Abstract
Whereas the adult gastrointestinal epithelium undergoes tremendous self-renewal through active proliferation in crypt stem cell compartments, the responsible growth factors regulating this continuous proliferation have not been defined. The exploration of physiologic functions of Wnt proteins in adult organisms has been hampered by functional redundancy and the necessity for conditional inactivation strategies. Dickkopf-1 (Dkk1) is a potent secreted Wnt antagonist that interacts with Wnt coreceptors of the LRP family. To address the contribution of Wnt signaling to gastrointestinal epithelial proliferation, adenoviral expression of Dkk1 was used to achieve stringent, conditional, and reversible Wnt inhibition in adult animals. Adenovirus Dkk1 (Ad Dkk1) treatment of adult mice repressed expression of the Wnt target genes CD44 and EphB2 within 2 days in both small intestine and colon, indicating an extremely broad role for Wnt signaling in the maintenance of adult gastrointestinal gene expression. In parallel, Ad Dkk1 markedly inhibited proliferation in small intestine and colon, accompanied by progressive architectural degeneration with the loss of crypts, villi, and glandular structure by 7 days. Whereas decreased Dkk1 expression at later time points (>10 days) was followed by crypt and villus regeneration, which was consistent with a reversible process, substantial mortality ensued from colitis and systemic infection. These results indicate the efficacy of systemic expression of secreted Wnt antagonists as a general strategy for conditional inactivation of Wnt signaling in adult organisms and illustrate a striking reliance on a single growth factor pathway for the maintenance of the architecture of the adult small intestine and colon.
The adult intestinal epithelium is characterized by continuous replacement of epithelial cells through a stereotyped cycle of cell division, differentiation, migration, and exfoliation occurring during a 5–7 day crypt-villus transit time (1). The putative growth factors regulating proliferation within the adult intestinal stem cell niche have not yet been identified (1, 2), although studies have implicated the cell-intrinsic action of β-catenin/Lef/Tcf signaling within the proliferative crypt compartment (3–7). Tcf-4-/- mice exhibit a single histologic defect in which late embryonic proximal small intestine exhibits loss of proliferative stem cell compartments with mild reduction in villus number, although rapid neonatal lethality has precluded addressing the role of Tcf-4 in adult mice (3). In chimeric transgenic mice allowing adult analysis, expression of constitutively active NH2-truncated β-catenin-stimulated proliferation in small intestine crypts, although either NH2-truncated β-catenin or Lef-1/β-catenin fusions induced increased crypt apoptosis as well (4, 7). Although these studies suggest the potential involvement of canonical Wnt signaling in the intestinal stem cell niche (8), modulation of β-catenin/Lef/Tcf-dependent transcription has also been described by diverse factors, including nonFrizzled G protein-coupled receptors and PTEN/PI-3-kinase (9–11).
The exploration of physiologic functions of Wnt proteins in adult organisms has been hampered by functional redundancy and the necessity for conditional inactivation strategies. Dickkopf-1 (Dkk1) has been recently identified as the founding member of a family of secreted proteins that potently antagonize Wnt signaling (12–14). Dkk1 associates with both the Wnt coreceptors, LRP5/6 (14–16), and the transmembrane protein, Kremen, with the resultant ternary complex engendering rapid LRP internalization and impairment of Wnt signaling through the absence of functional Frizzled/LRP Wnt receptor complexes (17). We have previously used adenoviral expression of soluble ectodomains of the vascular endothelial growth factor (VEGF) receptors, Flk1 and Flt1, to conditionally inactivate VEGF function in adult animals (18). In the current studies, we have used a similar strategy to achieve stringent, fully conditional Wnt inhibition in adult mice by adenoviral expression of Dkk1 (Ad Dkk1). Here, Ad Dkk1 treatment of adult mice rapidly ablated canonical Wnt signaling and epithelial proliferation in small intestine, cecum, and colon, accompanied by progressive architectural degeneration with loss of crypts, villi, and glandular structure to the extent of mucosal ulceration and lethality. During the preparation of this manuscript, Pinto et al. (19) reported that transgenic expression of Dkk1 in the intestine regulated by the villin promoter resulted in loss of proliferation and villi in small intestine. By using this fully conditional Ad strategy, we have observed a more severe phenotype involving small intestine, cecum, and colon. These data implicate Wnts as essential growth factors required for maintenance of the robust proliferation characteristic of both the adult small and large intestine, suggest the potential utility of Wnts in mucosal repair of the colon, and illustrate the efficacy of adenoviral expression of secreted Wnt inhibitors as a general strategy for defining physiologic functions of Wnt proteins in adult organisms.
Methods
Ad Construction and Production. Dkk1 cDNA was amplified from embryonic day (E)17.5 mouse embryo cDNA with C-terminal FLAG and/or His6 epitope tags, sequenced, and cloned into the E1 region of E1-E3- Ad strain 5 by homologous recombination, followed by Ad production in 293 cells and CsCl gradient purification of virus as described (18, 20). Detailed methods are presented in Supporting Methods, which is published as supporting information on the PNAS web site. The negative control virus Ad Fc expressing a murine IgG2a Fc fragment has been described (18).
Ad Administration and Detection of Plasma Transgene Expression. Adult (12–16 weeks old) male C57BL/6 or CB17 severe combined immunodeficient (SCID) mice received single i.v. tail vein injection of 109 pfu of the appropriate Ads. For low-dose studies, 3 × 108 plaque-forming units (pfu) were administered. At appropriate times after injection, whole blood was obtained by retroorbital phlebotomy followed by Western blot analysis of 1 μl of plasma using anti-His probe antibody (Santa Cruz Biotechnology) or anti-His C-term antibody (Invitrogen). Low-dose (3 × 108) administration was estimated to produce 10–20% of the circulating Dkk1 levels in high-dose animals (109 pfu).
Immunohistochemistry and Histology. The following antibodies were used: Rat anti-mouse CD44 (1:100; BD Pharmingen), rat anti-mouse Ki67 (1:100; DAKO), goat anti-mouse EphB2 (1:100; R & D Systems), rabbit anti-rat FABP (1:100; Novus Biologicals, Littleton, CO), and rabbit anti-human lysozyme (1:100; DAKO). Immunostainings of paraffin-embedded samples were performed according to standard procedures. Antigen retrieval was accomplished by boiling samples in Na-citrate buffer (10 mM, pH 6.0) for 20 min. Color development was performed by using diaminobenzidine (brown precipitate) with hematoxylin counterstain. For immunofluorescence, samples were cryoembedded in OCT compound and sectioned at 7-μM thickness. Stainings were visualized with Alexa 488-conjugated secondary anti-goat antibodies (Molecular Probes) and nuclei were counterstained with Hoechst 33342 (Molecular Probes). For histological analysis, hematoxylin/eosin and Alcian blue staining of paraffin-embedded sections was performed according to standard protocols. Gremelius staining was performed by using Pascual's modified method. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining on paraffin-embedded samples used 20 μM biotin-16-UTP and 0.4 units/μl terminal transferase followed by color development (Vectastain ABC kit, Vector Laboratories) and methyl green counterstaining.
Assay and Quantitation Details. Details of β-catenin-stabilization assay, TOPFLASH reporter assay, and quantitation of Ki67 immunoreactivity are presented in Supporting Methods.
Results
To achieve conditional Wnt inactivation in adult animals, an Ad-expressing murine Dkk1 cDNA bearing C-terminal His6 and Flag epitope tags was produced (Ad Dkk1) by conventional methods (refs. 18 and 20 and Fig. 1A). The transfected adenoviral Dkk1 shuttle plasmid inhibited Wnt3a-stimulated transcription of a TOPFLASH reporter gene (Fig. 1B), whereas recombinant Dkk1 purified from Ad Dkk1 supernatants inhibited recombinant Wnt3a-induced β-catenin stabilization in L cells (Fig. 1C), which is consistent with appropriate functional activity (13). Single i.v. injection of purified Ad Dkk1 (109 pfu) into tail veins of adult (12–16 weeks old) C57BL/6 mice resulted in liver transduction and produced transient Dkk1 expression in plasma peaking at day 2 and progressively diminishing over an 11-day period (Fig. 1D), which is in agreement with the typical expression kinetics of Ads in immunocompetent mice (18).
Fig. 1.
Analysis of Ads expressing murine Dkk1. (A) Construction of Ad Dkk1. Murine Dkk1 cDNA bearing N-terminal IgK signal peptide and C-terminal FLAG and His6 tags was inserted into E1-E3- Ad strain 5 by homologous recombination (7, 8). (B) Inhibition of Wnt3a-stimulated TOPFLASH luciferase reporter activity by transfected Dkk1. Wnt3a and/or Dkk1 expression vectors were cotransfected into 293T cells with pTOPFLASH followed by luciferase measurement. (C). β-Catenin stabilization assay. Purified recombinant Dkk1 (125 ng/ml) was added to L cells 2 h before recombinant Wnt3A (1:8,000; Nusse Laboratory, Stanford, CA), followed after 3 h by harvest and Western blot analysis for β-catenin (BD Transduction Laboratories, San Diego). (D) Time course of Dkk1 expression in the circulation. Adult C57BL/6 mice received single i.v. tail vein injection of Ad Dkk1 (109 pfu) followed by phlebotomy at the indicated times. Dkk1 was detected by Western blotting using anti-His probe, Ab (Santa Cruz Biotechnology), and migrated as a doublet of 38–34 kDa. (E) Survival analysis of C57BL/6 mice after i.v. administration of 109 pfu Ad Dkk1 (IgK signal/3′ FLAG/His), Ad Dkk1-HA (IgK signal/5′HA/3′ FLAG/His), or Ad Fc treatment. Ad Fc or Ad Dkk1 doses =< 3 × 108 pfu have not exhibited any mortality over a 120-day time course.
Single i.v. administration of Ad Dkk1 (109 pfu) to adult C57BL/6 mice produced progressive weight loss and frequent melena or hematochezia with >85% mortality by 10 days (Fig. 1E). An identical phenotype was observed with an independent Ad expressing an N-terminal hemagglutinin (HA)-tagged Dkk1 (Ad Dkk1-HA) (Fig. 1E). In contrast, significant weight loss, gastrointestinal bleeding, or mortality were not observed with control Ads expressing either an Ig IgG2α Fc fragment (Ad Fc) (Fig. 1E), the non-Wnt inhibitor Dkk3 (21), or the soluble VEGF receptor, Flk1-Fc (18), at levels comparable to, or exceeding that of, Ad Dkk1 (data not shown). Ad Dkk1 doses of 3 × 108 pfu or lower produced progressively less precipitous weight loss and were not associated with either hematochezia, melena, or mortality over a 120-day time course (data not shown).
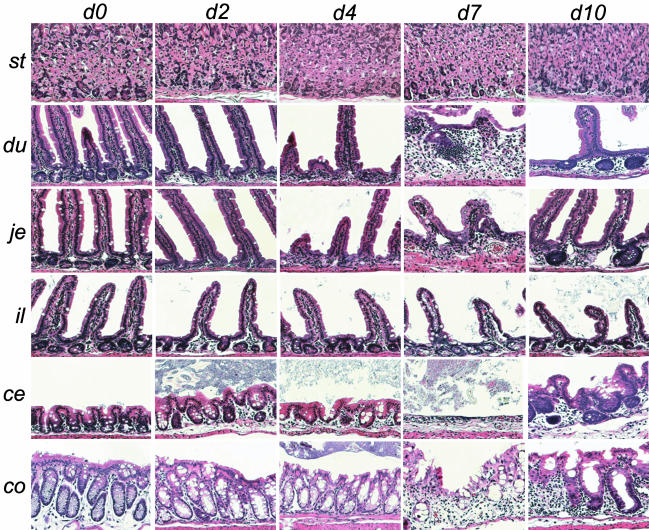
The ease of preparation of Ad combined with the convenience of single-injection dosing facilitated examination of synchronized cohorts of Ad Dkk1-treated animals (109 pfu) over defined intervals of a 10-day time course. Mucosal architecture in duodenum and proximal jejunum was severely distorted with rapid and near-total loss of crypts and decreased villus density by days 2 and 4, without inflammation or crypt necrosis (Fig. 2). In remnant crypts, Paneth cells predominated, and, by day 7, crypt loss was followed by villus blunting and fusion, loss of mucosal integrity, and frank ulceration and mucosal hemorrhage with mixed inflammatory infiltrate in the lamina propria. The small intestine exhibited a proximal-distal gradient of histologic effects with most severe phenotypes observed in duodenum and proximal jejunum, with the distal jejunum and ileum manifesting only mild crypt loss and villus blunting (Fig. 2).
Fig. 2.
Time course of histological changes in the gastrointestinal tract of Ad Dkk1-treated animals. Adult C57BL/6J mice (12–16 weeks old) received single i.v. tail vein injection of 109 pfu of either Ad Dkk1 or the negative control virus Ad Fc (109 pfu) followed by analysis of organs by hematoxylin/eosin staining at the indicated times. In duodenum and jejunum, Dkk1 induced crypt loss, villus blunting and fusion, and loss of mucosal integrity, followed by mucosal regeneration by day 10. The stomach was relatively unaffected. In cecum and colon, Dkk1 induced crypt loss with profound mucosal degeneration and ulceration by day 7 and regeneration by day 10 evidenced by irregular basophilic crypts at day 10. Stomach (st), duodenum (du), proximal jejunum (je), ileum (il), cecum (ce), and colon (co) are shown.
In the colon and cecum of C57BL/6J mice, only mild glandular thinning and/or crypt loss was observed at days 2 and 4, which was in contrast to striking crypt loss and villus blunting in the small intestine (Fig. 2). However, by day 7, the cecal and colonic epithelium exhibited multifocal mucosal degeneration and ulceration of a severity exceeding that of the small intestine, with the descending colon more severely affected than the ascending colon (Fig. 2). The spectrum of colonic lesions ranged from noninvolved foci particularly in ascending colon, to mild glandular thinning, focal ulceration, and extensive areas with complete effacement of architecture and replacement with mixed inflammatory infiltrates (Fig. 3). Ad Dkk1 treatment of CB17 SCID mice lacking B and T lymphocytes resulted in an identical spectrum of colon architectural lesions as in C57BL/6J mice, suggesting that the observed colitis in C57BL/6J mice was not inflammatory or autoimmune in nature. However, the ascending colon was more severely affected in SCID than C57 with more extensive and ulcerated lesions (Fig. 3), which was potentially consistent with higher level and more persistent adenoviral gene expression in immunocompromised SCID mice. Similarly, rectums of Ad Dkk1-treated SCID mice exhibited frequent ulceration as opposed to mild glandular thinning in C57BL/6J mice (data not shown). In contrast to the profound changes in small intestine and colon, the stomach of both strains exhibited only moderate glandular thinning at late time points that could not be distinguished from gastric atrophy secondary to inappetance (Fig. 2). Ad Dkk1 small intestine phenotypes were identical in both C57BL/6J and SCID mice, with severe involvement of duodenum and jejunum and notable absence of pathology in ileum. A summary table of gastrointestinal phenotypes in C57BL/6J and SCID mice is presented in Table 1, which is published as supporting information on the PNAS web site.
Fig. 3.
Spectrum of colonic lesions in Ad Fc- or Ad Dkk1-treated C57BL/6J (Upper) and SCID (Lower) mice. Colons were harvested for hematoxylin/eosin staining at day 7 after administration of 109 pfu of the appropriate Ads to 12- to 16-week-old mice. Moderate thinning of the ascending colon in C57BL/6J versus frequent ulceration in SCID is depicted. A spectrum of lesions was observed in descending colons of both strains, ranging from focal ulceration to frank effacement of architecture and replacement with inflammatory infiltrates, the latter being more severe in SCID animals.
Animals treated with lower doses of Ad Dkk1 (3 × 108 pfu) exhibited ≈80% lower plasma levels and displayed a less severe intestinal phenotype relative to high-dose (109 pfu) animals, illustrating dose dependency of Ad Dkk1. In these lower-dose animals, decreased small intestine crypt density with overall intact mucosal architecture was observed at day 4 in duodenum but not jejunum and ileum (data not shown). In cecum and colon of low-dose animals, ulceration, edema, and inflammation were less severe than with high dose, and these animals did not exhibit mortality over a 120-day time course (data not shown).
In both small and large intestine, decreased adenoviral transgene expression at day 10 (Fig. 1D) was accompanied by epithelial regeneration, which was consistent with a reversible effect. By day 10, duodenum and jejunum exhibited small numbers of regenerative basophilic, hyperplastic crypts, with more advanced reconstitution of villus structure in jejunum than duodenum (Fig. 2). In day 10 colon, hyperplastic regenerative crypts coexisted with persistent multifocal mucosal ulceration (Figs. 2 and 3). Despite this regenerative response, frequent mortality was observed with high doses of Ad Dkk1 (109 pfu) at days 8–10, which was likely secondary to colitis and systemic infection, with elevated WBC counts (>20 × 103/μl) and a left-shifted differential commonly noted in premorbid mice. Examination of adherens junctions in nonulcerated areas by electron microscopy and by immunofluorescence did not reveal significant alterations, whereas histologic examination of other solid organs including liver revealed them to be unaffected in a Dkk1-specific fashion, except for thymic atrophy, which could not be distinguished from systemic illness (data not shown).
Confirming functional blockade of canonical Wnt signaling by Dkk1, the β-catenin/TCF target gene, CD44 (5), was strongly and rapidly repressed within 2 days in duodenum and jejunum, with only nonepithelial lamina propria staining remaining (Fig. 4). Ad Dkk1 also potently repressed CD44 expression in ileum, despite the lack of gross architectural changes (Fig. 2). Epithelial CD44 expression was markedly reduced by Dkk1 in cecum and distal colon and partially reduced in proximal colon but was unaffected in stomach. Dkk1 also repressed the β-catenin/TCF target gene, EphB2 (6), in duodenum, jejunum, ileum, cecum, and descending colon, with mild repression in ascending colon, and little to no repression in stomach (Fig. 4). In contrast, the magnitude or location of expression of epithelial differentiation markers for absorptive enterocytes or secretory lineages was not altered by Dkk1 expression (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 4.
Expression analysis of Wnt/β-catenin target genes, CD44 and EphB2, in the gastrointestinal tract of Ad Dkk1- or Ad Fc-treated adult C57BL/6 mice (12–16 weeks old). Organs were harvested 2 days after Ad Dkk1 i.v injection (109 pfu). (Left) Ad Dkk1 repression of CD44 expression in proliferative zones of all levels of the gastrointestinal epithelium. Arrowheads indicate the absence of CD44 immunoreactivity in proliferative compartments of the intestinal epithelium in Ad Dkk1 animals. *, residual CD44 staining in nonepithelial lamina propria. (Right) Ad Dkk1 repression of EphB2 in small intestine and colon. Repression was weaker in ascending colon and no repression was observed in stomach. EphB2 immunofluorescence was performed with Alexa 488 detection of EphB2 immunoreactivity (green) and Hoechst 33342 nuclear counterstain (blue). Stomach (st), duodenum (du), jejunum (je), ileum (il), cecum (ce), ascending colon (ac), and descending colon (dc) are shown.
The proliferative status of the gastrointestinal epithelium in Ad Dkk1 mice was examined by immunohistochemistry for the S-phase marker, Ki67. Ad Dkk1 strikingly repressed enterocyte Ki67 immunoreactivity (>90%) within 2–4 days in duodenum and proximal jejunum, with any remnant crypts exhibiting diminished Ki67 staining and residual expression largely confined to nonepithelial cells of the lamina propria (Fig. 5). Proliferation in jejunum, along the proximal-distal axis, was progressively less affected by Ad Dkk1 (data not shown) to the extent that Ki67 staining in the ileum was not significantly inhibited by Ad Dkk1 (Fig. 5), despite effective repression of CD44 and EphB2 expression (Fig. 4). Epithelial Ki67 staining was also substantially reduced (70–80%) in cecum and descending colon, moderately reduced in ascending colon (≈60%), and not significantly affected in stomach. (Fig. 5). In contrast, TUNEL staining did not reveal increased apoptosis in either the proliferative crypts or differentiated villi/glands of the stomach, small intestine or colon (Fig. 7, which is published as supporting information on the PNAS web site.). In total, these data indicated that Dkk1 elicited stringent in vivo blockade of canonical Wnt signaling in both small intestine and colon, with repression of both Wnt target gene expression and epithelial proliferation in parallel.
Fig. 5.
Analysis of proliferative state in gastrointestinal epithelium in Ad Dkk1-treated C57BL/6J mice by Ki67 immunohistochemistry. Arrowheads indicate the absence of Ki67 immunoreactivity in proliferative compartments 2 days after i.v. injection of Ad Dkk1 or Ad Fc (109 pfu). Note the strong repression of Ki67 immunoreactivity in duodenum, jejunum, cecum, and descending colon and moderate reduction in ascending colon. Ileum and stomach were not significantly affected.
Discussion
The definition of functions of the Wnt family in adult physiology has been impeded by significant functional redundancy, with ≈20 Wnt proteins and 10 Frizzled receptors, as well as the necessity for conditional inactivation strategies (22). To overcome these obstacles, we have achieved stringent, fully conditional and reversible inactivation of Wnt signaling in adult mice by adenoviral expression of the soluble Wnt inhibitor Dkk1, which may function as a pan-inhibitor of canonical Wnt signaling through interactions with the Wnt coreceptors, LRP5/6 (14–16). The extensive Ad Dkk1 repression of proliferation and of β-catenin/TCF target genes, as well as the progressive loss of villi and glands in small intestine, cecum, and colon to the point of mucosal ulceration, implicates the Wnt receptor complex and canonical Wnt signaling in maintenance of gene expression and architecture throughout the intestinal epithelium, which is consistent with, but much more extensive than, the mild reduction of villus number in Tcf4-/- mouse small intestine (3, 5). The additional colon and cecum phenotypes observed in Dkk1 mice could result from either Dkk1 membrane-proximal interference with Wnt signaling versus membrane-distal effects in Tcf-4-/- animals, or from Tcf-3/Tcf-4 redundancy (23, 24). Analogous mechanistic redundancy with non-Wnt- or non-Dkk1-sensitive pathways may underlie the observed proximal-distal phenotypic gradient in Ad Dkk1 small intestine (Figs. 2 and 3), as well as the Dkk1 inhibition of Wnt target gene expression but not proliferation in ileum (Figs. 4 and 5). We cannot exclude regional differences in intestinal phenotypes resulting from local heterogeneity in Dkk1 expression levels, despite circulating Dkk1 in plasma. Given the direct action of Dkk1 on the LRP/frizzled receptor complex (14–16), as opposed to the membrane-distal action of Tcf-4, the current data strongly implicate Dkk1-sensitive Wnt signaling as essential for maintenance of both proliferation and architecture of the intestinal epithelium in adult animals.
During the preparation of this article, a related report by Clevers and coworkers (19) described transgenic Dkk1 overexpression regulated by the intestine-specific villin promoter. In these studies, proliferation in small intestine crypts was strongly attenuated with villus blunting, indicating a role for canonical Wnt signaling in maintenance of adult small intestine epithelial proliferation. The current data, using a distinct, fully conditional adenoviral approach, are fully consistent with those studies but suggest a much broader physiologic role for Wnt signaling in the adult gastrointestinal tract that is not restricted to the small bowel, but is a general property of the intestinal glandular epithelium, whether in small intestine or colon. Colon phenotypes in villin-Dkk1 mice were not commented on, but, because the villin promoter has been reported to be active in colon (25), the absence of such phenotypes could result from low-level Dkk1 expression in the transgenic animals. Indeed, heterozygous villin-Dkk1 mice did not display small intestine pathology and crossing of these animals to generate homozygous transgenics, with concomitant increase of gene expression, was required to obtain small intestine phenotypes (19). We have observed similar dose-dependency of the small intestinal phenotype with Ad Dkk1, although, with the lowest doses (3 × 108 pfu), which cause attenuation of duodenal crypts, colon pathology is still evident. Alternatively, the constitutive expression of Dkk1 from embryogenesis in villin-Dkk1 mice could elicit compensatory protective mechanisms in colon, a scenario not applicable to Ad Dkk1, which elicits conditional and transient overexpression in fully adult mice. Such differences between the two experimental systems could also underlie our inability to detect defects in enteroendocrine cell lineages or in goblet cell positioning (Fig. 7), which may be developmentally specified and thus not altered by transient Dkk1 expression in adult animals, or which may have longer turnover times than absorptive enterocytes.
The finding of Wnt-dependent proliferation in the colon raises several therapeutic and pathophysiologic correlates. The ability of Ad Dkk1 to produce colitis suggests its potential utility as a novel inducible animal model of inflammatory bowel disease (IBD). The current data, in which Wnt blockade results in inhibition of colonic epithelial proliferation and progressive architectural degeneration, indicate that Wnt proteins represent essential growth factors for proliferative compartments of the large intestine. In this regard, it should be interesting to assess the therapeutic potential of Wnt proteins to encourage mucosal regeneration during IBD, perhaps as an adjunctive therapy to antiinflammatory or immunosuppressive therapy, by analogy to recently described growth factor-based IBD therapy by using epidermal growth factor (26). Such attempts should be undertaken with caution, because it is further tempting to speculate that the normal physiologic dependence of colon proliferation on Wnt signaling, described herein, may underlie the efficiency with which mutations in Wnt pathway components such as adenomatous polyposis coli or β-catenin can predispose to colonic neoplasia (27).
Whereas Dkk1 represses Wnt target genes such as CD44 and EphB2 throughout the gastrointestinal epithelium, the onset of architectural lesions (small intestine > large intestine >> stomach) correlates well with cell-cycle time and epithelial turnover rates (28–30), which is consistent with crypt mitosis in Dkk1 animals being insufficient to compensate for rapid epithelial turnover. Our results do not rule out Dkk1 overexpression phenotypes in nongastrointestinal organ systems; however, the rapid lethality in high-dose Ad Dkk1-treated animals compromises the ability to detect changes in more slowly proliferating nongastrointestinal stem cell compartments. In this regard, the investigation of nongastrointestinal phenotypes in lower dose Ad Dkk1 animals, adenoviral expression of other classes of soluble Wnt inhibitors, or the use of regeneration models, may prove informative and reinforce the use of soluble Wnt antagonists as a general strategy for conditional Wnt inhibition in adult animals.
The current data reveal a striking reliance on a single signaling pathway for the maintenance of both the proliferation and architecture of an adult organ and indicate that a strict dependence on canonical Wnt signaling is a general property of intestinal epithelium, whether in small intestine or colon. The present findings, along with that of Pinto et al. (19), suggest that Wnt proteins represent primary candidates for the long-sought growth factor(s) responsible for maintenance of the adult intestinal epithelial stem cell niche (1), by analogy to the recent description of Wnt3a as a potent renewal factor for hematopoietic stem cells (31, 32). The stringent requirement for Dkk1-sensitive Wnt signaling demonstrated herein recalls the requirement of hematopoietic precursors for growth factors such as erythropoietin and G-CSF and suggests the potential utility of therapeutic manipulation of Wnt pathways, agonistic or antagonistic, for disorders of the intestinal epithelium.
Supplementary Material
Acknowledgments
We thank W. J. Nelson, J. Steiger, B. Tam, C. Chartier, A. Lowe, and C. Cartwright for helpful comments; A. Mikels and C. Logan for advice with in vitro Wnt assays; and K. Knox and J. Kao for assistance with Ad procedures. Animal procedures were carried out in accordance with Stanford University guidelines. R.N. is an Investigator of the Howard Hughes Medical Institute. C.J.K. is a Burroughs Wellcome Foundation New Investigator in the Pharmacological Sciences and a Kimmel Foundation Scholar in Translational Science. This work was supported by a National Institutes of Health Digestive Diseases Center Pilot Grant (to Stanford University and C.J.K.) and by National Institutes of Health Grant 5 R01 CA95654-01 (to C.J.K.).
Abbreviations: Dkk1, Dickkopf-1; Ad, adenovirus; pfu, plaque-forming unit; SCID, severe combined immunodeficient.
References
- 1.Marshman, E., Booth, C. & Potten, C. S. (2002) BioEssays 24, 91-98. [DOI] [PubMed] [Google Scholar]
- 2.Booth, C. & Potten, C. S. (2000) J. Clin. Invest. 105, 1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korinek, V., Barker, N., Moerer, P., van Donselaar, E., Huls, G., Peters, P. J. & Clevers, H. (1998) Nat. Genet. 19, 379-383. [DOI] [PubMed] [Google Scholar]
- 4.Wong, M. H., Rubinfeld, B. & Gordon, J. I. (1998) J. Cell Biol. 141, 765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Wetering, M., Sancho, E., Verweij, C., de Lau, W., Oving, I., Hurlstone, A., van der Horn, K., Batlle, E., Coudreuse, D., Haramis, A. P., et al. (2002) Cell 111, 241-250. [DOI] [PubMed] [Google Scholar]
- 6.Batlle, E., Henderson, J. T., Beghtel, H., van den Born, M. M., Sancho, E., Huls, G., Meeldijk, J., Robertson, J., van de Wetering, M., Pawson, T. & Clevers, H. (2002) Cell 111, 251-263. [DOI] [PubMed] [Google Scholar]
- 7.Wong, M. H., Huelsken, J., Birchmeier, W. & Gordon, J. I. (2002) J. Biol. Chem. 277, 15843-15850. [DOI] [PubMed] [Google Scholar]
- 8.Wielenga, V. J., Smits, R., Korinek, V., Smit, L., Kielman, M., Fodde, R., Clevers, H. & Pals, S. T. (1999) Am. J. Pathol. 154, 515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino, H. & Regan, J. W. (2001) J. Biol. Chem. 276, 12489-12492. [DOI] [PubMed] [Google Scholar]
- 10.Fujino, H., West, K. A. & Regan, J. W. (2002) J. Biol. Chem. 277, 2614-2619. [DOI] [PubMed] [Google Scholar]
- 11.Persad, S., Troussard, A. A., McPhee, T. R., Mulholland, D. J. & Dedhar, S. (2001) J. Cell Biol. 153, 1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glinka, A., Wu, W., Delius, H., Monaghan, A. P., Blumenstock, C. & Niehrs, C. (1998) Nature 391, 357-362. [DOI] [PubMed] [Google Scholar]
- 13.Fedi, P., Bafico, A., Nieto Soria, A., Burgess, W. H., Miki, T., Bottaro, D. P., Kraus, M. H. & Aaronson, S. A. (1999) J. Biol. Chem. 274, 19465-19472. [DOI] [PubMed] [Google Scholar]
- 14.Bafico, A., Liu, G., Yaniv, A., Gazit, A. & Aaronson, S. A. (2001) Nat. Cell Biol. 3, 683-686. [DOI] [PubMed] [Google Scholar]
- 15.Mao, B., Wu, W., Li, Y., Hoppe, D., Stannek, P., Glinka, A. & Niehrs, C. (2001) Nature 411, 321-325. [DOI] [PubMed] [Google Scholar]
- 16.Semenov, M. V., Tamai, K., Brott, B. K., Kuhl, M., Sokol, S. & He, X. (2001) Curr. Biol. 11, 951-961. [DOI] [PubMed] [Google Scholar]
- 17.Mao, B., Wu, W., Davidson, G., Marhold, J., Li, M., Mechler, B. M., Delius, H., Hoppe, D., Stannek, P., Walter, C., et al. (2002) Nature 417, 664-667. [DOI] [PubMed] [Google Scholar]
- 18.Kuo, C. J., Farnebo, F., Yu, E. Y., Christofferson, R., Swearingen, R. A., Carter, R., von Recum, H. A., Yuan, J., Kamihara, J., Flynn, E., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4605-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. (2003) Genes Dev. 17, 1709-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chartier, C., Degryse, E., Gantzer, M., Dieterle, A., Pavirani, A. & Mehtali, M. (1996) J. Virol. 70, 4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao, B. & Niehrs, C. (2003) Gene 302, 179-183. [DOI] [PubMed] [Google Scholar]
- 22.Cadigan, K. M. & Nusse, R. (1997) Genes Dev. 11, 3286-3305. [DOI] [PubMed] [Google Scholar]
- 23.Korinek, V., Barker, N., Willert, K., Molenaar, M., Roose, J., Wagenaar, G., Markman, M., Lamers, W., Destree, O. & Clevers, H. (1998) Mol. Cell. Biol. 18, 1248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker, N., Huls, G., Korinek, V. & Clevers, H. (1999) Am. J. Pathol. 154, 29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto, D., Robine, S., Jaisser, F., El Marjou, F. E. & Louvard, D. (1999) J. Biol. Chem. 274, 6476-6482. [DOI] [PubMed] [Google Scholar]
- 26.Sinha, A., Nightingale, J., West, K. P., Berlanga-Acosta, J. & Playford, R. J. (2003) N. Engl. J. Med. 349, 350-357. [DOI] [PubMed] [Google Scholar]
- 27.Bienz, M. & Clevers, H. (2000) Cell 103, 311-320. [DOI] [PubMed] [Google Scholar]
- 28.Potten, C. S. & Hendry, J. H. (1995) in Structure, Function, and Proliferative Organization of the Mammalian Gut: Radiation and Gut, eds. Potten, C. S. & Hendry, J. H. (Elsevier, Amsterdam), pp. 1-31.
- 29.Karam, S. M. (1999) Front. Biosci. 4, D286-D298. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal, N. G. & Bal, H. S. (1989) Lab. Anim. 23, 21-29. [DOI] [PubMed] [Google Scholar]
- 31.Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R., III, & Nusse, R. (2003) Nature 423, 448-452. [DOI] [PubMed] [Google Scholar]
- 32.Reya, T., Duncan, A. W., Ailles, L., Domen, J., Scherer, D. C., Willert, K., Hintz, L., Nusse, R. & Weissman, I. L. (2003) Nature 423, 409-414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.