Abstract
Objective
To evaluate the additive intraocular pressure (IOP)-lowering efficacy and safety of fixed-combination brimonidine 0.2%/timolol 0.5% compared with timolol 0.5% at peak and trough effect when used as therapy adjunctive to latanoprost 0.005% in patients with glaucoma or ocular hypertension who require additional IOP lowering.
Methods
In this prospective, randomized, multicenter, investigator-masked, parallel-group study, patients were treated with latanoprost monotherapy for at least four weeks prior to baseline. At baseline on latanoprost, patients with IOP ≥21 mmHg in at least one eye were randomized to twice-daily fixed brimonidine-timolol (n = 102) or timolol (n = 102), each adjunctive to latanoprost for 12 weeks. IOP was measured at 8 am and 10 am at baseline, week 6, and week 12 and evaluated in the per protocol population. The primary efficacy endpoint was peak IOP lowering at 10 am, week 12. Safety measures included adverse events.
Results
Baseline mean IOP was similar at 10 am in the treatment groups (brimonidine-timolol 23.4 mmHg; timolol 23.0 mmHg). The mean additional reduction from latanoprost-treated baseline IOP was 8.3 mmHg (35.5%) with fixed brimonidine-timolol and 6.2 mmHg (27.0%) with timolol at 10 am, week 12 (P < 0.001). Patients treated with fixed brimonidine-timolol adjunctive to latanoprost were significantly more likely than patients treated with adjunctive timolol to achieve an IOP <18 mmHg (P = 0.028) and a ≥20% reduction in IOP from baseline (P = 0.047) at both 8 am and 10 am in week 12. Adverse events occurred in 14.7% of fixed brimonidine-timolol patients and 12.7% of timolol patients. Biomicroscopy findings were similar between the treatment groups after 12 weeks of treatment.
Conclusion
Fixed-combination brimonidine-timolol reduced IOP significantly more effectively than timolol when used as adjunctive therapy to latanoprost in patients with glaucoma and ocular hypertension. Both fixed brimonidine-timolol and timolol were well tolerated as agents adjunctive to latanoprost.
Keywords: brimonidine, drug combinations, glaucoma, intraocular pressure, ocular hypertension, timolol
Introduction
Patients with glaucoma and ocular hypertension frequently require multiple intraocular pressure (IOP)-lowering medications. The topical once-daily prostaglandin analogs (latanoprost, bimatoprost, and travoprost) are the most commonly prescribed primary IOP-lowering therapy based on their safety and efficacy profile. However, some patients are unable to either achieve or maintain their target IOP with monotherapy alone. In the Ocular Hypertension Treatment Study, for example, 40% of treated patients required two or more medications to achieve a 20% reduction from baseline IOP by year 5.1 In a study by Covert and Robin,2 over 20% of patients treated with a once-daily prostaglandin analog added another IOP-lowering medication to their regimen within a year of initiating treatment.
The fixed combination of brimonidine 0.2%/timolol 0.5% has been demonstrated to reduce IOP more effectively than either brimonidine 0.2% or timolol 0.5% used alone and to be better tolerated than brimonidine 0.2% monotherapy.3 A fixed combination of brimonidine-timolol has also been shown to be as efficacious and well tolerated as concomitant use of separate bottles of brimonidine 0.2% and timolol 0.5%.4 When multiple drug therapy is needed, use of a fixed combination of two IOP-lowering medications in one bottle may be preferred to simplify the medication regimen and enhance patient convenience and adherence to treatment.5 Studies have suggested that adherence with topical medications may be reduced with the addition of each adjunctive glaucoma agent.6,7
The efficacy and safety of fixed brimonidine-timolol used as therapy adjunctive to prostaglandin analogs has been evaluated previously in open-label studies8,9 and in a comparison study of a fixed combination of dorzolamide and timolol.10 The purpose of the present study was to evaluate the additive IOP-lowering efficacy and safety of fixed-combination brimonidine-timolol compared with timolol alone at 8 am (trough effect) and 10 am (peak effect) when each is used as therapy adjunctive to latanoprost in patients requiring additional IOP lowering.
Methods
This prospective, randomized, multicenter (15 sites in the US and Canada), investigator-masked, parallel-group clinical study compared fixed brimonidine-timolol with timolol as adjunctive therapy for patients on latanoprost who required additional IOP lowering. The study was approved by the institutional review board at each site. All patients who participated in the study provided written informed consent. The study is registered at clinicaltrials.gov with the identifier NCT00735449.
Adult patients with a diagnosis of ocular hypertension or primary open-angle glaucoma, chronic angle-closure glaucoma with patent iridotomy or iridectomy, pseudoexfoliative glaucoma, or pigmentary glaucoma requiring treatment with IOP-lowering medication who had inadequate IOP control after at least four continuous weeks of latanoprost 0.5% (Xalatan®; Pfizer Inc, New York, NY) monotherapy were enrolled in the study. Patients were required to have IOP ≥21 mmHg and <34 mmHg in at least one eligible eye (the study eye) at both 8 am and 10 am on latanoprost-treated baseline. Patients were also required to have best-corrected visual acuity equivalent to a Snellen score of 20/100 or better in both eyes. Primary exclusion criteria included uncontrolled systemic disease or active ocular disease other than glaucoma or ocular hypertension that in the judgment of the investigator would interfere with study interpretation, any corneal abnormality that would preclude accurate IOP readings, history of or active ocular infection/inflammation, visual field loss indicative of end-stage glaucoma, history of intraocular surgery or glaucoma laser surgery within three months prior to baseline, any history of refractive surgery, any contraindication to beta-blocker or brimonidine therapy, presence of severe cardiovascular disease, and pregnant, lactating, or potential for pregnancy.
All patients were treated bilaterally with latanoprost monotherapy once daily in the evening for at least four weeks prior to the baseline visit (day 0). Patients on IOP-lowering medications at screening underwent a four-week washout of all medications other than latanoprost prior to baseline; patients not on latanoprost at screening were run in on latanoprost for four weeks prior to baseline; and patients on latanoprost monotherapy at screening were continued on latanoprost monotherapy for 2–28 days until baseline. At the baseline visit, patients who met all eligibility criteria for the study continued on latanoprost and were randomly assigned with a 1:1 allocation to bilateral adjunctive treatment with twice-daily brimonidine tartrate 0.2%/timolol maleate 0.5% ophthalmic solution (Combigan®; Allergan Inc, Irvine, CA) or timolol maleate 0.5% ophthalmic solution for 12 weeks. The randomization code was computer-generated, and personnel responsible for collection of efficacy and safety measures were masked to treatment assignment. To maintain masking of the investigators, bottles of the study drugs were overlabeled and provided to patients in identically appearing masked cartons labeled with the patient randomization number, and patients were instructed not to discuss their study medication with the investigator or office staff. An individual at each site, not otherwise involved in the study, was assigned to dispense study medications and retrieve them from patients.
Patients were instructed to instill one drop of latanoprost in each eye once daily at 8 pm (±15 minutes) and to instill one drop of the study medication in each eye twice daily, in the morning at 8 am (±15 minutes) and in the evening at five minutes after the instillation of latanoprost. Patients were scheduled for follow-up visits at weeks 6 and 12. At the week 6 and week 12 study visits, the study medication was instilled by office personnel after the 8 am IOP measurement.
The primary efficacy endpoint was mean IOP at peak effect (10 am) at week 12. IOP was measured in both eyes using a Goldmann applanation tonometer at 8 am and 10 am at baseline and at the week 6 and week 12 visits. Two consecutive measurements were taken for each eye, and the average value was used for analysis. For patients with both eyes eligible for the study, efficacy was evaluated in the worse eye (the eye with the higher IOP at baseline).
Safety outcome measures included adverse events and ocular signs on slit-lamp biomicroscopy. Biomicroscopic findings were graded on a scale of 0 = none, 0.5 = trace, 1 = mild, 2 = moderate, and 3 = severe. An adverse event was defined as any untoward medical occurrence in a patient during the course of a study. At each follow-up visit, patients were asked whether any adverse events had occurred since the previous visit. All adverse events observed by the investigator or reported by patients were recorded, and the investigator documented the severity of the adverse events and their potential relationship to study treatment. An adverse event was determined by the investigator to be treatment-related when there was a reasonable possibility of a causal relationship between the study medication and the event.
The preplanned analyses of IOP were based on observed data from the per protocol patient population with no imputation for missing values. The per protocol population was defined as all patients who had efficacy evaluations at baseline and during follow-up, and who used no prohibited medications and had no prohibited procedures during the study that could interfere with the study objectives. The a priori statistical plan for the study included analyses of mean IOP, mean change from baseline IOP, and the percentage of patients with a decrease from baseline IOP of 20% or more. Baseline differences in IOP between treatment groups were analyzed with t-tests, and differences in IOP between treatment groups at follow-up were analyzed using analysis of covariance with baseline IOP as the covariate. Post hoc analysis of the percentage of patients with IOP less than 14, 15, 16, 17, and 18 mmHg at both the 8 am and 10 am time points at week 12 and the within-group changes in IOP from baseline were also performed. The Fisher’s Exact test was used to compare the percentages of patients between treatment groups. Within-group changes in IOP from baseline were analyzed using paired t-tests.
Summary statistics were calculated for demographic and safety parameters. All randomized patients received at least one dose of study medication and were included in the safety analyses. Statistical analyses were performed using JMP 7.0 software (SAS Institute, Cary, NC). All statistical tests were two-tailed with the alpha level for significance set at 0.05. A sample size calculation estimated that 72 patients in each group would provide 80% power to detect a 1.5 mmHg difference between groups in the primary outcome. The planned sample size of 100 patients in each treatment group was chosen to ensure 80% power in the primary analysis, assuming a dropout rate of 15% and additional exclusions of patients from the per protocol population used for analysis.
Results
Patient baseline characteristics and disposition
A total of 204 patients with inadequate IOP control on latanoprost alone were enrolled in the study and randomized to adjunctive treatment with fixed brimonidine-timolol or timolol. Patient demographics at baseline were comparable between groups (P ≥ 0.123) and are listed in Table 1. The mean age of patients was 64 years, and almost all of the patients (196/204, 96.1%) were using at least one IOP-lowering medication at screening. Visual fields in the study eye were reported to be abnormal in the majority (63.6%) of patients.
Table 1.
Demographics and clinical characteristics of patients at baseline
| Fixed brimonidine-timolol and latanoprost (n = 102) | Timolol and latanoprost (n = 102) | Between-group P value | |
|---|---|---|---|
| Mean (SD) age, years | 63.4 (11.3) | 64.8 (10.8) | 0.813 |
| Sex, n (%) | 0.123 | ||
| Male | 45 (44.1%) | 57 (55.9%) | |
| Female | 57 (55.9%) | 45 (44.1%) | |
| Race/ethnicity, n (%) | 0.477 | ||
| Black | 26 (25.5%) | 24 (23.5%) | |
| White | 57 (55.9%) | 63 (61.8%) | |
| Hispanic | 17 (16.7%) | 15 (14.7%) | |
| Asian | 1 (1.0%) | 0 (0.0%) | |
| Other | 1 (1.0%) | 0 (0.0%) |
Abbreviation: SD, standard deviation.
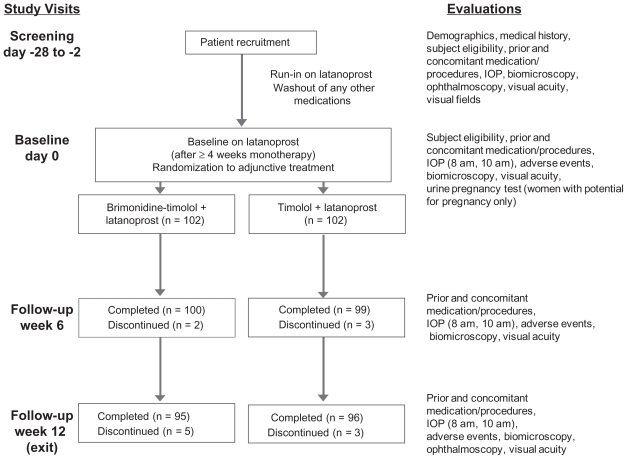
Figure 1 illustrates patient flow through the study. The study was completed by 93.1% (95/102) of patients in the fixed-combination plus latanoprost group and 94.1% (96/102) of patients in the timolol plus latanoprost group. Reasons for early discontinuation of patients from the study were adverse events (n = 5) and subject withdrawal (n = 2) in the fixed-combination plus latanoprost group and adverse events (n = 4) and noncompliance (n = 2) in the timolol plus latanoprost group. The per protocol patient population used for efficacy analyses represented 95.6% (195/204) of all randomized patients.
Figure 1.
Study design and patient flow through the study.
Abbreviation: IOP, intraocular pressure.
IOP-lowering efficacy
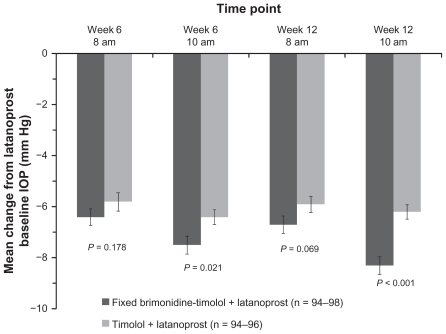
The mean IOP in each treatment group at each time point in the study is listed in Table 2. There were no statistically significant differences between groups at the baseline visit on latanoprost (P ≥ 0.229). At week 12, mean IOP was reduced at each time point in both treatment groups after the addition of adjunctive study medication (P ≤ 0.001). In the primary study endpoint, at 10 am, week 12, the mean (± standard deviation) IOP was 15.1 ± 2.6 mmHg in the eyes treated with fixed brimonidine-timolol and 16.9 ± 2.5 mmHg in the timolol-treated eyes (P < 0.001). The mean change from latanoprost-treated baseline IOP at 10 am, week 12, was 8.3 ± 3.4 mmHg (35.5%) in the fixed combination plus latanoprost group compared with 6.2 ± 2.8 mmHg (27.0%) in the timolol plus latanoprost group (P < 0.001, Figure 2). Adjunctive brimonidine-timolol also provided significantly greater IOP lowering than adjunctive timolol at the 10 am (peak effect) measurement at week 6 (between-group difference of 0.9 mmHg, P = 0.021, Figure 2). At the 8 am (trough effect) measurements, the mean reduction from baseline IOP on latanoprost was up to 6.7 mmHg with adjunctive brimonidine-timolol and up to 5.9 mmHg with adjunctive timolol; the between-group differences were not statistically significant (Figure 2).
Table 2.
Mean IOP at each time point and visit
| IOP, mmHg, mean (SD)
|
Between-group P value | ||
|---|---|---|---|
| Fixed brimonidine-timolol and latanoprost (n = 94–98) | Timolol and latanoprost (n = 94–97) | ||
| Baseline on latanoprost | |||
| 8 am | 23.7 (2.2) | 23.5 (2.4) | 0.534 |
| 10 am | 23.4 (2.3) | 23.0 (1.8) | 0.229 |
| Week 6 | |||
| 8 am | 17.3 (2.9) | 17.8 (3.2) | 0.178 |
| 10 am | 15.9 (3.1) | 16.7 (2.8) | 0.021 |
| Week 12 | |||
| 8 am | 17.0 (2.6) | 17.7 (2.6) | 0.069 |
| 10 am | 15.1 (2.6) | 16.9 (2.5) | <0.001 |
Abbreviations: SD, standard deviation; IOP, intraocular pressure.
Figure 2.
Mean change from latanoprost-treated baseline intraocular pressure at each time point after addition of fixed-combination brimonidine or timolol. Error bars, standard error of the mean.
Abbreviation: IOP, intraocular pressure.
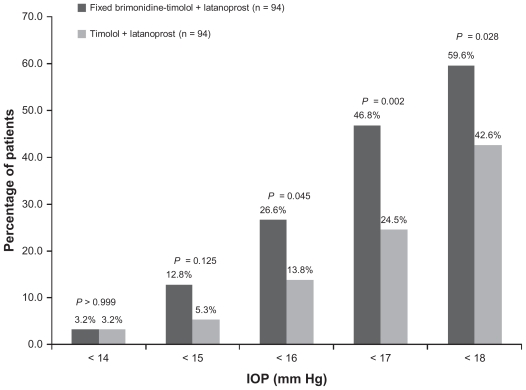
Response rates were higher with adjunctive brimonidine-timolol (Table 3). A significantly higher percentage of patients in the brimonidine-timolol plus latanoprost group than in the timolol plus latanoprost group achieved at least a 20%, 25%, 30%, and 35% reduction in IOP from the latanoprost baseline at both the 8 am and 10 am time points at week 12 (P ≤ 0.047). Further, at week 12, patients treated with fixed brimonidine-timolol plus latanoprost were significantly more likely than patients treated with timolol plus latanoprost to achieve IOP <18 mmHg, <17 mmHg, and <16 mmHg consistently at both 8 am and 10 am (Figure 3). The percentage of patients with IOP less than 18 mmHg at both peak and trough measurements was 59.6% in the brimonidine-timolol plus latanoprost group versus 42.6% in the timolol plus latanoprost group (P = 0.028).
Table 3.
Percentage of patients with at least a 20%, 25%, 30%, or 35% reduction in IOP from latanoprost baseline at both the 8 am and 10 am time points at week 12
| Patients, n (%)
|
Between-group P value | ||
|---|---|---|---|
| Fixed brimonidine-timolol and latanoprost (n = 94) | Timolol and latanoprost (n = 94) | ||
| ≥20% reduction in IOP from latanoprost baseline | 68 (72.3%) | 54 (57.5%) | 0.047 |
| ≥25% reduction in IOP from latanoprost baseline | 55 (58.5%) | 40 (42.6%) | 0.041 |
| ≥30% reduction in IOP from latanoprost baseline | 44 (46.8%) | 21 (22.3%) | <0.001 |
| ≥35% reduction in IOP from latanoprost baseline | 23 (24.5%) | 8 (8.5%) | 0.005 |
Figure 3.
Percentage of patients achieving specified intraocular pressure levels at both the 8 am and 10 am measurements at week 12.
Abbreviation: IOP, intraocular pressure.
Safety and tolerability
Both adjunctive study treatments were well tolerated and associated with a low incidence of adverse events (Table 4). Adverse events were reported for 15 patients (14.7%) in the adjunctive fixed brimonidine-timolol group and 13 patients (12.7%) in the adjunctive timolol group (P = 0.839). There was no statistically significant difference between treatment groups in the overall incidence of adverse events or in the incidence of ocular or treatment-related adverse events. Five patients treated with fixed brimonidine-timolol plus latanoprost discontinued from the study due to adverse events (one contact dermatitis/itching/redness, one allergic conjunctivitis, one allergic conjunctivitis/contact dermatitis, one itching/crusting/redness/swelling, and one allergy to study medication), and four patients in the timolol plus latanoprost group withdrew from the study due to adverse events (one severe punctate keratitis, one discomfort/swelling/redness, one exacerbation of chronic obstructive pulmonary disease/bronchitis/hypertension, and one progression of diabetic retinopathy). The treatment-related adverse events that occurred during the study are listed in Table 5. The most common treatment-related adverse events were ocular allergy in the fixed brimonidine-timolol plus latanoprost group (four patients, 3.9%) and punctate keratitis in the timolol plus latanoprost group (three patients, 2.9%). There were no reports of dry mouth. Only one patient had a serious adverse event. This patient was in the timolol plus latanoprost group and had acute exacerbation of chronic obstructive pulmonary disease and serious bacterial bronchitis.
Table 4.
Summary of adverse events
| Incidence, n (%)
|
Between-group P value | ||
|---|---|---|---|
| Fixed brimonidine-timolol and latanoprost (n = 102) | Timolol and latanoprost (n = 102) | ||
| Any adverse event | 15 (14.7%) | 13 (12.7%) | 0.839 |
| Treatment-related adverse event | 10 (9.8%) | 4 (3.9%) | 0.164 |
| Ocular adverse event | 9 (8.8%) | 7 (6.9%) | 0.796 |
| Treatment-related ocular adverse event | 8 (7.8%) | 4 (3.9%) | 0.373 |
| Serious adverse event | 0 (0.0%) | 1 (1.0%)a | >0.999 |
Notes: Acute exacerbation of chronic obstructive pulmonary disease and bacterial bronchitis.
Table 5.
Treatment-related adverse events
| Events (n)a |
||
|---|---|---|
| Fixed brimonidine-timolol and latanoprost (n = 102) | Timolol and latanoprost (n = 102) | |
| Superficial punctate keratitis | 1 | 3 |
| Itching | 3 | 0 |
| Redness | 2 | 1 |
| Allergic conjunctivitis | 2 | 0 |
| Contact dermatitis | 2 | 0 |
| Eye swelling | 1 | 1 |
| Allergy to study medication | 1 | 0 |
| Crusting | 1 | 0 |
| Discomfort | 0 | 1 |
| Dizziness/lightheadedness | 1 | 0 |
| Drowsiness | 1 | 0 |
| Fatigue | 1 | 0 |
| High intraocular pressure | 1 | 0 |
| Low heart rate | 1 | 0 |
| Ocular burning | 0 | 1 |
Notes: Multiple treatment-related adverse events were reported in individual patients.
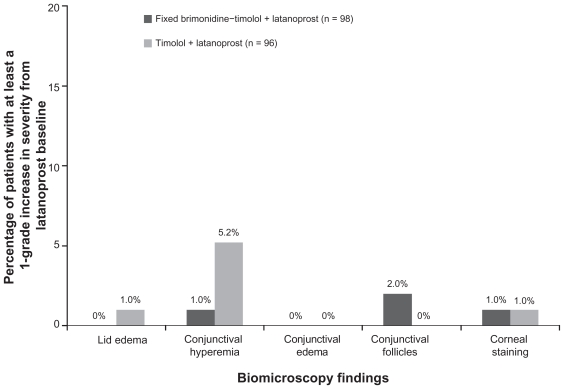
Findings on biomicroscopy following 12 weeks of treatment were similar in the treatment groups. Increases in the severity of findings (lids/lashes, conjunctiva, cornea, anterior chamber, and lens) from the latanoprost-treated baseline were rare in both treatment groups. Conjunctival hyperemia and conjunctival follicles were the most common findings, with 5% of patients in the timolol plus latanoprost group demonstrating at least a one grade increase in conjunctival hyperemia, and 2% of patients in the brimonidine-timolol plus latanoprost group demonstrating at least a one grade increase in conjunctival follicles (Figure 4).
Figure 4.
Percentage of patients with at least a one grade increase from latanoprost-treated baseline in severity scores on biomicroscopy at week 12.
Discussion
Achieving and maintaining a low target IOP minimizes the risk of glaucomatous progression and vision loss.11,12 Prostaglandin analogs are commonly used as first-line therapy in glaucoma and ocular hypertension because they reduce IOP effectively, have a favorable safety profile, and are conveniently dosed just once daily. However, many patients treated initially with a prostaglandin analog subsequently require adjunctive therapy to reach their target pressure.2 In the present study, both fixed brimonidine-timolol and timolol alone reduced IOP from the latanoprost baseline. However, use of fixed-combination brimonidine-timolol as adjunctive therapy to latanoprost provided significantly greater IOP lowering compared with adjunctive timolol and was well tolerated, with few discontinuations due to adverse events.
At 12 weeks, adjunctive fixed brimonidine-timolol provided an 8.3 mmHg (35.5%) reduction in IOP from latanoprost baseline at peak effect, approximately 2 mmHg larger than the 6.2 mmHg (27.0%) reduction from latanoprost baseline provided by adjunctive timolol (P < 0.001). These results are consistent with those of a previous randomized controlled study that compared fixed brimonidine-timolol with fixed dorzolamide-timolol as therapy adjunctive to a prostaglandin analog.10 In that study, the mean additional reduction from the prostaglandin analog-treated baseline IOP was 6.9 mmHg (29.3%) in the 37 patients who added fixed brimonidine-timolol to a prostaglandin analog and 5.2 mmHg (23.5%) in the 42 patients who added fixed dorzolamide-timolol to a prostaglandin analog.10
The efficacy of timolol as an agent adjunctive to latanoprost has been inconsistent in previous studies.13–16 In an early controlled randomized study evaluating the effectiveness of timolol given once daily in the morning as therapy adjunctive to latanoprost, adjunctive timolol reduced IOP from the latanoprost-treated baseline by approximately 4 mmHg at peak effect and 3 mmHg at trough effect.13
More recent studies comparing the fixed combination of timolol and latanoprost with latanoprost monotherapy have shown that the fixed combination provides approximately 1–2 mmHg additional IOP lowering compared with latanoprost alone.14–16 Randomized, controlled studies have also shown that brimonidine effectively reduces IOP when added to a prostaglandin analog.17,18 Three times daily brimonidine purite 0.1% provided additional IOP lowering of 4.8 mmHg at peak effect and 2.2 mmHg at trough effect when used as adjunctive therapy to latanoprost.17
The addition of fixed brimonidine-timolol to latanoprost in this study was as well tolerated as the addition of timolol alone. There were few adverse events in either treatment group, and the rate of discontinuations due to adverse events was comparable in the two treatment groups. For patients treated with adjunctive fixed brimonidine-timolol in this study, the overall incidence of treatment-related adverse events was 9.8%, approximately half the 20.2% overall incidence of treatment-related adverse events reported by Gõni4 in a previous 12-week, randomized controlled study that evaluated fixed brimonidine-timolol used alone in 188 patients with glaucoma or ocular hypertension. These results suggest that fixed brimonidine-timolol may be particularly well tolerated when used adjunctively to a prostaglandin analog.
Fixed combinations offer advantages of improved convenience and typically lower cost compared with separate use of the component medications. The addition of a fixed combination to a prostaglandin analog provides substantial additional IOP lowering while adding only one bottle to the patients’ daily regimen. Because use of more than two bottles of IOP-lowering medication may be associated with an increase in noncompliance, minimization of the number of bottles and drops used by patients is desirable to facilitate adherence with treatment and improve visual outcomes.
The primary limitation of this study was that the duration of treatment was only three months and IOP was measured at only two time points at each visit. Ocular allergy, a known side effect of brimonidine treatment, typically is a delayed response that presents after more than three months of therapy.3,19,20 In addition, the inclusion criteria specified that all patients required an IOP of at least 21 mmHg after at least four weeks of latanoprost therapy, and patients who were nonresponders to latanoprost were not specifically excluded. This may partially explain the greater IOP lowering for timolol than has been seen in previous trials.13–16 Further studies will be needed to determine the safety and efficacy of fixed brimonidine-timolol added to a prostaglandin analog over long-term treatment, as well as the effectiveness of adjunctive brimonidine-timolol in providing additional IOP lowering over 24 hours.
In summary, this study demonstrated that fixed-combination brimonidine-timolol reduces IOP significantly more effectively than timolol when used as therapy adjunctive to latanoprost. Adjunctive treatment with fixed brimonidine-timolol was also well tolerated. Given potential medication compliance issues, a regimen of a prostaglandin analog and the fixed combination of brimonidine and timolol, which requires instillation of just three drops in the eye each day, may represent optimal maximal medical therapy for many patients.
Acknowledgments
Renaissance Associates (Newport Beach, CA), a clinical research organization, was retained by Allergan to manage the study. Renaissance Associates personnel monitored the study sites and performed all data entry and analysis. RDF was supported, in part, by an unrestricted grant from Research to Prevent Blindness. Kate Ivins provided medical writing assistance in the development of the manuscript. Principal investigators in this study were Ike Ahmed (Toronto, ON, Canada), Louis Alpern (El Paso, TX), Edward Chang (Pittsburgh, PA), Anastasios Costarides (Atlanta, GA), Douglas Day (Atlanta, GA), Robert Fechtner (Newark, NJ), Brian Flowers (Fort Worth, TX), Paul Harasymowycz (Montreal, QC, Canada), Barry Katzman, (San Diego, CA), Norman Levy (Gainesville, FL), Donald Nixon (Orillia, ON, Canada), William Rand (Pompano Beach, FL), Steven Vold (Rogers, AK), Mark Weiss (Tulsa, OK), and Fiaz Zaman (Houston, TX).
Footnotes
Disclosure
This study was sponsored by Allergan Inc. RDF is a consultant to and has received research support from Allergan Inc. PH, DRN, SDV, and FZ have also received research support from Allergan Inc. JW and DAH are employees of Allergan Inc.
References
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Covert D, Robin AL. Adjunctive glaucoma therapy use associated with travoprost, bimatoprost, and latanoprost. Curr Med Res Opin. 2006;22(5):971–976. doi: 10.1185/030079906x104777. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimonidine–0.5% timolol fixed-combination therapy vs mono-therapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: A 12-month randomized trial. Arch Ophthalmol. 2006;124(9):1230–1238. doi: 10.1001/archopht.124.9.1230. [DOI] [PubMed] [Google Scholar]
- 4.Goñi FJ; Brimonidine/Timolol Fixed Combination Study Group. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005;15(5):581–590. [PubMed] [Google Scholar]
- 5.Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15(2):132–135. doi: 10.1097/00055735-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–868. doi: 10.1016/j.ophtha.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Neelakantan A, Vaishnav HD, Iyer SA, Sherwood MB. Is addition of a third or fourth antiglaucoma medication effective? J Glaucoma. 2004;13(2):130–136. doi: 10.1097/00061198-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Crichton ACS. Timolol/brimonidine combination therapy in glaucoma management. Clin Surg J Ophthalmol. 2005;23:356–359. [Google Scholar]
- 9.Ahmed I. CEED II: An in-depth look at the latest findings. Clin Surg J Ophthalmol. 2007;25:1–5. [Google Scholar]
- 10.Nixon DR, Yan DB, Chartrand JP, Piemontesi RL, Simonyi S, Hollander DA. Three-month, randomized, parallel-group comparison of brimonidine- timolol versus dorzolamide-timolol fixed-combination therapy. Curr Med Res Opin. 2009;25(7):1645–1653. doi: 10.1185/03007990902994041. [DOI] [PubMed] [Google Scholar]
- 11.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WC, Day DG, Sharpe ED, Dubiner HB, Holmes KT, Stewart JA. Efficacy and safety of timolol solution once daily vs timolol gel added to latanoprost. Am J Ophthalmol. 1999;128(6):692–696. doi: 10.1016/s0002-9394(99)00237-8. [DOI] [PubMed] [Google Scholar]
- 14.Higginbotham EJ, Feldman R, Stiles M, Dubiner H Fixed Combination Investigative Group. Latanoprost and timolol combination therapy vs monotherapy: One-year randomized trial. Arch Ophthalmol. 2002;120(7):915–922. doi: 10.1001/archopht.120.7.915. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer N European Latanoprost Fixed Combination Study Group. A comparison of the fixed combination of latanoprost and timolol with its individual components. Graefes Arch Clin Exp Ophthalmol. 2002;240(11):893–899. doi: 10.1007/s00417-002-0553-0. [DOI] [PubMed] [Google Scholar]
- 16.Olander K, Zimmerman TJ, Downes N, Schoenfelder J Xalacom/Latanoprost Study Group. Switching from latanoprost to fixed- combination latanoprost-timolol: A 21-day, randomized, double-masked, active-control study in patients with glaucoma and ocular hypertension. Clin Ther. 2004;26(10):1619–1629. doi: 10.1016/j.clinthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Day DG, Hollander DA. Brimonidine purite 0.1% versus brinzolamide 1% as adjunctive therapy to latanoprost in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2008;24(5):1435–1442. doi: 10.1185/030079908x301848. [DOI] [PubMed] [Google Scholar]
- 18.Feldman RM, Tanna AP, Gross RL, et al. Additivity Study Group. Comparison of the ocular hypotensive efficacy of adjunctive brimonidine 0.15% or brinzolamide 1% in combination with travoprost 0.004% Ophthalmology. 2007;114(7):1248–1254. doi: 10.1016/j.ophtha.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Williams GC, Orengo-Nania S, Gross RL. Incidence of brimonidine allergy in patients previously allergic to apraclonidine. J Glaucoma. 2000;9(3):235–238. doi: 10.1097/00061198-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Craven ER, Walters TR, Williams R, Chou C, Cheetham JK, Schiffman R Combigan Study Group. Brimonidine and timolol fixed-combination therapy versus monotherapy: A 3-month randomized trial in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2005;21(4):337–348. doi: 10.1089/jop.2005.21.337. [DOI] [PubMed] [Google Scholar]