Abstract
In this paper we describe new actions of nimesulide and paracetamol in cultured peripheral neurons isolated from rat dorsal root ganglia (DRG). Both drugs were able to decrease in a dose-dependent fashion the number of cultured DRG neurons showing translocation of protein kinase C epsilon (PKCɛ) caused by exposure to 1 μM bradykinin or 100 nM thrombin. In addition, the level of substance P (SP) released by DRG neurons and the level of preprotachykinin mRNA expression were measured in basal conditions and after 70 minutes or 36 hours of stimulation with nerve growth factor (NGF) or with an inflammatory soup containing bradykinin, thrombin, endothelin-1, and KCl. Nimesulide (10 μM) significantly decreased the mRNA levels of the SP precursor preprotachykinin in basal and in stimulated conditions, and decreased the amount of SP released in the medium during stimulation of neurons with NGF or with the inflammatory soup. The effects of paracetamol (10 μM) on such response was lower. Nimesulide completely inhibited the release of prostaglandin E2 (PGE2) from DRG neurons, either basal or induced by NGF and by inflammatory soup, while paracetamol decreased PGE2 release only partially. Our data demonstrate, for the first time, a direct effect of two drugs largely used as analgesics on DRG neurons. The present results suggest that PKCɛ might be a target for the effect of nimesulide and paracetamol, while inhibition of SP synthesis and release is clearly more relevant for nimesulide than for paracetamol mechanism of action.
Keywords: nociceptors, analgesia, hyperalgesia, dorsal root ganglia, PKCɛ
Introduction
In this paper we present a comparative study of mechanisms of action of nimesulide and paracetamol, drugs largely used as analgesics, in cultured rat dorsal root ganglia (DRG) neurons.
Nimesulide is a nonsteroidal anti-inflammatory drug (NSAID) with broad effects on inflammation and other biochemical processes leading to a multifactorial mode of action.1 Nimesulide is a preferential cyclo-oxygenase-2 (COX-2) inhibitor1–4 but its anti-inflammatory and analgesic action also involves activity on a wide range of inflammatory and pain mediators and intracellular pathways1,5 including an effect on synovial concentration of substance P (SP) in patients with knee osteoarthritis.6 While some evidence of analgesic action of nimesulide has been linked to actions in the central nervous system,7 to our knowledge direct effects of nimesulide in isolated sensory neurons have not yet been reported.
Paracetamol is one of the most popular and commonly used drugs for the treatment of moderate pain, which still presents a challenge in terms of a satisfactory explanation of its well-established effects. Despite its antipyretic and analgesic activities, it has almost no anti-inflammatory action and is a weak inhibitor of either COX-1 or COX-2.8,9 Contribution of several possible mechanisms has been proposed at a variety of levels in the nociceptive pathway from the periaqueductal gray to the periphery.10–16
The epsilon isoform of protein kinase C (PKCɛ) is a very important peripheral effector of a number of inflammatory mediators, including bradykinin, prostaglandins, proteases, prokineticins, SP and others,17–21 which causes increased activation of nociceptors via action on transient receptor potential vanilloid member1 (TRPV1) and on tetrodotoxin (TTX)-insensitive sodium channels.22
The neuropeptide SP has long been associated with transmission of noxious stimuli.23 In the peripheral nervous system it is expressed in a subset of unmyelinated nociceptive sensory neurons and is transported to central and peripheral axonal terminals. Centrally, SP is released in the superficial lamina of the spinal cord dorsal horn, while in the periphery it is implicated in neurogenic inflammation.24–26 Despite its importance in pain transmission, little is known about SP modulation by anti-inflammatory and analgesic drugs.
In this paper we investigate in cultured DRG neurons the ability of nimesulide and paracetamol to decrease translocation of PKCɛ, to reduce preprotachykinin synthesis and the release of SP induced by inflammatory mediators, and to reduce prostaglandin E2 (PGE2) release from activated neurons. We show that nimesulide and paracetamol share some novel mechanisms of action potentially relevant for their in vivo effects, although other effects are exclusively seen or are quantitatively stronger with nimesulide treatment.
Material and methods
Dorsal root ganglion primary cultures
Sprague Dawley rats (2–3 weeks old) were sacrificed under total anesthesia according to Italian and European legislation, with protocols in agreement with the guidelines of the Committee for Research and Ethical Issues of IASP.27 Experimental work was also reviewed and approved by local institutional animal care and use committee. DRGs were collected, incubated for 1 hour at 37°C with 0.125% collagenase (Worthington, Freehold, NJ), and mechanically dissociated, plated onto coverslips or Petri dishes pretreated with 10 μg/mL poly-L-lysine (Sigma-Aldrich, St Louis, MO) and 20 μg/mL laminin (Sigma-Aldrich, Milan, Italy), and cultured in DMEM containing 1% penicillin/streptomycin, 10% fetal bovine serum, 1% L-glutamine (Invitrogen, San Diego, CA), 1.5 μg/mL cytosine 1-d-arabinofuranoside (ARA-C, Sigma), as described previously.28 Neurons used for immunocytochemistry experiments were cultured in the presence of 100 ng/mL nerve growth factor (NGF) (Sigma) in order to improve bradykinin and thrombin receptor expression.29
Immunocytochemistry
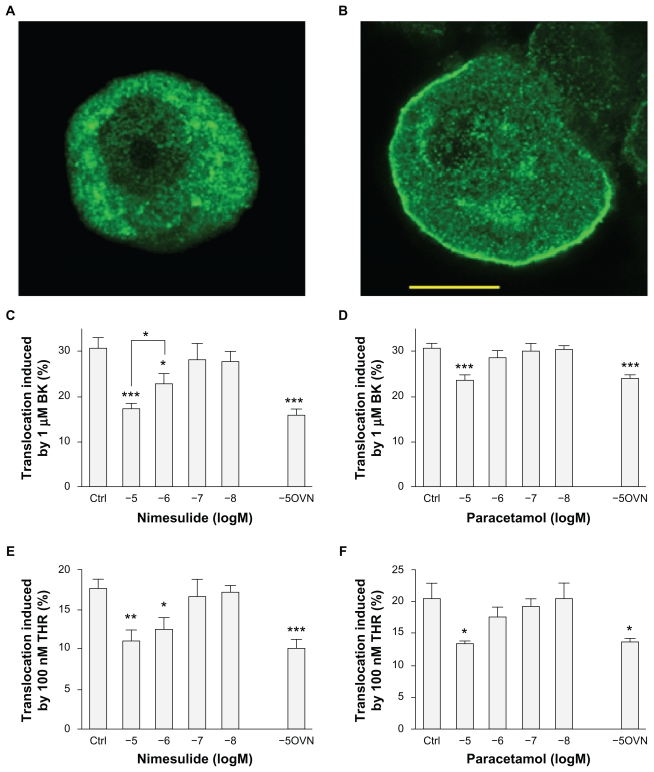
PKCɛ was visualized as previously described.18,19 In brief, rat DRG neurons cultured for 2–3 days in vitro were treated with bradykinin (BK, at 1 μM concentration) or thrombin (THR, 100 nM) for 30 seconds, and rapidly fixed for 10 minutes at room temperature with paraformaldehyde (4% formaldehyde and 4% sucrose, dissolved in phosphate-buffered saline (PBS)/distilled water 2:1). Stimulation solutions and then fixation solution were applied with an automated system (FSC-1, CV Scientific, Modena, Italy). In test experiments, nimesulide or paracetamol (from Sigma) at different concentrations were preapplied for 120 minutes or overnight (see Figure 1) and also added to BK and THR applied to coverslips. Dimethyl sulfoxide (DMSO) was used to prepare stock solutions of nimesulide and paracetamol, and the final concentration of DMSO applied to cells was always lower than 1:1000. Fixed cells were washed three times in PBS (with 0.1% fish skin gelatin to block nonspecific sites), permeabilized for 30 minutes at room temperature with Triton X-100 (0.2% in PBS), and incubated overnight at 4°C with a polyclonal anti-PKCɛ antibody17 diluted 1:1000 in PBS-T/gelatin (PBS with 0.05% Triton X-100). Coverslips were then incubated for 1 hour at room temperature with goat antirabbit IgG conjugated to the fluorophore Alexa Fluor 488 (1:200; Invitrogen), washed three times in PBS/gelatin, and visualized using a confocal microscope (Leica SP2, Leica, Switzerland). Activation of PKCɛ results in translocation from an entirely cytoplasmic location to the neuronal cell membrane (see Figure 1). Translocation was quantified by determining fluorescence intensity along a line positioned across the cell so as to avoid the nucleus (for details see Cesare et al17). Neurones in which intensity at the cell membrane was at least 1.5 times greater than the mean of cytoplasmic intensity were counted as positive.
Figure 1.
Nimesulide and paracetamol inhibit protein kinase C epsilon (PKCɛ) translocation in dorsal root ganglia (DRG) sensory neurons. (A–B) Confocal images of DRG neurons treated with bradykinin (BK, 1 μM) for 30 seconds and then fixed and stained for PCKɛ with a specific antibody. (A) typical behavior of an unresponsive neuron. (B) typical BK-responsive neuron showing translocation of PKCɛ to the plasma membrane. Neurons treated with 100 nM thrombin (THR) for 30 seconds would display similar behavior. Neurons fixed without prior treatment with BK or THR showed no sign of spontaneous translocation. Scale bar 10 μm. (C) The percentage of neurons showing PKCɛ translocation induced by 1 μM BK or (E) by 100 nM THR was significantly decreased by 2 hours preapplication of nimesulide at 1 or 10 μM concentration. Overnight treatment (OVN) did not result in larger inhibition (C–E). With paracetamol treatment only the largest concentration of drug tested (10 μM) significantly reduced translocation induced by BK (D) or by THR (F). Further explanations in the text. Values are means ± SEM obtained from 4–12 cultures.
Notes: *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control.
Dorsal root ganglia stimulation and drug treatment
After 2–3 days in vitro, DRG cultures were stimulated for experimental procedures using either NGF (100 ng/mL) or a cocktail of inflammatory/proalgesic mediators (inflammatory soup, IS) of the following composition: 1 μM BK, 100 nM THR, 100 nM endothelin-1, 25 mM KCl, dissolved in normal culture medium (DMEM + 10% FBS). Where appropriate, a concentration of 10 μM of tested chemicals (nimesulide or paracetamol, from Sigma) dissolved in DMSO was preapplied for 30 minutes to cultures before treatment with NGF and IS which was considered sufficient time to allow full onset of their effect before application of inflammatory mediators.
Cells were stimulated with either NGF or IS with or without the drugs for 70 minutes and 36 hours. At the end of these incubation periods, media and cells were collected for further processing as described below.
Measurement of SP and PGE2 in culture media
Quantitative determination of PGE2 was performed by the enzyme immunoassay using a commercially available EIA kit (Cayman Chemical Company, Ann Arbor, MI). The sensitivity of the PGE2 EIA kit was 15 pg/mL.
To measure SP, culture media were acidified with 1N acetic acid and SP was measured by radioimmunoassay (RIA) using antiserum and methods previously described and validated.30,31 The antibody was raised in rabbit against synthetic SP, and was directed towards the C terminal of the peptide. I125-SP was purchased from Perkin Elmer (Monza, Italy). Sensitivity of the RIA was 10 pg/tube and intra-assay and inter-assay variation coefficients were 8% and 11%, respectively.
RNA isolation and real-time RT-PCR
Total RNA from DRG cells was purified using TRIzol reagent (Invitrogen, Life Technologies, San Giuliano Milanese, Italy). Cells were lysed directly in the culture dish, according to the manufacturer’s instructions and RNA resuspended in 8 μL of water. After purification, total RNA concentrations were determined from the sample absorbance value at 260 nm. Total RNA (300 ng) was treated with DNase (DNA-free-Ambion) to avoid false-positive results due to amplification of contaminating genomic DNA. First-strand cDNA was synthesized from 1000 ng of total RNA in a final volume of 20 μL using M-MLV RT (Moloney Murine Leukemia Virus Reverse Transcriptase; Invitrogen, San Giuliano Milanese, Italy). cDNA (2 μL) was subjected to real-time quantitative PCR using ABI PRISM 7000 (Applied Biosystems, Forster City, CA). TaqMan PCR was performed in 25 μL volumes using Real Master Mix Probe ROX (Eppendorf, Hamburg, Germany). Custom probes were prepared by Applied Biosystems. The probes were the same as those used in a previous study31 and were designed to span an intron in order to avoid potential amplification of genomic DNA in the analyzed samples. The probes were labelled at the 5′ end with 6-carboxy fluorescein and at the 3′ end with 6-carboxy-tetramethyl rhodamine. The primers and probe sequence for preprotachykinin (PPT, Genbank accession number M15191) and GAPDH (Genbank accession number AF106860) are shown in Bianchi et al.31 All PCR assays were performed in duplicate. Before using the ΔΔCT method for relative quantification, we performed a validation experiment to demonstrate that the efficiencies of the two different probes (target and reference) are equal. The reaction conditions were as follows: 95°C for 2 minutes, followed by 40 cycles at 95°C for 15 seconds (denaturation) and 60°C for 1 minute (annealing and elongation). As controls, we used the reaction mixture without the cDNA. Threshold cycle numbers (CT) were determined with an ABI PRISM 7000 Sequence Detection System (version 1.1 software) and transformed using the ΔCT (2-ΔΔCT) comparative method. Gene-specific expression values were normalized to expression values of GAPDH (endogenous control) within each sample. The levels of preprotachykinin were expressed relative to the calibrator value control cells. Relative quantification was performed using the comparative method. The amount of target, normalized to an endogenous reference and relative to a calibrator, is given by 2-ΔΔCT. Briefly, the ΔCT value is determined by subtracting the average GAPDH CT value from the average PPT CT in the same sample. The calculation of ΔΔCT involves subtraction of the ΔCT calibrator value.
Statistical analysis
Data were analyzed by one way analysis of variance (ANOVA), followed by Bonferroni’s t-test for multiple comparison. An effect was determined to be significant if the P value was less than 0.05.
Results
Effects on PKCɛ translocation
Activation of PKCɛ by inflammatory mediators leads to its translocation from the cytoplasm to the surface membrane in DRG sensory neurons, which can be visualized directly with immunocytochemistry and confocal microscopy (Figure 1A and B).18,19,21 PKCɛ translocation can be quantified in terms of the number of neurons in which translocation is observed – with this approach reliable dose-response and time-course curves can be obtained.19,21 After application of 1 μM BK or 100 nM THR, which are saturating concentrations for these agonists, maximum translocation was consistently observed. At longer application times PKCɛ slowly became internalized into perinuclear vesicles as shown previously.17,19,21 As maximum translocation was consistently observed with the above agonist concentrations and at 30-second exposure, these parameters were adopted for all subsequent experiments. BK applied onto DRG cultures for 30 seconds at 1 μM before rapid fixation caused translocation in 30.8% ± 2% of neonatal rat neurons cultured in the presence of NGF. THR caused translocation in 17.8% ± 1% of neonatal rat DRG neurons, also cultured in the presence of NGF.
As shown in Figure 1C, the number of neurons in which 1 μM BK induced translocation was significantly decreased by 2 hours preapplication of nimesulide (10 μM) to a value of 17.9% ± 1.1% compared with neurons pretreated with vehicle solution (P < 0.001). Translocation was also significantly reduced by a 10-fold lower concentration of nimesulide (1 μM) to 22.9% ± 2.5%, and efficacy was significantly smaller compared with 10 μM nimesulide (P < 0.05), indicating that nimesulide effect was dose-dependent. Nimesulide had largely similar effects on translocation of PKCɛ induced by 100 nM THR (Figure 1E), and in this case inhibition was statistically significant although not significantly different at 10 μM (11.1 ± 1.4) and 1 μM concentrations (12.5 ± 1.7), which were preapplied for 2 hours before the experiment. Longer preapplication of nimesulide at 10 μM (overnight rather than of 2 hours) did not cause a larger inhibition of translocation, both for BK (14.3% ± 1.7%) and for THR (10.4% ± 1.2%) as shown in Figure 1C and E. Shorter preapplication of nimesulide for 15 minutes at 10 μM concentration did not cause significant differences compared with 120-minute application, and in this case 1 μM BK caused translocation in 17.4 ± 0.5% and 100 nM THR in 9.6% ± 1.2% of neurons (not shown in figure).
The effect of paracetamol on PKCɛ translocation was tested with an identical protocol as the one used for nimesulide. As shown in Figure 1D and F paracetamol was less effective than nimesulide, because only the highest concentration tested decreased the percentage of translocated neurons significantly. While nimesulide at 10 μM caused a percentage of decrease of translocation induced by BK and THR of ~42% and ~38%, paracetamol at the same concentration caused a decrease of ~23% and ~34% respectively. The effect of paracetamol, like that of nimesulide, did not increase with overnight preapplication.
Effects on preprotachykinin mRNA synthesis
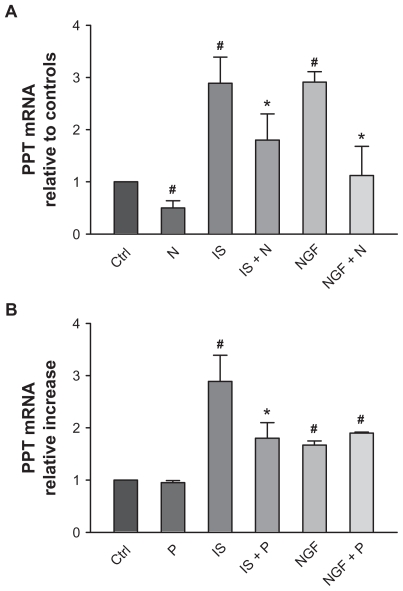
SP is synthesized from the precursor PPT. PPT mRNA was quantified in DRG cultures using real time PCR.31 We used as baseline, cultures in the absence of treatments and with no addition of growth factors 60 hours after plating, at which time relevant PTT mRNA levels were consistently above minimal levels for detection. PPT levels were significantly upregulated compared with control by 36 hours of treatment with NGF (100 ng/mL) or with a mixture of proinflammatory and proalgesic agents which we termed IS (see Materials and methods). Both treatments were effective in different trials, and upregulation by IS increased mRNA levels by about 2.9-fold compared with control while NGF-induced increase ranged from 1.7 to 2.9 compared with control (see Figure 2A and B).
Figure 2.
Effect of nimesulide and paracetamol (10 μM) on preprotachykinin (PPT) mRNA expression in cultured dorsal root ganglia neurons in basal conditions and following 36 hours treatment with inflammatory soup (IS) and with nerve growth factor (NGF). The amount of PPT is normalized to GAPDH by subtracting the average GAPDH cycle numbers (CT) value from the average PPT CT and then the comparative method (2-ΔΔCT ) was applied using untreated cells as calibrator (controls).
Notes: Values are means ± SE M of 4–6 experiments. # = P < 0.05 vs control; * = P < 0.05 vs respective stimulated cultures.
Abbreviations: P, paracetamol; N, nimesulide.
Nimesulide treatment (10 μM) applied 24 hours after plating for 36 hours significantly decreased basal level of PPT mRNA compared with untreated neurons. Nimesulide also significantly reduced upregulation of PPT mRNA caused by IS and NGF respectively of ~40 and ~60% (Figure 2A).
Effects of paracetamol (Figure 2B) were somewhat different: at 10 μM after 36 hours of treatment it did not cause any change in basal expression levels of PPT mRNA, and significantly reduced upregulation by IS (by ~40%), while upregulation by NGF was not changed by treatment. Both nimesulide and paracetamol were preapplied for 30 minutes to allow full onset of effect before treatment with IS and NGF.
Effects on basal and stimulated substance P release in medium
Levels of SP released in the medium by cultured DRG neurons were assessed by radioimmunoassay (see Materials and methods). Approximately 24 hours after plating, separate coverslips from the same DRG cultures were treated with either nimesulide, paracetamol (both 10 μM), with or without NGF (100 ng/mL) or IS, or with vehicle, for a total of 6 separate conditions (Figure 3A–D). Coverslips exposed to treatments containing nimesulide or paracetamol were pre-treated with the same concentration of these drugs for 30 minutes, and control coverslips were treated with vehicle medium for the same time. The treatments described remained on coverslips for either 70 minutes (Figure 3A and B) or 36 hours (Figure 3C and D), then the medium was removed and stored at –80°C until SP measurement.
Figure 3.
Effect of nimesulide and paracetamol (10 μM) on release of substance P (SP) measured in culture medium from DRG neurons in basal conditions and following 70 minutes (A, B) and 36 hours (C, D) treatment with inflammatory soup (IS) and nerve growth factor (NGF).
Notes: Values are means ± SEM of 4–6 experiments and are expressed as % of control cultures. # = P < 0.05 vs controls; * = P < 0.05 vs respective stimulated cultures.
Abbreviations: P, paracetamol; N, nimesulide.
Neither nimesulide nor paracetamol altered basal levels of release of SP, which were about 30 ± 2.19 (mean ± SEM) pg/mL of medium at 70 minutes and 77 ± 11 (mean ± SEM) pg/mL medium at 36 hours.
Treatment with both NGF and IS significantly increased SP level in medium after 70 minutes and after 36 hours ( Figure 3A–D). Such release was significantly decreased by nimesulide (Figure 3A and C) but was not changed by paracetamol, which was completely ineffective ( Figure 3B and D). Nimesulide effect was significant after 70 minutes, and was particularly strong after 36 hours, as SP release caused by NGF and IS was totally inhibited and reduced to values largely identical to basal levels.
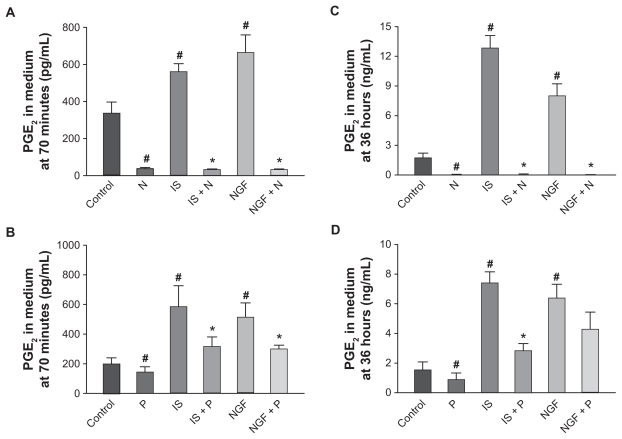
Effects on PGE2 release in medium
PGE2 was quantified in DRG cultures using an EIA (see Figure 4A).31,32 In culture medium there was little if any PGE2 as only traces were contributed by the 10% fetal bovine serum added to DMEM (see methods). Approximately 24 hours after plating, the medium was changed and cultures treated either with vehicle or with IS, NGF, with or without nimesulide or paracetamol (10 μM). After 70 minutes or 36 hours of treatment, the medium was collected and PGE2 released by cultures during this time measured. Basal release by unstimulated DRG cultures in different sets of experiment levels ranged between 199 ± 41 and 337 ± 37 pg/mL after 70 minutes and between 1.5 ± 0.5 and 1.7 ± 0.5 ng/mL after 36 hours. PGE2 levels were significantly increased compared with control by 70 minutes or 36 hours of treatment with NGF or IS. Both treatments were effective in different trials, and IS augmented PGE2 levels by about 1.6- to 2.3-fold compared with control, while NGF increase ranged between 1.5 and 2.0-fold after 70 minutes; the increase ranged between 4.8- and 7.4-fold (IS) and 4.6- and 4.2-fold (NGF) after 36 hours compared with control levels (see Figure 4) in different groups of experiments. Nimesulide treatment (10 μM) applied 24 hours after plating for 70 minutes or 36 hours (Figure 4A and C) completely inhibited PGE2 basal levels released in medium compared with untreated neurons (reduction by almost 100%). Nimesulide treatment also completely inhibited the release of PGE2 caused by IS and NGF. Both at 70 minutes and 36 hours nimesulide reduced PGE2 down to levels similar to those present in the medium before it was added to cultures (Figure 4A and C).
Figure 4.
Effect of nimesulide and paracetamol (10 μM) on release of prostaglandin E2 (PGE2) measured in culture medium from dorsal root ganglia neurons in basal conditions and following 70 minutes (A, B) and 36 hours (C, D) treatment with inflammatory soup (IS) and nerve growth factor (NGF). Values are means ± SE M of 4–6 experiments.
Notes: # = P < 0.05 vs controls; * = P < 0.05 vs respective stimulated cultures.
Abbreviations: P, paracetamol; N, nimesulide.
Paracetamol (Figure 4B and D) (10 μM) decreased basal levels of PGE2 both after 70 minutes and 36 hours. Similarly, release of PGE2 induced by IS was significantly reduced by paracetamol both at 70 minutes and 36 hours. Differently, PGE2 release induced by NGF was significantly decreased by paracetamol after 70 minutes, while reduction after 36 hours (about 30%) did not reach statistical significance.
Discussion
Specific involvement of PKCɛ in nociceptor sensitization and hyperalgesia has been described in many reports. Following a first paper by Cesare et al,17 showing that sensitization of heat-induced currents by bradykinin in nociceptive neurons is mediated by PKCɛ, a number of studies confirmed the importance, participation, and requirement of this enzyme in inflammatory pain and nociception at the cellular and whole-animal level.22,33,34 PKCɛ is preferentially expressed in nociceptive neurons where it plays a crucial role in chronic hyperexcitability, but its inhibition causes little disruption in normal sensory function or in other functions.34 PKCɛ therefore currently represents not only a well-validated target for both inflammatory and neuropathic pain in the preclinical scientific literature35,36 but also a novel and appealing target for therapeutic intervention in human patients. In this light, our novel finding of PKCɛ translocation inhibition by nimesulide and, although to a lesser extent, by paracetamol, appears to be of high relevance for a better understanding of the mechanisms of action of these drugs. Work is currently in progress to investigate whether interference on PKCɛ translocation is a common feature of other NSAIDs and analgesic drugs. PKCɛ inhibition by nimesulide, paracetamol, or possibly other NSAIDs and analgesics may be a relevant part of their pharmacological actions.
Prostaglandins work not only as mediators that directly activate downstream cascades leading to sensitization of nociceptor ion channels, but also function as paracrine mediators in nociceptor sensitization by other inflammatory agents (glutamate, bradykinin, thrombin etc). Prostaglandins may be produced by the inflamed tissues and by white bloods cells participating in inflammation, but also by sensory neurons and by peripheral glial cells. It is noteworthy that in DRG cultures, as in DRGs and in peripheral nerve endings, there is a significant presence of peripheral glial cells (satellite cells and Schwann cells) whose number, despite the presence of the cell replication inhibitor ARA-C (see Materials and methods), is in at least a 10:1 ratio compared with neurons. These cells express many of the receptors of inflammatory mediators expressed by nociceptors, and release prostaglandins as well as other mediators, including endocannabinoids37 or cytokines.38 Release of PGE2 from DRG cultures39 may be due to release from these non-neuronal cells. In fact in preliminary experiments (not shown here) we found a large release of PGE2 by cultures of non-neuronal peripheral glial cells after treatment with inflammatory mediators. Effects of PGE2 release inhibition from DRG cultures by nimesulide and paracetamol described in this paper may therefore be due to inhibition of non-neuronal and neuronal COXs. Both COX-1 and COX-2 are expressed in DRG neurons, with COX-1 especially expressed in small-sized sensory neurons,40 while the identity of COXs expressed in peripheral glia is less clear.39,41
DRG cultures released considerable amounts of PGE2 which increased significantly very quickly when IS and NGF were added to the medium. The blocking effect of nimesulide at the concentration used (10 μM) appeared to be maximum, as nimesulide completely suppressed both basal and induced PGE2 synthesis in DRG cultures. On the contrary, inhibition of PGE2 release caused by paracetamol was significantly smaller compared with the effect of nimesulide, consistent with current knowledge of paracetamol pharmacology and mechanisms of action.
Peripheral inflammatory pain is associated with a complex pattern of local changes, and after tissue injury many pronociceptive and proinflammatory mediators become activated; they lower nociceptive thresholds and increase neuronal membrane excitability, leading to hypernociception.42,43 The neuropeptide SP is present in C-fibers, is synthesized in DRG, and is transported to both central and peripheral endings of primary afferent neurons. In the central nervous system, SP plays a crucial role in spinal neuron sensitization. At the periphery, SP induces vasodilatation, increases the sensitivity to nociceptive stimuli, and contributes to neurogenic inflammation.24 In addition, SP can directly activate immune cells inducing the chemotaxis of monocytes/macrophages, and the production of different proinflammatory cytokines.30,44,45 These effects contribute to the spreading of sensitization leading to secondary hyperalgesia. The control of both central and peripheral SP release is therefore a critical step in determination of the threshold of pain perception in hyperalgesia during inflammation.
Upon nociceptor stimulation, SP is released through a very complex process involving several important intracellular effectors such as extracellular calcium influx, 1-4-5 inositol triphosphate induced calcium release, the activation of ERK, PKA, COX, and prostaglandins. In order to induce SP synthesis and release we have used two different stimuli: NGF and a mix of inflammatory mediators (IS), which have been used all together in order to mimic the inflammatory soup that is present in the inflamed tissue. Both stimuli are potent activators of SP, as we observed a significant increase of SP release already after 70 minutes of stimulation while, as expected, a longer time was needed to upregulate the synthesis of preprotachykinin.
It appears that while the IS stimulus induced a repetitive and consistent increase of both preprotakikinin mRNA and SP release across the different experiments, the modulation of the SP system by NGF, although always present, varied in the different cultures. It is likely that the IS, composed of 3 different proinflammatory agents, is more potent than NGF since it activates several pathways and induces an almost maximal stimulation of the system. On the other hand, the NGF effect might be more sensitive to slight variations in experimental conditions in different batches of experiments such as the age of the animals and the different months of the year in which experiments were performed, which might have influenced the basal state of activation of the DRG cells and/or the relative percentage of neurons and glial cells expressing NGF receptors in the cultures. Despite these quantitative variations in the amplitude of NGF response, the effects of the drugs tested appeared largely similar and consistent with other sets of experiments.
Our data show that the increase in synthesis and release of SP caused by IS and NGF was significantly reduced by nimesulide but not at all by paracetamol (Figure 3). Also basal levels of PPT mRNA were reduced by nimesulide, but again not by paracetamol. Given the in vitro system that we used in our experiment, and the rapid onset of the effect, the inhibition of SP release and production is likely to be due to a direct effect of nimesulide on nociceptors.
Our present data therefore suggest that sensory nerve fibers can be a target for nimesulide action and identify an important involvement of SP in the effects of nimesulide, indicating a further mechanism of action besides its well-known inhibition of peripheral COX-2. On the contrary, modulation of SP levels does not appear to be part of the effects of paracetamol.
These observations add other elements to the growing number of effectors targeted by nimesulide, confirming the multifactorial basis for the actions of nimesulide. The details of the mechanisms by which nimesulide causes a decrease both in SP synthesis and release remains to be clarified. At the moment we can only speculate about the possibility that the inhibition of COX-1/2 present in DRG cells, as discussed above, leading to a reduction of PGE2 production might mediate, at least in part, the modulatory effect of nimesulide on SP. Despite a contribution of nimesulide-induced prostaglandin, a reduction in inhibition of SP release is possible, but is unlikely to be the only mechanism responsible. In fact paracetamol can also cause significant inhibition of prostaglandin but this is not paralleled by any decrease in SP release from DRGs (Figures 3 and 4). Interestingly, it has been suggested recently that the activation of TRPV1 channel is a strong stimulus for SP synthesis and release.46 As the sensitization of TRPV1 is partly due to PKCɛ-dependent phosphorylation, the possibility that nimesulide could downregulate SP throughout the reduction of PKCɛ translocation can be hypothesized. Moreover the binding of SP to its NK-1 receptors in primary sensory neurons enhances TRPV1 activity via PKCɛ.20 We can therefore envisage a positive feedback mechanism where SP, prostaglandin, TRPV1, and PKCɛ act in parallel and contribute to induce and maintain inflammatory hyperalgesia.
On the other hand, considering that paracetamol is also a weak inhibitor of PKCɛ but does not affect SP, we cannot rule out the possibility that the inhibition of translocation and the reduction of SP release are unrelated events. However, the effects of nimesulide on several crucial interacting mediators can be important in controlling not only acute hyperalgesia but also in preventing progression of inflammatory hyperalgesia to chronicity. In a previous study nimesulide plasma concentrations were evaluated in patients after 2 weeks of treatment with an active dose of the drug. Interestingly, the measured nimesulide plasma levels were strictly in the range of the concentrations used in the present in vitro study, further indicating its relevance for in vivo effects of the drug.47
The effect of paracetamol and nimesulide investigated in this paper takes place in the peripheral nervous system. We need to remember that in vivo a large number of activities on several different targets may be involved in the effect of these drugs, and may be important in different, painful conditions. Supraspinal effects, which have been well described for paracetamol,48 are probably responsible for a large part of its analgesic activity. On the contrary, central mechanisms of nimesulide involved in its analgesic and antinflammatory effects are less recognized. We are also aware that our in vitro model is particularly representative of inflammatory hyperalgesia, and that further work is needed in order to evaluate the role of the pathways that we studied in other painful conditions, such as neuropathic pain.
Conclusion
Our data helped to clarify the pharmacological profile of nimesulide and paracetamol as analgesic drugs. From these results, nimesulide emerges as an NSAID with a multifactorial mode of action and with a growing number of targets including the most recently identified and appealing ones.
Acknowledgments
Work was supported by grants from Fondazione Cassa di Risparmio di Modena and Fondazione Cassa di Risparmio di Carpi and by an unrestricted research grant by Helsinn Healthcare SA (Lugano, Switzerland). We thank Maurizia Celario, Matteo Corradini, and Giuseppe Nespoli for invaluable technical assistance. The automated fast solution changer device used in immunocitochemistry experiments was a gift from CV Scientific (http://www.cvscientific.com).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rainsford KD. Members of the Consensus Report Group on Nimesulide. Nimesulide – a multifactorial approach to inflammation and pain: scientific and clinical consensus. Curr Med Res Opin. 2006;22:1161–1170. doi: 10.1185/030079906X104849. [DOI] [PubMed] [Google Scholar]
- 2.Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett A, Villa G. Nimesulide: an NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Expert Opin Pharmacother. 2000;1:277–286. doi: 10.1517/14656566.1.2.277. [DOI] [PubMed] [Google Scholar]
- 4.Suleyman H, Cadirci E, Albayrak A, et al. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem. 2008;15:278–283. doi: 10.2174/092986708783497247. [DOI] [PubMed] [Google Scholar]
- 5.Dogan MD, Ataoglu H, Akarsu ES. Nimesulide and diclofenac inhibit lipopolysaccharide-induced hypothermia and tumour necrosis factor-alpha elevation in rats. Fundam Clin Pharmacol. 2002;16:303–309. doi: 10.1046/j.1472-8206.2002.00093.x. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi M, Broggini M, Balzarini P, et al. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract. 2007;61:1270–1277. doi: 10.1111/j.1742-1241.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 7.Tassorelli C, Greco R, Sandrini G, et al. Central components of the analgesic/antihyperalgesic effect of nimesulide: studies in animal models of pain and hyperalgesia. Drugs. 2003;63:9–22. doi: 10.2165/00003495-200363001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ohki S, Ogino N, Yamamoto S, et al. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979;254:829–836. [PubMed] [Google Scholar]
- 9.Harvison PJ, Egan RW, Gale PH, et al. Acetaminophen as a cosubstrate and inhibitor of prostaglandin H synthase. Adv Exp Med Biol. 1986;197:739–747. doi: 10.1007/978-1-4684-5134-4_68. [DOI] [PubMed] [Google Scholar]
- 10.Duarte ID, Ferreira SH. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide- cGMP pathway. Eur J Pharmacol. 1992;221:171–174. doi: 10.1016/0014-2999(92)90789-7. [DOI] [PubMed] [Google Scholar]
- 11.Duarte ID, dos S, Lorenzetti BB, et al. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;217:225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- 12.Pini LA, Vitale G, Ottani A, et al. Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther. 1997;280:934–940. [PubMed] [Google Scholar]
- 13.Vaughan CW, Ingram SL, Connor MA, et al. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 14.Alloui A, Chassaing C, Schmidt J, et al. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol. 2002;443:71–77. doi: 10.1016/s0014-2999(02)01578-9. [DOI] [PubMed] [Google Scholar]
- 15.Vanegas H, Tortorici V. Opioidergic effects of nonopioid analgesics on the central nervous system. Cell Mol Neurobiol. 2002;22:655–661. doi: 10.1023/A:1021896622089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallet C, Daulhac L, Bonnefont J, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139:190–200. doi: 10.1016/j.pain.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Cesare P, Dekker LV, Sardini A, et al. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 18.Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vellani V, Colucci M, Lattanzi R, et al. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Cang CL, Kawasaki Y, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKC epsilon: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vellani V, Kinsey AM, Prandini M, et al. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurons. Mol Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang JJ, Zhang XM, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol. 2006;17:638–645. doi: 10.1016/j.semcdb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Cao YQ, Mantyh PW, Carlson EJ, et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 24.Maggi CA. Tachykinins and calcitonin-gene-related peptide (CGRP) as cotransmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- 25.White DM. Release of substance P from peripheral sensory nerve terminals. J Peripher Nerv Syst. 1997;2:191–201. [PubMed] [Google Scholar]
- 26.Tang HB, Li YS, Arihiro K, Nakata Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons. Mol Pain. 2007;3:42. doi: 10.1186/1744-8069-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 28.Vellani V, Mapplebeck S, Moriondo A, et al. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YJ, Zachrisson O, Tonge DA, et al. Upregulation of bradykinin B2 receptor expression by neurotrophic factors and nerve injury in mouse sensory neurons. Mol Cell Neurosci. 2002;19:186–200. doi: 10.1006/mcne.2001.1073. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi M, Martucci C, Biella G, et al. Increased substance P and tumor necrosis factor-alpha level in the paws following formalin injection in rat. Brain Res. 2004;1019:255–258. doi: 10.1016/j.brainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi M, Franchi S, Ferrario P, et al. Effects of the bisphosphonate ibandronate on hyperalgesia, substance P, and cytokine levels in a rat model of persistent inflammatory pain. Eur J Pain. 2008;12:284–292. doi: 10.1016/j.ejpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi M, Martucci C, Ferrario P, et al. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugs. Anesth Analg. 2007;104:949–954. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- 33.Aley KO, Messing RO, Mochly-Rosen D, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 36.Brandman R, Disatnik MH, Churchill E, et al. Peptides derived from the C2 domain of protein kinase C epsilon (epsilon PKC) modulate epsilon PKC activity and identify potential protein-protein interaction surfaces. J Biol Chem. 2007;282:4113–4123. doi: 10.1074/jbc.M608521200. [DOI] [PubMed] [Google Scholar]
- 37.Vellani V, Petrosino S, De Petrocellis L, et al. Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology. 2008;55:1274–1279. doi: 10.1016/j.neuropharm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Chopra B, Giblett S, Little JG, et al. Cyclooxygenase-1 is a marker for a subpopulation of putative nociceptive neurons in rat dorsal root ganglia. Eur J Neurosci. 2000;12:911–920. doi: 10.1046/j.1460-9568.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- 41.Capuano A, De Corato A, Lisi L, et al. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolf CJ, Allchorne A, Safieh-Garabedian B, et al. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha TM, Verri WA, Jr, Silva JS, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37:355–361. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharmacol. 2003;3:1901–1908. doi: 10.1016/j.intimp.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Tang HB, Nakata Y. The activation of transient receptor potential vanilloid receptor subtype 1 by capsaicin without extracellular Ca2+ is involved in the mechanism of distinct substance P release in cultured rat dorsal root ganglion neurons. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:325–332. doi: 10.1007/s00210-007-0211-5. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi M, Ferrario P, Balzarini P, et al. Plasma and synovial fluid concentrations of nimesulide and its main metabolite after a single or repeated oral administration in patients with knee osteoarthritis. J Int Med Res. 2006;34:348–354. doi: 10.1177/147323000603400402. [DOI] [PubMed] [Google Scholar]
- 48.Smith SH. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269–280. [PubMed] [Google Scholar]