Abstract
Fluorodeoxyglucose positron emission tomography (PET) studies have found that patients with Alzheimer's dementia (AD) have abnormally low rates of cerebral glucose metabolism in posterior cingulate, parietal, temporal, and prefrontal cortex. We previously found that cognitively normal, late-middle-aged carriers of the apolipoprotein E ε4 allele, a common susceptibility gene for late-onset Alzheimer's dementia, have abnormally low rates of glucose metabolism in the same brain regions as patients with probable AD. We now consider whether ε4 carriers have these regional brain abnormalities as relatively young adults. Apolipoprotein E genotypes were established in normal volunteers 20–39 years of age. Clinical ratings, neuropsychological tests, magnetic resonance imaging, and PET were performed in 12 ε4 heterozygotes, all with the ε3/ε4 genotype, and 15 noncarriers of the ε4 allele, 12 of whom were individually matched for sex, age, and educational level. An automated algorithm was used to generate an aggregate surface-projection map that compared regional PET measurements in the two groups. The young adult ε4 carriers and noncarriers did not differ significantly in their sex, age, educational level, clinical ratings, or neuropsychological test scores. Like previously studied patients with probable AD and late-middle-aged ε4 carriers, the young ε4 carriers had abnormally low rates of glucose metabolism bilaterally in the posterior cingulate, parietal, temporal, and prefrontal cortex. Carriers of a common Alzheimer's susceptibility gene have functional brain abnormalities in young adulthood, several decades before the possible onset of dementia.
Keywords: apolipoprotein E, positron emission tomography, glucose metabolism, brain mapping, surrogate markers
Alzheimer's dementia (AD) afflicts ≈10% of those over the age of 65 and almost half of those over the age of 85 (1). To develop and test effective primary prevention therapies, it would be helpful to characterize brain changes associated with the susceptibility to AD as early as possible before the onset of cognitive impairment (2).
Fluorodeoxyglucose positron emission tomography (PET) studies have found that patients with AD have abnormally low cerebral metabolic rates for glucose (CMRgl) in posterior cingulate, parietal, temporal, and prefrontal cortex and a progressive decline in these rates over time (3–8). We (2, 9, 10) and others (11, 12) have been using PET to detect and track these functional brain abnormalities before the onset of dementia in carriers of the apolipoprotein E (APOE) ε4 allele, a common Alzheimer's susceptibility gene associated with up to half of cases of late-onset AD (13–15). Previously, we found that cognitively normal 50- to 65-year-old ε4 carriers have abnormally low CMRgl in each of the same regions as patients with probable AD and abnormal rates of regional CMRgl decline over time (2, 9, 10). We now consider whether ε4 carriers have these regional brain abnormalities as relatively young adults, several decades before the possible onset of dementia. Our analysis of ε4 carriers was restricted to those with the ε3/ε4 genotype, found in ≈20–23% of Caucasian populations (16) and ≈11–36% of different ethnic groups (17).
Methods
Subjects. We used newspaper advertisements to recruit 135 20- to 39-year-old normal volunteers, who agreed that they would not be given information about their APOE genotype, provided their informed consent, and were studied under guidelines approved by human subjects committees at Banner Good Samaritan Medical Center and the Mayo Clinic (Rochester, MN). Venous blood samples were drawn, and APOE genotypes were characterized with analysis involving restriction fragment length polymorphisms (18).
Twelve ε4 heterozygotes and 15 control subjects without the ε4 allele satisfied our eligibility criteria and participated in our brain imaging studies. Thirteen of the control subjects had the ε3/ε3 genotype, and 2 had the ε2/ε3 genotype; 12 were individually matched to the ε4 carriers for sex, age (within 3 years), and educational level (within 2 years), and 3 were matched to the ε4 carriers for their mean age and educational level. Subjects denied an impairment in memory or other cognitive skills, did not satisfy criteria for a current psychiatric disorder, did not use centrally acting medications for at least 2 weeks before their PET session, and had a normal neurological examination. One subject in each group reported a history of hypertension. Investigators who were unaware of the subjects' APOE genotypes obtained data from medical and family histories, a neurologic examination, and a structured psychiatric interview (19). The subjects completed the Folstein modified Mini-Mental State Examination (20) and the Hamilton Depression Rating Scale (21), and all but one control subject completed a battery of neuropsychological tests (22).
Brain Imaging. Volumetric T1-weighted magnetic resonance imaging and fluorodeoxyglucose PET were performed as described (2, 9, 23). PET was performed with the 951/31 ECAT scanner (Siemens, Knoxville, TN), a transmission scan, the i.v. injection of 10 mCi of 18F-fluorodeoxyglucose, and a 60-min dynamic sequence of emission scans as the subjects, who had fasted for at least 4 h, lay quietly in a darkened room with their eyes closed and directed forward. For whole brain measurements, CMRgl (mg/min per 100 g) was calculated by using the PET images, an image-derived radiotracer input function, plasma glucose levels, and a graphic method (24). Regional analyses were performed by using the PET images (in counts) acquired during the last 30 min.
To test the hypothesis that young adult ε4 carriers have abnormally low CMRgl in the same regions as patients with AD, a fully automated algorithm, previously used in the study of patients with probable AD (6) and late-middle-aged ε4 carriers (9), was used to compare PET images in the two subject groups. Each subject's PET image was deformed according to the coordinates of a standard atlas of the brain (25). Measurements in each voxel were normalized to that in the pons, which has been reported to be the region least affected in patients with probable AD (26). Data on the outer and medial surface of each hemisphere were extracted. A 3D stereotactic surface projection t-score map of metabolic differences between groups was calculated and superimposed on a map of metabolic reductions in previously studied patients with probable AD (mean age 64) and a spatially standardized, volume-rendered MRI (Fig. 1) (6, 9). Significance levels of P < 0.001, uncorrected for multiple comparisons, were initially used to test the hypothesis that the ε4 carriers had abnormally low rates of glucose metabolism in posterior cingulate, parietal, temporal, and prefrontal cortex; a Monte Carlo procedure, implemented and tested in the Positron Emission Tomography Center (27), was then used to correct maximal significance levels for multiple comparisons in each of the regions of interest previously associated with metabolic reductions in the patients with probable AD. Data were extracted from the locations associated with the most significant differences between the ε4 carriers and controls (see Table 3) to graphically compare the distribution of regional/pontine CMRgl in the two subject groups (Fig. 2). (Data from some of the most superior and inferior regions of the brain were not sampled in every subject because of limitations in the PET system's field of view.)
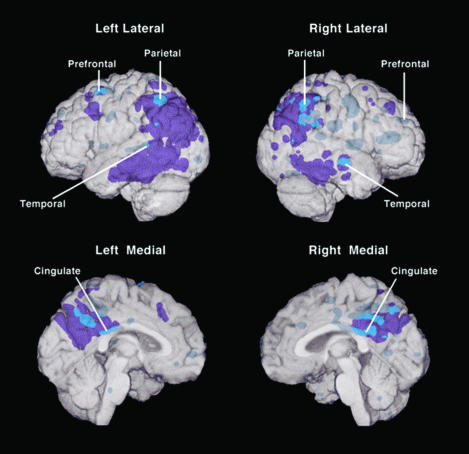
Fig. 1.
Regions of the brain with abnormally low CMRgl in young adult carriers of the APOE ε4 allele and their relation to brain regions with abnormally low CMRgl in patients with probable AD. In this analysis, a 3D surface-projection map of abnormally low CMRgl in the young adult ε4 carriers was superimposed on a map of abnormally low CMRgl in previously studied patients with the probable AD and a spatially standardized and volume-rendered MRI of the brain (P < 0.005, uncorrected for multiple comparisons) (6, 9). The purple areas are regions in which CMRgl was abnormally low only in the patients with AD, the bright blue areas are regions in which CMRgl was abnormally low in both the young adult ε4 carriers and patients with probable AD, and the muted blue areas are regions in which CMRgl was abnormally low only in the ε4 carriers. (Lines point to the locations of the ε4 carriers' most significant CMRgl reductions and correspond to the brain atlas coordinates in Table 3.) Like patients with AD, the young adult ε4 carriers had abnormally low CMRgl bilaterally in the posterior cingulate, parietal, temporal, and prefrontal cortex.
Table 3. Location and magnitude of most significant reductions CMRgI.
| Atlas coordinates,* mm
|
||||||
|---|---|---|---|---|---|---|
| Brain region | X | Y | Z | Percent reduction | T value† | Corrected P value‡ |
| Posterior cingulate | 2 | —32 | 25 | 10.5 | 3.77 | 0.0080 |
| Right parietal | 52 | —53 | 47 | 9.8 | 3.41 | 0.048 |
| Left parietal | —45 | —50 | 52 | 10.0 | 3.63 | 0.049 |
| Right temporal | 65 | —14 | —7 | 8.6 | 3.48 | 0.033 |
| Left temporal | —63 | —37 | 7 | 8.0 | 3.05 | 0.13 |
| Right prefrontal | 45 | 42 | 29 | 8.9 | 3.12 | 0.010 |
| Left prefrontal | —34 | 11 | 59 | 9.8 | 3.30 | 0.015 |
The reductions were identified by an automated search of posterior cingulate, parietal, temporal, and prefrontal cortex, regions previously found to be affected in patients with AD.
The coordinates were obtained from Talairach and Tournoux (25). x is the distance in mm to the right (+) or left (—) of the midline, y is the distance anterior (+) or posterior (—) to the anterior commissure, and z is the distance superior (+) or inferior (—) to a horizontal plane through the anterior and posterior commissures
P < 0.001 before correction for multiple comparisons
Significance levels were corrected for multiple comparisons in each of the regions of interest previously associated with CMRgI reductions in the patients with probable AD. Details of the correction procedure, which included 20,000 Monte Carlo simulations, are available on request
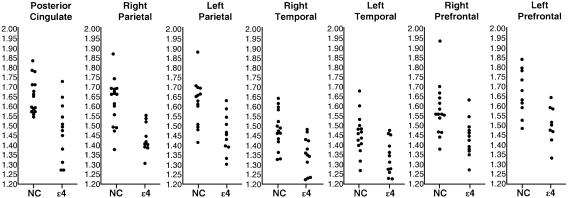
Fig. 2.
Regional/pontine CMRgl in young adult APOE ε4 carriers and controls. Measurements were normalized to that in the pons, which appears to be the region least affected in patients with the probable AD (25), and extracted from the locations specified in Table 3, which were associated with the most significant differences between the ε4 carriers (ε4) and controls who do not carry the ε4 allele (NC) (P < 0.001, uncorrected for multiple comparisons).
Results
Demographic characteristics and clinical ratings of the ε4 carriers and control subjects are shown in Table 1. Their neuropsychological scores are shown in Table 2. There were no significant differences in demographic characteristics or scores on the Hamilton Depression Rating Scale, modified Mini-Mental State Examination, or neuropsychological tests. There were no significant differences between the ε4 carriers and control subjects in their whole brain CMRgl (mean ± SD, 6.4 ± 0.8 vs. 6.5 ± 0.8 mg/min per 100 g; P = 0.82 by two-tailed, unpaired t test) or pons (5.9 ± 0.7 vs. 5.8 ± 0.6 mg/min per 100 g; P = 0.64).
Table 1. Characteristics of the young adult ε4 carriers and control subjects.
| Characteristic | Carriers, n = 12 | Controls, n = 15 | P value |
|---|---|---|---|
| Age, yr | 30.7 ± 5.4 | 31.2 ± 5.0 | 0.77* |
| Sex, female/male | 9/3 | 12/3 | 0.28† |
| Handedness right, yes/no | 12/0 | 14/1 | >0.99† |
| Years of education | 16.0 ± 1.7 | 16.1 ± 1.5 | 0.95* |
| Reported family history | |||
| Any first-degree relative, yes/no | 5/7 | 1/14 | 0.09† |
| Any first- or second-degree relative, yes/no | 6/6 | 5/10 | 0.63† |
| Score on Hamilton Depression Scale | 1.0 ± 1.9 | 1.1 ± 1.8 | 0.92* |
| Score on Mini-Mental State Examination | 29.9 ± 0.3 | 29.9 ± 0.3 | 0.88* |
Plus-minus values are means ± SD.
The P value was calculated with an unpaired two-tailed t test
The P value was calculated with Pearson χ2 two-tailed test, with continuity correction for n < 5
Table 2. Neuropsychological scores in young ε4 heterozygotes and control subjects.
| ε4 Carriers, n = 12
|
Controls, n = 14
|
||
|---|---|---|---|
| Test | Mean (±SD) score | P value | |
| AVLT | |||
| Total learning | 51.9 ± 6.7 | 52.6 ± 8.5 | 0.83 |
| Short-term recall | 11.2 ± 2.6 | 11.8 ± 2.5 | 0.54 |
| Long-term recall | 10.6 ± 3.1 | 11.7 ± 2.2 | 0.29 |
| Complex figure test | |||
| Copy | 35.6 ± 1.0 | 35.4 ± 0.9 | 0.67 |
| Recall | 20.3 ± 5.6 | 21.0 ± 7.6 | 0.77 |
| Boston naming test | 54.4 ± 3.2 | 56.1 ± 2.7 | 0.15 |
| WAIS-R | |||
| Information | 11.6 ± 1.5 | 11.0 ± 2.4 | 0.48 |
| Digit span | 10.8 ± 1.5 | 10.9 ± 3.3 | 0.93 |
| Block design | 11.3 ± 3.0 | 12.1 ± 2.5 | 0.46 |
| Mental arithmetic | 11.6 ± 2.2 | 11.2 ± 2.5 | 0.69 |
| Similarities | 11.6 ± 2.1 | 11.5 ± 2.1 | 0.92 |
| Controlled oral word association test | 43.3 ± 8.7 | 44.4 ± 10.0 | 0.79 |
| WMS-R Orientation Subtest | 13.9 ± 0.3 | 13.9 ± 0.3 | 0.91 |
AVLT, Auditory Verbal Learning Test; WAIS-R, Wechsler Adult Intelligence Scale-Revised; WMS-R, Wechsler Memory Scale-Revised. The P value was calculated with unpaired two-tailed t tests, uncorrected for multiple comparisons.
Like previously studied patients with probable AD (6, 8) and our previously studied 50- to 65-year-old cognitively normal ε4 homozygotes (9) and heterozygotes (10), the group of young adult ε4 carriers had abnormally low CMRgl in the posterior cingulate, parietal, temporal, and prefrontal cortex (Fig. 1 and Table 3). With the exception of the right temporal cortex, these abnormalities remained significant after correction for multiple comparisons (Table 3). Whereas ε4 carriers may have lower CMRgl in other cortical regions (P < 0.001, uncorrected for multiple comparisons, Fig. 1), these additional metabolic reductions need to be confirmed in an independent comparison. For each of the locations specified in Table 3, the mean CMRgl was 8.0–10.5% lower in the ε4 carriers than in the controls. These reductions were smaller than those previously reported in patients with probable AD (6), and there was considerable overlap between the ε4 carrier and noncarrier groups' individual measurements (Fig. 2). The ε4 carriers did not have abnormally high CMRgl, even at the more liberal significance level of P < 0.05, uncorrected for multiple comparisons.
In a post hoc analysis, the subjects' coregistered MRI and PET images, an automated brain mapping algorithm that permitted us to investigate the effects of partial volume averaging on PET measurements in each voxel (SPM99, Wellcome Department of Cognitive Neurology, University College, London) (28) and an algorithm intended to account for these effects (29) were used to generate and compare statistical brain maps of metabolic differences between the two subject groups with and without correction for individual differences in brain tissue volume. Because the pattern of metabolic differences between the two groups remained present after correction for partial volume averaging, and was not significantly different from the pattern identified without this correction, the functional brain abnormalities observed in the young adult ε4 carriers could not be solely attributed to alterations in the regional volume of brain tissue.
In another post hoc analysis, PET images from the 20- to 39-year-old subjects and previously studied 50- to 65-year-old subjects (10) were used to characterize and compare the effects of age on regional CMRgl in cognitively normal ε4 heterozygotes and controls. Overall, the ε4 carriers had significantly lower CMRgl than the controls in the posterior cingulate, parietal, temporal, and prefrontal cortex, and older age was significantly correlated with lower CMRgl in extensive areas of cerebral cortex, most prominently in the prefrontal and anterior cingulate cortex (P < 0.001, uncorrected for multiple comparisons). With possible exceptions in localized areas of the left prefrontal and posterior cingulate cortex (P < 0.005, uncorrected for multiple comparisons), the ε4 carriers did not have significantly steeper age-related CMRgl declines than those in the ε4 noncarriers. Thus, whereas 2-year follow-up studies have demonstrated abnormally high rates of regional CMRgl decline in older ε4 carriers before the onset of cognitive impairment (2, 12), this cross-sectional comparison did not demonstrate abnormally steep posterior cingulate, parietal, or temporal CMRgl declines in ε4 carriers between young adulthood (when the abnormalities are already present) and late middle age.
Discussion
We previously found that cognitively normal late-middle-aged carriers of the APOE ε4 allele, a common AD susceptibility gene, have functional brain abnormalities in the same brain regions as patients with probable AD. We now find that cognitively normal ε4 carriers have functional brain abnormalities as relatively young adults, several decades before the possible onset of dementia. These abnormalities were detected even though the young adult ε4 carriers were restricted to persons with one copy of this allele, who tend to have a lower risk of AD and a later age of onset of dementia than those with two copies of this allele ε4, even though at least half of ε4 heterozygotes are unlikely to develop AD, and even though the average age of onset of dementia in those ε4 heterozygotes who develop the disorder appears to be in the seventh decade of life, about four decades older than the average age of subjects in this study (13–15).
In separate studies, we have found abnormally low CMRgl bilaterally in the posterior cingulate, parietal, temporal, and prefrontal cortex in patients with AD (8), cognitively normal ε4 homozygotes and heterozygotes at 50–65 years of age (9, 10), and cognitively normal ε4 heterozygotes at 20–39 years of age at the time of their baseline scans. As noted (9, 10), the CMRgl abnormalities could reflect reductions in the activity or density of terminal neuronal fields that innervate the implicated regions (30), the activity of synaptic glial cells (31), an impairment in glucose metabolism unrelated to local neuronal activity (32, 33), or a combination of these factors. As in patients with probable AD (29), the CMRgl abnormalities in the young ε4 carriers could not be solely attributed to alterations in brain volume.
The CMRgl reductions in our young adult ε4 carriers may be the earliest brain abnormalities yet found in living persons at risk for late-onset AD. In postmortem studies, the characteristic histopathological features of AD include neuritic plaques, neurofibrillary tangles, and a loss of synapses. Whereas the type, density, and topographic distribution of this histopathology is likely to be extremely limited in normal young adults, postmortem studies have raised the possibility that the initial stage of AD histopathology [the selective involvement of neurofibrillary tangles in transentorhinal cortex (34, 35)] may be present several decades before the onset of dementia. In a study of young adults (mean age 38, range 22–46 years), this initial histopathological stage was observed in a higher frequency of persons with the ε3/ε4 genotype than in controls with no copies of the ε4 allele (36). Estimating the longitudinal progression of AD histopathology from cross-sectional data, a related study of persons 40–90 years of age suggested that this initial histopathological stage may precede AD by ≈50 years (37). MRI studies have found significantly smaller hippocampal volumes in patients with AD and mild cognitive impairment, correlations between reduced hippocampal volume and the severity of cognitive impairment, and progressive declines in hippocampal volume during the course of the illness (10, 23). Based on our study of late-middle-aged ε4 homozygotes and controls and a review of the MRI literature, we suggested that the reduction in posterior cingulate CMRgl is apparent before the onset of memory decline in persons at risk for AD and that hippocampal volumes begin to decline some time later, in conjunction with the onset of memory decline and shortly before the onset of dementia (23).
The causal connections, if any, between the CMRgl abnormalities and the histopathological features of AD remain to be clarified. For instance, it is possible that the CMRgl abnormalities are attributable to AD histopathology. In a PET study of baboons, neurotoxic lesions of entorhinal and perirhinal cortex were associated with reduced rates of glucose metabolism in posterior cingulate, parietal, and temporal cortex (38), raising the possibility that the PET abnormalities in these regions reflect a reduction in the activity or density of projections arising in the vicinity of entorhinal cortex, the location of earliest and most extensive AD-related histopathology (34, 39). Still, it remains to be shown that the CMRgl abnormalities could be attributed to the very limited histopathology that may or may not be present in the cognitively normal young adult ε4 carriers. Alternatively, it is possible that the CMRgl abnormalities found in young adult ε4 carriers increase vulnerability to AD histopathology. Because there may be some correspondence between the cortical association areas preferentially affected metabolically and histopathologically in AD (40, 41), our findings raise the possibility that functional alterations provide a foothold for the subsequent onset of neuropathology in brain regions that are preferentially vulnerable to this disorder. If so, it may be possible to identify neurobiological processes that are involved in the predisposition to AD and precede the onset of previously known neuropathology, providing particularly early targets for a prevention therapy.
The functional brain abnormalities observed in the young adult ε4 carriers could reflect a very early age-related decline in CMRgl or an abnormality in prenatal or early postnatal neurological development. In comparison with the other isoforms, the E4 isoform of APOE has been associated with higher cholesterol levels, increased aggregation of amyloid, less protection against amyloid-induced oxidative neurotoxicity, less efficient repair of neurons and synapses, less protection against the hyperphosphorylation of the microtubule-associated protein tau, the formation of neurofibrillary tangles, and a reduction in the outgrowth of neurons (13, 16, 42–45). Although any of these processes could have a role in the development of AD, some could have an additional role in neurological development.
Although it remains possible that the CMRgl abnormalities reflect aspects of the ε4 allele unrelated to AD, PET studies suggest that these abnormalities are related to the development of this disorder. Although there may be some differences (46, 47), patients with probable AD had a similar pattern of reductions in regional CMRgl whether or not they had the ε4 allele (48, 49). In patients with probable AD, the CMRgl abnormalities predicted the subsequent progression of dementia and the histopathological diagnosis of AD (7), were progressive (3–5, 8), and were correlated with dementia severity (6). In older ε4 carriers who did not have dementia, CMRgl continued to decline in these and other brain regions and did so at higher rates than in control subjects who did not have this allele (2, 12). In those ε4 carriers who had memory concerns, some of the metabolic abnormalities predicted a subsequent decline in memory (12). Our ongoing longitudinal PET study of late-middle-aged, cognitively normal ε4 carriers, and studies in patients with mild cognitive impairment who have an increased annual risk of AD (50), promise to clarify the extent to which the PET abnormalities are related to the risk of AD.
Although some studies do not detect a significant effect of early life factors (51, 52), it has been suggested that early life factors may contribute to the risk of late-onset AD (53–56). In a sample of nuns, those with lower measures of linguistic ability at a mean age of 22 years had lower cognitive function, a higher frequency of neuropathologically confirmed AD, and a higher density of neurofibrillary tangles in the hippocampus and cortex when they were assessed about six decades later (53). In a sample of Scottish residents, those with lower scores on a test of mental ability at the age of 11 had a higher frequency of late-onset dementia by the age of 75 (54). In several studies, lower educational levels were associated with an increased risk of AD (55, 56). The relationship between lower levels of intelligence and education and an increased risk of AD could be attributable to very early neurological alterations that lead to each of these conditions, differential cognitive reserve capacities in neuropathologically affected or unaffected brain regions (57), possible neuroprotective effects of cognitive stimulation (58), or a combination of these and other contributory factors.
In a post hoc cross-sectional analysis, PET images from our young adults and previously studied late-middle-aged subjects (10) were used to characterize and compare the effects of age on regional CMRgl in cognitively normal ε4 heterozygotes and controls. Consistent with previous studies (9, 59–63), the late-middle-aged adults had lower CMRgl than young adults in extensive areas of the cerebral cortex, most prominently in the medial frontal lobe. In contrast to 2-year follow-up findings that regional CMRgl continues to decline at an abnormally high rate in older ε4 carriers with and without memory concerns (2, 12), the CMRgl differences between young adulthood and late middle age were not significantly greater in the ε4 carriers than in the controls. Although there may be other explanations to reconcile these findings, we have previously postulated that the rates of age-related CMRgl decline in different regions vary depending on a person's age, AD risk and stage, and their interactions (10). Longitudinal studies are needed to further characterize the course of CMRgl declines across the adult lifespan, the extent to which they are accelerated in ε4 carriers and other persons at risk for AD, and the extent to which baseline and longitudinal CMRgl declines predict the subsequent onset of cognitive impairment and dementia.
This study has several limitations. For instance, because subjects in this study were selected by using newspaper advertisements, were mostly Caucasian and female, and had relatively high educational levels, additional studies are needed to determine whether our findings can be confirmed in young adult ε4 carriers with other demographic features. Although CMRgl abnormalities have been reported in some of the same brain regions in relatives of patients with an early-onset, autosomal dominant form of AD (64) and a patient with mild cognitive impairment who subsequently developed AD (65), additional studies are needed to determine whether the same pattern of functional brain abnormalities is related to the risk of AD in persons with no copies of the ε4 allele. Finally, whereas the ε4 carriers and control subjects had significant differences in their regional PET measurements, there was considerable overlap between groups in their individual measurements. Neither genetic testing for APOE alleles nor PET are clinically indicated to predict a cognitively normal person's risk of AD. This information does not yet determine with sufficient accuracy whether or when a person might develop AD; this information may be associated with psychological and social risks; and established prevention therapies are not yet available (66).
If, as we believe, the functional brain abnormalities observed in young adult ε4 carriers are associated with the risk of late-onset AD, it may be possible to discover primary prevention therapies that target the contributing processes at an unusually early age, decades before the onset of cognitive decline, and perhaps at a more tractable stage of disease or level of susceptibility.
Acknowledgments
We thank Anita Prouty, Christine Burns, Sandra Yee-Benedetto, Heather Wheeler, Debra Intorcia, Sandra Goodwin, Leslie Mullen, Susan Poulton, Dr. Wavrant-DeVrieze Fabienne, Louis Giordano, Alisa Domb, and Bernadette Romo for technical assistance; Dr. David Kuhl for permission to use PET data from the University of Michigan, Ann Arbor; Dr. Satoshi Minoshima for permission to use image-analysis software; and Dr. Michael Lawson and Connie Boker for their encouragement. This study was supported by Alzheimer's Association Grants IIRG-98-068 (to E.M.R.) and IIRG-98-078 (to R.J.C.), the Arizona Alzheimer's Research Center (E.M.R. and R.J.C.), National Institute of Mental Health and National Institute on Aging Grants RO1 MH57899-01 and P30 AG19610-02 (to E.M.R.), the Banner Health Foundation, and the Mayo Clinic Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PET, positron emission tomography; AD, Alzheimer's dementia; CMRgl, cerebral metabolic rate for glucose; APOE, apolipoprotein E.
References
- 1.Evans, D. A., Funkenstein, H. H., Albert, M. S., Scherr, P. A., Cook, N. R., Chown, M. J., Hebert, L. E., Hennekens, C. H. & Taylor, J. O. (1989) J. Am. Med. Assoc. 262, 2551-2556. [PubMed] [Google Scholar]
- 2.Reiman, E. M., Caselli, R. J., Chen, K., Alexander, G. E., Bandy, D. & Frost, J. (2001) Proc. Natl. Acad. Sci. USA 98, 3334-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGeer, E. G., Peppard, R. P., McGeer, P. L., Tuokko, H., Crockett, D., Parks, R., Akiyam, H., Calne, D. B., Beattie, B. L. & Harrop, R. (1990) Can. J. Neurol. Sci. 17, 1-11. [DOI] [PubMed] [Google Scholar]
- 4.Smith, G. S., de Leon, M. J., George, A. E., Kluger, A., Volkow, N. D., McRae, T., Golomb, J., Ferris, S. H., Reisberg, B., Ciaravino, J., et al. (1992) Arch. Neurol. 49, 1142-1150. [DOI] [PubMed] [Google Scholar]
- 5.Mielke, R., Herholz, K. & Grond, M. (1994) Dementia 5, 36-41. [DOI] [PubMed] [Google Scholar]
- 6.Minoshima, S., Frey, K. A., Koeppe, R. A., Foster, N. L. & Kuhl, D. E. (1995) J. Nucl. Med. 36, 1238-1248. [PubMed] [Google Scholar]
- 7.Silverman, D. H., Small, G. W., Chang, C. Y., Lu, C. S., Kung De Aburto, M. A., Chen, W., Czernin, J., Rapoport, S. I., Pietrini, P., Alexander, G. E., et al. (2001) J. Am. Med. Assoc. 286, 2120-2127. [DOI] [PubMed] [Google Scholar]
- 8.Alexander, G. E., Chen, K., Pietrini, P., Rapoport, S. & Reiman, E. M. (2002) Am. J. Psychiatry 159, 738-745. [DOI] [PubMed] [Google Scholar]
- 9.Reiman, E. M., Caselli, R. J., Yun, L. S., Chen, K., Bandy, D., Minoshima, S., Thibodeau, S. N. & Osborne, D. (1996) N. Engl. J. Med. 334, 752-758. [DOI] [PubMed] [Google Scholar]
- 10.Reiman, E. M., Caselli, R. J., Alexander, G. E. & Chen, K. (2001) Clin. Neurosci. Res. 1, 194-206. [Google Scholar]
- 11.Small, G. W., Mazziotta, J. C., Collins, M. T., Baxter, L. R., Phelps, M. E., Mandelkern, M. A., Kaplan, A., La Rue, A., Adamson, C. F., Chang, L., et al. (1995) J. Am. Med. Assoc. 273, 942-947. [PubMed] [Google Scholar]
- 12.Small, G. W., Ercoli, L. M., Silverman, D. H., Huang, S. C., Komo, S., Bookheimer, S. Y., Lavretsky, H., Miller, K., Siddarth, P., Rasgon, N. L., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 6037-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90, 1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L. & Pericak-Vance, M. A. (1993) Science 261, 921-923. [DOI] [PubMed] [Google Scholar]
- 15.Saunders, A. M., Strittmatter, W. J., Schmechel, D., George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., Rosi, B. L., Gusella, J. F., Crapper-MacLachlan, D. R., Alberts, M. J., et al. (1993) Neurology 43, 1467-1472. [DOI] [PubMed] [Google Scholar]
- 16.Mahley, R. W. (1988) Science 240, 622-630. [DOI] [PubMed] [Google Scholar]
- 17.Hallman, D. M., Boerwinkle, E., Saha, N., Sandholzer, C., Menzel, H. J., Csazar, A. & Utermann, G. (1991) Am. J. Hum. Genet. 49, 338-349. [PMC free article] [PubMed] [Google Scholar]
- 18.Hixson, J. E. & Vernier, D. T. (1990) J. Lipid Res. 31, 545-548. [PubMed] [Google Scholar]
- 19.Spitzer, R. L., Williams, J. B. W., Gibbon, M. & First, M. D. (1990) User's Guide for the Structured Clinical Interview for DSM-III-R (SCID) (Am. Psychiatric Press, Washington, DC).
- 20.Folstein, M. F., Folstein, S. E. & McHugh, P. R. (1975) J. Psychiatr. Res. 12, 189-198. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton, M. (1960) J. Neurol. Neurosurg. Psychiatry 23, 56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lezak, M. D. (1983) Neuropsychological Assessment (Oxford Univ. Press, New York), 2nd Ed.
- 23.Reiman, E. M., Uecke, A., Caselli, R. J., Lewis, S., Bandy, D., de Leon, M. J., De Santi, S., Convit, A., Osborne, D., Weaver, A., et al. (1998) Ann. Neurol. 44, 288-291. [DOI] [PubMed] [Google Scholar]
- 24.Chen, K., Bandy, D., Reiman, E., Huang, S. C., Lawson, M., Feng, D., Yun, L. S. & Palant, A. (1998) J. Cereb. Blood Flow Metab. 18, 716-723. [DOI] [PubMed] [Google Scholar]
- 25.Talairach, J. & Tournoux, P. (1988) Coplanar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 26.Minoshima, S., Frey, K. A., Foster, N. L. & Kuhl, D. E. (1995) J. Comput. Assist. Tomogr. 19, 541-547. [DOI] [PubMed] [Google Scholar]
- 27.Chen, K. C., Reiman, E. M., Bandy, D. & Alexander, G. E. (2003) in Modeling and Control in Biomedical Systems, eds. Feng, D. D. & Carson E. R. (Elsevier, Hong Kong), pp. 11-15.
- 28.Frackowiak, R. S. J., Friston K. J., Frith, C. D., Dolan, R. J. & Mazziotta, J. C. (1997) Human Brain Function (Academic, San Diego).
- 29.Ibanez, V., Pietrini, P., Alexander, G. E., Furey, M. L., Teichberg, D., Rajapakse, J. C., Rapoport, S. I., Schapiro, M. B. & Horwitz, B. (1998) Neurology 50, 1585-1593. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz, W. J., Smith, C. B., Davidsen, L., Savaki, H., Sokoloff, L., Mata, M., Fink, D. J. & Gainer, H. (1979) Science 205, 723-725. [DOI] [PubMed] [Google Scholar]
- 31.Magistretti, P. J. & Pellerin, L. (1996) Cereb. Cortex 6, 50-61. [DOI] [PubMed] [Google Scholar]
- 32.Piert, M., Koeppe, R. A., Giordani, B., Berent, S. & Kuhl, D. E. (1996) J. Nucl. Med. 37, 201-208. [PubMed] [Google Scholar]
- 33.Mark, R. J., Pang, Z., Geddes, J. W., Uchida, K. & Mattson, M. P. (1997) J. Neurosci. 17, 1046-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak, H. & Braak, E. (1991) Acta Neuropathol. 82, 239-259. [DOI] [PubMed] [Google Scholar]
- 35.Braak, H. & Braak, E. (1997) Neurobiol. Aging 18, 351-357. [DOI] [PubMed] [Google Scholar]
- 36.Ghebremedhin, E., Schultz, C., Braak, E. & Braak, H. (1998) Exp. Neurol. 153, 152-155. [DOI] [PubMed] [Google Scholar]
- 37.Ohm, T. G., Müller, H., Braak, H. & Bohl, J. (1994) Neuroscience 64, 209-217. [DOI] [PubMed] [Google Scholar]
- 38.Meguro, K., Blaizot, X., Kondoh, Y., Le Mestric, C., Baron, J. C. & Chavoix, C. (1999) Brain 122, 1519-1531. [DOI] [PubMed] [Google Scholar]
- 39.Hyman, B. T., Van Hoesen, G. W., Kromer, L. J. & Damasio, A. R. (1986) Ann. Neurol. 20, 472-481. [DOI] [PubMed] [Google Scholar]
- 40.Shoghi-Jadid, K., Small, G. W., Agdeppa, E. D., Kepe, V., Ercoli, L. M., Siddarth, P., Read, S., Satyamurthy, N., Petric, A., Huang, S. C., et al. (2002) Am. J. Geriatr. Psychiatry 10, 24-35. [PubMed] [Google Scholar]
- 41.Mathis, C. A., Bacskai, B. J., Kajdasz, S. T., McLellan, M. E., Frosch, M. P., Hyman, B. T., Holt, D. P., Wang, Y., Huang, G. F., Debnath, M. L., et al. (2002) Biorg. Med. Chem. Lett. 12, 295-298. [DOI] [PubMed] [Google Scholar]
- 42.Wisniewski, T., Castano, E. M., Golabek, A., Vogel, T. & Frangione, B. (1994) Am. J. Pathol. 145, 1030-1035. [PMC free article] [PubMed] [Google Scholar]
- 43.Miyata, M. & Smith, J. D. (1996) Nat. Genet. 14, 55-61. [DOI] [PubMed] [Google Scholar]
- 44.Strittmatter, W. J., Weisgraber, K. H., Goedert, M., Saunders, A. M., Huang, D., Corder, E. H., Dong, L. M., Jakes, R., Alberts, M. J., Gilbert, J. R., et al. (1994) Exp. Neurol. 125, 1663-1671. [DOI] [PubMed] [Google Scholar]
- 45.Nathan, B. P., Bellosta, S., Sanan, D. A., Weisgraber, K. H., Mahley, R. W. & Pitas, R. E. (1994) Science 264, 850-852. [DOI] [PubMed] [Google Scholar]
- 46.Mielke, R., Zerres, K., Uhlhaas, S., Kessler, J. & Heiss, W. D. (1998) Neurosci. Lett. 254, 49-52. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi, M., Arai, H., Nakagawa, T., Higuchi, S., Muramatsu, T., Matsushita, S., Kosaka, Y., Itoh, M. & Sasaki, H. (1997) NeuroReport 8, 2639-2643. [DOI] [PubMed] [Google Scholar]
- 48.Corder, E. H., Jelic, V., Basun, H., Lannfelt, L., Valind, S., Winblad, B. & Nordberg, A. (1997) Arch. Neurol. 54, 273-277. [DOI] [PubMed] [Google Scholar]
- 49.Hirono, N., Mori, E., Yasuda, M., Ishii, K., Ikejiri, Y., Imamura, T., Shimomura, T., Hashimoto, M., Yamashita, H. & Sasaki, M. (1998) Alzheimer Dis. Assoc. Disord. 12, 362-367. [DOI] [PubMed] [Google Scholar]
- 50.Petersen, R. C., Smith, G. E., Ivnik, R. J., Tangalos, E. G., Schaid, D. J., Thibodeau, S. N., Kokmen, E., Waring, S. C. & Kurland, L. T. (1995) J. Am. Med. Assoc. 273, 1274-1278. [PubMed] [Google Scholar]
- 51.Munoz, D. G., Ganapathy, G. R., Eliasziw, M. & Hachinski, V. (2000) Arch. Neurol. 57, 85-89. [DOI] [PubMed] [Google Scholar]
- 52.Caselli, R. J., Hentz, J. G., Osborne, D., Graff-Radford, N. R., Barbieri, C. J., Alexander, G. E., Hall, G. R., Reiman, E. M., Hardy, J. & Saunders, A. M. (2002) J. Am. Geriatr. Soc. 50, 49-54. [DOI] [PubMed] [Google Scholar]
- 53.Snowdon, D. A., Kemper, S. J., Mortimer, J. A., Greiner, L. H., Wekstein, D. R. & Markesbery, W. R. (1996) J. Am. Med. Assoc. 275, 528-532. [PubMed] [Google Scholar]
- 54.Whalley, L. J., Starr, J. M., Athawes, R., Hunter, D., Pattie, A. & Deary, I. J. (2000) Neurology 55, 1455-1460. [DOI] [PubMed] [Google Scholar]
- 55.Evans, D. A., Beckett, L. A., Albert, M. S., Hebert, L. E., Scherr, P. A., Funkenstein, H. H. & Taylor, J. O. (1993) Ann. Epidemiol. 3, 71-77. [DOI] [PubMed] [Google Scholar]
- 56.Stern, Y., Gurland, B., Tatemichi, T. K., Tang, M. X., Wilder, D. & Mayeux, R. (1994) J. Am. Med. Assoc. 271, 1004-1010. [PubMed] [Google Scholar]
- 57.Alexander, G. E., Furey, M. L., Grady, C. L., Pietrini, P., Brady, D. R., Mentis, M. J. & Schapiro, M. B. (1997) Am. J. Psychiatry 154, 165-172. [DOI] [PubMed] [Google Scholar]
- 58.Kesslak, J. P., So, V., Choi, J., Cotman, C. W. & Gomez-Pinilla, F. (1998) Behav. Neurosci. 112, 1012-1019. [DOI] [PubMed] [Google Scholar]
- 59.Kuhl, D. E., Metter, E. J., Riege, W. H. & Phelps, M. E. (1982) J. Cereb. Blood Flow Metab. 2, 163-171. [DOI] [PubMed] [Google Scholar]
- 60.Salmon, E., Maquet, P., Sadzot, B., Degueldre, C., Lemaire, C. & Franck G. (1991) Acta Neurol. Belg. 91, 288-295. [PubMed] [Google Scholar]
- 61.Loessner, A., Alavi, A., Lewandrowski, K. U., Mozley, D., Souder, E. & Gur, R. E. (1995) J. Nucl. Med. 36, 1141-1149. [PubMed] [Google Scholar]
- 62.Coffey, C. E., Wilkinson, W. E., Parashos, I. A., Soady, S. A., Sullivan, R. J., Patterson, L. J., Figiel, G. S., Webb, M. C., Spritzer, C. E. & Djang, W. T. (1992) Neurology 42, 527-536. [DOI] [PubMed] [Google Scholar]
- 63.Terry, R. D., DeTeresa, R. & Hansen, L. A. (1976) Arch. Psychiatr. Nervenkr. 223, 15-33.828039 [Google Scholar]
- 64.Kennedy, A. M., Frackowiak, R. S., Newman, S. K., Bloomfield, P. M., Seaward, J., Roques, P., Lewington, G., Cunningham, V. J. & Rossor, M. N. (1995) Neurosci. Lett. 186, 17-20. [DOI] [PubMed] [Google Scholar]
- 65.Pietrini, P., Azari, N. P., Grady, C. L., Salerno, J. A., Gonzales-Aviles, A., Heston, L. L., Pettigrew, K. D., Horwitz, B., Haxby, J. V. & Schapiro, M. B. (1993) Dementia 4, 94-101. [DOI] [PubMed] [Google Scholar]
- 66.American College of Medical Genetics, American Society of Human Genetics Working Group on ApoE and Alzheimer Disease (1995) J. Am. Med. Assoc. 274, 1627-1629. [PubMed] [Google Scholar]