeks. In brief, a phage preparation containing 107 transducing units/μl (total volume = 1 ml) was administered by four routes (i.v., i.p., intradermally, and s.c.). Mice were bled after each boost and ELISA was used to monitor anti-phage antibody titers in the serum. Animal experimentation involved standard established procedures reviewed and approved by the Institutional Animal Care and Use Committee from the University of Texas M. D. Anderson Cancer Center.
Derivation of Immortal Splenocytes. Mice were killed and their spleens were collected in DMEM. Cells were released by gentle pressure applied to the capsule of the organ, which was placed between two frosty glass slides. Next, splenocytes were resuspended in 15 ml of hybridoma medium containing 10% controlled-process serum replacement (Sigma–Aldrich, catalog no. C-0786), a quality-controlled bovine plasma that supports hybridoma growth, and hybridoma-enhancing supplements (Hybrimax; Sigma–Aldrich, catalog no. H-2900) to facilitate cell growth at low-density plating. Tissue debris were gravity-cleared by serially transferring spleen-derived cells to fresh 15-ml conic plastic tubes. A total of 2 × 108 cells were distributed in 6-, 24-, and 96-well plates and cultured at 33°C. The culture medium was changed completely three times during 2–3 weeks. Clones were observed in >90% of the wells after 3 weeks. For the next 2 months, the plates were monitored, and 100 μl of fresh medium was added to each well every 3 weeks. Positive wells were subcloned by limiting dilution (2); some of the clones were also expanded to 24-well and 96-well plates to monitor reactivity after a long term in culture. We minimized or avoided the problem of splenocyte “clumping” by carefully suspending the cells in each well and by using serum-free medium. After counting and plating the suspensions at 0.1–0.5 cells per well, we systematically inspected each 96-well plate visually under the microscope.
Screening and Generation of Clonal Antibody-Producing Splenocytes. ELISA against filamentous phage and against recombinant phage capsid pIII protein was performed as described (2). BSA, hybridoma medium alone, preimmune serum, and secondary antibody served as negative controls. Immune polyclonal serum and anti-phage antibody served as positive controls. Antibodies were plated directly from culture supernatants. Cells from the positive wells were subcloned by limiting dilution to obtain monoclonal lines. Subclones emerging after 2 months were tested against the entire phage particle and the pIII phage capsid protein by using ELISA. Reactivity was monitored in an ELISA reader.
Western Blot Analysis. Filamentous fd-tet phage (109 transducing units/lane) were boiled, resolved by a gradient 4–20% SDS/PAGE (Invitrogen) and electrotransferred to Immuno-Blot polyvinylidene fluoride membrane (Bio-Rad). The membrane was divided into strips, blocked by 5% nonfat milk in PBS for 1 h at room temperature followed by a single wash with PBS containing 0.1% Tween 20. Strips were incubated with preimmune serum (1:1,000), postimmune serum (1:1,000), antifd-tet phage (Sigma–Aldrich), supernatants containing anti-phage IgGs secreted from immortal splenocyte clones, or cell culture media alone for 2 h at room temperature. After three washes, a peroxidase-conjugated secondary antibody (Bio-Rad) was added to the strips and incubated for 1 h at room temperature. Strips were washed three times, and the reactivity was detected by enhanced chemiluminescence (Amersham Biosciences).
Results and Discussion
Many different types of conditionally immortal cell lines have been derived from H-2Kb-tsA58 mice (7, 9–12). Surprisingly, this well established mouse model has not been exploited in applications for the generation of monoclonal antibody-producing cells.
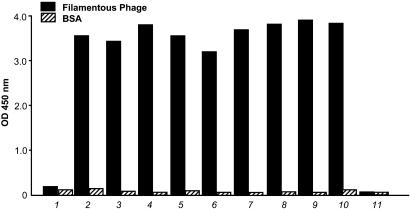
As proof of concept, H-2Kb-tsA58 mice were immunized with a defined antigen (filamentous phage; ref. 8), and anti-phage antibody titers in the serum were monitored by ELISA. Anti-phage IgG titers reached high levels (OD450 > 3 at 1:3,200 dilution, compared with <0.1 for preimmune serum) 7 days after a final boost (Fig. 1). Further testing of serial dilutions revealed that IgG titers against phage were on average ≈1:6,400; moreover, the serum titers against the pIII protein (coated at 10 μg per well) were on average ≈1:1,600 (data not shown). Mouse spleens were collected and cell suspensions were prepared in DMEM. The cells were distributed in 96-well plates and cultured at 33°C. The culture medium was changed completely three times during 2–3 weeks. Clones were observed in >90% of the wells after 3 weeks. To detect antibody reactivity, ELISA was performed with supernatants in microtiter well plates coated with phage particles. Up to 58% of the wells tested were positive for IgG reactivity against phage.
Fig. 1.
Screening and validation of antibodies obtained from H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes. Cells were released and resuspended in hybridoma medium (see Materials and Methods for details). ELISA against filamentous phage (fd-tet) and against recombinant phage capsid pIII protein was performed as described (2). BSA, hybridoma medium alone, preimmune serum, and secondary antibody served as negative controls. Immune polyclonal serum and anti-phage antibody served as positive controls. Antibodies were plated directly from culture supernatants and tested as follows: 1, preimmune serum at 1:400 dilution; 2, postimmune serum at 1:3,200 dilution; 3–10, supernatants derived different 6-, 24-, and 96-well plates, 2 weeks after plating of the spleen; 11, cultured medium alone as a negative control. Bars correspond to the mean. Standard errors of the mean were <1% of the mean.
We observed that splenocytes were healthy despite low cell density and yielded robust levels of reactivity in supernatants from most of the wells. To obtain monoclonal lines, cells from positive wells were cloned by limiting dilution, and most clones remained positive. Subcloning of monoclonal lines was repeated twice, and virtually all the resulting clones were positive, providing strong evidence that the lines generated were indeed derived from single clones.
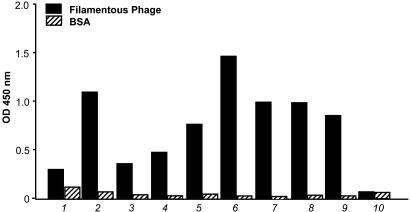
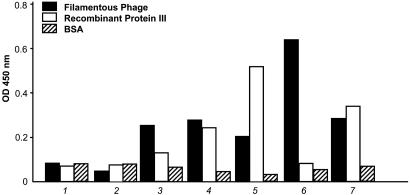
Clones emerging after 4–8 weeks were tested by ELISA against the phage particles and against the minor phage capsid protein (pIII). Again, most positive clones continued to react when expanded from 96-well to 24-well plates or after freeze and thaw (Fig. 2). Strong reactivity was observed against intact phage, and some clones also reacted against recombinant pIII fusion protein (Fig. 3). Original plates and clones at all stages were kept in culture for up to 3 months.
Fig. 2.
Characterization of H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes producing anti-phage antibodies. Clones 1–3 correspond to clones that underwent freeze–thaw. All thawed clones recovered and remained positive. Clones 4–9 correspond to different wells expanded from 96-well plates to 24-well plates and cultured for 6 weeks; 10 indicates cultured medium alone as a negative control. Other controls included were pre- and postimmune sera. Bars correspond to the mean. Standard errors of the mean were <1% of the mean.
Fig. 3.
Evaluation of H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes producing antibodies against intact phage particles and against the pIII phage capsid protein. 1, culture medium, negative control; 2, preimmune serum; 3–7, supernatants derived different monoclonal lines after 8 weeks in culture. 4, 5, and 7 react strongly with the pIII protein and, at this concentration, also react with intact filamentous phage particles. Bars correspond to the mean. Standard errors of the mean were <1% of the mean.
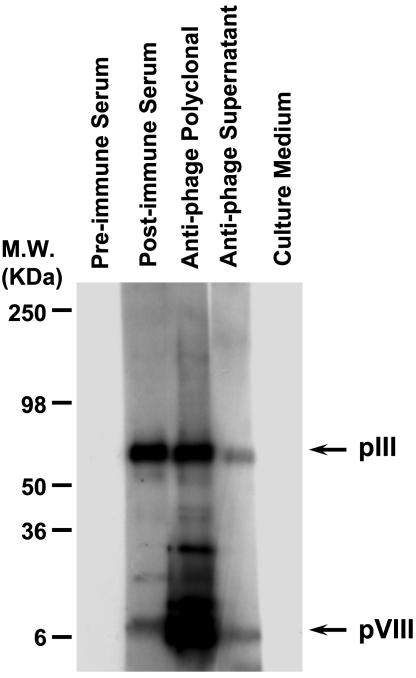
To determine whether antibodies screened by ELISA can recognize specific proteins in Western blots, we evaluated supernatants from H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes against the pIII and pVIII phage capsid proteins by resolving a filamentous phage preparation by SDS/PAGE. Polyvinylidene fluoride membranes containing phage proteins were incubated with preimmune serum, postimmune serum, a commercially available anti-phage, or supernatants containing anti-phage IgGs secreted from immortal splenocyte clones. Cell culture medium alone was used as an additional negative control. Antibodies reacting specifically with bands corresponding to the pIII and the pVIII phage capsid proteins were detected in the supernatants from H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes (Fig. 4). This result shows that antibodies produced by the methodology described here can also be used in applications such as immunoblotting (Fig. 4) or fluorescence-activated cell sorting of cell surface antigens (data not shown).
Fig. 4.
Reactivity of supernatants from H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes producing antibodies against phage proteins. Reactivity was evaluated after incubation with preimmune serum, postimmune serum, an anti-phage antibody, or supernatants containing anti-phage IgGs secreted from immortal splenocyte clones, as indicated. Cell culture medium alone served as an additional negative control. Antibodies reacting specifically against pIII and pVIII phage capsid proteins (arrows) were detected in supernatants from H-2Kb-tsA58 transgenic mouse-derived immortal splenocytes.
We conclude that splenocytes from H-2Kb-tsA58 transgenic mice can yield high titers of IgG against defined antigens. This cell culture system ensures a reliable and reproducible source of monoclonal antibodies and eliminates the need for hybridoma generation.
Several advantages of this methodology merit further comment. First, the antibody-synthesizing cells are stable for at least several months in culture, tolerate limiting dilution cloning, and survive freeze–thaw without loss or inactivation of antibody production. We have frozen polyclonal populations and recovered viable clones secreting a given IgG (data not shown). Second, immortal clones grow slowly at 33°C and are genetically stable, allowing for timely processing of numerous samples (and, logically, the possibility of obtaining “rare” antibodies). H-2Kb-tsA58-derived splenocytes enable the production of large amounts of specific polyclonal IgGs from wells containing clones that have been cultured long term. In contrast, hybridomas are problematic because, in a random mixture of clones, nonsecreting clones generally will overtake the secreting ones. Preliminary data suggest that the proliferation rate between IgG secreting and nonsecreting splenocytes derived from an H-2Kb-tsA58 transgenic mouse is similar (unpublished observations). Third, in vitro immunization is enhanced through the presence of other spleen-derived immortal cell types, such as macrophages, dendritic cells, and fibroblasts, that facilitate antibody production (2), whereas ex vivo immunization is inefficient with mortal splenocytes or hybridomas. Given the recent restrictions placed on ascites production, this new technology favors convenient large-scale manufacture of antibodies ex vivo. Fourth, crossing H-2Kb-tsA58 mice with mice expressing the genetic complement for human antibody production (13–15) will also enable production of human monoclonal antibodies.
In summary, the strategy described here is likely to replace hybridoma generation and streamline the production of mouse and human antibodies, with profound and immediate scientific and medical benefits.
Acknowledgments
We thank Drs. Richard L. Sidman for critical reading of the manuscript, Akihiko Kunyiasu for helpful insights, and Robert R. Langley and Isaiah J. Fidler for reagents, and Ms. Connie Sun for technical assistance. This work was supported by National Institutes of Health Grants CA90270 and CA82976 (to R.P.) and CA90270 and CA90810 (to W.A.) and by awards from AngelWorks and the Gilson–Longenbaugh Foundation.
Abbreviation: ts, temperature-sensitive.
References
- 1.Köhler, G. & Milstein, C. (1975) Nature 256, 495-497. [DOI] [PubMed] [Google Scholar]
- 2.Harlow, E. & Lane, D. (1998) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 3.Winter, G., Griffiths, A. D., Hawkins, R. E. & Hoogenboom, H. R. (1994) Annu. Rev. Immunol. 12, 433-455. [DOI] [PubMed] [Google Scholar]
- 4.Barbas, C. F., III, Burton, D. R., Scott, J. K. & Silverman, G. J. (2000) Phage Display: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 5.Winter, G. & Milstein, C. (1991) Nature 349, 293-299. [DOI] [PubMed] [Google Scholar]
- 6.Karpas, A., Dremucheva, A. & Czepulkowski, B. H. (2001) Proc. Natl. Acad. Sci. USA 98, 1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jat, P. S., Noble, M. D., Ataliotis, P., Tanaka, Y., Yannoutsos, N., Larsen, L. & Kioussis, D. (1991) Proc. Natl. Acad. Sci. USA 88, 5096-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zacher, A. N., III, Stock, C. A., Golden, J. W., II & Smith, G. P. (1980) Gene 9, 127-132. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead, R. H., Van Eeden, P. E., Noble, M. D., Ataliotis, P. & Jat, P. S. (1993) Proc. Natl. Acad. Sci. USA 90, 587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, T., Owens, J., Hattersly, G., Jat, P. & Noble, M. D. (1993) Proc. Natl. Acad. Sci. USA 90, 5578-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holley, M. C., Nishida, Y. & Grix N. (1997) Int. J. Dev. Neurosci. 15, 541-552. [DOI] [PubMed] [Google Scholar]
- 12.Langley, R. R., Ramirez, K. M., Tsan, R. Z., Van Arsdall, M., Nilsson, M. B. & Fidler, I. J. (2003) Cancer Res. 63, 2971-2976. [PubMed] [Google Scholar]
- 13.Mendez, M. J., Green, L. L., Corvalan, J. R., Jia, X. C., Maynard-Currie, C. E., Yang, X. D., Gallo, M. L., Louie, D. M., Lee, D. V., Erickson, K. L., et al. (1997) Nat. Genet. 15, 146-156. [DOI] [PubMed] [Google Scholar]
- 14.Lonberg, N., Taylor, L. D., Harding, F. A., Trounstine, M., Higgins, K. M., Schramm, S. R., Kuo, C. C., Mashayekh, R., Wymore, K, McCabe, J. G., et al. (1994) Nature 368, 856-859. [DOI] [PubMed] [Google Scholar]
- 15.Kuroiwa, Y., Tomizuka, K., Shinohara, T., Kazuki, Y., Yoshida, H., Ohguma, A., Yamamoto, T., Tanaka, S., Oshimura, M. & Ishida, I. (2000) Nat. Biotechnol. 18, 1086-1090. [DOI] [PubMed] [Google Scholar]