Abstract
Despite significant advances in intensive care therapy and antibiotics, severe sepsis accounts for 9% of all deaths in the United States annually. The pathological sequelae of sepsis are characterized by a systemic inflammatory response, but experimental therapeutics that target specific early inflammatory mediators [tumor necrosis factor (TNF) and IL-1β] have not proven efficacious in the clinic. We recently identified high mobility group box 1 (HMGB1) as a late mediator of endotoxin-induced lethality that exhibits significantly delayed kinetics relative to TNF and IL-1β. Here, we report that serum HMGB1 levels are increased significantly in a standardized model of murine sepsis, beginning 18 h after surgical induction of peritonitis. Specific inhibition of HMGB1 activity [with either anti-HMGB1 antibody (600 μg per mouse) or the DNA-binding A box (600 μg per mouse)] beginning as late as 24 h after surgical induction of peritonitis significantly increased survival (nonimmune IgG-treated controls = 28% vs. anti-HMGB1 antibody group = 72%, P < 0.03; GST control protein = 28% vs. A box = 68%, P < 0.03). Animals treated with either HMGB1 antagonist were protected against the development of organ injury, as evidenced by improved levels of serum creatinine and blood urea nitrogen. These observations demonstrate that specific inhibition of endogenous HMGB1 therapeutically reverses lethality of established sepsis indicating that HMGB1 inhibitors can be administered in a clinically relevant time frame.
Severe sepsis is a systemic inflammatory response to infection associated with coagulopathy, multiple organ failure, and death. Despite significant advances in intensive care therapy and antibiotics, the overall mortality due to severe sepsis is ≈30%, and sepsis is associated with an annual health care cost of nearly $17 billion (1-3). During the past 20 years, a series of basic scientific observations have focused sepsis research on products of the innate immune system. Bacterial toxins induce host cells to release cytokines [e.g., tumor necrosis factor (TNF) and IL-1β] and other factors that activate specific immune responses. The kinetics and magnitude of cytokine release influence the development of sepsis (4-9). TNF and IL-1β are released early in systemic inflammatory responses and can be acutely toxic, but the acute kinetics of most cytokines provide an extremely narrow therapeutic window for effective use of specific cytokine inhibitors. Typically, the early cytokine response has resolved before sepsis is diagnosed and treatment initiated. For example, the majority of patients with sepsis in large-scale trials of anti-TNF were not enrolled until many hours or days into their clinical course, after the early proinflammatory cytokine response had peaked (10).
High mobility group box 1 (HMGB1) was recently identified as a late mediator of systemic inflammation (11). Originally described as an intracellular transcription factor, it has become clear that HMGB1 is released from endotoxin-stimulated macrophages after a significant delay, beginning 8-12 h after the release of the early cytokines (e.g., TNF and IL-1β). Similar delays in elevated serum HMGB1 are observed in animals after exposure to endotoxin (11). Cytokine activities of HMGB1 include activation of macrophages and pituicytes to release TNF and IL-1β (11-13), stimulation of neutrophil and smooth muscle cell chemotaxis (14, 15), and induction of epithelial cell permeability (16). Systemic administration of HMGB1 is lethal, and anti-HMGB1 antibodies confer significant protection against the lethality of intratracheal or i.p. endotoxin even when anti-HMGB1 antibodies are delivered after early TNF release (11, 14). Ethyl pyruvate, an experimental antiinflammatory agent, inhibits systemic HMGB1 release and rescues animals from the lethal sequelae of systemic inflammation, even when the first dose is given 24 h after the induction of endotoxemia or peritonitis (17).
The identification of a cytokine role for HMGB1 and its downstream action in diseases of systemic inflammation renew the potential for specific cytokine inhibitors in the treatment of severe sepsis in a significantly wider treatment window (≈24 h) than has been available for TNF- and IL-1β-targeted strategies. In recent structure-function analyses, we localized the active cytokine domain of HMGB1 to the DNA-binding B box (18). As described here, a similar approach has revealed that the other DNA-binding domain of HMGB1, the A box, competes with HMGB1 for binding sites on the surface of activated macrophages and attenuates HMGB1-induced release of proinflammatory cytokines. Administration of the A box or anti-HMGB1 antibodies significantly protects against sepsis lethality, even when they are first administered as late as 24 h after induction of peritonitis. Both therapeutic approaches significantly protect against end-organ damage associated with endotoxemia or sepsis, suggesting that specific HMGB1 antagonists may be effective in the clinical management of sepsis.
Materials and Methods
Materials. Recombinant mouse TNF and IL-1β were obtained from R & D Systems. Isopropyl d-thiogalactopyranoside was from Pierce. Polymyxin B, lipopolysaccharide (LPS; Escherichia coli O111:B4), and nonimmune rabbit IgG (catalog no. I5006) were purchased from Sigma. DNase I and 2-YT medium were obtained from Life Technologies (Grand Island, NY). Tryptic soy agar was from Difco.
Cell Culture. Murine macrophage-like RAW 264.7 cells (American Type Culture Collection) were cultured in RPMI medium 1640 (Life Technologies) supplemented with 10% FBS (Gemini Biological Produces, Catabasas, CA), penicillin, and streptomycin (Life Technologies). Cells were used at 90% confluence, and treatment was carried out in serum-free Opti-MEM I medium (Life Technologies).
Cloning, Expression, and Purification of HMGB1 Constructs. The cDNAs encoding full-length or various truncated forms of human HMGB1 were amplified by PCR from a human brain Quick-Clone cDNA (Clontech) by using primers as described (18). The PCR products were subcloned into an expression vector (pGEX) with a GST tag (Amersham Pharmacia). The recombinant plasmids were transformed into the protease-deficient E. coli strain BL21 (Novagen) and grown in 2-YT medium, and protein expression was induced by isopropyl d-thiogalactopyranoside; fusion proteins were purified by using the Glutathione Sepharose affinity column (Amersham Pharmacia) as described (18). HMGB1 and mutants were further purified by using a polymyxin B column (Pierce) to remove endotoxin. The purity and integrity of all recombinant proteins were verified by Coomassie blue staining after SDS/PAGE to reveal purity >85%.
LPS Content. The LPS content of recombinant proteins was measured by the chromogenic Limulus amoebocyte lysate assay (Associates of Cape Cod or BioWhittaker). LPS content in HMGB1 was 219 pg/μg of protein; B box, 19 pg/μg; A box, 16 pg/μg; and GST vector, 4 pg/μg. For stimulation experiments, polymyxin B was added to cell culture medium at 6 units of polymyxin B per pg of LPS. Separate controls confirmed that this amount of polymyxin B neutralized the maximal amount of contaminating endotoxin (11).
125I-labeling of HMGB1 and Cell Surface Binding. Purified HMGB1 (10 μg) was radiolabeled with 0.2 mCi (1 Ci = 37 GBq) of carrier-free 125I (NEN Life Science Products) by using Iodo-beads (Pierce) according to the manufacturer's instructions. 125I-HMGB1 was separated from unreacted 125I by gel chromatography columns (P6 Micro Bio-Spin Chromatography Columns, Bio-Rad) previously equilibrated with 300 mM sodium chloride/17.5 mM sodium citrate, pH 7.0/0.1% BSA. The specific activity of the eluted HMGB1 was 2.8 × 106 cpm/μg of protein. The cell surface binding of HMGB1 or A box to macrophage cultures was performed according to a described protocol (19). In brief, RAW 264.7 cells were plated in 24-well plates and grown to confluence. Cells were washed twice with ice-cold PBS containing 0.1% BSA, and binding was carried out at 4°C for 2 h or as indicated in 0.5 ml of binding buffer containing 120 mM sodium chloride, 1.2 mM magnesium sulfate, 15 mM sodium acetate, 5 mM potassium chloride, 10 mM Tris·HCl (pH 7.4), 0.2% BSA, 5 mM glucose, and 25,000 cpm of 125I-HMGB1. At the end of the incubation, the cells were washed three times with 0.5 ml of ice-cold PBS/0.1% BSA, and subsequently lysed with 0.5 ml of 0.5 M NaOH/0.1% SDS for 20 min at room temperature. The radioactivity in the lysate was measured by using a γ-counter (Packard 36621).
Antibody Production. Polyclonal antibodies against HMGB1 B box were raised in rabbits (Cocalico Biologicals, Reamstown, PA), and titers were determined by immunoblotting. Anti-HMGB1 B box antibodies were affinity-purified by using cyanogen bromide-activated Sepharose beads following standard procedures. Neutralizing activity of anti-HMGB1 was confirmed in HMGB1-stimulated macrophage cultures by assay of TNF release. In the presence of anti-HMGB1 antibody, neutralizing antibody was defined as inhibiting >80% of HMGB1-induced TNF release.
Cytokine Measurements. The concentrations of TNF were determined by using a standard cytotoxicity bioassay by L929 cells as described (20), with the minimum detectable concentration of TNF of 30 pg/ml or by a commercially obtained ELISA kit according to the instructions of the manufacturer (R & D Systems). Levels of IL-1β, IL-6, and IL-10 were determined by using ELISA kits (R & D Systems).
HMGB1 Measurements. HMGB1 levels were measured by Western immunoblotting analysis as described (11). In brief, serum samples or cell-conditioned medium (100-200 μl) was ultrafiltered with Centricon 100 (Millpore). The eluate was fractionated by SDS/PAGE, transferred to a poly(vinylidene difluoride) immunoblot membrane (Bio-Rad), and probed with either specific anti-HMGB1 antiserum (1:250 dilution) or purified IgG from anti-HMGB1 antiserum (5 μg/ml) for Western blot analysis. Polyclonal anti-HMGB1 IgG was purified by using protein A agarose according to the manufacturer's instructions (Pierce). Western blots were scanned with a silver image scanner (Silver-scanner II, Lacie Limited, Beaverton, OR), and the relative band intensity was quantified by using the NIH IMAGE 1.59 software. The levels of HMGB1 were determined by reference to standard curves generated with purified HMGB1.
Animal Experiments. Male 6- to 8-week-old BALB/c mice were purchased from Harlan-Sprague-Dawley and allowed to acclimate for 7 days before use. Mice were housed in the North Shore University Hospital Animal Facility under standard temperature and light and dark cycles. All procedures were performed under approval of North Shore-Long Island Jewish Research Institute Institutional Animal Care and Use Committee.
Cecal Ligation and Puncture (CLP). To establish live intraabdominal infection and sepsis, mice were subjected to the CLP procedure as described (21). After anesthesia with an intramuscular injection of ketamine (75 mg/kg, Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (20 mg/kg, Boehringer Ingelheim), a 15-mm midline incision was made to expose the cecum. After ligation 5.0 mm from the tip, the cecal stump was punctured once with a 22-gauge needle, and small amount of stool (1-mm length) was extruded. The cecum was placed back into its normal intraabdominal position, and the wound was closed with two layers of running suture. All animals received saline-solution (0.9% s.c., 20 ml/kg of body weight) resuscitation, and a single dose of antibiotic (0.5 mg of imipenem per mouse in 200 μl of sterile saline injected s.c.) (Primaxin, Merck) 30 min after the surgery. To determine levels of HMGB1 in the circulation, blood was collected from mice at various time points (0-72 h) after surgery. Peritoneal fluid was obtained 48 h after surgery by rinsing the peritoneal cavity twice with a total of 1.5 ml of PBS and collecting the exudate fluid.
LPS Lethality. Mice were given an LD75 dose of LPS (15 mg/kg) injected i.p. and were treated with A box or GST control (600 μg per mouse) or with nonimmune IgG or anti-HMGB1 antibody (600 μg per mouse), administered i.p. or i.v. either immediately after LPS injection or, in some experiments, after a 2-h delay. Additional doses of A box or GST control proteins were administered at 12 and 24 h after the first treatment. Mortality was monitored for up to 2 weeks after the procedure to ensure no late death occurred.
Splenic Bacterial Counts. Mice received either anti-HMGB1 IgG or nonimmune control IgG (n = 7 in each group), or A box or GST control (n = 10 in each group), given at 24 and 31 h after surgery, and were killed at 32 h (A box-treated) or 35 h (antibody-treated). Splenic bacteria were recovered as described (22). In brief, spleen was removed by using sterile technique and homogenized in 2 ml of PBS. After serial dilutions with PBS, the homogenate was plated as 0.15-ml aliquots on tryptic soy agar plates, and colony-forming units were counted after overnight incubation at 37°C. Data are expressed as colony-forming units per gram tissue.
Serum Measurements. BALB/c mice, subjected to an LD75 dose of LPS or cecal perforation, received A box treatment (600 μg/mouse i.p.) at 0 and 12 h after LPS injection or 24 and 31 h after surgery of cecal perforation and were killed at 14 h (LPS model) or 33 h (CLP model) by a CO2 overdose. Blood was obtained by cardiac puncture. Serum levels of blood urea nitrogen, creatinine, and lactate dehydrogenase were measured by using commercially available clinical assay kits.
Statistical Analysis. Data are presented as mean ± SEM unless otherwise indicated. Differences between treatment groups were determined by Student's t test, one-way ANOVA followed by the least-significant difference test, or Fisher's exact test; P values <0.05 were considered statistically significant.
Results and Discussion
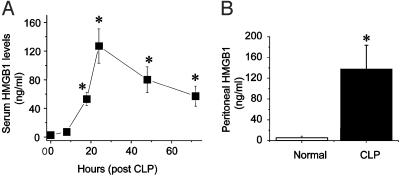
Serum and Peritoneal HMGB1 Levels in Sepsis. To determine whether HMGB1 is produced in sepsis, and whether it exhibits a “late” kinetic profile as occurs in endotoxemia (11), HMGB1 levels were measured in a standardized mouse model of sepsis induced by CLP. In this model, a surgically created diverticulum of the cecum is punctured; this procedure reproducibly results in polymicrobial peritonitis, bacteremia, and sepsis (21). Serum HMGB1 levels were not significantly altered for the first 8 h after cecal perforation, but then increased significantly by 18 h (Fig. 1A). Increased serum HMGB1 remained significantly elevated for at least 72 h, a time course that is similar to the delayed HMGB1 kinetics in endotoxemia described in ref. 11. HMGB1 was also significantly increased in peritoneal exudate fluid collected 48 h after cecal perforation (Fig. 1B). The observed kinetics of increased serum HMGB1 levels corresponded closely with the development of clinical signs of sepsis. During the first 8 h after cecal perforation, animals showed only minimal signs of illness, including diminished activity and loss of exploratory behavior. During the ensuing 18 h, however, as HMGB1 levels increased into the pathological range (>50 ng/ml), animals became gravely ill, huddled together in groups with piloerection, did not seek water or food, and were minimally responsive to external stimuli. Thus, systemic HMGB1 release occurs late in severe sepsis as compared with TNF and IL-1 and correlates with clinical prognosis of the disease.
Fig. 1.
Serum and peritoneal HMGB1 levels are elevated in septic mice. BALB/c mice (male, 20-25 g) were subjected to CLP, and sera were collected at various time points and assayed for HMGB1 levels as described (11). (A) Serum HMGB1 was not detectable until 18 h after surgery and then remained elevated from 24 to 72 h after surgery. Data are shown as mean ± SEM (n = 3-6 mice per time point). *, P < 0.05 vs. control as tested by one-way ANOVA followed by the least-significant difference test. (B) Peritoneal exudate fluid was collected 48 h after cecal perforation or sham surgery (normal), and HMGB1 was measured. Data are mean ± SEM of 7 and 11 mice per group. *, P < 0.05 vs. normal as tested by Student's t test.
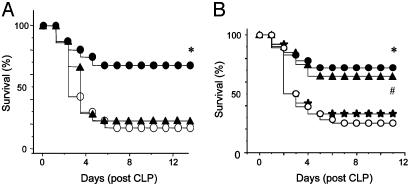
Anti-HMGB1 Antibodies Neutralize HMGB1 in Vitro and Protect Against Sepsis Lethality. To identify neutralizing anti-HMGB1 antibodies, we measured TNF and IL-6 release in macrophage-like RAW 264.7 cells stimulated with HMGB1, IL-1β, or TNF in the absence or presence of anti-HMGB1 antibodies (Fig. 2). HMGB1 antibodies significantly inhibited HMGB1-induced TNF and IL-6 release, without altering IL-1β-induced TNF release or TNF-induced IL-6 release, indicating that the anti-HMGB1 antibodies were specific. To determine whether endogenous HMGB1 is a mediator of lethality in sepsis, neutralizing anti-HMGB1 antibodies were administered to mice with established sepsis (Fig. 3A). Treatment with anti-HMGB1 antibodies (600 μg per mouse) beginning 24 h after CLP surgery significantly increased survival (survival in nonimmune IgG-treated controls = 28% vs. survival in anti-HMGB1 = 72%; n = 18 mice in each group, P < 0.03 by Fisher's exact test). This survival advantage is striking for several reasons: (i) therapy did not begin until animals were morbidly ill; (ii) anti-TNF antibodies actually worsen the outcome in this model; and (iii) no other cytokine-based therapy has proved effective when administered >8 h after CLP (23).
Fig. 2.
Anti-HMGB1 antibodies specifically inhibit HMGB1-induced cytokine release. Murine macrophage-like RAW 264.7 cells were stimulated with HMGB1, IL-1β, or TNF as indicated for 16 h in serum-free Opti-MEM medium in the absence or presence of purified rabbit anti-HMGB1 IgG antibodies or nonimmune rabbit IgG (as control). Conditioned media were collected and assayed for TNF and IL-6 levels by use of ELISA kits. Data represent mean ± SEM of three to six independent experiments, each done in duplicate. *, P < 0.05 vs. control; **, P < 0.05 vs. HMGB1 alone.
Fig. 3.
Anti-HMGB1 antibodies protect against lethality caused by cecal perforation. (A) BALB/c mice were randomly grouped (18-25 mice per group) and subjected to CLP. Mice received neutralizing IgG-purified (or, in some experiments, affinity-purified) anti-HMGB1 antibodies twice daily for 3 days at 60 or 600 μg per mouse (i.p.) beginning 24 h after surgery. Irrelevant nonimmune rabbit IgG was used as control. *, P < 0.03 vs. control, as tested by two-tailed Fisher's exact test. •, anti-HMGB1 antibody, 600 μg per mouse (24-h delay); ▴, anti-HMGB1 antibody, 60 μg per mouse (24-h delay); ○, IgG control. (B) Anti-HMGB1 antibody treatment (600 μg per mouse) was initiated at 12, 24, or 36 h after surgery, twice a day, and continued for 3 days. * and #, P < 0.05 vs. IgG control group. ▴, anti-HMGB1 antibody (12-h delay); •, anti-HMGB1 antibody (24-h delay); ★, anti-HMGB1 antibody (36-h delay); ○, IgG control.
Anti-HMGB1 antibody-treated animals were significantly more alert and active and resumed feeding behavior more quickly than either controls treated with irrelevant antibodies or animals treated with 10-fold lower antibody doses (60 μg per mouse), which did not confer protection (Fig. 3A). A time-course analysis of delayed antibody administration showed that antibody treatment beginning either 12 or 24 h after cecal puncture significantly improved survival; no survival advantage occurred when dosing was initiated after 36 h (Fig. 3B). All animals were observed for at least 2 weeks and late deaths did not occur, indicating that anti-HMGB1 antibody did not merely delay death but conferred lasting protection against lethal sepsis. Anti-HMGB1 antibody administration was associated with significantly reduced serum TNF levels in mice with sepsis (Table 1). Administration of anti-HMGB1 antibodies at this late stage in disease progression “rescued” animals from the lethal sequelae of sepsis, because animals in both the antibody- and vehicle-treated groups had already begun to succumb to the disease at the time of the first antibody dose. Anti-HMGB1 antibodies did not significantly alter bacterial counts in spleens of infected, septic animals, indicating that the survival advantage conferred by anti-HMGB1 antibodies was specific and not associated with bacterial proliferation (Table 1). Anti-HMGB1 antibody treatment did not significantly decrease serum HMGB1 levels, because neutralizing HMGB1 antibodies did not increase serum clearance of HMGB1, but neutralized HMGB1 activity to increase survival.
Table 1. Effect of A box or HMGB1 antibodies on splenic bacterial counts and on serum concentrations of TNF, HMGB1, and IL-6 in septic mice.
| Measurement | Control (GST) | A box | Control (IgG) | Anti-HMGB1 |
|---|---|---|---|---|
| Splenic bacterial counts, CFU/g | 10.0 ± 2.0 × 104 | 17.0 ± 8.0 × 104 | 5.7 ± 1.9 × 104 | 4.0 ± 0.9 × 104 |
| Serum TNF, pg/ml | 1,150 ± 103 | 171 ± 33* | 1,500 ± 345 | 560 ± 300* |
| Serum HMGB1, ng/ml | 115 ± 44 | 10 ± 3* | 127 ± 24 | 108 ± 20 |
| Serum IL-6, pg/ml | 130 ± 47 | 804 ± 398* | 87 ± 20 | 958 ± 388* |
BALB/c mice received A box or anti-HMGB1 antibodies administered (600 μg per mouse, i.p.) at 24 and 31 h after cecal perforation and were killed at 32 h (for A box) or 35 h (for anti-HMGB1 antibody) after surgery. Control mice received matched doses of GST or nonimmune IgG. Data are mean ± SEM of 4—10 animals per group. *, P < 0.05 vs. control group. CFU, colony-forming unit.
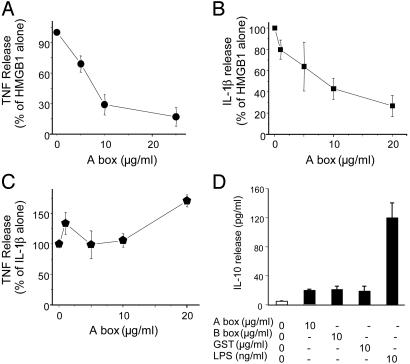
The HMGB1 A Box Inhibits HMGB1 Activity. Structure-function analyses were performed with full-length and truncated forms of HMGB1 to identify key functional domains within the protein. HMGB1 and HMGB1-derived peptides were expressed, purified, and tested for cytokine activity in macrophage cultures (see Materials and Methods). As shown here and in ref. 12, full-length (wild-type) HMGB1 significantly stimulated TNF release in macrophage cultures (Fig. 4). Mutants lacking the carboxyl terminus and the isolated recombinant B box each significantly stimulated TNF release in murine macrophage-like RAW 264.7 cells as compared with protein isolated from bacteria transfected with vector plasmid devoid of HMGB1-coding sequences (Fig. 4). The HMGB1 A box (A box) did not induce significant TNF release, suggesting that despite a 40% sequence homology to B box, A box possesses no intrinsic proinflammatory activity. HMGB1 binding to macrophages exhibited saturable first-order kinetics; binding was specific because unlabeled HMGB1 competitively displaced bound 125I-HMGB1 (see Fig. 7, which is published as supporting information on the PNAS web site). A box significantly displaced saturable 125I-labeled HMGB1 binding to macrophage cultures at 4°C (Fig. 7), indicating that A box is a competitive antagonist of HMGB1. To determine whether A box can antagonize the cytokine activity of HMGB1, we measured TNF and IL-1β release in HMGB1-stimulated macrophage cultures exposed to A box (Fig. 5 A and B). A box dose-dependently inhibited HMGB1-induced TNF and IL-1β release by >75%; the effective concentration of A box that inhibited 50% of released TNF and IL-1β (EC50) was 7.5 μg/ml (in the presence of 1 μg of HMGB1 per ml). A box did not inhibit IL-1β-induced TNF release (Fig. 5C) and did not induce release of the antiinflammatory cytokine IL-10 (Fig. 5D). Together, these observations provide evidence that A box specifically antagonizes the proinflammatory activity of HMGB1.
Fig. 4.
Schematic representation of HMGB1 constructs and their activity in TNF stimulation. Subconfluent cultures of RAW 264.7 cells were incubated with various HMGB1 mutant proteins (1 μg/ml) for 8 h. The conditioned medium was assayed for TNF by a standard murine fibroblast L929 (ATCC) cytotoxicity bioassay (20). Wild-type HMGB1, carboxyl-truncated, and B box significantly stimulated TNF release (*, P < 0.05 vs. GST control, one-way ANOVA followed by the least-significant difference test), but A box stimulation of TNF was not statistically significant. Full-length HMGB1 = 216 aa; carboxyl-truncated mutant corresponds to amino acids 1-182 of HMGB1; B box corresponds to amino acids 88-162 of HMGB1; and A box corresponds to amino acids 1-85 of HMGB1. n = 6-10 separate experiments.
Fig. 5.
A box antagonizes HMGB1 in vitro. Subconfluent RAW 264.7 cells in 24-well dishes were treated with HMGB1 or IL-1β and various concentrations of A box (as indicated) for 16 h in Opti-MEM I medium in the presence of polymyxin B. The TNF- or IL-1β-stimulating activity was expressed as percent of maximal response, and inhibition by A box was expressed as percent of HMGB1 or IL-1β alone. (A) HMGB1 (1 μg/ml) induced the release of 13,344 ± 2,335 pg of TNF per ml. A box dose-dependently inhibited HMGB1-induced TNF release with an apparent EC50 of 7.5 μg/ml. (B) Similarly, HMGB1-induced IL-1β was dose-dependently inhibited by the addition of A box. (C) In contrast, A box did not have any significant effect on IL-1β-induced TNF release. TNF release stimulated by IL-1β (100 ng/ml) was 640 ± 160 pg of TNF per ml. (D) RAW 267.4 cells were stimulated with LPS and A box, B box, or GST vector, or LPS at the concentration indicated for 16 h, and IL-10 released in the conditioned medium was measured with an ELISA kit. Data represent three or four separate experiments.
A Box Protects Against Endotoxin and Sepsis Lethality. To determine whether A box can neutralize HMGB1 toxicity in vivo, we used a standardized model of endotoxemia, in which BALB/c mice were given an LD75 dose of LPS and treated with A box or purified GST vector protein devoid of A box. The i.p. administration of A box significantly increased survival when given concurrently with or 2 h after administration of LPS (P < 0.05; Fig. 6A). The i.v. administration of A box (600 μg per mouse, given at 0, 12, and 24 h after LPS administration) was protective; survival in GST control i.v. was 2 of 5 alive, and survival in A box i.v. was 5 of 5 alive. We also compared these results with i.v. antibody dosing (600 μg per mouse) and observed similar results; survival in nonimmune IgG-treated i.v. was 2 of 5 mice alive, and anti-HMGB1 antibody i.v. was 5 of 5 alive. We then assessed the effect of A box administration on mice with established sepsis and observed that administration of A box rescued mice from the lethal effects of sepsis, even when treatment was initiated as late as 24 h after cecal perforation (survival in animals treated with GST vector only = 28% vs. survival in animals receiving A box = 68%, P < 0.03; Fig. 6B). Similar results were obtained when A box treatment was initiated 12 h after surgery (21% survival in control group compared with 64% in A box-treated group, n = 25 mice per group, P < 0.03 by Fisher's exact test). The rescuing effects of A box in this sepsis model were dose-dependent; animals treated with 600 μg per mouse of A box were significantly more alert and active, and resumed feeding behavior more quickly than either controls treated with vector-derived preparations or animals treated with 60 μg of A box per mouse. The latter animals remained gravely ill, with depressed activity and feeding for several days before death. All animals were observed for at least 2 weeks, indicating that A box did not merely delay death but conferred lasting protection against lethal sepsis.
Fig. 6.
A box protects against lethality caused by LPS or cecal perforation in mice. (A) BALB/c mice were randomly grouped (18 mice per group) and challenged with an LD75 dose of LPS (15 mg/kg of body weight, i.p.). A box or GST control was administered at 600 μg per mouse (in 300 μl of sterile PBS) either immediately or 2 h after LPS injection. Two additional doses were given (i.p.) at 12 and 24 h after the first treatment. * and #, P < 0.05 vs. GST control group as determined by two-tailed Fisher's exact test. •, A box (0-h delay); ▴, A box (2-h delay); ○, GST control. (B) BALB/c mice (15-25 mice per group) were subjected to CLP. At 24 h after the operation, mice were given recombinant HMGB1 A box or GST control (60 or 600 μg per mouse per injection, i.p.) twice daily for 3 days, beginning 24 h after surgery. Animal survival was monitored for up to 2 weeks to ensure that no late death occurred. *, P < 0.03 vs. GST control as tested by two-tailed Fisher's exact test. ▴, A box 60 μg per mouse (24-h delay); •, A box 600 μg per mouse (24-h delay); ○, GST control.
The survival advantage provided by A box treatment was associated with a reduction of serum TNF levels as compared with controls at 32 h after cecal puncture (Table 1). In contrast, serum levels of IL-6 were significantly increased in septic mice treated with A box (P < 0.05 vs. control). Splenic bacterial counts measured in animals treated with A box or irrelevant GST vector protein were not significantly different 32 h after surgery (Table 1). Significantly lower serum HMGB1 levels were observed in A box-treated mice. The mechanism of this A box-mediated decrease of HMGB1 is unknown, but it is plausible that reduced cell death decreases HMGB1 release, because dying cells are a major source of proinflammatory HMGB1 (24). Evidence of reduced tissue and organ damage in A box-treated mice was obtained by measuring significantly reduced serum levels of lactate dehydrogenase, blood urea nitrogen, and creatinine in A box-treated endotoxemic or septic mice, as compared with control groups treated with GST vector alone (Table 2). A box was not toxic to animals, even at doses as high as 4 mg per mouse (i.p.) (data not shown). Thus, the HMGB1 antagonist A box confers significant protection against sepsis-induced tissue injury and lethality, even when first administered 24 h after operation to induce peritonitis.
Table 2. Effects of A box on serum levels of blood urea nitrogen (BUN), creatinine (CRE), and lactate dehydrogenase (LDH) in mice subjected to endotoxemia or peritonitis.
| Assay | Normal | LPS | LPS + A box | CLP | CLP + A box |
|---|---|---|---|---|---|
| BUN, mg/dl | 15 ± 2 | 73 ± 14 | 24 ± 3* | 47 ± 9 | 27 ± 3* |
| CRE, mg/dl | 0.2 ± 0.08 | 1.3 ± 0.7 | 0.3 ± 0.04* | 0.4 ± 0.07 | 0.2 ± 0.05* |
| LDH, units/liter | 594 ± 68 | 2,277 ± 179 | 1,187 ± 137* | 2,778 ± 404 | 1,756 ± 113* |
BALB/c mice, subjected to an LD75 dose of LPS or cecal perforation, received A box treatment (600 μg per mouse, i.p.) at 0 and 12 h after LPS injection or 24 and 31 h after surgery, and were killed at 14 h (LPS model) or 33 h (CLP model) after surgery. Control mice received injections of matched doses of GST protein. Data are presented as mean ± SEM with three mice in the normal group and five to seven mice with each treatment. *, P < 0.05 vs. control (LPS or CLP alone) group.
It now appears that HMGB1 contributes directly to the development of lethality in sepsis. Moreover, the kinetics of serum HMGB1 accumulation enables therapy against this cytokine to be delayed for as much as 24 h after surgery to induce peritonitis. To date, no other pathogenic mediators of sepsis have been specifically targeted this late in the course of sepsis to rescue animals from lethality. By comparison, administration of anti-TNF antibodies can actually increase mortality in cecal perforation, and anti-macrophage migration inhibitory factor antibodies are ineffective if administered >8 h after cecal perforation (23, 25). Widening the therapeutic window to 24 h is a critical necessity. It is interesting to consider that HMGB1 activates tissue-type plasminogen activator (26); recently, the focus on the development of drugs that interfere with activation of blood coagulation has been intense (27). It will be important to delineate the connection between neutralization of HMGB1 and coagulation mechanisms, because these two systems occupy a critical final common pathway to tissue injury and death from sepsis. It is not known whether the A box is produced endogenously as a discrete protein in vivo, but the formation of this product by proteolytic degradation at the site of inflammation could provide a regulatory influence to protect against HMGB1-mediated toxicity. Combined with our previous findings that HMGB1 is an important late mediator in endotoxemia (11) and arthritis (28-30), the data presented here indicate a critical role of HMGB1 in pathogenesis of sepsis. Antibodies or A box can neutralize the cytokine activity of HMGB1 in sepsis, and either treatment is successful in rescuing animals with elevated HMGB1 levels and sepsis. It should now be possible to design clinical studies of sepsis patients who have elevated HMGB1 levels to assess the effects of HMGB1 antagonists.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institute of General Medical Sciences, the Defense Advanced Research Projects Agency, and Critical Therapeutics, Inc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CLP, cecal ligation and puncture; LPS, lipopolysaccharide; TNF, tumor necrosis factor; HMGB1, high mobility group box 1.
References
- 1.Munford, R. S. & Pugin, J. (2001) Am. J. Respir. Crit. Care Med. 163, 316-321. [DOI] [PubMed] [Google Scholar]
- 2.Angus, D. & Wax, R. S. (2001) Crit. Care Med. 29, Suppl. 7, S109-S116. [DOI] [PubMed] [Google Scholar]
- 3.Friedman, G., Silva, E. & Vincent, J. L. (1998) Crit. Care Med. 26, 2078-2086. [DOI] [PubMed] [Google Scholar]
- 4.Tracey, K. J., Beutler, B., Lowry, S. F., Merryweather, J., Wolpe, S., Milsark, I. W., Hariri, R. J., Fahey, T. J., III, Zentelia, A., Albert, J. D., et al. (1986) Science 234, 470-474. [DOI] [PubMed] [Google Scholar]
- 5.Tracey, K. J., Fong, Y., Hesse, D. G., Manogue, K. R., Lee, A. T., Kuo, G. C., Lowry, S. F. & Cerami, A. (1987) Nature 330, 662-664. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello, C. A. (1994) FASEB J. 8, 1314-1325. [PubMed] [Google Scholar]
- 7.Nathan, C. F. (1987) J. Clin. Invest. 79, 319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong, Y., Tracey, K. J., Moldawer, L. L., Hesse, D. G., Manogue, K. B., Kenney, J. S., Lee, A. T., Kuo, G. C., Allison, A. C., Lowry, S. F. & Cerami, A. (1989) J. Exp. Med. 170, 1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riedemann, N. C., Guo, R.-F. & Ward, P. A. (2003) Nat. Med. 9, 517-524. [DOI] [PubMed] [Google Scholar]
- 10.Abraham, E., Laterre, P. F., Garbino, J., Pingleton, S., Butler, T., Dugernier, T., Margolis, B., Kudsk, K., Zimmerli, W., Anderson, P., et al. (2001) Crit. Care Med. 29, 503-510. [DOI] [PubMed] [Google Scholar]
- 11.Wang, H., Bloom, O., Zhang, M., Vishnubhakat, J. M., Ombrellino, M., Che, J., Frazier, A., Yang, H., Ivanova, S., Borovikova, L., et al. (1999) Science 285, 248-251. [DOI] [PubMed] [Google Scholar]
- 12.Andersson, U., Wang, H., Palmblad, K., Aveberger, A.-C., Bloom, O., Erlandsson-Harris, H., Janson, A., Kokkola, R., Yang, H. & Tracey, K. J. (2000) J. Exp. Med. 192, 565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, H., Vishnubhakat, J. M., Bloom, O., Zhang, M., Ombrellino, M., Sama, A. & Tracey, K. J. (1999) Surgery 126, 389-392. [PubMed] [Google Scholar]
- 14.Abraham, E., Arcaroli, J., Carmody, A., Wang, H. & Tracey, K. J. (2000) J. Immunol. 165, 2950-2954. [DOI] [PubMed] [Google Scholar]
- 15.Degryse, B., Bonaldi, T., Scaffidi, P., Muller, S., Resnati, M., Sanvito, F., Arrigoni, G. & Bianchi, M. E. (2001) J. Cell Biol. 152, 1192-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sappington, P., Yang, R., Yang, H., Tracey, K. J., Delude, R. L. & Fink, M. P. (2002) Gastroenterology 123, 790-802. [DOI] [PubMed] [Google Scholar]
- 17.Ulloa, L., Ochani, M., Yang, H., Tanovic, M., Halperin, D., Yang, R., Czura, C. J., Fink, M. P. & Tracey, K. J. (2002) Proc. Natl. Acad. Sci. USA. 99, 12351-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, J., Kokkola, R., Tabibzadeh, S., Yang, R., Ochani, M., Qiang, X., Harris, H. E., Czura, C. J., Wang, H.-C., Ulloa, L., et al. (2003) Mol. Med. 9, 37-45. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, H., Egan, J. M., Wang, Y., Moyes, C. D., Roth, J., Montrose, M. H. & Montrose-Rafizadeh, C. (1998) Am. J. Physiol. 275, C675-C683. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi, M., Bloom, O., Raabe, T., Cohen, P. S., Chesney, J., Sherry, B., Schmidtmayerova, H., Calandra, T., Zhang, X., Bukrinsky, M., et al. (1996) J. Exp. Med. 183, 927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wichmann, M. W., Haisken, J. M., Ayala, A. & Chaudry, I. H. (1996) J. Surg. Res. 65, 109-114. [DOI] [PubMed] [Google Scholar]
- 22.Villa, P., Meazza, C., Sironi, M., Bianchi, M., Ulrich, P., Botchkina, G., Tracey, K. J. & Ghezzi, P. (1997) J. Endotoxin Res. 4, 197-204. [Google Scholar]
- 23.Calandra, T., Echtenacher, B., Roy, D. L., Pugin, J., Metz, C. N., Hultner, L., Heumann, D., Mannel, D., Bucala, R. & Glauser, M. P. (2000) Nat. Med. 6, 164-170. [DOI] [PubMed] [Google Scholar]
- 24.Scaffidi, P., Misteli, T. & Bianchi, M. E. (2002) Nature 418, 191-195. [DOI] [PubMed] [Google Scholar]
- 25.Remick, D., Manohar, P., Bolgos, G., Rodriguez, J., Moldawer, L. & Wollenberg, G. (1995) Shock 4, 89-95. [DOI] [PubMed] [Google Scholar]
- 26.Parkkinen, J. & Rauvala, H. (1991) J. Biol. Chem. 266, 16730-16735. [PubMed] [Google Scholar]
- 27.Bernard, G. R., Vincent, J. L., Laterre, P. F., LaRosa, S. P., Dhainaut, J. F., Lopez-Rodriguez, A., Steingrub, J. S., Garber, G. E., Helterbrand, J. D., Ely, E. W. & Fisher, C. J., Jr. (2001) N. Engl. J. Med. 8, 759-762. [DOI] [PubMed] [Google Scholar]
- 28.Kokkola, R., Sundberg, E., Ulfgren, A. K., Palmblad, K., Li, J., Wang, H., Ulloa, L., Yang, H., Yan, X. J., Furie, R., et al. (2002) Arthritis Rheum. 46, 2598-2603. [DOI] [PubMed] [Google Scholar]
- 29.Ulloa, L., Batliwalla, F. M., Andersson, U., Gregersen, P. K. & Tracey, K. J. (2003) Arthritis Rheum. 48, 876-881. [DOI] [PubMed] [Google Scholar]
- 30.Kokkola, R., Li, J., Sundberg, E., Aveverger, A.-C., Palmblad, K., Yang, H., Tracey, K. J., Andersson, U. & Harris, H. E. (2003) Arthritis Rheum. 48, 2052-2058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.