Abstract
Background and Purpose
Using a rodent model of ischemia (permanent middle cerebral artery occlsion; pMCAO), our lab previously demonstrated that 4.27 minutes of patterned single whisker stimulation delivered over 120 minutes can fully protect from impending damage when initiated within two hours of pMCAO (“early”). When initiated three hours post-pMCAO (“late”), stimulation resulted in irreversible damage. Here we investigate the effect of altering pattern, distribution, or amount of stimulation in this model.
Methods
We assessed the cortex using functional imaging and histological analysis with altered stimulation treatment protocols. In two groups of animals we administered the same number of whisker deflections but in a random rather than patterned fashion, distributed either over 120 minutes or condensed into 10 minutes post-pMCAO. We also tested increased (full whisker array versus single whisker) stimulation.
Results
Early random whisker stimulation (condensed or dispersed) resulted in protection equivalent to early patterned stimulation. Early full whisker array patterned stimulation also resulted in complete protection, but promoted faster recovery. Late full whisker array patterned stimulation however, resulted in loss of evoked function and infarct volumes larger than those sustained by single whisker counterparts.
Conclusions
When induced early on after ischemic insult, stimulus-evoked cortical activity, irrespective of the parameters of peripheral stimulation that induced it, seems to be the important variable for neuroprotection.
Keywords: neuroprotection, brain ischemia, brain recovery, basic science, animal models, imaging
INTRODUCTION
Middle cerebral artery (MCA) occlusion is used to model the most clinically relevant type and location of stroke in humans: ischemic stroke in MCA1. We have previously shown that, if initiated within 1 and in most cases 2 hours following permanent MCA occlusion (pMCAO) in a rat model, single whisker stimulation completely protects against the expected stroke related structural and functional damage and behavioral deficits according to a host of techniques. Treatment consisted of 4.27 minutes of patterned (5 Hz) stimulation of a single whisker delivered over 120 minutes (see Lay et al., 2010 for details). Animals that did not receive the same stimulation until 3 hours post-pMCAO not only had eliminated function, behavioral deficits and large infarcts, but had larger infarcts than animals that never received post pMCAO stimulation2. These results led us to ask the following questions: Given that patterns are generally important in terms of experience and learning, is random stimulation as protective as patterned? The 120 minute stimulation period overlaps the 2 hour window for protection, if we condense the stimulation into 10 minutes, can it still protect? Does increased cortical activity (resulting from full whisker array versus single whisker stimulation) alter the early protective or late damaging effects of stimulation?
To determine whether patterned stimulation was critical for protection, we administered the same number of whisker deflections as in the previous +0h group (stimulation delivered immediately following pMCAO), but in a random rather than 5Hz (patterned) fashion. To address the distribution question, this random stimulation was either spread out over the first 120 minutes following pMCAO (“dispersed +0h group”; Figure 1A) or condensed into the first 10 minutes (“condensed +0h group”; Figure 1B). To determine whether more cortical activity would alter results, we ran a +0h group (Figure 1C) and a +3h (stimulation delivered starting at 3 hours post-pMCAO; Figure 1D) group using the patterned stimulation protocol from the previous manuscript except that single whisker stimulation was replaced with full whisker array stimulation; “+0h full whisker array” and “+3h full whisker array” (See methods for stimulation protocol details).
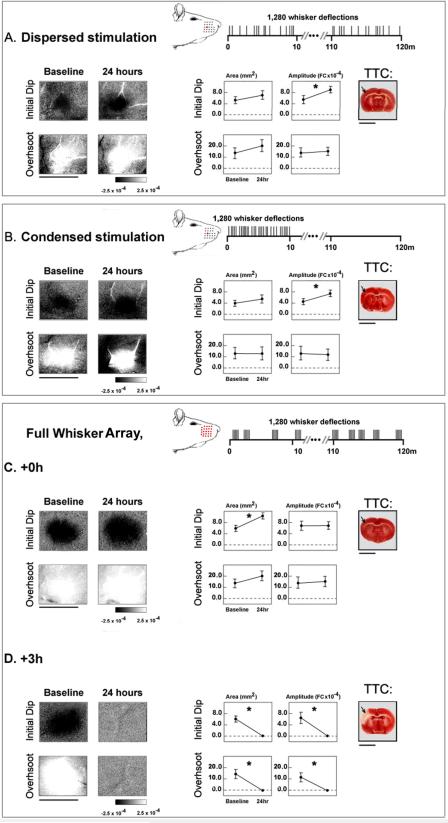
Figure 1. Experimental group stimulation and assessment.
Representative (A) dispersed and (B) condensed +0h single whisker subjects', and (C) full whisker array +0h and (D) +3h subjects' initial dip and overshoot before and 24 hours post-pMCAO. Linear grayscale bar indicates intrinsic signal strength ×10−4 FC. Streaks correspond to large surface vessels. Illustration depicts timing, location and number of whiskers stimulated during treatment (temporal axis not to scale). Group baseline and +24 hour data are plotted in each graph. Means and standard errors are provided for the area (left) and amplitude (right) of the initial dip (first row) and overshoot (second row). A value of zero indicates no response. Asterisks indicate significant differences between baseline and 24 hour values. Right, representative TTC stained coronal sections. Arrow indicates approximate region vulnerable to pMCAO infarct. Scale bars indicate 5mm.
Utilizing Intrinsic Signal Optical Imaging (ISOI) and 2,3,5- triphenyltetrazolium chloride (TTC) staining, we found that changes in pattern or distribution of stimulation did not diminish protection. Full whisker array stimulation resulted in a faster recovery in +0h animals and increased infarct volume in +3h group compared to single whisker counterparts. Thus it appears that once the window of opportunity to protect the cortex has closed, the more stimulation given, the worse the damage. On the other hand, when given early, alterations in pattern or distribution still lead to complete protection and more stimulation accelerates recovery.
METHODS
All procedures are in compliance with UC Irvine Animal Care and Use Committee. For detailed descriptions, see Lay et al, 20102.
Subjects and surgical preparation
Experimental subjects, 295–400g male Sprague Dawley rats were individually housed in standard cages. Animals were injected with a sodium pentobarbital bolus (55 mg/kg b.w.), and supplemental injections (27.5 mg/kg b.w.) were given as necessary. A ~5 mm × 6 mm `imaging' area of skull over the left somatosensory cortex was thinned. 5% dextrose (3mL) and atropine (0.05 mg/kg, b.w.) were administered initially and for every six hours after, during the experiment. Body temperature was maintained at 37 °C.
Baseline data collection was followed by pMCAO: double ligature and transection of the stem (M1 segment; just distal to lenticulostriate branch) of the left proximal MCA3–5.
Stimulation protocols
“random dispersed”
A total of 1,280 whisker deflections delivered individually at varying intervals of 4.8±3.6 seconds between onsets of consecutive deflections. This stimulation is distributed over about 120 minutes (see Figure 1A).
“random condensed”
A total of 1,280 whisker deflections delivered individually at varying intervals of 0.5±0.25 seconds between onsets of consecutive deflections. This stimulation is distributed over about 10 minutes (see Figure 1B).
“patterned” (single [previous study] or full whisker array [current study])
A total of 1,280 whisker deflections delivered in 256 events (5 whisker deflections per event in a 5Hz pattern) at varying intervals of 21±5 seconds between onsets of consecutive events. This stimulation is distributed over about 120 minutes (see Figure 1C).
Histology (TTC staining for infarct)
24 hours post- pMCAO, the brain was removed, sectioned into 2mm coronal slices, and incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) at 37° C for 20 minutes in the dark6. Infarct volume was determined by an observer blind to experimental condition. A small amount of damage occasionally produced at the surgical site was excluded from infarct analysis3.
Intrinsic Signal Optical Imaging (ISOI) and Analysis
We used ISOI to assess evoked functional response to single whisker stimulation (whisker functional representation; WFR) or full whisker array stimulation (whisker array functional representation; WAFR). For a recent review of ISOI see7. A detailed description of ISOI8–10 data acquisition and analysis can be found elsewhere11, 12. Briefly, a charge coupled device (CCD) camera was used for imaging with red light illumination. Post-stimulus ratio images were created by calculating fractional change (FC) values relative to intrinsic activity collected immediately before stimulus onset. The first two phases of evoked functional representation, the `initial dip' and `overshoot', were analyzed. The ratio image containing the maximum areal extent was quantified at a threshold level of 2.5 × 10−4 away from zero. Peak amplitude was quantified in FC units from the pixel with peak activity within the maximum areal extent for each of the two phases.
Statistical Analysis
Two-sample T-tests were run on raw baseline imaging values to ensure no significant differences prior to pMCAO between condensed and dispersed stimulation groups, or between +0h and +3h full whisker array groups existed.
For the randomized stimulation groups, paired sample t-tests were employed to compare between baseline and 24 hour WFRs.
Because there were no responses to quantify in +3h full whisker array animals, post-pMCAO imaging evoked area and amplitude were converted to difference score values (post-occlusion - baseline), with values away from 0 signifying a change from baseline. A constant was added to difference values, which were then transformed with a natural log function to better satisfy the assumptions of an ANOVA and inferential statistics were performed on the transformed data. Following the repeated measures ANOVA, specific contrasts were performed to identify at which post-pMCAO time points WAFRs differed from baseline. Separate ANOVAs followed by respective contrasts were performed for the two phases of the WAFR. The alpha level was set to 0.05 and Bonferroni adjustment applied to account for the 5 contrasts (p=0.05/5= 0.01).
Finally, infarct volume comparisons were performed by employing two-sample T-tests.
RESULTS
Random single whisker stimulation
Prior to pMCAO and random stimulation treatment, there were no differences between groups in area or peak amplitude of initial dip (area: t(12)=0.76. p>0.05; amplitude: t(12)=1.00, p>0.05) or overshoot (area: t(12)=−0.02, p>0.05; amplitude: t(12)=0.34, p>0.05). 24 hours post-pMCAO, both dispersed (n=7) and condensed (n=7) groups maintained WFR at or above baseline (Figure 1A, B). Specifically, initial dip area was equivalent to baseline (dispersed: t(6)= −2.16, p>0.05; condensed: t(6)=−2.03, p>0.05), whereas initial dip amplitude increased (dispersed: t(6)= −5.02, p<0.01; condensed: t(6)=−5.48, p<0.01). The increased WFR at 24 hours post-pMCAO matches our previous findings for patterned stimulation +0h animals and may be evidence of changes in the system that facilitated protection2. For both groups, overshoot area (dispersed: t(6)= −2.37, p>0.05; condensed: t(6)= −0.003, p>0.05) and amplitude (dispersed: t(6)= −0.05, p>0.05; condensed: t(6)=0.31, p>0.05) maintained baseline levels. Finally, there was no sign of infarct in any subject in either group.
Full whisker array stimulation
When the full whisker array was stimulated immediately following pMCAO (+0h full whisker array; n=7), the corresponding WAFRs regained baseline or greater levels and no cortical infarct was sustained (area: F1,12=112.58, p<0.001; amplitude: F1,12=0.13, p>0.05; Figures 1C). In contrast, the identical stimulation, when delivered three hours post-pMCAO (area: F1,12=138.61, p<0.001; amplitude: F1,12=66.59, p<0.001;+3h full whisker array; n=7), failed to restore WAFR and resulted in a substantial infarct (mean=91.85 ± 9.8mm3; Figures 1D).
To further characterize the effect of full whisker array stimulation, initial dip and overshoot phases of WAFRs were examined quantitatively at four points during stimulation treatment in addition to the 24-hour time point (Figures 1CD, and 2). Prior to pMCAO, no differences between groups in initial dip (area: t(12)=−0.347, p>0.05; amplitude: t(12)=0.344, p>0.05) or overshoot (area: t(12)= −0.310, p>0.05; amplitude: t(12)=0.692, p>0.05) were observed. Post-pMCAO, however, an interaction between assessment time point and experimental group was found for both area (F4,48= 22.66, p<0.001, ANOVA) and amplitude (F4,48= 13.13, p<0.001, ANOVA) of the initial dip. In the +0h subjects, both were reduced at 0– 30 minutes of treatment (area: F1,12=22.53, p<0.001; amplitude: F1,12=16.76, p<0.001), but by 60 minutes the area surpassed (F1,12=11.87, p<0.01), and the amplitude reached baseline level (amplitude: F1,12=5.27, p>0.05). The area of the initial dip continued to increase and remained above baseline the following day (60–90m: F1,12=18.09, p<0.01; 90–120m: F1,12=71.97, p<0.001; following day: F1,12=112.58, p<0.001) while the amplitude remained at baseline level (Figure 2, blue bars).
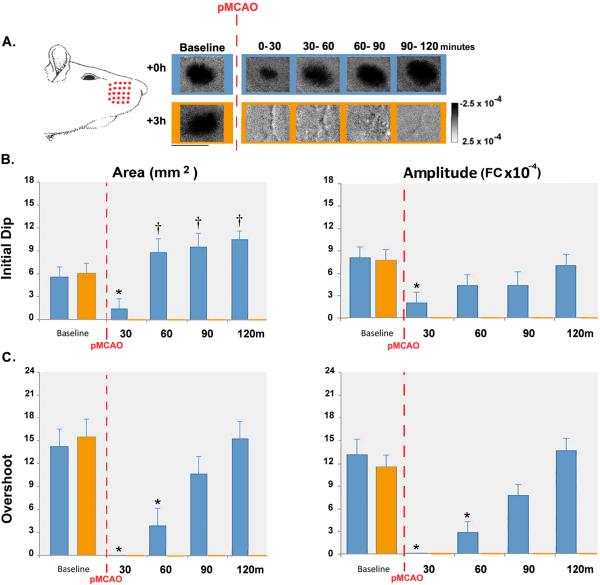
Figure 2. +0h and +3h full whisker array stimulation data.
A. Initial dip images from representative +0h and +3h full whisker array animals. Illustration depicts whiskers stimulated during treatment. All +0h animals regained baseline level cortical function by 90 minutes of stimulation and sustained no infarct, while all +3h animals permanently lost cortical activity and sustained infarct. Linear scale bar indicates intrinsic signal strength ×10−4 FC. Streaks correspond to large surface vessels. Scale bar indicates 5mm. (B–C) Graphs of group baseline and post-occlusion data. Means and standard errors are provided for the area (left) and amplitude (right) of the initial dip (B) and overshoot (C). A value of zero indicates no response. For +0h animals, asterisks indicate values significantly below and daggers indicate values significantly above baseline. For +3h animals, all post-pMCAO values are unmarked and were zero.
Similar to the initial dip data, there was also an interaction between assessment time point and experimental group for both the area (F4,48=26.47, p<0.01, ANOVA) and amplitude (F4,48=43.49, p<0.01, ANOVA) of the overshoot. In +0h full whisker array subjects, overshoot was reduced for the first 60 minutes of treatment (area: 0–30 minutes: F1,12=17.71, p<0.01; 30–60 minutes: F1,12=7.39, p<0.05; amplitude: 0–30 minutes: F1,12=51.6, p<0.01; 30–60 minutes: F1,12=23.47, p<0.01) but regained baseline level area by 90 minutes (Figure 2, blue bars).
Current work in our lab (Davis et al., unpublished data, 2010) shows that +0h animals demonstrate complete recovery of initial dip prior to the overshoot. Although the +0h full whisker array group in the present study recover earlier than their above mentioned single whisker counterparts, the sequence of recovery of the phases replicate the earlier finding: the initial dip recovers prior to the overshoot.
Single versus full whisker array +0h groups
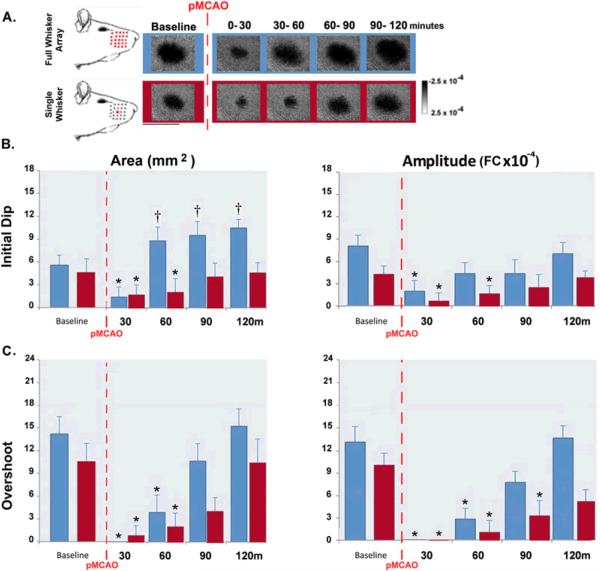
To study the effect of increased stimulation, we compared the rate of recovery of cortical function in the present study's +0h full whisker array animals to their single whisker counterparts (+0h single whisker group: data from a previous study; Davis et al., unpublished data, 2010). By 90 minutes of treatment in single whisker +0h animals, initial dip area and amplitude and overshoot area had regained baseline values, while overshoot amplitude required 120 minutes (Figure 3, red bars). In the present study, the initial dip area (F1,12=11.87, p<0.05) and amplitude (F1,12=5.27, p>0.05) of full whisker array +0h subjects recovered pre-pMCAO baseline values or greater by 60 minutes of stimulation treatment, and overshoot area (F1,12=1.41, p>0.05) and amplitude (F1,12=6.96, p>0.05) recovered pre-pMCAO baseline values by 90 minutes of stimulation treatment (see previous results section for detailed statistical analysis of the full whisker array group). Thus, full whisker array treatment resulted in full recovery 30 minutes faster than single whisker stimulation (Figure 3, blue bars).
Figure 3. +0h full whisker array versus +0h single whisker data.
A. Initial dip images from representative +0h full whisker array and +0h single whisker groups. Illustration depicts whiskers stimulated during treatment. Linear scale bar indicates intrinsic signal strength ×10−4 FC. Streaks correspond to large surface vessels. Scale bar indicates 5mm. (B–C) Graphs of group baseline and post-occlusion data. Means and standard errors are provided for the area (left) and amplitude (right) of the initial dip (B) and overshoot (C). A value of zero indicates no response. Asterisks indicate values significantly below and daggers indicate values significantly above baseline.
Ischemic infarct in single versus full whisker array +3h groups
Previous results demonstrated that late whisker stimulation increased the volume of infarct in +3h subjects compared to non-stimulated controls2. Here, we compare infarct in +3h full whisker array group to +3h single-whisker infarct. +3h full whisker array infarct volume was found to be significantly larger than that sustained by single-whisker counterparts (meansingle=63.4 ± 3.9mm3; meanfull=91.9 ± 9.8mm3; t(12)=2.69, p<0.05; Figure 4). Thus, when delivered too late, increased evoked cortical activity results in increased infarct volume.
Figure 4. +3h full whisker array versus +3h single whisker infarct volumes.
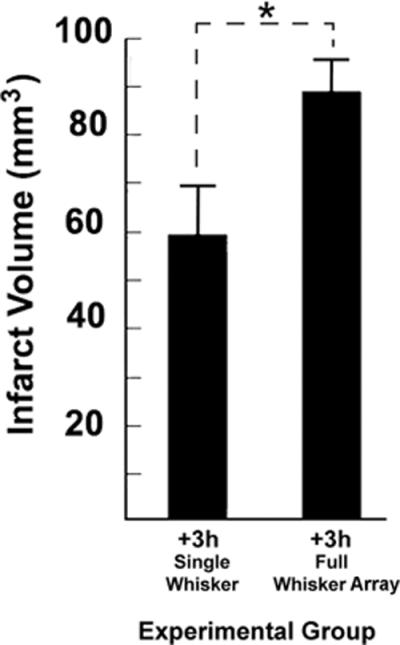
The total infarct volume sustained by +3h single whisker and +3h full whisker array animals according to TTC. Asterisk indicates significant difference in infarct volume between groups.
DISCUSSION
In the current study we attempted to identify important aspects of protective whisker stimulation by altering components we thought might affect our results: pattern and distribution of stimulation and number of whiskers stimulated. Based on our previous work we suspected that cortical activity was the critical factor in producing protection, but were unsure whether an increase resulting from stimulating the full whisker array would alter our results. Additionally we considered the possibility that a patterned stimulus might be more salient to the rat cortex and also wondered whether condensing the stimulation (previously spread out over 120 minutes) into 10 minutes might alter our results.
It appears that neither the pattern nor the distribution of stimulation is an important factor for protection, but that the amount of cortical activation can augment the speed of recovery (or exacerbate injury when delivered late). Thus early administration seems to be critical. This fits with current research which suggests that the greatest opportunity for recovery exists within a limited window of time following a stroke13.
Though still debated, the initial dip is generally associated with evoked neuronal activity and the overshoot with blood flow response; in any case the recovery of both phases, and their underlying signal sources, have the same profile whether single or full whisker array stimulation is delivered. This similar progress of recovery of the initial dip and overshoot in both groups further supports this idea that activating the cortex early on is of more importance than the type of stimulation administered. Not all parameters of stimulation were explored, however, and it is possible that other, untested variables of whisker deflection might alter the outcome. Furthermore, it remains to be determined whether generating cortical activity in non-anesthetized animals, could also be protective.
Previous research has demonstrated that direct electrical stimulation of the brain, spinal cord, or peripheral nerves can temper impending stroke damage14–16. It has been suggested that this stimulation may help maintain neurovascular coupling under ischemic conditions via collateral flow and that this is an important potential avenue for future stroke therapy17, 18. Targeting this coupling mechanism has also been suggested as a strategy more likely to succeed in translation to humans19. Indeed, blood flow data from our previous research2 suggest that protected animals have collateral vessel supported reperfusion. Additionally, in reference to the current results, it is reasonable that if stimulation induced blood flow increases allow protection, patterned stimulation would not be more protective than random and increased stimulation could increase blood flow and accelerate recovery. Clearly long term re-establishment of blood flow is necessary for protection, but perhaps an acute reperfusion resulting from early stimulation induced collateral flow maintains the cortex in a less compromised position during acute ischemia, allowing complete protection. Other possible mechanisms have also been proposed; some direct stimulation methods have been shown to induce various neuroprotective effects and agents20–22. Whether initiation of neuroprotective agents or early, acute induction of collateral flow or both are necessary for activity induced complete protection from ischemia is yet unknown and requires more research.
Sensory induced protection from ischemic stroke nonetheless appears to be a robust phenomenon unperturbed by alterations in pattern or distribution of stimulation which can be accelerated with increased cortical activation. These results may simplify future investigations regarding the mechanism and though there is still much research to be done, this might also mean that translational application could be straightforward as long as sensory stimulation is delivered early on after ischemic onset.
SUMMARY
Previous research in our lab demonstrated that patterned whisker stimulation given within 2 hours of pMCAO can fully protect from stroke damage. In the current manuscript we sought to determine whether altering various parameters of stimulation would alter recovery. Results demonstrate that altering the pattern and distribution of whisker stimulation does not perturb the protective effect, but that increasing the number of whiskers stimulated in early treatment speeds recovery. Increasing the number of whiskers stimulated outside of the protective window (3 hours post-pMCAO) is not only no longer protective, but increases cortical damage compared to single whisker counterparts.
Acknowledgments
FUNDING: This work was supported by (NIH-NINDS) NS-55832, NS-066001, and an American Heart Association Predoctoral Fellowship 788808-41910.
Footnotes
DISCLOSURES: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS ONE. 2010;5:e11270. doi: 10.1371/journal.pone.0011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 4.Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: Methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- 5.Wang-Fischer Y. Manual of stroke models in rats. CRC Press; Boca Raton: 2009. [Google Scholar]
- 6.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 7.Frostig RD, Chen-Bee CH. Visualizing adult cortical plasticity using intrinsic signal optical imaging. In: Frostig RD, editor. In vivo optical imaging of brain function. CRC Press; 2009. pp. 255–287. [PubMed] [Google Scholar]
- 8.Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- 9.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ts'o DY, Frostig RD, Lieke EE, Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990;249:417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- 11.Chen-Bee CH, Polley DB, Brett-Green B, Prakash N, Kwon MC, Frostig RD. Visualizing and quantifying evoked cortical activity assessed with intrinsic signal imaging. J Neurosci Methods. 2000;97:157–173. doi: 10.1016/s0165-0270(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: Implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 14.Burnett MG, Shimazu T, Szabados T, Muramatsu H, Detre JA, Greenberg JH. Electrical forepaw stimulation during reversible forebrain ischemia decreases infarct volume. Stroke. 2006;37:1327–1331. doi: 10.1161/01.STR.0000217305.82123.d8. [DOI] [PubMed] [Google Scholar]
- 15.Reis DJ, Berger SB, Underwood MD, Khayata M. Electrical stimulation of cerebellar fastigial nucleus reduces ischemic infarction elicited by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 1991;11:810–818. doi: 10.1038/jcbfm.1991.139. [DOI] [PubMed] [Google Scholar]
- 16.Sagher O, Huang DL, Keep RF. Spinal cord stimulation reducing infarct volume in a model of focal cerebral ischemia in rats. J Neurosurg. 2003;99:131–137. doi: 10.3171/jns.2003.99.1.0131. [DOI] [PubMed] [Google Scholar]
- 17.Armitage GA, Todd KG, Shuaib A, Winship IR. Laser speckle contrast imaging of collateral blood flow during acute ischemic stroke. J Cereb Blood Flow Metab. 2010;30:1432–1436. doi: 10.1038/jcbfm.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF, Lyden PD, Kleinfeld D. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 2006;4:e22. doi: 10.1371/journal.pbio.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 20.Galea E, Glickstein SB, Feinstein DL, Golanov EV, Reis DJ. Stimulation of cerebellar fastigial nucleus inhibits interleukin-1beta-induced cerebrovascular inflammation. Am J Physiol. 1998;275:H2053–2063. doi: 10.1152/ajpheart.1998.275.6.H2053. [DOI] [PubMed] [Google Scholar]
- 21.Galea E, Golanov EV, Feinstein DL, Kobylarz KA, Glickstein SB, Reis DJ. Cerebellar stimulation reduces inducible nitric oxide synthase expression and protects brain from ischemia. Am J Physiol. 1998;274:H2035–2045. doi: 10.1152/ajpheart.1998.274.6.H2035. [DOI] [PubMed] [Google Scholar]
- 22.Golanov EV, Zhou P. Neurogenic neuroprotection. Cell Mol Neurobiol. 2003;23:651–663. doi: 10.1023/A:1025088516742. [DOI] [PMC free article] [PubMed] [Google Scholar]