Abstract
Neurogenesis, which persists in the adult mammalian brain, may provide a basis for neuronal replacement therapy in neurodegenerative diseases like Alzheimer's disease (AD). Neurogenesis is increased in certain acute neurological disorders, such as ischemia and epilepsy, but the effect of more chronic neurodegenerations is uncertain, and some animal models of AD show impaired neurogenesis. To determine how neurogenesis is affected in the brains of patients with AD, we investigated the expression of immature neuronal marker proteins that signal the birth of new neurons in the hippocampus of AD patients. Compared to controls, Alzheimer's brains showed increased expression of doublecortin, polysialylated nerve cell adhesion molecule, neurogenic differentiation factor and TUC-4. Expression of doublecortin and TUC-4 was associated with neurons in the neuroproliferative (subgranular) zone of the dentate gyrus, the physiological destination of these neurons (granule cell layer), and the CA1 region of Ammon's horn, which is the principal site of hippocampal pathology in AD. These findings suggest that neurogenesis is increased in AD hippocampus, where it may give rise to cells that replace neurons lost in the disease, and that stimulating hippocampal neurogenesis might provide a new treatment strategy.
Alzheimer's disease (AD), a common cause of dementia (1), is characterized by senile plaques containing β-amyloid peptide (Aβ) derived from amyloid precursor protein (APP), and neurofibrillary tangles (NFTs) containing hyperphosphorylated τ-protein. Aβ and phospho-τ may be neurotoxic, leading to progressive neuronal degeneration and death. Despite progress in understanding molecular mechanisms in AD, effective treatment remains elusive.
One potential approach to treating AD involves using endogenous neuronal precursors to replace lost or damaged cells, based on the capacity of the rostral subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) to generate neurons into adulthood (2, 3). Neuronal stem cells in the SVZ give rise predominantly to committed progenitor cells that migrate into the olfactory bulb (OB) via the rostral migratory stream and differentiate into local interneurons (4); progenitors in the SGZ migrate into the granular cell layer and also differentiate into neurons (5). However, the migration of newborn neurons is not restricted to these sites. Endogenous neuronal precursors can proliferate in response to cerebral ischemia (6, 7) and migrate into ischemic regions of striatum and cerebral cortex (8, 9), and into the CA1 region of hippocampus, where they may integrate into existing brain circuitry and contribute to repair (10). These neurons may have a physiological role, because blockade of hippocampal neurogenesis is reported to inhibit hippocampus-dependent learning (11), and because reducing the population of new interneurons in the OB impairs odor discrimination (12).
The proliferation of neuronal stem or progenitor cells in the adult brain is also affected by growth factors, including epidermal growth factor (EGF) (13), fibroblast growth factor-2 (FGF-2) (14, 15), brain-derived neurotrophic factor (16, 17), stem cell factor (18), heparin-binding EGF (HB-EGF) (19) and vascular endothelial growth factor (20). Restoring insulin-like growth factor-I (IGF-I) levels enhances neurogenesis in the aged brain (21), suggesting that neurogenesis might be augmented by growth factors in vivo in neurodegenerative diseases like AD.
Aβ disrupts neurogenesis in SVZ and hippocampus in mouse models of AD (22, 23), but the status of neurogenesis in neurodegenerative disorders in humans is unknown. We examined the expression of neurogenesis marker proteins in hippocampus of brains from AD patients and neurologically normal subjects. In contrast to findings in animal models, the hippocampus of patients with AD showed increased expression of neurogenesis markers and an increased number of cells expressing these markers, which is consistent with enhanced neurogenesis in human AD. This finding suggests that in AD, as in experimental cerebral ischemia, hippocampal neurogenesis is increased, and might therefore serve to replace hippocampal neurons. As a corollary, measures designed to enhance neurogenesis could have therapeutic value in AD.
Materials and Methods
Human Brain Tissue. Tissue was from the Harvard Brain Tissue Resource Center, McLean Hospital (Belmont, MA), the Neuropathology Core Facility, Massachusetts Alzheimer's Disease Research Center, Massachusetts General Hospital (Boston), and the Brain and Tissue Bank for Developmental Disorders at the University of Maryland at Baltimore (Baltimore). Research was conducted in compliance with the policies and principles contained in the Federal Policy for the Protection of Human Subjects. Twenty-five postmortem brains were used: 14 from individuals with a clinical diagnosis of probable AD and 11 (controls) from individuals without neurological disorders (Table 1). The diagnosis of AD was confirmed and its severity was rated by a standardized neuropathology protocol developed by the Consortium to Establish a Registry for AD (24).
Table 1. Patient and tissue information.
| Brain no. | Neuropathological diagnosis | Age, yr | Sex | Postmortem interval, h |
|---|---|---|---|---|
| Western blotting | ||||
| 1474 | Normal | 50 | M | 8 |
| 1503 | Normal | 53 | F | 5 |
| 1065 | Normal | 15 | M | 12 |
| 1670 | Normal | 13 | M | 5 |
| 1712 | Normal | 20 | M | 8 |
| 1713 | Normal | 23 | M | 8 |
| B5463 | Normal | 74 | F | 19 |
| B2751 | Early AD | 85 | M | 12 |
| B2933 | Early AD | 70 | M | 16 |
| B3266 | Early AD | 75 | M | 14 |
| B4734 | Moderate AD | 68 | M | 13 |
| B4697 | Moderate AD | 80 | M | 20 |
| B5099 | Moderate AD | 89 | M | 14 |
| B3791 | Severe AD | 88 | M | 9 |
| B3855 | Severe AD | 75 | M | 16 |
| B3975 | Severe AD | 69 | M | 18 |
| Immunohistochemistry | ||||
| M232 | Normal | 45 | M | 15 |
| M552 | Normal | 70 | M | 17 |
| M555 | Normal | 70 | M | 17 |
| M451 | Normal | 74 | F | 13 |
| M238 | AD | 62 | F | 4 |
| M445 | AD | 90 | ? | 13 |
| M456 | AD | 77 | ? | 12 |
| M522 | AD | 68 | ? | 14 |
| M457 | AD | 77 | ? | 12 |
?, Unknown.
Western Blotting. Hippocampi were isolated from fresh brains and cell lysates extracted in PBS containing 0.05% Nonidet P-40, 0.25% sodium deoxycholate, 50 mM Tris·HCl (pH 8.5), 100 nM NaCl, 1 mM EDTA (pH 8.0), 1 μg/ml aprotinin and 100 μg/ml phenylmethylsulfonyl fluoride. Protein (50 μg), determined by using a Bio-Rad assay, was boiled at 100°C in SDS sample buffer for 5 min, electrophoresed on SDS/12% PAGE gels, and transferred to polyvinyldifluoridine membranes, which were incubated overnight at 4°C with one of the following primary antibodies: (i) affinity-purified goat polyclonal anti-doublecortin (DCX) (Santa Cruz Biotechnology; 1:200), (ii) mouse monoclonal anti-polysialylated neural cell adhesion molecule (PSANCAM) (Chemicon; 1:500), (iii) mouse monoclonal anti-neuronal nuclear antigen (NeuN) (Chemicon; 1:250), (iv) rabbit polyclonal anti-TUC-4 (1:10,000; Chemicon), (v) affinity-purified goat polyclonal anti-neurogenic differentiation factor (NeuroD) (Santa Cruz Biotechnology; 1:200), (vi) mouse monoclonal anti-calbindin (Oncogene Science; 1:10,000), or (vii) mouse monoclonal anti-actin (Oncogene Science; 1:20,000). Membranes were washed with PBS/0.1% Tween 20, incubated at room temperature for 60 min with horseradish peroxidaseconjugated anti-mouse, anti-rabbit, or anti-goat secondary antibody (Santa Cruz Biotechnology; 1:3,000), and washed three times for 15 min with PBS/Tween 20. Peroxidase activity was visualized by chemiluminescence (NEN Life Science Products, Boston). Antibodies were removed with stripping buffer (100 mM 2-mercaptoethanol/2% SDS/62.5 mM Tris·HCl, pH. 6.7) at 50°C for 30 min, followed by washing with PBS/Tween 20, and membranes were reprobed. Differences in protein expression were quantified by using a GS-710 calibrated imaging densitometer and QUANTITY ONE software (Bio-Rad).
Immunohistochemistry. Tissue was immersion-fixed in 4% paraformaldehyde in PBS (pH 7.5) and embedded in paraffin, and 6-μm sections were deparaffinized with xylene and rehydrated with ethanol. Immunocytochemistry was performed as described (7, 25). After blocking peroxidase activity with 1% H2O2, sections were incubated in blocking buffer (2% horse serum/0.2% Triton X-100/0.1% BSA in PBS) for 1 h at room temperature. Primary antibodies were those listed above, plus rabbit polyclonal anti-Aβ1–17 and mouse monoclonal antibodies 5A3 and 1G7, which recognize nonoverlapping epitopes within residues 380–665 (extracellular domain) of human APP (provided by E. Koo, University of California, San Diego; 1:5,000), rabbit polyclonal anti-phospho-τ (BioSource International, Sunnyvale, CA; 1:2000) and mouse monoclonal anti-cleaved caspase-8 (Cell Signaling Technology, Beverly, MA; 1:2,000). Primary antibodies were added in blocking buffer and incubated with sections at 4°C overnight. The second antibody was biotinylated goat anti-mouse or donkey anti-rabbit or anti-goat IgG (Vectastain Elite ABC, Vector Laboratories; 1:200). Sections were processed with ABC reagents by using a Vector ABC kit (Vector Laboratories). After two washes with PBS, sections were incubated for 10 min at room temperature with 0.5% biotinylated tyramine and 0.01% H2O2 in PBS (25), washed with PBS, and treated with ABC reagents as above. The horseradish peroxidase reaction was detected with diaminobenzidine and H2O2. Alternating sections were incubated without primary antibody, as a control.
Double-label immunohistochemistry was used to detect coexpression of neuronal marker proteins. The secondary antibodies were FITC-conjugated goat anti-mouse IgG, rhodamineconjugated rat-absorbed donkey anti-rabbit IgG and rhodamineconjugated donkey anti-goat IgG (Jackson ImmunoResearch; 1:200). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) and fluorescence signals were detected with a Nikon E800 microscope at excitation/emission wavelengths of 535/565 nm (rhodamine, red), 470/505 (FITC, green) and 360/400 (DAPI, blue). Results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI). Controls included omitting or preabsorbing primary or omitting secondary antibody. Selected images were viewed at high magnification by using a Nikon PCM-2000 laser-scanning confocal microscope, and Simple PCI imaging software (Compix, Cranberry Township, PA) was used to confirm colocalization of markers.
Results
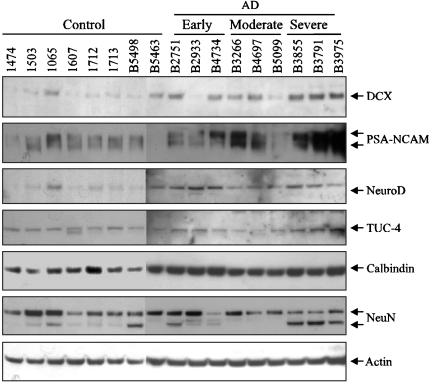
Neurogenesis Marker Proteins Are Overexpressed in AD Hippocampus. Histopathological hallmarks of AD in the hippocampus of patients with clinically probable, autopsy-documented AD, including neuronal degeneration, neuritic plaques and NFTs, were confirmed in our laboratory by immunocytochemistry with anti-Aβ, anti-APP and anti-phospho-τ antibodies (Fig. 1). To investigate endogenous neurogenesis in AD hippocampus, we first used protein from AD and control hippocampus to perform Western blots with antibodies against neurogenesis markers and mature neuronal proteins. The expression of several neurogenesis marker proteins was increased in hippocampus of severely affected AD patients (Fig. 2). These included DCX (2.1 ± 0.2 × same-blot control, n = 3), a microtubule-associated protein found in somata and processes of migrating and differentiating neurons (26); PSA-NCAM (54.7 ± 19.5 × same-blot control, n = 3), a plasma membrane glycoprotein expressed by neuronal progenitors and by differentiating neurons and astroglia in response to a variety of toxic insults (27); and TUC-4 (turned on after division/Ulip-1/CRMP-4; 4.6 ± 1.0 × same-blot control, n = 3), a protein expressed early in neuronal differentiation in the rat (28). NeuroD (1.5 ± 0.4 × same-blot control, n = 3), a basic helix–loop–helix protein expressed during terminal neuronal differentiation (29), was also up-regulated in AD, but to a lesser extent. In some instances (DCX, PSA-NCAM, and TUC-4), there appeared to be a tendency for expression to increase with increasing disease severity. In contrast, expression of calbindin D28k and NeuN, which identify mature neurons, was not increased. On Western blots, NeuN migrates as a triplet of Mr ≈46–48 kDa (30), and it is therefore of interest that the lowest-Mr band tended to be relatively more prominent in the brains of patients with severe AD, although the significance of different NeuN banding patterns is unknown. Actin expression was used as a control for protein loading. The increased expression of neurogenesis markers suggested that neurogenesis might be enhanced in the hippocampus of AD patients.
Fig. 1.

Plaques and NFTs in hippocampus of AD brain. Plaques, detected with antibodies against Aβ (blue) and APP (brown) (A), were surrounded by phospho-τ-immunopositive nerve terminals (B). NFTs delineated with anti-phospho-τ are shown at higher magnification in C.
Fig. 2.
Expression of neuronal marker proteins in AD hippocampus. Protein from control (Con) and AD hippocampus was used for Western blotting with antibodies against the indicated proteins and actin was used as a control for consistency of protein loading. Early, Moderate, and Severe refer to AD of increasing histopathological severity. Band intensities were quantified by computer-assisted densitometry to give average values (fold increase over same-gel control), as reported in Results.
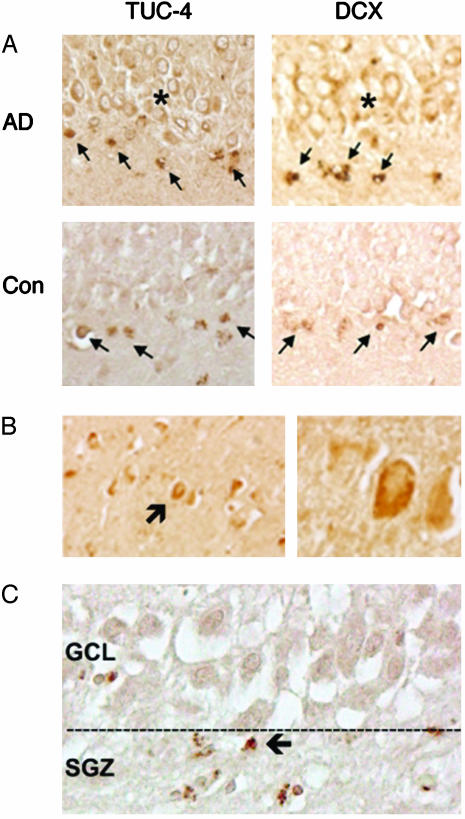
Neurogenesis Markers Are Localized to the Neuroproliferative Zone (SGZ) of AD Hippocampus. Next, we used immunohistochemistry to determine whether increased expression of immature neuronal markers in AD brain was associated with known sites of hippocampal neurogenesis. Both control and AD brains showed TUC-4 and DCX immunolabeling in the dentate SGZ (Fig. 3), which is the site of neuronal precursor proliferation in hippocampus. However, these cells were shrunken and stained for the 10-kDa cleavage product of caspase-8, which is consistent with evidence that proliferating neuronal precursors in the adult brain undergo programmed cell death in situ (31). The granule cell layer (GCL), the usual destination of surviving neuronal precursors that arise in the SGZ, contained numerous TUC-4- and DCX-immunopositive cells in AD, but not control brains, indicating that the GCL in AD brains had been partly repopulated by new neurons that survived to transit from the SGZ.
Fig. 3.
Immunohistochemical evidence for increased neurogenesis in hippocampus of AD brains. (A) TUC-4 and DCX are expressed in the SGZ of control and AD brain (arrows), but only in AD are large numbers of TUC-4- and DCX-immunopositive cells observed in GCL (*). Immunoreactive cells in the SGZ show shrunken cytoplasm and condensed nuclei, which is consistent with death of cells that do not transit to the GCL; a similar finding was observed in aged control brains (data not shown). (B) DCX-immunopositive cells (arrow) can also be detected in CA1 (Left, low power; Right, high power) of AD hippocampus. (C) Cells in SGZ express the 10-kDa caspase-8 cleavage product (arrow), suggesting caspase-dependent programmed cell death.
New Neurons Are Increased in CA1, the Major Site of Neuronal Loss in AD Hippocampus. The primary site of hippocampal pathology in AD is the pyramidal cell layer of Ammon's horn, especially the CA1 sector of Lorente de Nó, which exhibits the most severe neuronal loss (32) and the largest number of NFTs (33). In contrast, DG is relatively spared, so the stimulus to enhanced hippocampal neurogenesis in AD is likely to arise elsewhere. In addition, the paucity of neuronal loss in the DG of AD brains suggests another ultimate destination for the new neurons seen in the GCL. Therefore, we examined the CA1 sector of AD hippocampus for evidence of recruitment of new neurons, and found that DCX-immunoreactive cells were present in this region (Fig. 3), indicating that one destination for new neurons that arise in AD is CA1. Whether these new neurons originate in the dentate SGZ or elsewhere cannot be resolved in our postmortem tissue.
Putative New Neurons in AD Hippocampus Express Multiple Neuronal Markers. To confirm that the TUC-4- or DCX-immunopositive cells that we identified in AD hippocampus were genuinely of neuronal lineage (and not nonneuronal cells aberrantly expressing a single neuronal marker), we performed double-label immunohistochemistry with multiple neuronal markers. Laser-scanning confocal imaging showed that TUC-4 colocalized with Hu, a neuron-specific RNA-binding protein that begins to be expressed in neuronal nuclei and somata soon after differentiation (34), and DCX colocalized with PSA-NCAM (Fig. 4). Therefore, cells identified by TUC-4 or DCX immunoreactivity in our AD hippocampal sections express a more extensive repertoire of neuronal lineage markers.
Fig. 4.
Colocalization of multiple markers of neuronal lineage in TUC-4- and DCX-immunopositive cells from DG of AD brains. Confocal imaging shows colocalization of TUC-4 (green) and Hu (red) (A) and of DCX (red) and PSANCAM (green) (B). Nuclei were counterstained with DAPI (blue).
Discussion
Here, we report increased expression of immature neuronal marker proteins in hippocampus of AD brains, with immunohistochemical localization to hippocampal sites of neurogenesis (DG) and of AD pathology (CA1). The up-regulated proteins included DCX, PSA-NCAM, TUC-4, and NeuroD. Our findings are consistent with increased hippocampal neurogenesis in AD, as has been documented in other neuropathological states such as ischemia (6), and which may represent a mechanism directed toward the replacement of dead or damaged neurons.
Although brain atrophy, which occurs in AD, can spuriously affect measurements of protein concentration, this factor is unlikely to explain our results. Actin expression, used as a control, was unchanged in AD brains, and the expression patterns of the neuronal marker proteins that we studied varied. For example, DCX and PSA-NCAM expression increased with increasing disease severity, calbindin expression was unchanged, and NeuN expression was characterized by an altered banding pattern in which lower-Mr bands became more and higher-Mr bands less prominent as the severity of AD increased. Moreover, immunohistochemistry showed increased expression of DCX and TUC-4 in the dentate GCL from patients with AD; in contrast to Ammon's horn, this area of hippocampus is not prominently affected in AD.
Prior studies (35) establish that neurogenesis occurs in the adult mammalian brain, although at a reduced rate with advancing age. Nevertheless, the aged brain retains the capacity to up-regulate neurogenesis in response to physiological (3, 36) and pathological (37) factors. This finding contrasts with the hematopoietic system, where basal production of new cells remains fairly constant with age, whereas the hematopoietic response to stress is attenuated (38). In conditions like AD, which occurs at increasing frequency with advancing age, the ability of the aged brain to mobilize new neurons offers the prospect of cell replacement. This result could have especially important consequences in the hippocampus, because this brain region is disproportionately affected in AD, and because memory function, which is prominently impaired in AD, may depend on hippocampal neurogenesis (11). Although no current evidence connects neurogenesis with improved function or slower disease progression in AD, it is tempting to speculate that the ability of N-methyl-d-aspartate (NMDA) receptor inhibition to enhance dentate neurogenesis (39) might contribute to the therapeutic effects of NMDA antagonist drugs like memantine in patients with AD (40).
Our results contrast with findings in transgenic models of AD, in which mice expressing mutant forms of APP (22, 23) or presenilin-1 (41) show impaired, rather than increased, neurogenesis. However, none of these models fully reproduces the features of familial AD, let alone those of sporadic AD, which our patients are statistically most likely to have had. Because the molecular stimulus to neurogenesis in AD (or other neurological disorders) is unknown, it is impossible to predict whether animal models that may more closely resemble human AD, such as APP/τ double transgenic mice (42), would be more likely to show enhanced neurogenesis, but this possibility is worth exploring.
Notwithstanding that neurogenesis appears to be increased in the brains of patients with AD, progressive cell loss is still observed. There are several possible reasons for the limited repair capacity of neurogenesis in AD. First, the rate or extent of cell loss may be too great for quantitatively significant replacement to be achieved. Second, the neurons that are produced may be ineffectual because they do not develop into fully mature, functional neurons, because they do not develop into the right type of neurons, or because they are incapable of integrating into the surviving brain circuitry. Third, the micro-environment of the AD brain may be toxic to new neurons (43). If the human AD brain can support neurogenesis, as appears to be the case, then measures that increase neurogenesis could have therapeutic value. One such measure may be environmental enrichment, which stimulates neurogenesis in aged mice (36). Another is the administration of growth factors, such as IGF-I (21), FGF-2, or HB-EGF (44), which also increase neurogenesis in the aged rodent brain.
Acknowledgments
This work is dedicated to the memory of June Elizabeth Starwald Greenberg (1920–2003). This work was supported by Public Health Service Grant NS44921 and the Buck Institute for Age Research. The Harvard Brain Tissue Resource Center is supported in part by Public Health Service Grant MH/NS31862.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Aβ, β-amyloid peptide; AD, Alzheimer's disease; APP, amyloid precursor protein; DCX, doublecortin; DG, dentate gyrus; GCL, granule cell layer; NeuN, neuronal nuclear antigen; NeuroD, neurogenic differentiation factor; NFTs, neurofibrillary tangles; PSA-NCAM, polysialylated neural cell adhesion molecule; SGZ, subgranular zone.
References
- 1.Hardy, J. & Selkoe, D. J. (2002) Science 297, 353-356. [DOI] [PubMed] [Google Scholar]
- 2.Gage, F. H., Kempermann, G., Palmer, T. D., Peterson, D. A. & Ray, J. (1998) J. Neurobiol. 36, 249-266. [DOI] [PubMed] [Google Scholar]
- 3.Cameron, H. A. & McKay, R. D. (1999) Nat. Neurosci. 2, 894-897. [DOI] [PubMed] [Google Scholar]
- 4.Luskin, M. B. (1993) Neuron 11, 173-189. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A. & Gage, F. H. (1998) Nat. Med. 4, 1313-1317. [DOI] [PubMed] [Google Scholar]
- 6.Liu, J., Solway, K., Messing, R. O. & Sharp, F. R. (1998) J. Neurosci. 18, 7768-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin, K., Minami, M., Lan, J. Q., Mao, X. O., Batteur, S., Simon, R. P. & Greenberg, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 4710-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 8, 963-970. [DOI] [PubMed] [Google Scholar]
- 9.Jin, K., Sun, Y., Xie, L., Peel, A., Mao, X. O., Batteur, S. & Greenberg, D. A. (2003) Mol. Cell. Neurosci. 24, 171-189. [DOI] [PubMed] [Google Scholar]
- 10.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429-441. [DOI] [PubMed] [Google Scholar]
- 11.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. [DOI] [PubMed] [Google Scholar]
- 12.Gheusi, G., Cremer, H., McLean, H., Chazal, G., Vincent, J.-D. & Lledo, P.-M. (2000) Proc. Natl. Acad. Sci. USA 97, 1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, C. G., Tropepe, V., Morshead, C. M., Reynolds, B. A., Weiss, S. & van der Kooy, D. (1996) J. Neurosci. 16, 2649-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. (1997) J. Neurosci. 17, 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner, J. P., Black, I. B. & DiCicco-Bloom, E. (1999) J. Neurosci. 19, 6006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencea, V., Bingaman, K. D., Wiegand, S. J. & Luskin, M. B. (2001) J. Neurosci. 21, 6706-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benraiss, A., Chmielnicki, E., Lerner, K., Roh, D. & Goldman, S. A. (2001) J. Neurosci. 21, 6718-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, K., Mao, X. O., Sun, Y., Xie, L. & Greenberg, D. A. (2002) J. Clin. Invest. 110, 311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, K., Mao, X. O., Sun, Y., Xie, L., Jin, L., Nishi, E., Klagsbrun, M. & Greenberg, D. A. (2002) J. Neurosci. 22, 5365-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, K., Zhu, Y., Sun, Y., Mao, X. O., Xie, L. & Greenberg, D. A. (2002) Proc. Natl. Acad. Sci. USA 99, 11946-11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenwalner, R. J., Forbes, M. E., Bennett, S. A., Lynch, C. D., Sonntag, W. E. & Riddle, D. R. (2001) Neuroscience 107, 603-613. [DOI] [PubMed] [Google Scholar]
- 22.Haughey, N. J., Nath, A., Chan, S. L., Borchard, A. C., Rao, M. S. & Mattson, M. P. (2002) J. Neurochem. 83, 1509-1524. [DOI] [PubMed] [Google Scholar]
- 23.Haughey, N. J., Liu, D., Nath, A., Borchard, A. C. & Mattson, M. P. (2002) Neuromolecular Med. 1, 125-135. [DOI] [PubMed] [Google Scholar]
- 24.Mirra, S., Heyman, A., McKeel, D., Sumi, S., Crain, B., Brownlee, L., Vogel, F., Hughes, J., van Belle, G. & Berg, L. (1991) Neurology 41, 479-486. [DOI] [PubMed] [Google Scholar]
- 25.Adams, J. (1992) J. Histochem. Cytochem. 40, 1457-1463. [DOI] [PubMed] [Google Scholar]
- 26.Magavi, S. S., Leavitt, B. R. & Macklis, J. D. (2000) Nature 405, 951-955. [DOI] [PubMed] [Google Scholar]
- 27.Rousselot, P., Lois, C. & Alvarez-Buylla, A. (1995) J. Comp. Neurol. 351, 51-61. [DOI] [PubMed] [Google Scholar]
- 28.Minturn, J. E., Fryer, H. J., Geschwind, D. H. & Hockfield, S. (1995) J. Neurosci. 15, 6757-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, J. E., Hollenberg, S. M., Snider, L., Turner, D. L., Lipnick, N. & Weintraub, H. (1995) Science 268, 836-844. [DOI] [PubMed] [Google Scholar]
- 30.Mullen, R. J., Buck, C. R. & Smith, A. M. (1992) Development (Cambridge, U.K.) 116, 201-211. [DOI] [PubMed] [Google Scholar]
- 31.Morshead, C. M. & van der Kooy, D. (1992) J. Neurosci. 12, 249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies, D. C., Horwood, N., Isaacs, S. L. & Mann, D. M. (1992) Acta Neuropathol. 83, 510-517. [DOI] [PubMed] [Google Scholar]
- 33.Fukutani, Y., Kobayashi, K., Nakamura, I., Watanabe, K., Isaki, K. & Cairns, N. J. (1995) Neurosci. Lett. 200, 57-60. [DOI] [PubMed] [Google Scholar]
- 34.Marusich, M. F., Furneaux, H. M., Henion, P. D. & Weston, J. A. (1994) J. Neurobiol. 25, 143-155. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn, H. G., Dickinson-Anson, H. & Gage, F. H. (1996) J. Neurosci. 16, 2027-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempermann, G., Gast, D. & Gage, F. H. (2002) Ann. Neurol. 52, 135-143. [DOI] [PubMed] [Google Scholar]
- 37.Gray, W. P., May, K. & Sundstrom, L. E. (2002) Neurosci. Lett. 330, 235-238. [DOI] [PubMed] [Google Scholar]
- 38.Globerson, A. (1999) Exp. Gerontol. 34, 137-146. [DOI] [PubMed] [Google Scholar]
- 39.Bernabeu, R. & Sharp, F. R. (2000) J. Cereb. Blood Flow Metab. 20, 1669-1680. [DOI] [PubMed] [Google Scholar]
- 40.Reisberg, B., Doody, R., Stoffler, A., Schmitt, F., Ferris, S. & Mobius, H. J. (2003) N. Engl. J. Med. 348, 1333-1341. [DOI] [PubMed] [Google Scholar]
- 41.Wen, P. H., Shao, X., Shao, Z., Hof, P. R., Wisniewski, T., Kelley, K., Friedrich, V. L., Jr., Ho, L., Pasinetti, G. M., Shioi, J., et al. (2002) Neurobiol. Dis. 10, 8-19. [DOI] [PubMed] [Google Scholar]
- 42.Lewis, J., Dickson, D. W., Lin, W. L., Chisholm, L., Corral, A., Jones, G., Yen, S. H., Sahara, N., Skipper, L., Yager, D., et al. (2001) Science 293, 1487-1491. [DOI] [PubMed] [Google Scholar]
- 43.Rapoport, M., Dawson, H. N., Binder, L. I., Vitek, M. P. & Ferreira, A. (2002) Proc. Natl. Acad. Sci. USA 99, 6364-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin, K., Sun, Y., Xie, L., Batteur, S., Mao, X. O., Smelick, C., Logvinova, A. & Greenberg, D. A. (2003) Aging Cell 2, 175-183. [DOI] [PubMed] [Google Scholar]