Abstract
Many neurological insults and neurodegenerative disorders are accompanied by an acute inflammatory reaction that can contribute to neuronal damage. This inflammation involves infiltration of bloodborne polymorphonuclear leukocytes (PMNs) into the injured brain area. The role of inflammation in brain injury, however, is controversial, because recent studies suggest that inflammation may actually be beneficial in the recovery from brain damage. Therefore, we investigated the effects of pathophysiologically relevant concentrations of PMNs in vitro on mixed hippocampal primary cultures. Rat PMNs and peripheral blood lymphocytes were isolated by density centrifugation and cocultured with hippocampal cells for 24-72 h plus or minus an excitotoxic insult (50 μM kainic acid) or 6-h oxygen glucose deprivation. Cell death was analyzed by immunocytochemistry, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, and neuron-specific [2,2′-azino-bis(ethylbenzothiazoline-6-sulfonic acid)] assay. After 3 days of coculture in the absence of insult, PMNs caused massive neuron loss and dramatic morphological changes in glial cells (astrocyte detachment, aggregation). Furthermore PMNs exacerbated kainic acid- and oxygen glucose deprivation-induced neuron death by 20-30%. The cytotoxic effect of PMNs required heterocellular contact and were ameliorated by protease inhibitors. Lymphocytes, on the other hand, were not neurotoxic, but, instead, increased astrocyte proliferation. These findings suggest that PMN might represent a harmful part of inflammation after brain injury that can contribute to secondary damage.
Abundant evidence exists that an inflammatory reaction is mounted in the CNS after trauma, stroke, and seizure. The inflammation, in response to brain injury, involves infiltration of neutrophils and monocytes/macrophages into the injured brain parenchyma, activation of resident brain cells (e.g., microglia and astrocytes) and expression of proinflammatory cytokines, adhesion molecules, and other inflammatory mediators (1, 2). The role of inflammation (harmful, beneficial, or nonrelevant) in the pathogenesis of brain injury is controversial (3, 4).
Activated polymorphonuclear leukocytes (PMNs), also referred to as neutrophils, play a prominent role in the neuropathology of neurological insults (5-9). As evidence, PMNs infiltrate injured CNS tissue at the time that cell death occurs, and neutropenias as well as prevention of PMN vascular adhesion/evasions is neuroprotective (10-13). However, other studies indicate that there is not a clear cause-effect relationship between PMN recruitment and CNS pathogenesis (14-16). The causative role of PMNs in ischemia-reperfusion damage in other tissues (e.g., myocardium), on the other hand, is widely accepted (17, 18).
Activated PMNs can contribute to tissue damage by (i) release of oxygen radicals; (ii) release of proteolytic enzymes such as elastase or metalloproteases; and (iii) stimulation and/or release of proinflammatory cytokines like TNF-α (18, 19). Considering the controversial role of PMNs during a neurological insult, strikingly few studies have evaluated whether and how PMNs interact with CNS cells: only one report demonstrates cytotoxic effects of PMNs on astrocyte cultures (20). To elucidate the role of PMNs during brain injury, one must characterize potential cellular interactions between these blood and CNS cells in the absence and presence of an insult. In this study, we investigate whether PMNs interact with hippocampal cell cultures and exacerbate neuron death during kainic acid (KA)-induced excitotoxicity or oxygen glucose deprivation (OGD).
Materials and Methods
Animals and Materials. Male Sprague-Dawley rats (250-300 g; Simonsen Laboratories, Gilroy, CA) were housed under a 12-h light-dark cycle. KA was purchased from Ocean Produce (Shelburne, NS, Canada), α1-antitrypsin (aTr), BSA, and diaminobenzidine (DAB)-FAST were purchased from Sigma.
Stereotaxic Microinfusion. Anesthesized rats (1 ml/kg of body weight of “rodent mixture” (76 mg/ml ketamine, 1.5 mg/ml promace, and 7.7 mg/ml xylazine, i.p.) were placed in a stereotaxic frame. Area CA3 of the hippocampus (anterior/posterior -3.85, lateral/medial ± 3.35 from bregma, dorsal/ventral -2.55 from dura) was microinfused. One side was injected with 0.06 μg of KA dissolved in PBS, and PBS alone was injected contralaterally as a control.
Immunohistochemistry. At indicated times after microinfusion, rats (n = 6 per group and time) were killed by halothane inhalation. Brains were quick-frozen in 2-methylbutane at -42°C for 3 min. Cryostat sections (15 μm) were cut, mounted, dried, and stored at -70°C. Slides were fixed in ice-cold acetone at -20°C for 3 min, treated with 0.03% H2O2 for 10 min at room temperature (RT) to block endogenous peroxidase activity and blocked with 5% normal goat serum or 3% BSA solution for 15 min at RT. Sections were incubated with granulocyte-specific Ab HIS48 (BD Pharmingen) and diluted in PBS 3% BSA for 2 h at RT in a humid chamber. Slides were rinsed three times in PBS and incubated with the respective biotinylated secondary Ab for 40 min at 37°C in a humid chamber. Slides were rinsed and treated with a horseradish peroxidase-streptavidin solution (1:400 in PBS 3% BSA) for 45 min at RT. Peroxidase labeling was visualized by incubation with DAB-FAST solution as a substrate for 2-4 min.
Hippocampal Cell Culture. Primary mixed neuronal/glial cultures were prepared from the hippocampi of embryonic day 18 rats. Hippocampi were dissected and cells dissociated by incubation in papain (10 units/ml) solution (Sigma) for 20 min. Solution was removed, tissue was resuspended in Hanks' balanced salt solution (GIBCO) and 10% FCS, and was dissociated by trituration with an 18-gauge needle. The cell suspension was centrifuged at 800 × g for 8 min and the pellet was resuspended in modified MEM (University of California, San Francisco) and 10% horse serum (HyClone). Sixty thousand cells per well were plated in poly-d-lysine-coated 96-well plates (Sigma) and maintained in 5% CO2 at 37°C. Cultures used at day 11 were 30-40% neuronal, as assessed by immunocytochemical staining using neuron-specific MAP-2 and glia-specific GFAP Abs (Sigma).
Isolation of Blood Cells. PMNs. A modified version of the hetastarch exchange transfusion protocol (21) was used. The jugular vein of a rat was cannulated and 2-ml aliquots of blood withdrawn in alternation with the infusion of 2-ml heparinized (25 units/ml) hydroxyethyl starch (HET; Sigma) by means of a three-way tap until the rat expired. The HET/blood mixture was sedimented for 40 min. Aliquots (5 ml) of the leukocyte-rich supernatant were mixed with 8 ml of Percoll (Amersham Pharmacia, Uppsala; specific gravity was adjusted to 1.120 g/ml at RT) and centrifuged at 360 × g for 30 min. The buffy coat of PMNs on top of the erythrocytes was collected by pipetting. Contaminating erythrocytes were removed by hypotonic lysis for 20 sec followed by addition of the same volume 2× PBS to restore osmolarity. PMNs were washed two times in Hanks' balanced salt solution and resuspended in MEM 10% HS. Cell yield (≈1-2 × 107 cells per rat) and viability (>95%) were estimated by hemocytometry/Trypan blue exclusion. Purity of the isolated PMN fraction was determined by using an Accustain Wright stain (Sigma) on smears; only fractions containing >90% PMNs were used.
Peripheral blood lymphocytes (Lys). One thousand units heparin was injected, and 15 ml of blood was obtained through the jugular vein and mixed with 15 ml of RPMI medium 1640 (GIBCO) and 10% FCS (GIBCO). Ten milliliters of the blood/medium mix was layered on an equal volume of Ficoll-Paque plus (Amersham Pharmacia) and centrifuged at 400 × g for 40 min at 20°C. The Ly layer was collected, washed three times with RPMI medium 1640, and resuspended in MEM 10% HS. Cell counts (≈1-2 × 107 cells per rat) and viability (>98%) were determined by hemocytometry/Trypan blue exclusion. As determined by Wright stain, Ly preparations contained >92% lymphocytes and monocytes. Monocytes represent only a minor fraction (<1-2%) of this Ly preparation.
Coculture of Blood and CNS Cells. Isolated blood cells (PMNs or Lys) were added to 11-day-old hippocampal cultures in effector-to-target cell (E/T) ratio representing blood cell/CNS cell ranging from 0.5/1 to 3/1; 1/1 is the pathophysiological E/T ratio found with in vivo kainate insult. Cocultures were maintained for 1-3 days. Viability of isolated blood cells was assessed by Trypan blue exclusion performed in separate wells on days 0, 1, 2, and 3. In experiments involving an excitotoxic insult, mixed cultures were treated with 50 μM KA 2 h before addition of blood cells, and cultures were analyzed 24 h later. In some experiments, cocultures were treated 1 h after addition of blood cells with protease inhibitor cocktail (PIC) (Boehringer-Mannheim; 0.2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride/1 μg/ml aprotinin/1 mM benzamidine/1 mM EDTA/10 μM leupeptin/10 μg/ml pepstatin) or 12.5 μg/ml serineprotease inhibitor aTr (Sigma). To investigate whether toxicity depended on heterocellular contact, tissue culture inserts for 96-well plates (Nunc) were used. Inserts were filled with 60 μl of the respective cultured medium before addition of blood cells. Cultures were then washed two times with MEM.
OGD and Reoxygenation Model. Medium was replaced with glucose- and serum-free MEM (plus essential vitamins, amino acids, and inorganic salts). Cultures were placed in a modular incubator chamber (Billups-Rothenberg, Del Mar, CA) flushed with anaerobic gas (5% CO2,5%H2, 90% N2; Bioblend PraxAir, San Ramon, CA). After a 6-h incubation at 37°C, reperfusion/normoxia was modeled by adding glucose to a final concentration of 5 mM as well as freshly isolated PMNs or Lys in a 2/1E/T ratio, was and subsequently incubated 18 h at 37°C under regular atmospheric conditions. Control cultures (no blood cells) received an equivalent amount of serum-containing MEM.
Immunocytochemistry. Briefly, cells were fixed with ice-cold methanol, washed four times with PBS and blocked in PBS 5% skim milk overnight (22). Cells were incubated for 30 min with either neuron-specific anti-MAP-2 or glia-specific anti-GFAP Ab (Sigma) diluted 1/1,000 in PBS 5% skim milk. Cells were washed four times with PBS and rat-adsorbed biotinylated secondary Ab (Vector Laboratories) was added for 30 min. After washing, a 30-min incubation with ABC reagent (Vector Laboratories), and a final PBS wash, cells were visualized by light microscope after incubation with DAB-FAST solution as a substrate.
Assessment of Cytotoxicity. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. After washing, 100 μl of fresh MEM-Pak and then 20 μl of a 5 mg/ml solution MTT (Sigma) in MEM-Pak were added to each well (23). After incubation at 37°C in 5% CO2 air for 1 h, medium was removed. Twenty microliters of 1.0 M NaOH and 100 μl of isopropanol/0.04 M HCl were added. After 10 min on a rocker platform, the plate was read on a microplate reader by using a test wavelength of 570 nm and a reference wavelength of 630 nm. Controls were vehicle-treated and the respective mean OD was set to 100% viability. All sample values are given as a percent of this control. 2,2′-Azino-bis(ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay. A slightly modified neuron-specific ABTS-ELISA was performed (22) to quantitate neuron loss. Cells were gently washed twice in PBS before fixation. The staining protocol for ABTS was performed as described in the immunocytochemistry section until the last step when ABTS reagent (Vector Laboratories) was used as a substrate. A green color developed over 5-20 min in the dark; plates were read immediately in an ELISA reader at 405 nm. Some wells containing vehicle-treated control cells were not incubated with primary Ab to determine a blank value that was subtracted from all sample values. The mean OD reading of vehicle treated control cells (- blank value) was set to 100% viability. All sample values are given as a percent of this control.
Statistical Analysis. Data are given as mean ± SD and were analyzed by paired t test or one-way ANOVA followed by post hoc comparisons (Tukey's test).
Results
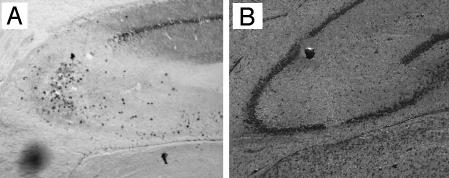
PMN Infiltration After KA Insult in Vivo. We first investigated whether KA-induced injury in vivo was associated with PMN infiltration. KA was injected locally into the hippocampus and caused the typical CA3 lesion. Injection of KA caused marked PMN infiltration (Fig. 1A). No PMNs were detected after PBS injection (Fig. 1B). PMNs were most concentrated at area CA3. Thus, KA-induced excitotoxic insult was clearly associated with acute PMN trafficking/migration to the hippocampus in vivo.
Fig. 1.
Immunohistochemical analysis of in vivo PMN infiltration after hippocampal KA injection. Coronal frozen sections were immunostained for PMNs 40 h after KA (A) or PBS (B) injection after Cresyl violet counterstaining.
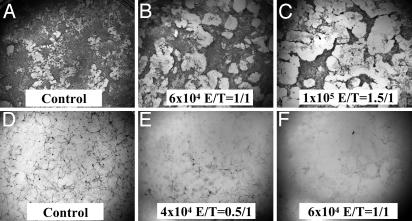
Three-Day Coculture of PMNs and Hippocampal Cells. To determine the effects of PMNs on CNS cells, we examined the in vitro interaction of PMNs and hippocampal cells. We first examined the effects of PMNs on CNS cells in the absence of insult. Primary hippocampal cells were incubated for 3 days with increasing numbers of freshly isolated PMNs. Immunocytochemical analysis of glial cells (Fig. 2 A-C) revealed that PMNs caused glial cells to aggregate and/or detach from the substrate. This marked effect on the glial cell layer was more pronounced with increasing concentration of PMNs. Immunocytochemical staining of neurons (Fig. 2 D-F) indicated a strong neurotoxic effect of PMNs because, even at lower PMN concentrations (4 × 104 cells, Fig. 2E) very few neurons could be detected; higher (6-8 × 104 cells) PMN concentrations (Fig. 2F) caused a complete loss of neurons.
Fig. 2.
Immunocytochemical analysis of PMNs and hippocampal cells after 3 days of coculture in the absence of an insult. Control, untreated cells; E/T (PMNs/CNS cells) ratio. Cultures were stained for glia (GFAP+; A-C) or neurons (MAP-2+; D-F).
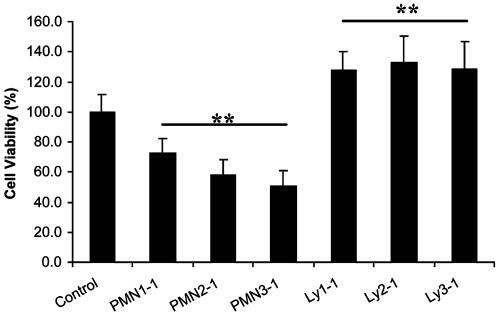
In addition to this qualitative analysis, we quantified the effect of PMNs on CNS cell viability by using a non-cell-specific MTT assay (Fig. 3). Compared to the control group, PMNs significantly decreased overall (thus including neurons and glia) cell viability after 3 days of coculture (P < 0.001, F = 108.25, df = 6/112). The cytotoxic PMN effect was detected after 3 days of coculture, but not after 1 or 2 days (data not shown). To eliminate the possibility that the observed cytotoxic effect could simply be caused by nutrient depletion (due to the increased number of cells per well), equivalent numbers of Lys were used as a control. Three days coculture of hippocampal cells with increasing numbers of Lys did not cause any cytotoxic effect; in fact, Lys seemed to cause an increase in cell numbers (Fig. 3; P < 0.001) compared with untreated cells. This result suggested a mild mitogenic effect of Lys on glial cells. Because the MTT assay measures mitochondrial activity that correlates with the number of viable cells, a distinction between glial and neuronal cell death could not be made.
Fig. 3.
Quantitation of PMN cytotoxic effect on CNS cells. PMNs or Lys were cocultured at different E/T ratios (1/1, 2/1, and 3/1) with hippocampal cells for 3 days. Cell viability was determined by MTT assay. (**, P < 0.001 vs. control; n = 22-42 per bar.)
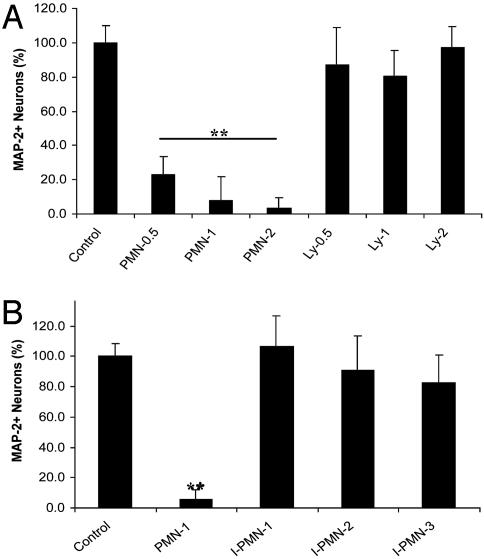
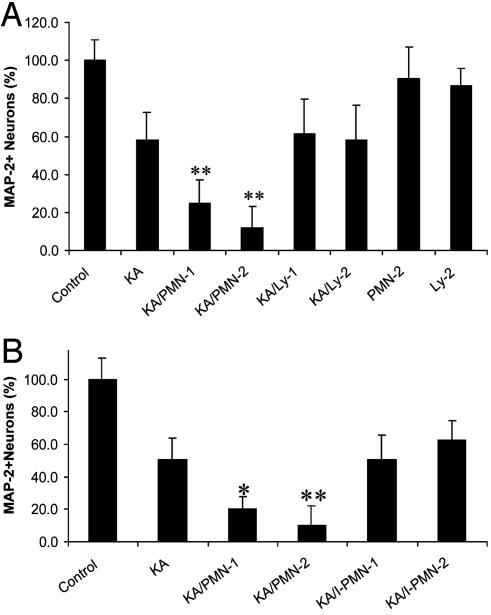
To quantitate the neurotoxic (see Fig. 2 F-H) effects of PMNs on CNS cells, an assay specific for neuron loss was used: MAP-2 Ab-based ABTS-ELISA (ABTS assay). After PMNs or Lys were cocultured at different E/T ratios (0.5/1, 1/1, and 2/1) with hippocampal cells for 3 days, PMNs significantly decreased neuron viability (Fig. 4A; P < 0.001, F = 124.9, df = 6/68, PMN 0.5, 1, and 2 vs. control). Lys, in contrast, did not significantly alter neuron viability at any E/T ratio. To exclude the possibility that PMN cytotoxicity was due to PMN death and release of toxic substances, we performed a Trypan blue exclusion assay on days 0, 1, 2, and 3. PMN viability was >95% throughout the experimental procedure (data not shown).
Fig. 4.
(A) Quantitation of PMN neurotoxic effect on CNS cells. PMNs or Lys were cocultured at 0.5/1, 1/1, and 2/1E/T ratios with hippocampal cells for 3 days. Neuron viability was determined by ABTS assay. (**, P < 0.001 vs. control; n = 20 per bar.) (B) Neurotoxic effects of PMNs on CNS cells after 3 days are dependent on heterocellular contact. PMNs or I-PMNs were cocultured at 1/1 and 2/1E/T ratios with CNS cells for 3 days. Neuron viability was determined as for A. (**, P < 0.001 vs. control; n = 20 per bar.)
We then investigated whether the PMN neurotoxic effects were dependent on heterocellular contact. PMNs (1/1 E/T ratio) or PMNs in tissue culture inserts [(I-PMNs), 1/1, 2/1, and 3/1 E/T ratios] were cocultured with hippocampal cells for 3 days. The I-PMNs were in close proximity to the CNS cells; medium or other soluble mediators could be exchanged freely between the two cell populations but PMNs had no direct contact with CNS cells. Neuron viability was determined by ABTS assay (Fig. 4B). PMNs significantly decreased neuron viability (P < 0.001), but I-PMNs did not have a significant neurotoxic effect at any E/T ratio. These data show that PMNs, even in the absence of an insult, have the potential to cause contact-dependent neurotoxic effects. Furthermore, it was confirmed that PMN neurotoxicity is not an unspecific effect secondary to PMN cell death. The fact that the neurotoxicity of PMNs was not evident before day 3 (data not shown) of coculture suggests that, in the absence of an insult, PMN activation takes 3 days.
Effect of PMN on Neuron Viability in Hippocampal Cells After Neurological Insult. During KA-induced excitotoxic insult, cells dying from necrosis release various substances (e.g., ROS and cytokines) that are potential activators of PMNs. We were interested in examining whether PMNs' neurotoxic potential after 3 days could be triggered within 24 h in the presence of KA. Therefore, we investigated whether PMNs exacerbate KA-induced neuron death. PMNs or Lys (1/1 and 2/1 E/T ratios) were cocultured with KA-treated hippocampal cells for 24 h. Neuron viability was determined by ABTS assay (Fig. 5A). PMNs significantly exacerbated KA-induced neurotoxicity (P < 0.001, F = 173.7, df = 6/317, KA/PMN 1 and 2 vs. KA). These data clearly show that the neurotoxic potential of PMNs could be activated by a neurological insult within 24 h. KA/Ly did not significantly alter neuron viability at any E/T ratio compared to KA alone. PMNs or Lys (2/1 E/T ratio) did not significantly alter neuron viability compared with the control (no KA) after 24 h of coculture in the absence of KA.
Fig. 5.
(A) PMNs exacerbate KA-induced excitotoxic neuron death. PMNs or Lys were cocultured at 1/1 and 2/1 E/T ratios with KA-treated hippocampal cells for 24 h. Neuron viability was determined by ABTS assay. (**, P < 0.001 vs. KA; n = 30-50 per bar.) (B) PMN exacerbation of KA-induced neuron death depends on heterocellular contact. PMNs or I-PMNs were cocultured at 1/1 and 2/1E/T ratios with KA-treated CNS cells. Neuron viability was determined as in A. (**, P < 0.001 vs. KA; n = 30-50 per bar.)
As with the PMN effect observed after 3 days of coculture, we also tested whether the PMN neurotoxic effects detected 24 h after KA insult were contact-dependent. KA/PMNs (1/1 E/T ratio) or KA/I-PMNs (1/1 and 2/1 E/T ratios) were cocultured with hippocampal cells. Neuron viability was determined by ABTS assay (Fig. 5B) after 24 h. KA/PMN significantly exacerbated neuron loss (P < 0.001 KA/PMN vs. KA), but KA/I-PMN did not have a significant neurotoxic effect at any E/T ratio compared with KA alone. These data show that PMN exacerbated KA-induced hippocampal neuron loss and that this exacerbation depends on heterocellular contact.
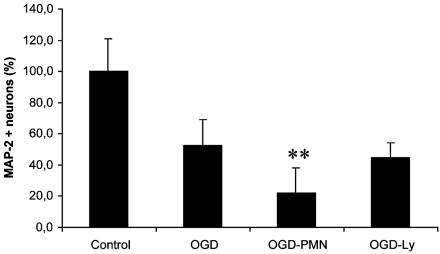
We further assessed whether PMN exacerbation of neuronal death could be observed in a second model of neurological insult, OGD, which is a physiologically relevant in vitro global ischemia model. After 6 h of OGD, hippocampal cells were cocultured with PMNs or Lys (2/1 E/T ratio) with glucose and oxygen levels normalized. Neuron viability was determined by ABTS assay (Fig. 6) after 18 h. PMNs significantly exacerbated OGD-induced neurotoxicity (P < 0.001, F = 45.9, df = 1/54, OGD/PMN vs. OGD), whereas Lys did not significantly alter neuron viability (P = 0.207; OGD-Ly vs. OGD). Thus, we demonstrated that PMNs clearly have the potential to worsen the outcome of neurological insults.
Fig. 6.
PMNs exacerbate neuron loss after OGD. After 6 h of OGD, hippocampal cells were cocultured with PMNs or Lys at 2/1E/T ratios with glucose and oxygen levels normalized. Neuron viability was determined by ABTS assay. (**, P < 0.001 vs. OGD; n = 30 per bar.)
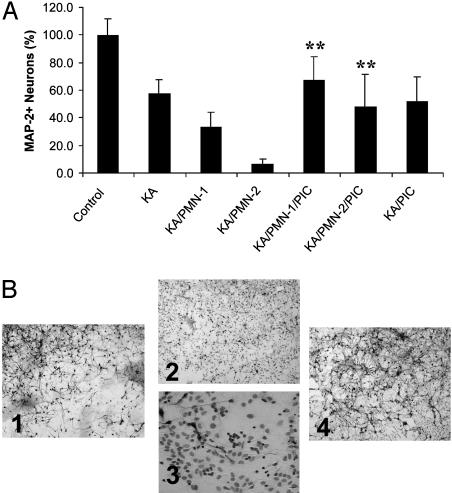
Blocking of PMN Exacerbation of KA-Induced Neuron Loss by Protease Inhibitors. We then addressed the question of how PMNs exacerbate KA-induced neuron loss. Activated PMNs could contribute to tissue damage by releasing toxic substances such as free radicals and proteolytic enzymes; thus, suppression of these potentially noxious substances should lead to neuroprotection. Two hours after adding PMNs to KA-treated hippocampal cells, cultures were treated with protease inhibitors or various anti-oxidants. The addition of different radical scavengers (riboflavine, tiron, and ascorbic acid) did not have a significant effect on neuron viability (data not shown). In contrast, treatment with PIC resulted in complete blocking of the PMN exacerbation of neurotoxicity (Fig. 7A). PMNs significantly exacerbated neuron loss (P < 0.001, KA/PMN-1,2 vs. KA); the addition of PIC caused a significant increase in neuronal survival (P < 0.001, F = 99.8, df = 6/159, KA/PMN-1,2 vs. KA/PMN-1,2/PIC). PIC did not provide neuroprotection against KA alone (P = 0.114; KA/PIC vs. KA). These findings were further confirmed by immunocytochemistry (Fig. 7B). Cultures were immunostained for neurons followed by a Wright stain to visualize PMN. Compared with KA alone (Fig. 7B1) the addition of PMNs caused a dramatic increase in neuron loss and the almost complete destruction of the neuronal network consisting of axons and dendrites (Fig. 7B2). A higher magnification revealed that PMNs were concentrated around the few remaining intact neurons (Fig. 7B3). In the presence of PIC, however, this strong neurotoxic effect of PMNs could be successfully blocked (Fig. 7B4). These data suggest that proteases seem to play a major role in PMN exacerbation of KA-induced neurotoxicity.
Fig. 7.
(A) PMN exacerbation of KA-induced neuron death is blocked by protease inhibitors. PMNs were cocultured at 1/1 and 2/1 E/T ratios (± PIC) with KA-treated hippocampal cells for 24 h. Neuron viability was determined by ABTS assay. Addition of PIC caused a significant increase in neuronal survival (**, P < 0.001 vs. KA/PMN-1,2; n = 30-40 per bar). (B) Immunocytochemical analysis of PIC treatment and blocking of PMN exacerbation of neuron death. Cells were treated as described in A. Cultures were then immunostained for neurons (MAP-2) followed by a Wright stain to visualize PMN (arrows in B3). The addition of PIC successfully prevented PMN exacerbation of KA-induced neuron loss (B1, KA alone; B2 and B3, KA plus PMN-2/1 E/T ratio; B4, KA plus PMN plus PIC).
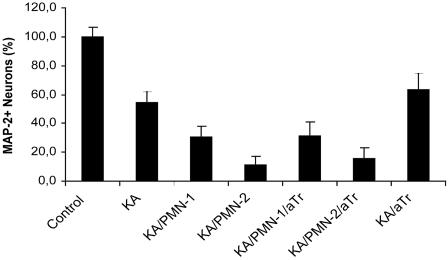
Inhibition of Serine Protease Elastase. Because PIC is a mixture of five different protease inhibitors with different targets, it caused a broad and general protease inhibition in these experiments. Whereas PMNs can release several proteolytic enzymes, the serine protease elastase is known to be a major contributor to PMN-related injury. To elucidate the potential role of PMN elastase, we investigated whether the serine protease inhibitor aTr could prevent the PMN exacerbation after KA-induced insult. PMNs (1/1 and 2/1 E/T ratios) were cocultured with KA-treated hippocampal cells for 24 h. Two hours after adding the PMNs, cells were treated with aTr (Fig. 8). PMNs significantly exacerbated KA-induced neuron death (P < 0.001). PMN exacerbation was not prevented by the elastase inhibitor aTr (P > 0.238, F = 189.3, df = 6/103, KA/PMN 1,2/aTr vs. KA/PMN 1,2), however, aTr significantly increased neuron viability compared with KA alone (P < 0.03; KA/aTr vs. KA). These results indicate either that elastase does not play a major role in PMN exacerbation of neurotoxicity or that administration of aTr alone is not sufficient to provide the neuroprotective effects seen with PIC.
Fig. 8.
PMN exacerbation of KA-induced neuron death is not blocked by serine protease inhibitor aTr. PMNs were cocultured at 1/1 and 2/1E/T ratios (± aTr) with KA-treated hippocampal cells for 24 h. Neuron viability was determined by ABTS assay (n = 24-32 per bar).
Discussion
Insults such as trauma, stroke, and seizure are accompanied by a marked inflammatory reaction within the CNS (1, 24), which is believed to contribute to brain damage (2, 25). Because PMNs rapidly infiltrate the injured brain parenchyma, it has been hypothesized that PMNs can exacerbate postinsult injury by releasing noxious substances such as cytokines, oxygen radicals and proteases (19, 26). The present study investigated potential interactions of bloodborne PMN and hippocampal CNS cells under various conditions in vitro.
We first confirmed that PMN infiltration was indeed involved in our experimental model of a neurological insult in vivo. KA-induced excitotoxicity in vivo caused a massive and rapid PMN infiltration into the CA3 area of the hippocampus. We then studied PMN-CNS cell interactions in vitro, analyzing the effects of PMN on primary mixed hippocampal cultures by using an approximate 1/1 PMN/neuron ratio that resembles pathophysiological in vivo conditions (27, 28).
We first investigated the effect of PMN on CNS cell cultures in the absence of insult. After 3 days of coculture, PMNs caused glial cells to aggregate and detach from the substrate; this result was probably due to the release of extracellular matrix degrading proteases from PMN. As determined by MTT assay, PMNs had an overall cytotoxic effect on these cultures, whereas Lys had a mild mitogenic effect on glial cells (Lys had no effect on the total number of neurons as determined by neuron-specific assays). These results agree with findings investigating PMN effects on astrocyte cultures (20). We further showed that, in contrast to Lys, PMNs had a dramatic concentration-dependent cytotoxic effect on neurons in these hippocampal cultures. After 3 days of coculture, even with relatively low concentrations (0.5/1 E/T ratio) of PMNs, the cultures were nearly depleted of neurons. This finding demonstrates that neurons are particularly sensitive to PMN-mediated toxicity. Interestingly, no cytotoxic PMN effects were detected before day 3, suggesting the requirement of further stimulatory signals (such as mediators released during a neurological insult). It is also possible that 3-day proliferation of the respective cell types was needed before showing the observed PMN-induced neurotoxicity or the Ly-induced mild mitogenic effect on glia. The hetastarch exchange transfusion used in this study to isolate PMNs harvests a large pool of noncirculating PMNs that reflect unstimulated PMNs better than peritoneally derived PMNs (21). Unstimulated PMNs undergo spontaneous apoptosis on in vitro culture (29, 30), whereas different stimuli (e.g., platelets, red blood cells, or IL-6) extended PMN lifespan (31, 32). PMNs in our coculture experiments were viable for at least for 3 days, strongly indicating an interaction between PMN and CNS cells.
We also showed that heterocellular contact was needed for the neurotoxic PMN effect, because no neuron loss was detected in experiments without direct PMN-CNS cell contact. This finding suggests that 3 days of direct cell contact activate PMN to trigger the respective neurotoxic effector pathway (e.g., release of cytotoxic substances). These data showed that PMNs can cause neuronal loss in the absence of a neurological insult.
We then investigated PMN-CNS cell interactions during KA-induced excitotoxicity. In many neurological disorders, neuron death is caused by glutamatergic excitotoxicity (33). A massive increase of extracellular glutamate results in prolonged depolarization of neurons, inducing further glutamate release and in turn increased intracellular Ca2+-levels, which then activate Ca2+-dependent enzymes (e.g., proteases degrading the cytoskeleton), eventually leading to necrotic cell death. PMNs exacerbated KA-induced neuron loss, whereas Lys did not alter neuron viability. This neurotoxic PMN effect was detectable as early as 24 h post-KA, suggesting that in the presence of a neurological insult, PMNs were rapidly activated. In response to excitotoxic brain injury, neurons and glia release potent proinflammatory mediators such as oxygen-free radicals or cytokines (25, 34), which can directly or indirectly activate PMNs (35-37). As seen in cocultures without insult, we showed that the observed PMN-mediated exacerbation of KA insult depended on PMN concentration and cell contact with CNS cells. PMNs also exacerbated neuron loss after OGD, which is an established in vitro global ischemia model. However, the OGD results need to be interpreted with caution because the in vivo PMN/neuron ratio in the whole ischemic infarct area might be smaller than that determined in the KA model.
There are several potential mechanisms/effectors that could be involved in this PMN-mediated neurotoxicity. PMN activation causes spontaneous release of proteolytic enzymes and generation of reactive oxygen species (37). To test which components were responsible for the observed PMN neurotoxic effect, we investigated whether blocking certain substances would be neuroprotective. Treatment of the cocultures with various anti-oxidants (riboflavine, tiron, and ascorbic acid) was not beneficial, suggesting that free radicals may not be the main effectors of PMN toxicity. Even though the use of three anti-oxidant compounds suggested this possibility, there still remains the possibility of oxygen radical involvement. In contrast, treatment of the cultures with proteases inhibitors was protective, indicating that the cytotoxic PMN effect was mediated by protease activity. Even though all relevant cell types were present in our in vitro model, interactions may be different in vivo. Because the mechanisms of inflammation-mediated neurotoxicity are likely to be model-specific, in vivo correlates will be critical to define the biological relevance of the observations. Of the various proteases that are released by activated PMN (elastase, metalloproteases, and gelatinase), neutrophil elastase is particularly relevant. Neutrophil elastase, a granule serine protease, degrades extracellular matrix components (38) and has been linked to excessive injury during inflammation of various tissues (39, 40), cerebral ischemic damage (41), and spinal cord injury (42). Furthermore, plasma neutrophil elastase levels are increased by acute cerebral infarct (43). Treatment of the cocultures with aTr, a serine protease inhibitor and endogenous inhibitor of neutrophil elastase, was not protective, indicating that elastase is not a key enzyme in PMN neurotoxicity. However matrix metalloproteases readily degrade aTr (44); therefore, in our system, aTr inhibition might only be effective in combination with other protease inhibitors. Furthermore, the biological activity of aTr is affected by PMN oxidative products and other chemical modifications (39, 45).
Our findings suggest that PMNs can be a harmful part of the acute inflammatory response and can contribute to secondary damage after a neurological insult. Understanding the molecular detail of PMN-CNS cell interactions may be key to effective therapeutic intervention.
Acknowledgments
We thank Angela Lee for manuscript assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant DI-866 (to K.D.), National Institutes of Health Grants RO1 MH53814 and PO1 NS37520 (to R.M.S.), and National Institutes of Health Grant RO1 49885 (to F.S.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PMN, polymorphonuclear leukocyte; I-PMN, PMNs in tissue culture inserts; Ly, lymphocyte; PIC, protease inhibitor cocktail; aTr, α1-antitrypsin; KA, kainic acid; ABTS, 2,2′-azino-bis(ethylbenzothiazoline-6-sulfonic acid); OGD, oxygen glucose deprivation; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; RT, room temperature; E/T, effector-to-target cell.
References
- 1.Dirnagl, U., Iadecola, C. & Moskowitz, M. A. (1999) Trends Neurosci. 22, 391-397. [DOI] [PubMed] [Google Scholar]
- 2.Feuerstein, G., Wang, X. & Barone, F. (1998) Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management (Blackwell Scientific, Oxford).
- 3.del Zoppo, G. J., Becker, K. J. & Hallenbeck, J. M. (2001) Arch. Neurol. (Chicago) 58, 669-672. [DOI] [PubMed] [Google Scholar]
- 4.Feuerstein, G. Z. & Wang, X. (2001) Arch. Neurol. (Chicago) 58, 672-674. [DOI] [PubMed] [Google Scholar]
- 5.Wang, X. & Feuerstein, G. Z. (2000) Drug News Perspect. 13, 133-140. [DOI] [PubMed] [Google Scholar]
- 6.Jean, W. C., Spellman, S. R., Nussbaum, E. S. & Low, W. C. (1998) Neurosurgery 43, 1382-1397. [DOI] [PubMed] [Google Scholar]
- 7.Ember, J. A., del Zoppo, G. J., Mori, E., Thomas, W. S., Copeland, B. R. & Hugli, T. E. (1994) J. Cereb. Blood Flow Metab. 14, 1046-1054. [DOI] [PubMed] [Google Scholar]
- 8.Kochanek, P. M. & Hallenbeck, J. M. (1992) Stroke (Dallas) 23, 1367-1379. [DOI] [PubMed] [Google Scholar]
- 9.Prestigiacomo, C. J., Kim, S. C., Connolly, E. S., Jr., Liao, H., Yan, S. F. & Pinsky, D. J. (1999) Stroke (Dallas) 30, 1110-1117. [DOI] [PubMed] [Google Scholar]
- 10.Heinel, L. A., Rubin, S., Rosenwasser, R. H., Vasthare, U. S. & Tuma, R. F. (1994) Brain Res. Bull. 34, 137-141. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo, Y., Onodera, H., Shiga, Y., Nakamura, M., Ninomiya, M., Kihara, T. & Kogure, K. (1994) Stroke (Dallas) 25, 1469-1475. [DOI] [PubMed] [Google Scholar]
- 12.Yanaka, K., Camarata, P. J., Spellman, S. R., McCarthy, J. B., Furcht, L. T., Low, W. C. & Heros, R. C. (1996) J. Cereb. Blood Flow Metab. 16, 1120-1125. [DOI] [PubMed] [Google Scholar]
- 13.Connolly, E. S., Jr., Winfree, C. J., Springer, T. A., Naka, Y., Liao, H., Yan, S. D., Stern, D. M., Solomon, R. A., Gutierrez-Ramos, J. C. & Pinsky, D. J. (1996) J. Clin. Invest. 97, 209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerich, D. F., Dean, R. L., III, & Bartus, R. T. (2002) Exp. Neurol. 173, 168-181. [DOI] [PubMed] [Google Scholar]
- 15.Fassbender, K., Ragoschke, A., Kuhl, S., Szabo, K., Fatar, M., Back, W., Bertsch, T., Kreisel, S. & Hennerici, M. (2002) Cerebrovasc. Dis. 13, 198-203. [DOI] [PubMed] [Google Scholar]
- 16.Hayward, N. J., Elliott, P. J., Sawyer, S. D., Bronson, R. T. & Bartus, R. T. (1996) Exp. Neurol. 139, 188-202. [DOI] [PubMed] [Google Scholar]
- 17.La, M., Tailor, A., D'Amico, M., Flower, R. J. & Perretti, M. (2001) Eur. J. Pharmacol. 429, 263-278. [DOI] [PubMed] [Google Scholar]
- 18.Jordan, J. E., Zhao, Z. Q. & Vinten-Johansen, J. (1999) Cardiovasc. Res. 43, 860-878. [DOI] [PubMed] [Google Scholar]
- 19.Barone, F. C., Hillegass, L. M., Price, W. J., White, R. F., Lee, E. V., Feuerstein, G. Z., Sarau, H. M., Clark, R. K. & Griswold, D. E. (1991) J. Neurosci. Res. 29, 336-345. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Flores, M. T., Bovolenta, P. & Nieto-Sampedro, M. (1993) Glia 7, 146-157. [DOI] [PubMed] [Google Scholar]
- 21.Williams, J. H., Jr., Moser, K. M., Ulich, T. & Cairo, M. S. (1987) J. Leukocyte Biol. 42, 455-462. [DOI] [PubMed] [Google Scholar]
- 22.Brooke, S. M., Bliss, T. M., Franklin, L. R. & Sapolsky, R. M. (1999) Neurosci. Lett. 267, 21-24. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann, T. (1983) J. Immunol. Methods 65, 55-63. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. M., Zipfel, G. J. & Choi, D. W. (1999) Nature 399, A7-A14. [DOI] [PubMed] [Google Scholar]
- 25.Barone, F. C. & Feuerstein, G. Z. (1999) J. Cereb. Blood Flow Metab. 19, 819-834. [DOI] [PubMed] [Google Scholar]
- 26.Hallenbeck, J. M., Dutka, A. J., Tanishima, T., Kochanek, P. M., Kumaroo, K. K., Thompson, C. B., Obrenovitch, T. P. & Contreras, T. J. (1986) Stroke (Dallas) 17, 246-253. [DOI] [PubMed] [Google Scholar]
- 27.Dinkel, K., MacPherson, A. M. & Sapolsky, R. M. (2003) J. Neurochem. 84, 705-716. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky, R. M. & Stein, B. A. (1989) Neurosci. Lett. 97, 157-162. [DOI] [PubMed] [Google Scholar]
- 29.Athens, J. W., Raab, O. P. & Raab, S. O. (1961) J. Clin. Invest. 40, 989-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savill, J. S., Wyllie, A. H., Henson, J. E., Walport, M. J., Henson, P. M. & Haslett, C. (1989) J. Clin. Invest. 83, 865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andonegui, G., Trevani, A. S., Lopez, D. H., Raiden, S., Giordano, M. & Geffner, J. R. (1997) J. Immunol. 158, 3372-3377. [PubMed] [Google Scholar]
- 32.Aoshiba, K., Nakajima, Y., Yasui, S., Tamaoki, J. & Nagai, A. (1999) Blood 93, 4006-4010. [PubMed] [Google Scholar]
- 33.Lipton, S. A. & Rosenberg, P. A. (1994) N. Engl. J. Med. 330, 613-622. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell, N. J., Luheshi, G. & Toulmond, S. (1996) Pharmacol. Ther. 69, 85-95. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, K., Hino, M., Hato, F., Tatsumi, N. & Kitagawa, S. (1999) Blood 93, 341-349. [PubMed] [Google Scholar]
- 36.Yuo, A., Kitagawa, S., Ohsaka, A., Saito, M. & Takaku, F. (1990) Biochem. Biophys. Res. Commun. 171, 491-497. [DOI] [PubMed] [Google Scholar]
- 37.Labro, M. T. (2000) Clin. Microbiol. Rev. 13, 615-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss, S. J. & Regiani, S. (1984) J. Clin. Invest. 73, 1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujie, K., Shinguh, Y., Inamura, N., Yasumitsu, R., Okamoto, M. & Okuhara, M. (1999) Eur. J. Pharmacol. 374, 117-125. [DOI] [PubMed] [Google Scholar]
- 40.Carden, D., Xiao, F., Moak, C., Willis, B. H., Robinson-Jackson, S. & Alexander, S. (1998) Am. J. Physiol. 275, H385-H392. [DOI] [PubMed] [Google Scholar]
- 41.Shimakura, A., Kamanaka, Y., Ikeda, Y., Kondo, K., Suzuki, Y. & Umemura, K. (2000) Brain Res. 858, 55-60. [DOI] [PubMed] [Google Scholar]
- 42.Tonai, T., Shiba, K., Taketani, Y., Ohmoto, Y., Murata, K., Muraguchi, M., Ohsaki, H., Takeda, E. & Nishisho, T. (2001) J. Neurochem. 78, 1064-1072. [DOI] [PubMed] [Google Scholar]
- 43.Iwatsuki, K., Kumura, E., Yoshimine, T., Yamamoto, K., Sato, M. & Hayakawa, T. (1998) Neurol. Res. 20, 397-402. [DOI] [PubMed] [Google Scholar]
- 44.Chandler, S., Cossins, J., Lury, J. & Wells, G. (1996) Biochem. Biophys. Res. Commun. 228, 421-429. [DOI] [PubMed] [Google Scholar]
- 45.Moraga, F., Lindgren, S. & Janciaskiene, S. (2001) Arch. Biochem. Biophys. 386, 221-226. [DOI] [PubMed] [Google Scholar]