Abstract
This study examined how task (implicit vs. explicit) and semantic relationship (direct vs. indirect) modulated hemodynamic activity during lexico‐semantic processing. Participants viewed directly related, indirectly related, and unrelated prime‐target word‐pairs as they performed (a) an implicit lexical decision (LD) task in which they decided whether each target was a real word or a nonword, and (b) an explicit relatedness judgment (RJ) task in which they determined whether each word‐pair was related or unrelated in meaning. Task influenced both the polarity and neuroanatomical localization of hemodynamic modulation. Semantic relationship influenced the neuroanatomical localization of hemodynamic modulation. The implicit LD task was primarily associated with inferior prefrontal and ventral inferior temporal/fusiform hemodynamic response suppression to directly related (relative to unrelated) word‐pairs, and with more widespread temporal–occipital response suppression to indirectly related (relative to unrelated) word‐pairs. In contrast, the explicit RJ task was primarily associated with left inferior parietal hemodynamic response enhancement to both directly and indirectly related (relative to unrelated) word‐pairs, as well as with additional left inferior prefrontal hemodynamic response enhancement to indirectly related (relative to unrelated) word‐pairs. These findings are discussed in relation to the specific neurocognitive processes thought to underlie implicit and explicit semantic processes. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, semantic priming, semantic memory, language, fusiform: left inferior frontal, temporal cortex, lexical decision, relatedness judgment
INTRODUCTION
It has long been recognized that words and concepts that have been frequently encountered together and/or that share perceptual or functional features are structured and organized according to such associations and common features within semantic memory. There have been two main approaches to understanding how this organized structure impacts upon processing new incoming words. The first is to present participants with word‐pairs or lists but not to alert them to the presence of any semantic relationship between these words, and to examine the effects of any semantic relationship between them on their performance of an incidental implicit task. The second is to alert participants to the existence of potential semantic relationships between word‐pairs or lists and to ask them to use such relationships to perform a more explicit task. The current study used functional magnetic resonance imaging (fMRI) to examine the neural basis of implicit and explicit semantic processing of the same words in the same participants. We tested the overall hypothesis that both the task performed by subjects (implicit vs. explicit) and the nature of the semantic relationship between pairs of words (directly related, indirectly related, and unrelated) would influence both the neuroanatomical localization and the polarity of hemodynamic modulation. Understanding these relationships between task, semantic relationship, and brain activity may give new insights into whether distinct semantic cognitive processes are mediated by distinct neuroanatomical networks. This, in turn, may help explain the cognitive basis of abnormal patterns of hemodynamic activity observed during semantic processing in patients with neuropsychiatric disorders including schizophrenia [Kuperberg et al., 2007] and Alzheimer's disease [Grossman et al., 2003].
Implicit Semantic Processing
The most common paradigm used to explore implicit semantic processing is the semantic priming paradigm in conjunction with an implicit task. The semantic priming effect describes the shorter reaction times [Meyer and Schvaneveldt, 1971; Neely, 1991] or the attenuated electrophysiological response (the N400 event‐related potential) [Bentin et al., 1985; Rugg, 1985] to target words (e.g. “tiger”) that are preceded by semantically related prime words (e.g. “lion”) relative to semantically unrelated prime words (e.g. “truck”). One implicit task often used to study semantic priming is lexical decision (LD). In this task, letter‐strings are randomly introduced as targets to unrelated primes, and participants are simply asked to decide whether or not each target that they encounter is a real word or a nonword [Meyer and Schvaneveldt, 1971]. The semantic priming effect observed during LD can reflect the operation of automatic processes such as a spread of activation across semantic memory [Anderson, 1983; Collins and Loftus, 1975], and/or controlled processes such as the generation of expectancies of which word will be presented next [Becker, 1980], and postlexical attempts to match prime and target according to prior associations or shared semantic features that are stored within semantic memory [Neely et al., 1989]. The degree to which each of these processes contribute to the semantic priming effect depends on various experimental parameters such as the proportion of related prime‐target pairs in a stimulus set (the relatedness proportion, RP) and the time interval between the onset of the prime and the onset of the target (the stimulus onset asynchrony, SOA). It also depends on the nature of the semantic relationship between the prime and the target. For example, when the SOA is relatively long (more than ∼400 ms), and the prime and target are directly related, controlled expectancy and semantic matching processes are the main contributors to the semantic priming effect. However, when the SOA is long and the prime and target are only indirectly related (connected through an unseen mediator word, e.g. “lion” and “stripes”, connected via “tiger”), behavioral semantic priming is usually not seen [Balota and Lorch, 1986; Chwilla and Kolk, 2002; Chwilla et al., 2000; Hill et al., 2002; McNamara and Altarriba, 1988; Silva‐Pereyra et al., 1999]. This is because an indirectly related target cannot easily be predicted from its prime, and because, in many trials, the semantic relationship between the prime and target is not obvious and the mediator word cannot easily be retrieved from semantic memory and used to bias the lexical decision [discussed by Neely, 1991]. Interestingly, however, some electrophysiological indirect priming may still be detected, even in the absence of behavioral indirect priming [Chwilla et al., 2000], perhaps indexing some effects of automatic spreading activation at the neural level.
More recently, several functional neuroimaging studies have described an attenuation of hemodynamic activity across widespread, but variable, regions within temporal and/or inferior prefrontal cortices in response to directly related, relative to unrelated, word‐pairs during semantic priming paradigms [Copland et al., 2003; Giesbrecht et al., 2004; Gold et al., 2006; Matsumoto et al., 2005; Rissman et al., 2003; Wheatley et al., 2005]. This attenuation of hemodynamic activity—termed 'hemodynamic response suppression' [Henson, 2003; Henson and Rugg, 2003]—has often been interpreted as reflecting the reduced neurocognitive activity required to process primed targets [Wiggs and Martin, 1998] and mirrors the attenuation of the behavioral and electrophysiological responses to primed targets.
There is some variability between fMRI studies in the precise neuroanatomical localization of hemodynamic response suppression observed during semantic priming. Several factors may account for such variability, including the modality of stimulus presentation [visual, e.g. Rossell et al., 2003, vs. auditory, e.g. Kotz et al., 2002], the nature of the semantic relationship between prime and target [associative vs. categorical, e.g. Kotz et al., 2002], as well as experimental parameters, such as the SOA, that bias towards automatic versus controlled processes. For instance, there is some evidence that, at short SOAs, hemodynamic response suppression within the temporal fusiform cortices (Brodmann area, BA 37) reflects the effects of an automatic spreading activation [Gold et al., 2006; Wheatley et al., 2005], while at longer SOAs, hemodynamic response suppression within the left anterior inferior prefrontal cortex (BA 47) may reflect the facilitory effects of controlled semantic expectations [Gold et al., 2006]. These distinctions, however, are not absolute. For example, Copland et al. [2003] and Wheatley et al. [2005] reported response suppression within the left inferior frontal cortex at short SOAs. And, at long SOAs, several studies have reported modulation within temporal cortices [Gold et al., 2006; Matsumoto et al., 2005; Rissman et al., 2003; Wible et al., 2006]. Moreover, Rossell et al. [2003] failed to show any effect of SOA on suppression within either the inferior prefrontal or fusiform cortices.
Hemodynamic response suppression is not the only pattern of hemodynamic modulation seen in neuroimaging studies of semantic priming using a LD task. In some studies, at long SOAs, it is accompanied by increases in the hemodynamic response to semantically related relative to unrelated word‐pairs in other regions [Kotz et al., 2002; Mummery et al., 1999; Rossell et al., 2003; Wible et al., 2006]. Indeed, sometimes such increases are the only pattern of modulation observed [Raposo et al., 2006]. These increases in the hemodynamic response to primed relative to unprimed targets are known as hemodynamic response enhancement and are thought to reflect the engagement of neurocognitive processes that occur selectively on primed targets [Henson, 2003; Henson and Rugg, 2003]. There are some consistencies in the localization of such hemodynamic response enhancement reported in semantic priming paradigms: for example, several studies have reported response enhancement within parietal cortices (BAs 40 and 7) [Kotz et al., 2002; Raposo et al., 2006; Rossell et al., 2003; Wible et al., 2006]. This is interesting because such regions constitute part of an attentional circuitry [Behrmann et al., 2004; Chein et al., 2003; Cristescu et al., 2006] that may be specifically engaged as participants attempt to match semantic associations and common semantic features between prime and target after both have been recognized, i.e. postlexically.
Explicit Semantic Processing
In a relatedness judgment (RJ) task, participants are explicitly told to search for semantic features or associations that are shared between pairs or groups of words and to use such associations or features to determine whether or not the words are related to each other. Such a task therefore taps directly into postlexical semantic matching processes. When participants perform RJ, it takes longer to conclude that a word‐pair is unrelated than that it is semantically related [Faust and Lavidor, 2003; Zwaan and Yaxley, 2003]. And, in ERP studies, asking participants to attend to the semantic relationships between prime and target tends to increase the magnitude of the N400 effect produced, both using word stimuli [Holcomb, 1988] and picture stimuli [McPherson and Holcomb, 1999]. More recently, we have also shown that, in comparison with an implicit semantic word‐monitoring task, an explicit RJ task also increases the size of the N400 effect produced by indirectly related, relative to unrelated, targets [Kreher et al., 2006].
In fMRI studies, a RJ task has been used in a variety of different paradigms exploring the functional neuroanatomy underlying semantic processing. In most such studies, however, activity has been collapsed across different types of semantic relationships [Thompson‐Schill et al., 1997; Vandenberghe et al., 1996], making it difficult to infer which brain regions are recruited in specific association with related vs. unrelated word‐pairs during explicit semantic processing. Nonetheless, there is some evidence that inferior parietal regions (BA 40) are engaged as participants make explicit similarity judgments about objects with common semantic features [Grossman et al., 2002; Koenig et al., 2005]. And there is also evidence that, during a RJ task, the left inferior prefrontal cortex (BAs 45 and 47) is recruited in association with word‐pairs [Fletcher et al., 2000] and word‐triplets [Sabsevitz et al., 2005] that are more distantly (vs. more closely) semantically related.
The Present Study
In summary, there is some evidence from previous neuroimaging studies that hemodynamic response suppression during implicit semantic processing might reflect the effects of a spread of activation under automatic experimental conditions (fusiform suppression), and of predictive strategies under controlled experimental conditions (left inferior frontal suppression), while hemodynamic response enhancement (within left inferior parietal and inferior frontal cortices) might reflect postlexical semantic matching processes, also occurring under controlled experimental conditions. Such postlexical matching processes would be maximally engaged as participants carry out explicit RJs. To date, however, no study has examined the effects of task and semantic relationship on the hemodynamic modulation in the same participants, using the same stimuli and the same experimental parameters.
In the present study, the same participants viewed the same directly related, indirectly related, and unrelated word‐pair stimuli as they performed both an implicit LD task and an explicit RJ task, using a long SOA that biased towards controlled processing. Both semantic relatedness and task, however, were counterbalanced such that no single participant saw the same word more than once. Based on the findings by Gold et al. [2006], we predicted that, during the LD task, the directly related (relative to unrelated) word‐pairs would be associated with both faster RTs and with hemodynamic response suppression within the left anterior inferior prefrontal cortex (BA 47), reflecting the facilitory effects of strategic semantic expectancies on processing. We predicted that indirectly related (relative to unrelated) word‐pairs would neither be associated with faster RTs nor with left inferior prefrontal cortex response suppression because such expectancy strategies would be ineffective. Based on the findings of Grossman et al. [2002] and Koenig et al. [2005], during the explicit RJ task, we predicted that participants' attention to semantic relationships between the directly and indirectly related word‐pairs (relative to the unrelated word‐pairs) would be reflected by hemodynamic response enhancement within the left inferior parietal cortex (BA 40). Based on the findings by Fletcher et al. [2000] and Sabsevitz et al. [2005], we also predicted that participants' explicit attempts to retrieve the mediator linking the indirectly related word‐pairs would be additionally associated with response enhancement within the left inferior prefrontal cortex.
METHODS
Design and Stimulus Materials
The stimuli were designed such that they could be counterbalanced both across the two tasks (LD and RJ) and across the three relatedness conditions (directly, indirectly, and unrelated). To counterbalance in this way, three hundred word triplets were developed such that target words (e.g. “stripes”) were paired with directly related primes (e.g. “tiger”) and indirectly related primes (e.g. “lion”). Word‐triplets were taken from those used in previous published studies [Balota and Lorch, 1986; McNamara and Altarriba, 1988; Weisbrod et al., 1999] or else were developed for the current study.
In 113 of these triplets, we conducted a free association experiment [described by Kreher et al., 2006, Experiment 3] in which 30 participants who did not participate in the fMRI experiment generated five associates to either the primes from the directly related word‐pairs, the primes from the indirectly related word‐pairs, or to the target words. The directly related targets were almost always generated as associates while the unrelated targets were never generated as associates from the primes of the directly related word‐pairs. The theoretical mediating words or the primes of the directly related word‐pairs were often generated from the primes of the indirectly related word‐pairs, while the targets of the indirectly related word‐pairs were almost never generated from the primes of the indirectly related word‐pairs [for details, see Kreher et al., 2006]. In the additional 187 triplets, all the directly related word‐pairs, but none of the indirectly or unrelated word‐pairs, had an associative strength on the Edinburgh Associative Thesaurus [Coltheart, 1981] of greater than zero; and, again, the associative strength of the indirect primes to their theoretical mediating words was much greater than the associative strength between the indirectly related word‐pairs.
A second norming study established that, although individuals generally generated the mediator words of the indirectly related word‐pairs when given both prime and target, they did not generate the mediator word when they were just given the prime. Finally, 15 subjects who did not take part in the fMRI experiment conducted a RJ task on the word‐pairs in which they were asked to rate how related in meaning they were on a five‐point scale using three counterbalanced lists; the directly‐related word‐pairs the (mean = 4.41, SD = 0.56) were rated as being more related in meaning than the indirectly related word‐pairs (mean = 3.11, SD = 0.60) [t(299) = 27.207, P < 0.01], which were, in turn, rated as more related in meaning than the unrelated word‐pairs (mean = 1.45, SD = 0.37) [t(299) = 40.579, P < 0.01].
These word triplets were then used to counterbalance targets across the six lists in a Latin Square design. Each participant saw one list during the LD task and one list during the RJ task. This ensured that no individual would see the same prime or target more than once (avoiding repetition priming effects), but that, across all participants, exactly the same targets would be seen in all three relatedness conditions in both tasks, and that, across all participants, exactly the same primes would be viewed in the directly related and the unrelated conditions. Thus, in each of the six lists there were 150 pairs: 50 directly related pairs, 50 indirectly related pairs, and 50 unrelated pairs. In addition, the frequency and number of letters of both primes and targets across the six lists (and the three relatedness conditions) was the same (no main effect of list or no list by relatedness interaction, Ps > 0.5). Then, to each list, 50 word–nonword trials were added. All nonword targets were phonologically permissible strings in English and they were all derived from words that were unrelated to their primes. The nonwords were also counterbalanced across the LD and RJ tasks (they were included in the RJ task so that, counterbalanced across all participants, exactly the same stimulus lists could be used in both tasks).
Given that, on the RJ task, participants classified 50% of the indirectly related word‐pairs as related and 50% as unrelated (reported in the Results), the RP was ∼0.5. The nonword ratio (the number of word–nonword‐pairs/word–nonword‐pairs + unrelated pairs) [Neely et al., 1989] was 0.4. An example stimulus set is given in Table I.
Table I.
Example of word pairs, counterbalanced across conditions, derived from the triplet “lion–tiger‐stripes”
| Experimental condition | Example | Frequency | Word length |
|---|---|---|---|
| Directly related | tiger‐stripes | Prime: 70 (133) | Prime: 5 (1) |
| Target: 101 (431) | Target: 5 (1) | ||
| Indirectly related | lion‐stripes | Prime: 75 (253) | Prime: 5 (1) |
| Target: 101 (431)a | Target: 5 (1)a | ||
| Unrelated | chair‐stripes | Prime: 70 (133)a | Prime: 5 (1)a |
| Target: 101 (431)a | Target: 5 (1)a | ||
| Word–nonword | skate‐soble | Prime: 74 (121) | Prime: 5 (1) |
| Target: NA | Target: 5 (1) |
NA: not applicable. Means are shown with standard deviations in brackets.
These values are exactly the same as the directly related condition because of how, across subjects, these words were counterbalanced.
FMRI Study
Participants
Participants were recruited by advertisement. All were right‐handed as assessed using the modified Edinburgh Handedness Inventory [Oldfield, 1971; White and Ashton, 1976]. Selection criteria required all participants to have normal or corrected‐to‐normal vision, to be native speakers of English, and to have learned no other language before the age of five. In addition, volunteers were not taking any medication and were screened to exclude the presence of psychiatric and neurological disorders and to exclude contraindications for MRI. Written consent was obtained from all subjects before participation according to the established guidelines of the Massachusetts General Hospital Institutional Review Board. Two subjects were excluded because of scanning artifacts and one subject was excluded because his behavioral performance was at chance. This left sixteen participants in total (14 males and 2 females; mean age: 42).
Stimulus presentation and tasks
During scanning, each participant viewed one list during the LD task and one list during the RJ task (lists were fully counterbalanced across participants as explained above). Each list was divided into three functional runs, each lasting 4 min and 10 s. The LD task was performed during the first three functional runs, and the RJ task was performed during the second three functional runs. The LD task always took place before the RJ task so that participants were not explicitly alerted to the semantic relationships between the word‐pairs that could potentially bias their lexical decisions.1
During the LD task, subjects decided as quickly and as accurately as possible whether the target was a real English word or a nonword. During the RJ task, subjects decided as quickly and as accurately as possible whether the target was related or unrelated in meaning to the prime. Participants were explicitly told that, when they saw target nonwords during the RJ task, they should indicate that these were not related in meaning to the primes. In both tasks, participants indicated their decisions by pressing one of two buttons using the index or middle fingers of their left hand (counterbalanced across subjects). Participants were practiced on the LD task before scanning and on the RJ task inside the scanner after carrying out the LD task. Subjects' accuracy and reaction times (RTs) on both tasks were recorded.
In both tasks, each trial began with the prime (500 ms), a blank screen (300 ms), a target (500 ms), and then another blank screen (300 ms). Thus, the SOA was 800 ms. Between word‐pairs, a question mark appeared (1,100 ms) followed by a blank screen (300 ms). The four trial types appeared in pseudorandom order, in all runs, interspersed among 100 visual fixation trials (fixate on a “+” for variable durations of 1,000–8,000 ms, mean: 3,000 ms). The random interleaving of these fixation or “null‐events” among the word‐pairs enabled the efficient estimation and deconvolution of the entire hemodynamic response [Burock et al., 1998].
MRI data acquisition
Subjects underwent two structural scans on a 1.5 T scanner (Siemens Medical Solutions, Iselin, NJ), each constituting a 3D MPRAGE sequence (128 sagittal slices, 1.3 mm thickness, TR: 7.25 ms, TE: 3 ms, flip angle: 7°, bandwidth: 195 Hz/pixel, in‐plane resolution: 1.3 mm × 1 mm). Functional imaging took place in a 3.0 T head‐only Siemens Allegra scanner. Blood oxygen level dependent (BOLD) signal was imaged using a T2*‐weighted gradient‐echo pulse sequence (TR: 2 s, TE: 25 ms, flip angle: 90°) with 33 transverse slices covering the whole brain (125 images per slice, 3 mm thickness, 0.9 mm between slices). The in‐plane resolution was 3.13 mm × 3.13 mm (64 × 64 matrix, 200 mm FOV). One hundred and twenty five images were acquired during each functional run for a total run time of 4 min 10 s. Head motion was minimized using pillows and a forehead strap. The first four volumes of each functional run were discarded to allow the magnetization to equilibrate.
Behavioral Data Analysis
Accuracy
On the LD task, the frequencies with which nonwords were classified as words (false positive errors) and with which words were classified as nonwords (false negative errors) are reported. On the RJ task, the frequency with which the unrelated words were classified as related (false positive errors) and with which the related words were classified as unrelated (false negative errors) are reported. In addition, the frequency with which the indirectly related words were classified as unrelated are reported. Note that the judgments of the indirectly related word‐pairs were subjective—they could be judged as related or unrelated depending on whether, within the time period given, participants were able to retrieve a potential mediator. They therefore cannot be considered correct responses or errors per se.
Reaction times
Given our a priori predictions, we performed planned repeated‐measures 2 (task) × 2 (relatedness) ANOVAs that contrasted (a) the directly related and the unrelated word‐pairs, (b) the indirectly related and the unrelated word‐pairs, and (c) the directly related and the indirectly related word‐pairs. Planned paired t‐tests within the LD or RJ tasks were conducted to examine the source of any interactions between task and relatedness. Both subjects analyses (in which RTs were averaged over all items in each relatedness condition) and items analyses (in which RTs were averaged over all subjects in each relatedness condition) were conducted. In both subjects and items analyses, task and relatedness were within‐subject factors. In all ANOVAs and t‐tests, the dependent variable was RTs to the correctly‐answered trials: For the LD task, these were the trials on which the targets were correctly classified as words; for the RJ task, these were the trials on which participants classified the directly related and the indirectly related word‐pairs as related, and the unrelated word‐pairs as unrelated. Because, as discussed above, during the RJ task, the decision as to whether the indirectly related words were related or unrelated was subjective, all analyses that involved the RJ task were repeated (a) including all RTs to indirectly related word‐pairs, regardless of how they were classified in the RJ task, and (b) including RTs to indirectly related word‐pairs that were judged as unrelated in the RJ task.
Alpha was set to 0.05. All analyses were repeated after logarithmically transforming the data and yielded the same pattern of findings.
fMRI Analysis
To increase the signal:noise ratio, the two structural scans for each participant were averaged together, after motion correction, to create a single volume.2 This resulting high signal:noise volume was then subject to an automated segmentation procedure by which the surface representing the gray/white border was reconstructed and inflated to yield a 2D representation of the cortical surface [Dale et al., 1999; Dale and Sereno, 1993; Fischl et al., 2001] using FreeSurfer software developed at the Martinos Center, Charlestown, MA (http://surfer.nmr.mgh.harvard.edu/).
Functional images were motion corrected using the AFNI algorithm [Cox, 1996; Cox and Jesmanowicz, 1999]. Images were corrected for temporal drift, normalized and spherically smoothed using a 3D spatial filter (full‐width‐half‐max: 8.7 mm). The functional images were then analyzed with a General Linear Model (GLM) using a finite impulse response (FIR) model, using FreeSurfer Functional Analysis Stream (FS‐FAST). The FIR model gave estimates of the hemodynamic response every 1 s as stimuli were allowed to onset on half as well as the full 2 s TR. It allowed us to address our hypotheses without assumptions about the shape of the hemodynamic response [Burock and Dale, 2000; Burock et al., 1998; Dale, 1999].
The cortical surface of each individual was morphed/registered on to an average spherical surface representation to align sulci and gyri across subjects [Fischl et al., 1999a, b]. This structural spherical transform was used to map the GLM parameter estimates and residual error variances of each participant's functional data to a common spherical coordinate system [Fischl et al., 1999a, b]. Each participant's data was then smoothed on the surface tessellation using an iterative nearest‐neighbor averaging procedure, equivalent to applying a two‐dimensional Gaussian smoothing kernel with a FWHM of ∼8.5 mm. Because this smoothing procedure was restricted to the cortical surface, averaging data across sulci or outside gray matter was avoided.
BOLD activity to correctly‐answered trials was examined in the LD task (i.e. the trials on which the targets were correctly classified as words). In the RJ task, BOLD activity was examined to correctly‐answered unrelated and related trials and to the indirectly related trials that were classified as related. However, because relatedness decisions to these indirectly related word‐pairs is subjective, we also examined BOLD activity in the RJ task to all indirectly related word‐pairs (regardless of how they were classified), as well as to indirectly related word‐pairs that were judged as unrelated. We note any differences in the findings revealed by these different analyses.
Because the LD and RJ tasks may have engaged neural processes at different latencies, we first examined the hemodynamic time courses that were generated during each of these tasks and to each type of word‐pair, without any assumption about their overall shapes. These hemodynamic time courses were generated by averaging activity across voxels within temporal and prefrontal regions of interest at each TR (using the FIR model) and across all participants, see Figure 1. The time window that captured the peak of this hemodynamic response across the two tasks and the three relatedness conditions was ∼3–6 s. Therefore, all the statistical maps described below were constructed by summing activity at each voxel across this time‐epoch.
Figure 1.
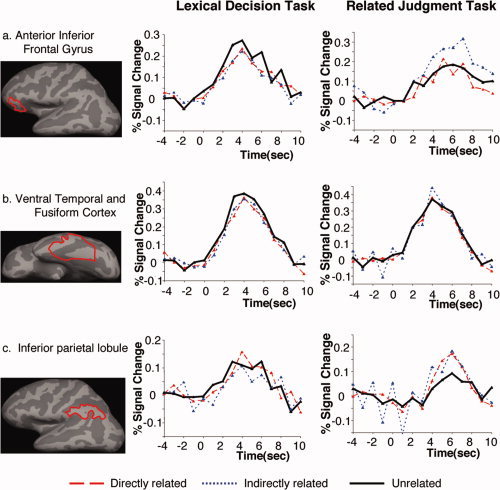
Hemodynamic time courses within a priori regions of interest, showing modulation of activity to directly related, indirectly related, and unrelated word‐pairs in the LD and RJ tasks.
We first constructed a statistical map examining the regions activated across both tasks relative to the low‐level baseline fixation condition. We also determined whether any of these regions were differentially modulated across the two tasks. We then constructed statistical maps based on planned 2 (Relatedness: directly related vs. unrelated, or indirectly related vs. unrelated) × 2 (Task: LD vs. RJ) repeated measures ANOVAs to show the main effects of Relatedness as well as Task by Relatedness interactions. In these analyses, only “highest order” effects are shown/reported. In other words, clusters that we report as showing main effects for a particular factor are those that failed to show task by relatedness interactions. To determine the sources of any significant task by relatedness interactions as well as to examine hemodynamic modulation by semantic relationship within the LD and RJ tasks within regions that did not show significant main effects or interactions in the overall ANOVA maps, we also constructed statistical maps comparing the directly related and unrelated word‐pairs and comparing the indirectly related and unrelated word‐pairs for the LD and RJ tasks separately. Finally, we constructed statistical maps that directly contrasted the directly and indirectly related word‐pairs for each of the LD and RJ tasks. This enabled us to determine the specificity of any hemodynamic modulation to directly vs. indirectly related word‐pairs.
Correction for multiple comparisons depended on whether voxels fell within or outside a priori regions of interest (Fig. 1). Within regions of interest (Fig. 1), P values for sets of contiguous voxels (clusters) were computed using a permutation [Nichols and Holmes, 2002] with 10,000 iterations; a cluster was only considered significant if, on this permutation, its significance was less than P = 0.05. These clusters are indicated with a * in Tables VII and VIII. Outside regions of interest, we also report clusters that covered at least 300 mm2, with a corrected threshold for rejection of the null hypothesis of P < 0.05, identified on the basis of a Monte Carlo simulation across the whole cortical surface [Doherty et al., 2004]. These clusters are indicated with a # in Tables VII and VIII.
Table VII.
Hemodynamic modulation: Directly related vs unrelated
|
A (numbers correspond to clusters in Figure 3A) |
Main effects: Response Suppression | Lexical Decision: Response Suppression | Relatedness Judgment: Response Suppression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Region | Lat. | BA | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p |
| 1 | Inferior temporal/fusiform gyrus | L | 37 | 162 | −54, −49, −18 | 0.0022* | 164 | −54, −51, −14 | 0.0005* | 95 | −47, −48, −5 | 0.00012† |
| 2 | Occipito‐parietal junction | R | 19/7 | 382 | 29, −83, 34 | 0.0056# | 570 | 26, −75, 40 | 0.0005# | — | — | NS |
| 3 | Interiror frontal gyrus | L | 47 | — | — | NS | 205 | −44, 33, −10 | 0.0012* | — | — | NS |
| 4 | Anterior orbito‐frontal cortex** | R | 10/11 | — | — | NS | 363 | 19, 53, −9 | 0.00001# | NS | ||
|
B (number corresponds to clusters in Figure 4A) |
Task by Relatedness Interaction | Lexical Decision | Relatedness Judgment: Response Enhancement | |||||||||
| 5 | Inferior parietal lobule | L | 40 | 661 | −50, −45, 30 | 0.0024*# | — | — | NS | 920 | −59, −49, 36 | 5.1 e−5*# |
When clusters or Brodmann areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a slash sign. Talairach coordinates of peak activation correspond to the local minimum P values for each cluster of activated vertices on the cortical surface.
Regions marked with ** if they also showed significant response suppression in a direct comparison between related and indirectly related word‐pairs. Clusters indicated in P column marked * if reached significance on small surface correction (P < 0.05) and/or marked # if reached cluster‐level significance (P < 0.05) across whole cortical surface. Cluster is marked † if failed to reach significance on small surface correction but reached an uncorrected voxel‐wise significance across the cortex, P < 0.05. P value indicated is the minimum P value within that cluster. NS: nonsignificant.
Table VIII.
Hemodynamic modulation: Indirectly‐related vs unrelated
|
A (numbers correspond to clusters in Figure 3B) |
Main effects: Response Suppression | Lexical Decision: Response Suppression | Relatedness Judgment: Response Suppression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Region | Lat. | BA | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p |
| 1 | Ventral temporal: Inf. temp. gyrus | L | 20 | 200 | −56, −20, −19 | 0.0014* | 196 | −58, −21, −18 | 0.0026* | — | — | NS |
| 2 | Fusiform gyrus | L | 37/19 | 141 | −30, −58, −8 | 0.0069* | 523 | −35, −55, −13 | 0.00055# | — | — | NS |
| 3a | Lateral temporal: Inf. temp. gyrus** | L | 20 | 366 | −59, −20, −20 | 0.00093# | 463 | −63, −20, −26 | 0.00116# | — | — | NS |
| 3b | Lateral temporal: Mid. temp. gyrus | L | 21 | — | — | NS | 359 | −53, 5, −13 | 0.00027# | — | — | NS |
| 4 | Parahipp. gyrus | R | 30/35 | — | — | NS | 762 | 14, −43, −2 | 0.00086# | — | — | NS |
| 5a | Extrastriate cortex | L | 18/19 | — | — | NS | 723 | −25, −72, 6 | 0.0004# | — | — | NS |
| 5b | R | 18 | 366 | 28, −86, 17 | 0.00021# | 432 | 30, −84, 18 | 0.0032# | — | — | NS | |
| 5c | 441 | 33, −83, −9 | 0.00075# | 497 | 37, −78, −6 | 0.0013# | — | — | NS | |||
| 6 | Superior parietal lobule/ precuneus | R | 7 | 1029 | 29, −69, 42 | 7.1 e−5# | 360 | 36, −75, 30 | 0.0014# | — | — | NS |
| 7 | Retrosplenial cortex | L | 30 | — | — | NS | 351 | −12, −45, 5 | 0.0005# | — | — | NS |
| 8 | R | 23 | 309 | 20, −54, 20 | 0.0012# | 436 | 16, −53, 20 | 0.0007# | — | — | NS | |
| 9 | Anterior cingulate** | L | 32 | — | — | NS | 361 | −9, 38, −3 | 0.003# | — | — | NS |
| 10a | Insula | L | — | — | — | NS | 543 | 24, 24, 9 | 3.1 e−6# | — | — | NS |
| 10b | R | — | — | NS | 407 | 32, −16, 1 | 0.00036# | — | — | NS | ||
| 11 | Para‐central lobule | L | 4 | — | — | NS | — | — | NS | 389 | −4, −32, 61 | 0.0001# |
|
B (numbers correspond to clusters in Figure 4B) |
Task by Relatedness Interactions | Lexical Decision: Response Suppression | Relatedness Judgment: Response Enhancement | |||||||||
| 12 | Superior & Inferior parietal lobule | L | 1/7/40 | 461 | −33, −65, 46 | 0.0002# | — | — | NS | 382 | −35, −66, 34 | 0.00005# |
| 13a | Inferior frontal gyrus (ant.)** | L | 47 | 185 | −48, 15, 1 | 0.0023* | — | — | NS | 101 | −48, 15, 0 | 0.003* |
| 13b | Inferior frontal gyrus (post.)** | L | 45 | 92 | −26, 18, −13 | 0.0004* | — | — | NS | 143 | −26, 19, −12 | 7.1 e−5* |
When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a slash sign. Talairach coordinates of peak activation correspond to the local minimum P values for each cluster of activated vertices on the cortical surface.
In Panel A, regions marked with ** also showed significant response suppression to the indirectly related word‐pairs than to the directly related word‐pairs. In Panel B, regions marked with ** also showed significant response enhancement to indirectly related word‐pairs than to directly related word‐pairs. Clusters indicated in P column marked * if reached significance on small surface correction (P < 0.05) and/or marked # if reached cluster‐level significance (P < 0.05) across whole cortical surface. P value indicated is the minimum P value within that cluster. NS: non‐significant. Of note, cluster number 11 (the paracentral lobule) only reached cluster‐level significance on RJ analyses that included indirectly‐related word‐pairs that were classified as directly related; it did not reach cluster‐level significance on RJ analyses that included indirectly‐related trials that participants' classified as unrelated or on RJ analyses that included all indirectly‐related trials.
RESULTS
Behavioral Data
Behavioral classifications (Table II)
Table II.
Task accuracy: percentages of errors
| Direct | Indirect | Unrelated | Word–nonwords | |
|---|---|---|---|---|
| Lexical decision (LD) | 3.2 (7.0) | 2.9 (6.0) | 3.4 (7.15) | 6.0 (8.5) |
| Relatedness judgment (RJ) | 10.8 (17.7) | 50.4 (21.0) | 4.6 (4.6) | 0.5 (0.9) |
In the case of the indirectly related word‐pairs where the responses are somewhat subjective and cannot be considered “correct” or “errors,” the percentage of indirectly‐related word‐pairs that were classified as unrelated is given. Means are shown with standard deviations in brackets.
There were no differences in errors (falsely classifying a nonword as a word or erroneously classifying words as nonwords) in the LD task across the four conditions, F(3,42) = 1.50, P = 0.24. A′ scores in all participants in all conditions were more than 0.8, suggesting that there were no response biases. In the RJ task, there was no significant difference in errors to the related word‐pairs (falsely classifying them as unrelated) and the unrelated word‐pairs (falsely classifying them as related), t(15) = 1.221, P = 0.241. The range of errors in the LD task was between 0–8% and in the RJ task was between 0–20%, with the exception of one participant who had on average 26% errors in the LD task and 33% errors in the RJ task. Exclusion of this participant made no difference to the pattern of behavioral findings reported in Table IV. The judgment of the indirectly related word‐pairs was subjective and depended on whether participants were able to identify the mediating word in the time‐interval given: on average, 50% of the indirectly related word‐pairs were classified as unrelated (range: 28–94%).
Table IV.
Reaction times ANOVAs
| Direct vs unrelated | Indirect vs unrelated | Direct vs indirect | ||||
|---|---|---|---|---|---|---|
| Subjects (DOF 1,14) | Items (DOF 1,291) | Subjects (DOF 1,14) | Items (DOF 1,203) | Subjects (DOF 1,14) | Items (DOF 1,202) | |
| Relatedness | 7.26* | 28.32*** | 1.79 (NS) | 0.23 (NS) | 24.2** | 16.09*** |
| Task × Relatedness | 4.77* | 12.64*** | 3.56# | 8.157** | 51.74*** | 36.03*** |
| Task | 44.7*** | 458.2*** | 66.89*** | 501.3*** | 37.57*** | 320.7*** |
P < 0.05.
P < 0.005.
P < 0.0005.
P < 0.1.
NS: not significant.
DOF: Degrees of Freedom.
F values are shown.
Reaction times (Tables III and IV)
Table III.
Reaction times to correctly answered trials (as defined in Table II)
| Subjects analysis | Items analysis | |||||
|---|---|---|---|---|---|---|
| Direct | Indirect | Unrelated | Direct | Indirect | Unrelated | |
| Lexical decision (LD) | 825.6 (164.7) | 838.4 (180.8) | 841.7 (167.1) | 812.3 (176.9) | 827.3 (181.6) | 832.7 (185.2) |
| Relatedness judgment (RJ) | 1007.3 (171.0) | 1163.1 (203.7) | 1103.0 (151.3) | 1006.5 (214.0) | 1152.2 (252.3) | 1107.1 (177.1) |
Mean reaction times in subjects and items analyses in each condition for the LD and RJ tasks are shown with standard deviations in brackets. In the items analysis, RTs to each correctly‐answered word‐pair item were averaged over all subjects. In the subjects analysis, RTs in each subject were averaged over all correctly‐answered items.
Comparison between the unrelated and the directly related word‐pairs revealed a main effect of relatedness and a task by relatedness interaction (Table IV). Follow ups showed that the direct priming effect was greater in the RJ task (significant on both items and subjects analyses, P < 0.0001) than in the LD task (significant on the items analysis, P < 0.05 but not on the subjects analysis).3
Comparison between the unrelated and the indirectly related word‐pairs (in the RJ task, those indirectly‐related word‐pairs that were classified as related) revealed a task by relatedness interaction that approached significance on the subjects analysis and that reached significance on the items analysis. Follow‐ups failed to show priming effects on the LD task but showed reverse priming effects on the RJ task with longer RTs to the indirectly related than to the unrelated word‐pairs that reached significance on the items analysis.4
A direct comparison between RTs to the directly and indirectly related word‐pairs also revealed significant task by relatedness interactions, with follow‐ups confirming longer RTs to the indirectly related word‐pairs than to the related word‐pairs on the RJ task (P < 0.001) but no significant differences on the LD task.
Finally, all three 2 × 2 ANOVAs showed main effects of task reflecting longer RTs in the RJ task than in the LD task.
fMRI Data
As expected, a large network was activated (Table VA) and another network was deactivated (Table VB) in comparing all word‐pairs with the fixation condition (Fig. 2). Additionally, a few of these regions were modulated by task (Table VI). Of most interest, however, were the comparisons between the three relatedness conditions.
Table V.
Hemodynamic modulation to all word‐pairs (versus fixation): main effects across LD and RJ tasks
| A | Main effects: All words > Fixation | Lexical Decision: All words > Fixation | Relatedness Judgment: All words > Fixation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Region | Lat. | BA | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p |
| 1a | Inferior frontal gyrus (ant.) | L | 47/45 | 1176 | −43, 34, 5 | 3.9 e−5 | 927 | −43, 27, −3 | 8.5 e−5 | 840 | −50, 28, 12 | 0.0007 |
| 1b | Inferior frontal cortex (post.) | L | 44/45 | 1388 | −51, 11, 17 | 2.8 e−5 | 542 | −48, 15, 14 | 0.0001 | 366 | −42, 18, 21 | 6.9 e−6 |
| R | 544 | 35, 34, 13 | 0.0008 | 330 | 29, 36, 17 | 0.0012 | — | — | NS | |||
| 2 | Precentral gyrus | L | 6 | 2681 | −43, −1, 34 | 2.1 e−8 | 5257 | −46, −1, 37 | 1.1 e−7 | 3937 | −32, −1, 29 | 5.1 e−7 |
| R | 2582 | 37, −20, 55 | 2.2 e−6 | 2042 | 41, −13, 49 | 1.9 e−5 | 1220 | 38, −20, 56 | 2.4 e−7 | |||
| 3 | Superior parietal lobule & intrapariet.sulcus (post.) | L | 7 | 1142 | −29, −49, 45 | 3.2 e−5 | 1139 | −35, −46, 50 | 1.2 e−5 | 625 | −27, −66, 32 | 0.0014 |
| 4 | Central sulcus & post‐central gyrus | R | 4 | 2050 | 37, −20, 56 | 2.2 e−6 | 5591 | 41, −11, 43 | 0.2 e−7 | 1954 | 41, −12, 48 | 2.1 e−5 |
| 5 | Inferior temporal gyrus (lateral) | L | 37 | 1320 | −55, −46, −9 | 3.5 e−5 | 800 | −55, −44, −6 | 0.0003 | 875 | −52, −48, −3 | 7.7 e−5 |
| 6 | Lateral occipital cortex | L | 18 | 1450 | −42, −85, 7 | 5.6 e−7 | 1100 | −47, −84, 1 | 7.7 e−8 | 1100 | −50, −69, −5 | 0.0007 |
| R | 1349 | 36, −90, −2 | 5.7 e−6 | 1250 | 46, −86, −2 | 0.0002 | 950 | 45, −80, −1 | 0.0001 | |||
| 7 | Superior frontal gyrus | L | 6 | 1641 | −3, 9, 46 | 6.1 e5 | 1728 | −6, 0, 49 | 3.5 e−5 | 470 | −8, 18, 48 | 0.0006 |
| R | 1111 | 7, −8, 45 | 0.0005 | 410 | 9, −1, 51 | 0.0002 | 332 | 27, 15, 47 | 0.0003 | |||
| 8 | Inferior temporal gyrus (ventral) & fusiform gyrus | L | 37 | 2000 | −44, −63, −10 | 2.1 e−7 | 1200 | −47, −56, −6 | 0.8 e−5 | 1000 | −44, −73, −5 | 0.2 e−6 |
| R | 1500 | 39, −49, −14 | 5.9 e−7 | 800 | 41, −47, −9 | 2.8 e−6 | 400 | 38, −62, −9 | 8.1 e−5 | |||
| 9 | Ventral occipital cortex | L | 18 | 8000 | −27, −96, 5 | 2.2 e−8 | 5000 | −49, −84, 0 | 2.2 e−6 | 7403 | −17, −96, 7 | 2.8 e−5 |
| R | 6241 | 11, −99, −4 | 3.5 e−8 | 6044 | 47, −73, −9 | 5.1 e−6 | 988 | 47, −74, 25 | 0.0003 | |||
| B | Main effects: Fixation > All words | Lexical Decision: Fixation > All words | Relatedness Judgment: Fixation > All words | |||||||||
| 10 | Inferior parietal lobule & posterior superior temporal gyrus | R | 39 | 1665 | 44, −67, 29 | 9.1 e−5 | 293 | 44, −69, 31 | 0.022* | 1450 | 42, −42, 40 | 0.0002 |
| 11 | Insula | R | — | 332 | 42, −21, 2 | 0.0015 | — | — | NS | 546 | 44, −16, 0 | 0.0005 |
| 12 | Precuneus & retro— splenial cortex | R | 31/30 | 917 | 10, −64, 25 | 0.0002 | 642 | 9, −90, 32 | 0.0031* | 1200 | 15, −49, 14 | 6.3 e−5 |
| 13 | Posterior cingulated | L | 31 | 3306 | −8, −32, 35 | 4.4 e−5 | 275 | 11, −36, 34 | 0.00035* | 3000 | −3, −45, 49 | 0.0003 |
| R | 1449 | 12, −42, 40 | 0.0003 | 274 | 11, −36, 34 | 0.0004* | 1200 | 12, −32, 40 | 0.0003 | |||
| 14 | Anterior cingulated | L | 32/24 | 2628 | −4, 34, 8 | 0.0004 | 193 | −9, 33, 14 | 0.0002* | 2162 | −5, 40, 9 | 0.00005 |
| R | 865 | 10, 39, −4 | 0.0003 | — | — | NS | 780 | 10, 35, −11 | 4.1 e−5 | |||
No. corresponds directly to cluster labels in Figure 2. When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a slash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in P column reached cluster‐level significance, P < 0.05 corrected across the whole cortex, except for those marked with * that reached a voxel‐wise significance of P < 0.05, uncorrected across the cortex. NS: nonsignificant.
Figure 2.
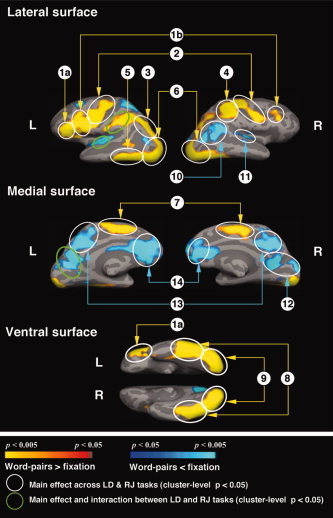
Cortical statistical maps comparing all word‐pairs (correctly answered responses) across both LD and RJ tasks with the fixation condition. Yellow–red: More activity to the word‐pairs than to the fixation condition. Blue: Less activity to the word‐pairs than to the fixation condition. All clusters circled are significant at a cluster‐level P < 0.05. Cluster numbers correspond directly to those regions reported in Table V.
Table VI.
Interactions with task: differences between the LD and RJ tasks in hemodynamic modulation across all word‐pairs relative to fixation
| A No. | Region | Lat. | BA | Interactions with Task | Lexical Decision: All words > Fixation | Relatedness Judgment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p | Area (mm2) | Tal. (x, y, z) | p | ||||
| 1 | Post central sulcus | L | 2 | 461 | −40, −30, 37 | 0.0005 | 1100 | −47, −30, 40 | 4.1 e−5 | — | — | NS |
| 2 | Lingual gyrus | L | 19 | 576 | −12, −54, 7 | 0.0005 | 250 | −19, −57, 4 | 0.0005* | — | — | NS |
| B | Interactions with Task | Lexical Decision | Relatedness Judgment: Fixation > All words | |||||||||
| 3 | Middle/inferior temporal (ant. lat) | L | 20/21 | 709 | −61, −16, −21 | 0.0045 | — | — | NS | 502 | −48, −5, −21 | 0.0008 |
| 4 | Para‐hippocamp. | R | 28/36 | 380 | 18, −11, −22 | 0.0002 | — | — | NS | 212 | 21, −15, −23 | 0.001* |
| 5 | Post‐central gyrus & central sulcus | L | 4/3 | 661 | −16, −30, 69 | 0.0002 | — | — | NS | 710 | −16, −30, 70 | 1.6 e−5 |
| 6 | Insula | L | — | 402 | −36, 2, −11 | 0.0006 | — | — | NS | 831 | −42, −20, −46 | 1.2 e−5 |
When clusters or Brodmann areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a slash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in P column reached cluster‐level significance of P < 0.05, corrected across the whole cortex, except for those marked with * that reached a voxel‐wise significance of P < 0.05, uncorrected across the cortex. NS: nonsignificant.
Hemodynamic responses suppression
In contrasting the directly related with the unrelated word‐pairs, a cluster within the left inferior temporal/fusiform gyrus and a cluster at the right occipito‐parietal junction showed main effects due to response suppression across both the LD and RJ tasks (Table VIIA, Fig. 3A). These clusters failed to show task by relatedness interactions, suggesting that they were modulated to the same degree across both tasks. In addition, a left anterior inferior prefrontal and a right anterior orbitofrontal cluster showed hemodynamic response suppression during the LD task (Table VIIA, Fig. 3A). These two clusters did not show main effects of relatedness across both tasks or task by relatedness interactions.
Figure 3.

Cortical statistical maps comparing directly related word‐pairs with unrelated word‐pairs (A) and indirectly related word‐pairs with unrelated word‐pairs (correctly answered responses) across both LD and RJ tasks (top row), during the LD task (middle row) and during the SM task (bottom row). Clusters indicated in yellow–red showed hemodynamic response suppression, i.e. a greater hemodynamic response to unrelated word‐pairs than to related word‐pairs. Directly related vs. unrelated: Cluster numbers correspond to those indicated in Table VIIA. Indirectly related vs. unrelated: cluster numbers correspond to those indicated in Table VIIIA.
In contrasting the indirectly related with the unrelated word‐pairs, there was fairly widespread hemodynamic response suppression within inferior temporal, occipital, parietal, and cingulate cortices during the LD task (Table VIIIA, Fig. 3B). Some of these regions also showed main effects of relatedness across the LD and RJ tasks, but none showed main effects on the RJ task alone and none showed task by relatedness interactions.
A direct comparison between the indirectly and the directly related word‐pairs indicated that the left lateral anterior inferior temporal cortex and left anterior cingulate clusters showed significantly more suppression to the indirectly related than to the directly related word‐pairs (indicated with ** in Table VIIIA).
Hemodynamic response enhancement
During the RJ task, other regions showed response enhancement (more activity to the directly or indirectly related word‐pairs than to the unrelated word‐pairs). In comparing both the directly and the indirectly related word‐pairs with the unrelated word‐pairs, a cluster within the left inferior parietal lobule showed response enhancement during the RJ task, but no significant modulation during the LD task. This difference in modulation across the two tasks was reflected by a task by relatedness interaction (Tables VIIB and VIIIB, Fig. 4). A direct comparison between the related and the unrelated word‐pairs did not reveal any difference in modulation within this cluster.
Figure 4.
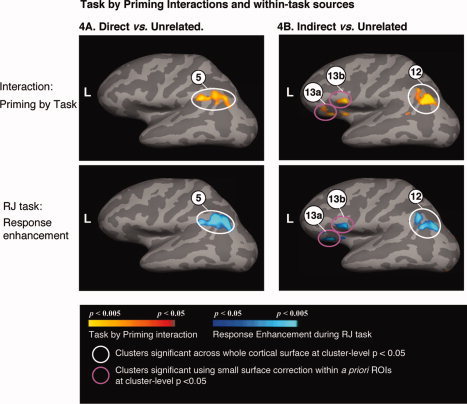
Row 1: Cortical statistical maps showing task (LD vs. RJ) by priming interactions in comparing directly related word‐pairs with unrelated word‐pairs (A) and indirectly related word‐pairs with unrelated word‐pairs (B). Row 2: Cortical statistical maps showing sources of these interactions: Regions that showed response enhancement during the RJ task, i.e. more hemodynamic activity to directly related word‐pairs than to unrelated word‐pairs (A) and more hemodynamic activity to indirectly related word‐pairs than to unrelated word‐pairs (B). Directly related vs. unrelated: Cluster numbers correspond to those indicated in Table VIIB. Indirectly related vs. unrelated: Cluster numbers correspond to those indicated in Table VIIIB.
In addition, comparing the indirectly related word‐pairs with the unrelated word‐pairs during the RJ task, response enhancement was also seen within the left inferior frontal cortex (Table VIIIB, Fig. 4B). A direct comparison between the indirectly and directly related word‐pairs during this task confirmed that this region showed more activity to the indirectly related word‐pairs than to the directly related word‐pairs (indicated with ** in Table VIIIB).
DISCUSSION
This study contrasted the effects of an implicit and an explicit task on the modulation of the hemodynamic response to the same directly related and indirectly related word‐pairs, relative to unrelated word‐pairs. In the implicit LD task, participants simply decided whether a target word was a word or a nonword. In the explicit RJ task, participants determined whether or not the prime and target were related in meaning. The results were striking. The task affected both the polarity of hemodynamic modulation as well as the neuroanatomical regions that were modulated. The semantic relationship between the word pairs (direct or indirect) affected the neuroanatomical regions that were modulated. The LD task led to hemodynamic response suppression within bilateral anterior inferior prefrontal cortices (BA 47 on the left; BA 10/11 on the right) and within the left inferior temporal/fusiform cortex (BA 37) to directly related (relative to unrelated) word‐pairs, and to hemodynamic response suppression within more widespread temporal and occipital regions to indirectly related (relative to unrelated) word‐pairs. The RJ task, on the other hand, was mainly associated with hemodynamic response enhancement. This response enhancement was observed within the left inferior parietal lobule (BA 40) to both directly and indirectly related word‐pairs as well as within the left inferior prefrontal cortex (BAs 47 and 45) to indirectly related word‐pairs, each relative to unrelated word pairs. Below we consider these patterns of hemodynamic response suppression and enhancement in relation to participants' behavioral responses, the potential cognitive processes engaged, and previous neuroimaging studies of semantic processing.
Hemodynamic Response Suppression
We predicted that, during LD task, the left anterior inferior prefrontal cortex (BA 47)—a region known to mediate controlled semantic retrieval processes [Wagner et al., 2001]—would show response suppression to the directly related relative to unrelated word‐pairs. This prediction was confirmed. Response suppression within the left inferior prefrontal cortex is consistent with several previous neuroimaging studies of direct semantic priming using a LD task [Copland et al., 2003; Giesbrecht et al., 2004; Gold et al., 2006; Matsumoto et al., 2005; Wheatley et al., 2005; Wible et al., 2006]. Consistent with the interpretation of Gold et al. [2006], we suggest that its response suppression reflected the relative ease of accessing target words that had been predicted from their directly related primes through controlled semantic expectancy strategies [Neely, 1991]. In the current study, the additional response suppression within the right anterior orbitofrontal cortex (BA 10/11) to related (relative to unrelated) word‐pairs is less consistent with previous fMRI studies of semantic priming, but may reflect the more general involvement of right inferior prefrontal regions in inhibitory processes [Aron et al., 2004], possibly in inhibiting predictions that did not match unrelated targets.
A failure of inferior prefrontal suppression and the absence of a significant behavioral priming effect to the indirectly related word‐pairs, relative to unrelated word‐pairs, was also predicted. The absence of indirect priming under controlled experimental conditions with long SOAs [Balota and Lorch, 1986; Chwilla and Kolk, 2002; Chwilla et al., 2000; Hill et al., 2002; McNamara and Altarriba, 1988; Silva‐Pereyra et al., 1999] has been explained by positing that any expectancy strategies in which participants engage are just as ineffective in predicting indirectly related targets as in predicting unrelated targets [Neely, 1991]. If, as discussed above, hemodynamic response suppression within the inferior and ventral prefrontal cortices reflects the reduced retrieval effort that results from such predictions, this would explain why it was not suppressed to the indirectly related word‐pairs: it was engaged to the same degree as to the unrelated word‐pairs.
Interestingly, despite the long SOA, temporal fusiform cortices (BA 37) that previous studies have implicated in the storage of lexico‐semantic representations [Nobre and McCarthy, 1995; Price, 2000; Van Petten and Luka, 2006] and their automatic access through processes such as spreading activation [Gold et al., 2006; Wheatley et al., 2005], also showed hemodynamic response suppression during the LD task in response to the directly related, relative to the unrelated, word‐pairs. This is not, however, the first time that response suppression within the temporal fusiform cortex has been described at long SOAs [Gold et al., 2006; Matsumoto et al., 2005; Rissman et al., 2003; Rossell et al., 2003; Wible et al., 2006]. Although, under these controlled experimental conditions, any spreading activation is unlikely to contribute to behavioral priming, it is still possible that BOLD suppression within this region reflected the effects of spreading activation at the neural level.
In comparing the indirectly related and unrelated word‐pairs, hemodynamic suppression was not confined to the temporal fusiform cortex, but was also observed within other temporal–occipital regions, including the right medial temporal cortex and the left lateral anterior temporal cortex, and within bilateral extrastriate cortices. Although there was no indirect behavioral priming during the LD task, it is still possible that this hemodynamic response suppression to the indirectly related word‐pairs reflected a spread of activation that was picked up at a neural level. This would be consistent with a previous report of some neurophysiological priming in the absence of behavioral indirect priming [Chwilla et al., 2000]. Indeed, the relatively widespread suppression may have reflected the longer time such activation had to build up, spread and hemodynamically prime lexico‐semantic representations stored within these cortices before participants made their lexical decisions.
Hemodynamic Response Enhancement
During the RJ ask, hemodynamic response enhancement was observed within the left inferior parietal lobule (BA 40) in response to both the directly and the indirectly related, relative to the unrelated, word‐pairs. We suggest that the recruitment of these regions reflected participants' attention to semantic associations or common semantic features between prime and target, as they attempted to find semantic matches between them.5 This interpretation accords with the findings of previous studies that have reported parietal activation in association with the acquisition and application of semantic categorical rules to classify novel objects with common semantic features, as well as with its activation as participants make similarity judgments about objects with common semantic features [Grossman et al., 2002; Koenig et al., 2005]. It is also consistent with views that the left inferior parietal cortex plays a role in attentional focus and shifting [Behrmann et al., 2004], aspects of working memory maintenance [Ravizza et al., 2004], and the integration of semantic features across words [Grossman et al., 2002; Koenig et al., 2005]. Indeed, there is evidence that some of these functions may be related. For example, in addition to its known role in spatial attention [Corbetta and Shulman, 2002], the left parietal cortex is recruited when participants are specifically cued to attend to semantic attributes of a word target [Cristescu et al., 2006].
In addition to hemodynamic response enhancement within the left inferior parietal cortex, the indirectly related word‐pairs also led to hemodynamic response enhancement within the left inferior frontal cortex (BA 47 and 45). This is consistent with previous studies that have demonstrated response enhancement within the left inferior prefrontal cortex in association with semantic relatedness decisions on word‐pairs [Fletcher et al., 2000] and word‐triplets [Sabsevitz et al., 2005] that were more distantly (vs. more closely) semantically related. Unlike the contrast between the directly related and unrelated word‐pairs, RTs to the indirectly related word‐pairs were longer than to the unrelated word‐pairs. We suggest that both these longer RTs and the recruitment of the left inferior prefrontal cortex to the indirectly related word‐pairs reflected participants' attempts to retrieve the specific mediators linking indirectly related primes and targets in order to make their relatedness judgments.
Notably, no response enhancement, either to the directly or to the indirectly related (relative to the unrelated) word‐pairs, was observed during the LD task. This contrasts with some other fMRI semantic priming studies that have used a LD task and that have reported some hemodynamic response enhancement in addition to, or even instead of, response suppression [Kotz et al., 2002; Mummery et al., 1999; Raposo et al., 2006; Rossell et al., 2003; Wible et al., 2006]. In the current study, the absence of hemodynamic response enhancement during the LD task does not imply that postlexical semantic matching processes were not contributing to priming at all. However, it is possible that these matching processes were not operating to the same degree as in some of these previous fMRI studies. First, in the present study, the RP was relatively low (∼0.5 given that 50% of the word‐pairs were classified as related) and this may have reduced the efficacy of any semantic matching processes in speeding up lexical decisions to primed targets. Second, although the SOA in the current study (800 ms) was long enough to allow some semantic matching, it was not as long as in a study by Raposo et al. [2006] that reported only response enhancement to related (relative to unrelated) word‐pairs during a LD task and in which the 2,500 ms between prime and target is likely to have encouraged participants to attend to any semantic relationships between them. Future studies will determine whether experimental parameters such as RP and SOA can predict the degree of hemodynamic response enhancement to related relative to unrelated word‐pairs during an implicit LD task.
CONCLUSIONS
In sum, the current study demonstrates that, as the same participants viewed the same directly related, indirectly related, and unrelated word‐pairs, the task they performed influenced both the polarity and neuroanatomical localization of hemodynamic modulation. We have suggested that, during the LD task at a long SOA, inferior prefrontal response suppression reflected participants' successful predictions of directly related targets and that this was the primary determinant of the behavioral priming effect. We have also suggested that the temporal (and occipital) hemodynamic response suppression reflected some effect of spreading activation across stored neural word representations, even though, at this SOA, such automatic activation did not contribute significantly to behavioral priming. Finally, we have suggested that, during the RJ task, the left parietal response enhancement reflected participants' attention to semantic relationships as they attempted to semantically match primes and targets, and that the additional left inferior prefrontal response enhancement to the indirectly related word‐pairs reflected participants' attempts to retrieve the specific words that mediated between indirectly related primes and targets.
Although the explanations of these patterns of response enhancement and suppression are still relatively hypothetical, they lead to specific predictions about the time course of activation within these regions during semantic priming under controlled experimental conditions: they predict that response suppression due to prelexical automatic spreading activation and controlled expectancy generation will occur before response enhancement due to postlexical semantic matching processes. Such hypotheses cannot be tested directly using fMRI that has an inherently poor temporal resolution. However, it may be possible to combine its excellent spatial resolution with techniques such as ERPs and magneto‐encephalography (MEG) that do have the temporal resolution to examine the precise time courses of these neurocognitive processes. Encouragingly, there is already some convergence of findings across studies that have used fMRI and ERP/MEG techniques. For example, intracranial ERP studies have implicated the anterior fusiform cortex [Halgren et al., 1994b; Nobre and McCarthy, 1995] as well as the left inferior prefrontal cortex [Halgren et al., 1994a]—both regions that were modulated in the current study—as sources of the N400 evoked in single word paradigms. And MEG studies have also demonstrated modulation within temporal and inferior prefrontal cortices within the N400 time window during word repetition priming [Marinkovic et al., 2003] and sentence anomaly [D'Arcy et al., 2004; Halgren et al., 2002; Helenius et al., 1998] paradigms. Future studies combining the spatial resolution of fMRI with the temporal resolution of ERP and MEG techniques [Dale et al., 2000] will be able to test the model outlined in the present study more directly.
Acknowledgements
Gina R. Kuperberg was also supported by NARSAD (with the Sidney Baer Trust) and by a Claflin Distinguished Scholars Award from Massachusetts General Hospital. We thank Kristin Girasa for her help in developing the stimuli, Thilo Deckersbach and Daphne Holt for their help with scanning, and Sarah Groff for her help with data analysis.
Footnotes
It is theoretically possible that a systematic difference in noise between the first three and the last three runs could have confounded our assessment of hemodynamic modulation across the two tasks. We therefore computed the difference in the residual variance at each voxel (a direct measure of both scanner and physiological noise) between the LD task (the first three runs) and the RJ task (the last three runs) in each participant, averaged this difference across all participants in standardized space and then used t‐tests to determine whether these differences were significant at any voxel. This analysis failed to reveal any significant differences in noise at any voxel across the cortex at P < 0.01, uncorrected for multiple comparisons.
We now use a higher sensitivity coil to acquire these high‐resolution structural scans, and signal averaging is therefore no longer necessary to achieve the required signal:noise for the automated reconstruction procedures.
Behavioral data collected from a larger sample of controls (n = 36) outside the scanner using exactly the same paradigm, however, did reveal a significant behavioral direct priming effect on the subjects analysis t(35) = 2.8, P < 0.009.
When ANOVAs were repeated including RTs to indirectly related word‐pairs that were judged as unrelated in the RJ task or using all RTs to indirectly related word‐pairs regardless of how they were classified in the RJ task, both task by relatedness interactions and reverse priming effects in the RJ task reached significance on both subjects and items analyses.
Our piloting studies indicated that, even though participants failed to generate indirectly related targets to primes on a word association task, they recognized some semantic relationship between them and tended to rate the indirectly related word‐pairs as more related than the unrelated word‐pairs.
REFERENCES
- Anderson JR ( 1983): A spreading activation theory of memory. J Verb Learn Verb Behav 22: 261–295. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Balota DA, Lorch RF Jr ( 1986): Depth of automatic spreading activation: Mediated priming effects in pronunciation but not in lexical decision. J Exp Psychol Learn Mem Cogn 12: 336–345. [Google Scholar]
- Becker CA ( 1980): Semantic context effects in visual word recognition. Mem Cognit 8: 493–512. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S ( 2004): Parietal cortex and attention. Curr Opin Neurobiol 14: 212–217. [DOI] [PubMed] [Google Scholar]
- Bentin S, McCarthy G, Wood CC ( 1985): Event‐related potentials, lexical decision and semantic priming. Electroencephalogr Clin Neurophysiol 60: 343–355. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM ( 1998): Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM ( 2000): Estimation and detection of event‐related fMRI signals with temporally correlated noise: A statistically efficient and unbiased approach. Hum Brain Mapp 11: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA ( 2003): Using neuroimaging to evaluate models of working memory and their implications for language processing. J Neurolinguistics 16: 315–339. [Google Scholar]
- Chwilla DJ, Kolk HH ( 2002): Three‐step priming in lexical decision. Mem Cognit 30: 217–225. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Kolk HHJ, Mulder G ( 2000): Mediated priming in the lexical decision task: Evidence from event‐related potentials and reaction time. J Mem Lang 42: 314–341. [Google Scholar]
- Collins AM, Loftus EF ( 1975): A spreading activation theory of semantic processing. Psychol Rev 82: 407–428. [Google Scholar]
- Coltheart M ( 1981): MRC psycholinguistic database. Quart J Exp Psychol 3A: 497–505. [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ ( 2003): Brain activity during automatic semantic priming revealed by event‐related functional magnetic resonance imaging. Neuroimage 20: 302–310. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A ( 1999): Real‐time 3D image registration for functional MRI. Magn Reson Med 42: 1014–1018. [DOI] [PubMed] [Google Scholar]
- Cristescu TC, Devlin JT, Nobre AC ( 2006): Orienting attention to semantic categories. Neuroimage 33: 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcy RC, Connolly JF, Service E, Hawco CS, Houlihan ME ( 2004): Separating phonological and semantic processing in auditory sentence processing: A high‐resolution event‐related brain potential study. Hum Brain Mapp 22: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM ( 1999): Optimal Experimental Design for Event‐Related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI ( 1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E ( 2000): Dynamic statistical parametric mapping: Combining fMRI and MEG for high‐resolution imaging of cortical activity. Neuron 26: 55–67. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI ( 1993): Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cogn Neurosci 5: 162–176. [DOI] [PubMed] [Google Scholar]
- Doherty CP, West WC, Dilley LC, Shattuck‐Hufnagel S, Caplan D ( 2004): Question/statement judgments: An fMRI study of intonation processing. Hum Brain Mapp 23: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Lavidor M ( 2003): Semantically convergent and semantically divergent priming in the cerebral hemispheres: Lexical decision and semantic judgment. Cogn Brain Res 17: 585–597. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM ( 2001): Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999a): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM ( 1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ ( 2000): “Sculpting the response space”—an account of left prefrontal activation at encoding. Neuroimage 12: 404–417. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY ( 2004): Separable effects of semantic priming and imageability on word processing in human cortex. Cereb Cortex 14: 521–529. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH ( 2006): Dissociation of automatic and strategic lexical‐semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci 26: 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Detre J, Alsop D, Gee J ( 2003): Neural basis for semantic memory difficulty in Alzheimer's disease: An fMRI study. Brain 126 (Part 2): 292–311. [DOI] [PubMed] [Google Scholar]
- Grossman M, Smith EE, Koenig P, Glosser G, DeVita C, Moore P, McMillan C ( 2002): The neural basis for categorization in semantic memory. Neuroimage 17: 1549–1561. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M ( 1994a): Spatio‐temporal stages in face and word processing. 2. Depth‐recorded potentials in the human frontal and Rolandic cortices. J Physiol Paris 88: 51–80. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M ( 1994b): Spatio‐temporal stages in face and word processing. I. Depth‐recorded potentials in the human occipital, temporal and parietal lobes. J Physiol Paris 88: 1–50. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM ( 2002): N400‐like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage 17: 1101–1116. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly J ( 1998): Distinct time courses of word and context comprehension in the left temporal cortex. Brain 121: 1133–1142. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD ( 2003): Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia 41: 263–270. [DOI] [PubMed] [Google Scholar]
- Henson RNA.( 2003). Neuroimaging studies of priming In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human Brain Function, 2nd ed. Academic Press, London. [Google Scholar]
- Hill H, Strube M, Roesch‐Ely D, Weisbrod M ( 2002): Automatic vs. controlled processes in semantic priming—Differentiation by event‐related potentials. Int J Psychophysiol 44: 197–218. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ ( 1988): Automatic and attentional processing: An event‐related brain potential analysis of semantic priming. Brain Lang 35: 66–85. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M ( 2005): The neural basis for novel semantic categorization. Neuroimage 24: 369–383. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD ( 2002): Modulation of the lexical‐semantic network by auditory semantic priming: An event‐related functional MRI study. Neuroimage 17: 1761–1772. [DOI] [PubMed] [Google Scholar]
- Kreher DA, Holcomb PJ, Kuperberg GR ( 2006): An electrophysiological investigation of indirect semantic priming. Psychophysiology 43: 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G, Deckersbach T, Holt D, Goff D, West WC ( 2007): Increased temporal and prefrontal activity to semantic associations in schizophrenia. Arch Gen Psychiatry 64: 138–151. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E ( 2003): Spatiotemporal dynamics of modality‐specific and supramodal word processing. Neuron 38: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N ( 2005): Linking semantic priming effect in functional MRI and event‐related potentials. Neuroimage 24: 624–634. [DOI] [PubMed] [Google Scholar]
- McNamara TP, Altarriba J ( 1988): Depth of spreading activation revisited: Semantic mediated priming occurs in lexical decisions. J Mem Lang 27: 545–559. [Google Scholar]
- McPherson WB, Holcomb PJ ( 1999): An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology 36: 53–65. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW ( 1971): Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval operations. J Exp Psychol 20: 227–234. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ ( 1999): Dual‐process model in semantic priming: A functional imaging perspective. Neuroimage 9: 516–525. [DOI] [PubMed] [Google Scholar]
- Neely JH.( 1991). Semantic priming effects in visual word recognition: A selective review of current findings and theories In: Besner D, Humphreys GW, editor. Basic Processes in Reading and Visual Word Recognition. Hillsdale, NJ: Erlbaum; pp 264–333. [Google Scholar]
- Neely JH, Keefe DE, Ross K ( 1989): Semantic priming in the lexical decision task: Roles of prospective prime‐generated expectancies and retrospective semantic matching. J Exp Psychol Learn Mem Cogn 15: 1003–1019. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G ( 1995): Language‐related field potentials in the anterior‐medial temporal lobe. II. Effects of word type and semantic priming. J Neurosci 15: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197: 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK ( 2006): Repetition suppression and semantic enhancement: An investigation of the neural correlates of priming. Neuropsychologia 44: 2284–2295. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA ( 2004): Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE ( 2003): An event‐related FMRI investigation of implicit semantic priming. J Cogn Neurosci 15: 1160–1175. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC ( 2003): The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia 41: 550–564. [DOI] [PubMed] [Google Scholar]
- Rugg MD ( 1985): The effects of semantic priming and word repetition on event‐related potentials. Psychophysiology 22: 642–647. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR ( 2005): Modulation of the semantic system by word imageability. Neuroimage 27: 188–200. [DOI] [PubMed] [Google Scholar]
- Silva‐Pereyra J, Harmony T, Villanueva G, Fernandez T, Rodriguez M, Galan L, Diaz‐Comas L, Bernal J, Fernandez‐Bouzas A, Marosi E, Reyes A ( 1999): N400 and lexical decisions: Automatic or controlled processing? Clin Neuropsychol 110: 813–824. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ ( 2006): Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang 97: 279–293. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ ( 1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare‐Blagoev EJ, Clark J, Poldrack RA ( 2001): Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Weisbrod M, Kiefer M, Winkler S, Maier S, Hill H, Roesch‐Ely D, Spitzer M ( 1999): Electrophysiological correlates of direct versus indirect semantic priming in normal volunteers. Cogn Brain Res 8: 289–298. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A ( 2005): Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci 17: 1871–1885. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R ( 1976): Handedness assessment inventory. Neuropsychologia 14: 261–264. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor P ( 2006): Connectivity among semantic associates: An fMRI study of semantic priming. Brain Lang 97: 294–305. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A ( 1998): Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8: 227–233. [DOI] [PubMed] [Google Scholar]
- Zwaan RA, Yaxley RH ( 2003): Spatial iconicity affects semantic relatedness judgments. Psychon Bull Rev 10: 954–958. [DOI] [PubMed] [Google Scholar]


