Abstract
The review focuses on the application of supercritical fluids as antisolvents in the pharmaceutical field and demonstrates the supercritical antisolvent method in the use of drug encapsulation. The main factors for choosing the solvent and biodegradable polymer to produce fine particles to ensure effective drug delivery are emphasized and the effect of polymer structure on drug encapsulation is illustrated. The review also demonstrates the drug release mechanism and polymeric controlled release system, and discusses the effects of the various conditions in the process, such as pressure, temperature, concentration, chemical compositions (organic solvents, drug, and biodegradable polymer), nozzle geometry, CO2 flow rate, and the liquid phase flow rate on particle size and its distribution.
Keywords: supercritical antisolvent method, drug encapsulation, particle size, drug release mechanisms, drug delivery
Introduction
Drug delivery includes important situations such as the slow release of soluble drugs in water, the rapid release of low-solubility drugs, drug delivery to specific sites, and the delivery of more than one agent with the same formulation and system based on soluble or degradable carriers that are easily eliminated. The ideal drug delivery method should be safe, inert, and comfortable for the patients. It should also be biocompatible, and easily administered or removable, with high drug loading and easy fabrication/sterilizing ability. Using biodegradable polymers for drug encapsulation is one of the best ways to achieve this ideal method. The biodegradable polymer first combines with the drug and then coats it; therefore, if the drug is released from the encapsulated material in a predesigned manner, controlled drug delivery will occur. Drug release can be constant or cyclic over a long-term period, or it may be activated by the environment or other external events. Therefore, drug delivery control provides more effective therapies, and avoids the potentials above or below the dosing range. Besides, the coating polymer protects the susceptible active substance from degradation. However, there are some limitations, such as the possible nonbiocompatibility or toxicity of the polymers, an unwanted byproduct of degradation, and higher costs.1–3 Biodegradable polymer drug nanoencapsulation reduces drug side effects, and increases the bioavailability and sustained release. Bioavailability of pharmaceutical compounds depends on their absorption by the gastrointestinal tracts which is affected by both the dissolution rate and membrane permeation rate. During the supercritical antisolvent (SAS) process the surface area will be increased, which leads to improvement of bioavailability. It is also crucial in controlling the particle size and its distribution for efficient drug delivery. Obviously, the smaller particles with narrower particle size distribution result in better flexibility of administration. Further, increasing the bioavailability decreases the required drug dosage and raises the control over a sustained period.4–9 Smaller-sized particles can accelerate toward the target organs, and distribute drug evenly throughout the body. Additionally, the drug dosage can be controlled by biodegradable polymers, so that the polymers can actually control the periodic time of release. The particle size must be between 1 and 5 μm for inhalation delivery, between 0.1 and 0.3 μm for intravenous delivery, and between 0.1 and 100 μm for oral delivery.1,4,10–12 Therefore, drug nanoencapsulation becomes crucial in successful drug delivery. The usage of supercritical fluid for the purpose of drug nanoencapsulation is a clean and effective method compared with other techniques.4,5,7–9
Supercritical fluid properties
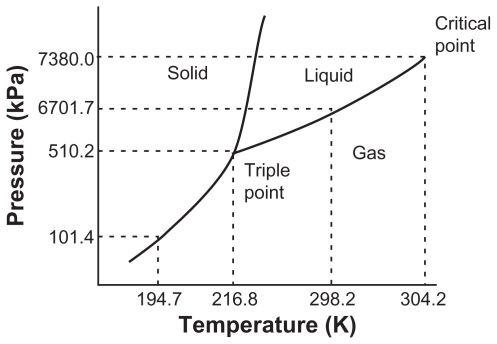
A supercritical fluid is a solvent whose temperature and pressure are greater than its critical temperature and pressure, while it remains as a single phase, as shown in Figure 1.13 CO2 supercritical fluid is the best choice, from among the others available for pharmaceutical processes, as it is affordable, nontoxic, and inflammable. Further, it has high volatility, mild critical temperature (304°K), low critical pressure (7.38 MPa), low cohesive energy density, low polarizability per unit volume, and poor solubility for many polymers and drugs,8,10,13,15,16 and it has low viscosity like a gas, although its density is similar to that of a liquid. Around the critical point, its properties such as density, viscosity, solvency, and diffusivity can be manipulated by adjusting the pressure and temperature.13,14,17
Figure 1.
Triple point phase diagram for pure CO2.7,14
Note: Adapted with permission from: Ginty PJ, Whitaker MJ, Shakesheff KM, Howdle SM. Drug delivery goes supercritical. Materials Today. 2005;8(8) Suppl 1: 42–48. Copyright 2005 American Chemical Society; and: reprinted from International Journal of Pharmaceutics, vol 364, Are pharmaceutics really going supercritical?, pages 176–187, copyright 2008, with permission from Elsevier.
Due to its low viscosity, it reveals a high mass transfer ratio during the SAS process. Besides, it has high diffusivity, typically 10−3 cm2/second in organic solvents, which promotes rapid mixing with the solvent in the nucleation step, for approximately 10−4 to 10−5 seconds. Further, the solvating power can be controlled by adjusting both pressure and temperature, so that it produces dry particles by removing the organic solvents in a continuous single step of the SAS process.6,14,17,18 The interaction between the solute and the solvent in supercritical fluids is explained in a similar manner to the three-density region solvation model.13,19 Another important advantage of the CO2 supercritical fluid (ScCO2) lies in its ability to provide a nondegrading and nonoxidizing environment for sensitive compounds. Also, its drying process prevents damage to the drug particles.14
The solubility of polymers in ScCO2 and conversely the solubility of ScCO2 in polymers are the two main aspects that need further study. CO2 is a nonpolar molecule possessing a small polarity due to its quadruple moment. Thus, nonpolar and light molecules with higher vapor pressure can be easily dissolved in the CO2 compared with heavy molecules, and polar molecules with lower vapor pressure. Most polymers and drug compounds have low solubility in ScCO2, whereas ScCO2 easily dissolves in most biodegradable polymers, and dramatically reduces the glass transition temperature and melting temperature of the polymers; thus, the viscosity of polymers will be reduced.5,15,17,19,20 Drug solubility in the ScCO2 depends on the vapor pressure of the drug, the interaction between the drug and CO2, and the density of the supercritical fluid.17,21
The antisolvent application
Bleich and coworkers firstly discovered the use of antisolvent techniques in encapsulation.15 In this technique, CO2 acts as an antisolvent and causes the precipitation of a solute from an organic solvent. The base of this technique is:
The possibility of dissolving a large volume of a supercritical fluid by an organic solvent.
The reciprocal miscibility of the supercritical fluid CO2 and an organic solvent.
The low affinity of the supercritical fluid for the solute.
CO2 is diffused in the solvent and evaporates in the gas phase. The droplets are expanded and stabilized by surface tension. The mass transfer between the supercritical fluid and liquid phase decreases the surface tension which is strong enough to control droplet shape. Diffusion phenomenon increases the volume of the solvent, reduces the density of the solvent, thus decreasing the solvating power of the solvent, and precipitates the solute.1,4,6,12,14,19,20,22–24 Different densities between the liquid phase and the supercritical fluid phase significantly affect the mass transfer. Besides, the high diffusivity of the supercritical fluid is another factor that produces the high rate of mass transfer. The high pressure vapor–liquid equilibrium phase of the ternary system controls the precipitation of the solute in the SAS process.19
SAS
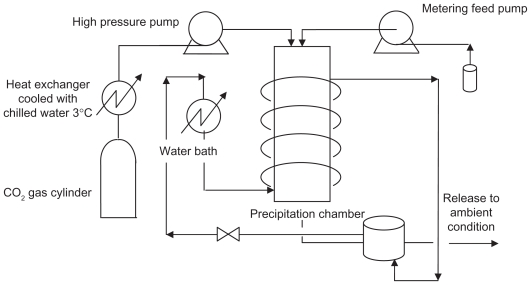
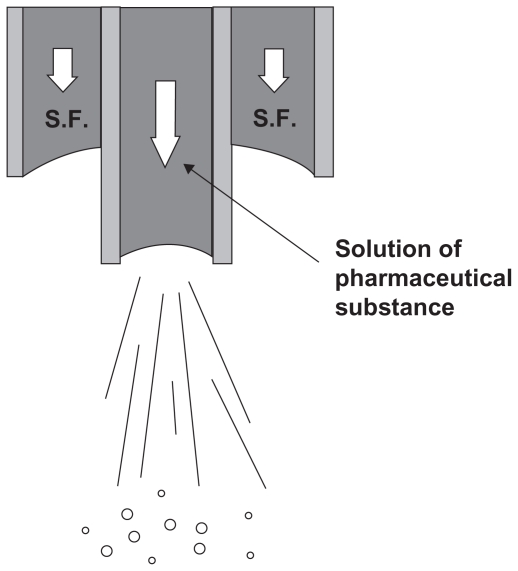
This process refers to the precipitation in a supercritical fluid due to particle formation. The supercritical antisolvent must be miscible with the solution solvent, and the solute must also be insoluble in the supercritical antisolvent. In the SAS process, the supercritical CO2 is pumped into a high-pressure vessel to a specific pressure. Then the solution, including the drug, biodegradable polymer, and organic solvent, is sprayed in the reactor via a suitable nozzle. The solvent diffuses rapidly from the solution droplets into the bulk supercritical fluid, precipitating the solute. Formed particles are collected on a filter washed by supercritical fluid to remove the residual solvent.5,13,14,19,22,25,26 Therefore, the supercritical fluid dissolving into liquid droplets, together with the evaporation of the organic solvent in the supercritical fluid phase, provides a supersaturated solute in the liquid phase, which will be later precipitated. A schematic diagram of the apparatus for the SAS process is shown in Figure 2. The advantages of this method are:
Figure 2.
Schematic diagram of the apparatus for the supercritical antisolvent process.
During this process a very fine dispersion of liquid phase occurs, so there is a very fine droplet and a high specific surface area for mass transfer.5,20,23,24,28,29
Freshly precipitated particles will remain in the system and the supercritical fluid and organic solvent drain from the system continuously.20,27,30,31
High supersaturation is achieved and, therefore, small particle size is attained due to the rapid mixing of the supercritical fluid and solution (liquid phase).5,17,24,25,31,32
By controlling the operating condition, it is possible to produce narrower particles.25,32
By reducing the pressure or depressurizing, the supercritical fluid is more easily removed from the system.20,21,24,29,32–34
The process can take place at near ambient temperatures, thus avoiding thermal degradation of the particles by choosing a suitable antisolvent.32,33
Before recovering the solid, relatively high amounts of liquid solution can be processed.27
This process can prepare drug-encapsulated particles with high polymorphic purity, enhanced dissolution rate, and acceptable residual solvent.35
This method is adaptable for continuous operations, and this property is very important for the large-scale mass production of nanoencapsulated drug particles.24
Some experiments are summarized in Table 1.
Table 1.
Summary of literature reviews
| Compound | Solvent | Polymer | Particle size | Ref |
|---|---|---|---|---|
| Bupivacaine HCl | DCM/acetonitrile/potassium phosphate and sodium azide | PLGA/PLLA | 4–10 μm | 31 |
| Diuron | DCM | PLLA | Needle-like crystals mean length 500 μm | 27 |
| Amoxicillin | NMP | – | 0.3–1.2 μm | 26 |
| Europium acetate | DMSO | – | 0.2–10 μm | 36 |
| Cilostazol | DCM | Irregular crystals 0.9–4.52 μm | 37 | |
| Gadolinium acetate | DMSO | – | 0.2–10 μm | 36 |
| Amoxicillin | NMP | – | 0.25–1.2 μm | 38 |
| Fluconazole | DCM, acetone and ethanol | – | Needle like crystals several hundred μm | 39 |
| Nalmefene hydrochloride | Ethanol | – | Above the MCP 200–300 nm, near and below the MCP 0.5–2 μm | 40 |
| Zinc acetate | – | – | 50 nm | 41 |
| Salbutamol sulphate | DMSO | – | Length 1–3 mm and diameters 0.2–0.35 mm | 43 |
| Tetracycline | NMP | – | Needle-like particles irregular amorphous particles 0.6–0.8 μm 150 nm | 44 |
| Rifampicin | DCM | PLLA | <5 μm | 11 |
| Methylprednisolone acetate | Tetrahydrofuran | – | 4–10 μm | 45 |
| Amoxicillin | DMSO | – | Amorphous spherical particles 0.2–1.6 μm | 34 |
| Chlorpropamide | EtAc | – | Platy crystals several tenths μm | 46 |
| Chlorpropamide | Acetone | – | Columnar habit crystals several tenths μm | 46 |
| Sulfathiazole | Acetone | – | Prismatic crystals >750 μm | 46 |
| Sulfathiazole | MeOH | – | Needle-like, tabular crystal habit >750 μm | 46 |
| Ampicillin | NMP | – | Aggregate and separated amorphous spherical particles 0.26 μm | 47 |
| Ampicillin | EtOH | – | Aggregate and separated amorphous spherical particles 1.26 μm | 47 |
| Rifampicin | DMSO | – | Amorphous particles, coalescent nanometric spherical separated icrometric mean 0.4–1 μm 2.5–5 μm | 36 |
| Arbutine | EtOH | – | 2.4–4.7 μm | 48 |
| L-PLA | DCM | – | Agglomerate particle <4 μm | 28 |
| Oxeglitizar | EtOH+ CHCl3, EtOH hydrocortisone | PEG/PVP | Needle crystals, polymorphic form A size >50 μm | 35 |
| Oxeglitizar | THF, DCM | PEG/PVP | Needle-like crystals, polymorphic form A, traces form B size >50 μm | 35 |
| Oxeglitizar | EtOH/THF(50:50), EtOH | PEG/PVP | Needle-like crystals, polymorphic form A and form B size >50 μm | 35 |
| Cefonicid | DMSO | – | Spherical submicroparticles and empty shells from 0.2 μm to >50 μm | 29 |
| Sulfamethizole | Acetone | – | Thin platy <56 μm/tabular <220 μm | 49 |
| Silica | – | Eudragit RL100 | 50 μm | 50 |
| – | DMSO | Dextran | Spherical particles mean 5–100 μm | 38 |
| – | DMSO | HPMA | Spherical particles 100–200 nm | 38 |
| – | DMSO | Inulin | Irregular particles 5–50 mm | 38 |
| – | DCM | L-PLA | Spherical particles mean 1–4 mm | 38 |
| Cyclotrimethylenetrinitramin | DMSO | – | Granular mean size 12.8 μm | 51 |
| RDX | ACN | – | Granular mean size 6.6 μm | 51 |
| RDX | Acetone | – | Rob shaped granular 17.7 μm | 51 |
| RDX | DMF | – | Granular mean size 5.1 μm | 51 |
| Cyclotrimethylenetrinitramin | NMP | – | Irregular mean size 11.4 μm | 51 |
| Cefoperazone | DMSO | – | Submicro particles, micropetric particles, large crystals 0.25–0.5 μm | 52 |
| Cefuroxime | DMSO | – | Submicro particles, wrinkled microparticles, balloons 0.1–0.9 μm, 1–3 μm, 5–20 μm | 52 |
| Trypsin/lysozyme | DMSO | – | Irregular coalescing particles 1–5 μm | 53 |
| Theophylline | EtOH/DCM | – | lamellar crystals and rosette crystals L/d = 5–300 μm/1–100 μm | 54 |
| – | DCM + DMSO | Ethylcellulose/methylcellulose | Spherical coalescing particles 5 μm | 55 |
| – | DCM | L-PLA | Spherical particles or fibers 1–5 μm | 27 |
| – | DCM | L-PLA | Fibers and/or microspheres mean 1–3 μm | 56 |
| – | DCM | L-PLA | Coalescing particles 3–15 μm | 28 |
| Nimesulide | CHCl3, DCM | – | Needle and thin rod-shaped crystals Form I | 57 |
| Nimesulide | Acetone | – | Needle and thin rod-shaped crystals, meta-stable Form II | 57 |
| Rifampicin | DCM | L-PLA | Spherical particle <5 μm | 11 |
Abbreviations: CHCl3, chloroform; DCM, di-chloromethane (methylene chloride); PLGA, polylactic-co-glycolic acid; EtAc, ethyl acetate; EtOH, ethanol; MeOH, methanol; PLLA, L poly lactic acid; NMP, N-methyl-2-pyrrolidone; oxeglitazar, (2E, 4E)-5-(7-methoxy-3,3-dimethyl-2,3-dihydro-1-benzoxepin-5-yl)-3-methylpenta-2,4-dienoic acid; DMSO, dimethyl sulfoxide; MCP, melting critical point; THF, tetrahydrofuran; PEG, polyethylene glycol; PVP, polyvinylpyrrolidone; HPMA, N-(2-hydroxypropyl) methacrylamide; L-PLA, L-polylactic acid; ACN, acetonitrile; DMF, dimethylformamide.
Despite all these advantages, there is a limitation to the success of this method for drugs and biodegradable polymers that occur as solids.20 The major disadvantage of this method is the long washing period prior to the agglomeration and aggregation of particles. This problem can be minimized by intensively mixing the supercritical antisolvent and the solution, which increases the mass transfer and thus produces smaller particle size. One of the methods to achieve intensive mixing is by using ultrasonic nozzles. During this process, 10 to 100 kHz ultrasonic waves produce ultrasonic vibrations which enhance the mass transfer rate between the supercritical fluid and solution; therefore, smaller droplets will be formed, which results in smaller particles.1,13,23,58 Using additional solvents in the SAS process causes a broad range of solutes to dissolve in the organic solvents. Therefore, the presence of residual toxic solvents in the final product is the only disadvantage of this process.7,24
Drug
It is possible to encapsulate pharmaceutical compounds using the SAS process.36 The structure of drugs and their properties are important factors in produced particle size in the SAS process.59 Drug loading efficiency has been observed to be strongly related to the nature of the drug. For example, lipophilic drugs or CO2-soluble drugs are difficult to load.24 The process parameters have less effect on the drug loading because of the solute particles that are precipitated from the solvent.23,60 By decreasing the ratio of the polymer to drug, supercritical fluid is saturated with the drug and the drug loading efficiency will be enhanced.11 Drug loading in drug encapsulation is explained by the ratio of mass fraction of a nanoencapsulated drug to the total mass of the sample, according to the following equation:4,9,11,61–63
| (1) |
and,
| (2) |
The particle size of the microparticles is determined by the volume mean diameter. The microparticles’ polydispersity is expressed by the span value:11
| (3) |
where D90%, D10%, and D50% are the equivalent volume diameters at 90%, 10%, and 50% of the cumulative volume.11
Solvent
Most polymers have a limited solubility in the supercritical fluid, although they have high solubility in the organic solvents. Thus, a critical factor in the SAS process is the selection of the correct combination of a suitable organic solvent and a supercritical fluid as antisolvent.24 Further, selecting a suitable solvent for drug nanoencapsulation is very important, as the molecules could be polar and multifunctional with a tendency toward hydrogen bonding. This will create a special interaction between the solvent and solute.21
The pharmaceutical agents must also be soluble in a suitable organic solvent that is miscible with the supercritical fluid. Thus, there is a limitation in the choice of compounds and solvents, which usually causes failure in the SAS process. In reality, there is no problem for the solubility of hydrophobic compounds of low molecular weight in the organic solvent; however, complex hydrophilic compounds are mostly insoluble in most of organic solvents. Therefore, supercritical fluid miscible organic solvents such as dimethyl sulfoxide (DMSO) are suitable to dissolve the biological molecules. When these compounds are dissolved in such solvents, the molecules irreversibly change their structure and lose their functional activity and immunogenicity risk. To overcome this setback, a suitable cosolvent, such as ethanol, can be used to enable the water to mix with the supercritical fluid CO2.15 The organic solvent should be reasonably soluble in the polymer and show high mutual solubility with the supercritical fluid under moderate operating pressure and temperature. The complete miscibility or high mutual solubility with CO2 in the near and supercritical region is observed by most organic solvents to dissolve a particular polymer.24 The volumetric expansion of the organic solvent in the precipitation process clearly plays an important role. This expansion results from an expanded dissolution of the supercritical fluid in the liquid phase.24,41 The volume expansion can be calculated as follows:
| (4) |
where V(P,T) is the volume of the liquid phase (organic solvent) loaded with the supercritical fluid as antisolvent, at the operating pressure and temperature, and V0 is the volume of the pure liquid (pure organic solvent) at atmospheric conditions.41,42 When the volume expansion is low, the precipitated particles from the liquid phase will form at the bottom of the vessel. Incomplete dissolution of the solvent liquid occurs in the supercritical fluid as antisolvent produces the liquid phase in the precipitator. When the volume expansion is intermediate, dried expansion droplets will be formed and an empty shell of solute will be produced. In a very large volume expansion, the precipitated particles are very small and the particle size distribution is narrow. The aim of encapsulation is to choose an organic solvent with high volume expansion.41 Sometimes, in spite of an asymptotic expansion of the liquid organic solvent obtained according to the pure solvent curve, a liquid phase can be observed at the bottom of the chamber. This failure is due to the presence of a solute that modifies the phase behavior of the solvent–antisolvent mixture. In this case, a film or large solute crystals will be produced in the precipitator instead of small particles.41
According to the drug and biodegradable polymer structure and operating conditions, selecting a suitable solvent is crucial to the SAS process. These two key points must be considered: At first, an organic solvent with high volatility which induces high volume of expansion and which can also be removed from the system easily needs to be selected. The solubility of the biodegradable polymer in the organic solvent needs to be higher than the solubility of the drug in the solvent, because the drug first precipitates in the chamber, then it is coated by the biodegradable polymer by precipitation, and finally drug encapsulation by the biodegradable polymer occurs.27 Therefore, the selection of suitable solvent is an important factor to produce fine particles in SAS process.
Biodegradable polymer
The selection of a suitable biodegradable polymer is another important factor in the nanoencapsulation of drug that attracts a lot of attention due to biodegradable polymer’s ability to be reabsorbed by the body.64 Compatibility between the drug and polymer is vitally significant. These nanoencapsulated particles decrease the side effects of the drug, and also extend the circulation time in the bloodstream and target the drugs to specific organs.65 Furthermore, biodegradable drug delivery mechanisms can be designed to deliver vaccines in a number of pulses from a single injection of microencapsulated drug.64 The degree of polymer degradation can be increased by adding more hydrophilic backbone or end groups. The higher number of the reactive hydrophilic groups in the backbone, the less the degree of crystallinity; and the higher porosity, the smaller the size of the device.62,66
Supercritical CO2 decreases the glass transition temperature of biodegradable polymers, acting like a plasticizer. Therefore, these polymers with a low glass transition temperature tend to form sticky and aggregated particles.30,67 However, the presence of residual organic solvents in the product increases the plasticizing effects.15,68 However, the crystalline biodegradable polymers are better suited for drug delivery of some extremely potent drugs such as vaccines and drug-eluting medical devices. Their restrictions are because of their very long in vivo degradation time, slow releasing period, and application that is drawn out and infrequent, whereas most drugs require frequent delivery over a few weeks. Dose frequency of the drugs is controlled by the stability of the drugs in the biodegradable polymeric system and their therapeutic potency. For faster drug delivery, amorphous polymers are used.15,35,64,68,69 The polymer chains of biodegradable polymers usually hydrolyze into biologically acceptable progressive smaller compounds, and degrade so that they can be removed easily from the body by metabolic pathways. Degradation phenomenon may occur through bulk hydrolysis and the polymers degrade uniformly throughout the matrix. Factors affecting the biodegradation of polymers are:2
Chemical composition of polymer.
Chemical structure of polymer.
Configuration structure.
Morphology of polymer.
Molecular weight of polymer.
Molecular weight distribution.
Shape of polymer.
Physicochemical factors such as ionic strength and pH.
Annealing.
Processing condition.
Physical factors such as changes in shape and size, mechanical stress, changes in diffusion factor.
Adsorbed and absorbed compounds.
Mechanism of hydrolysis.
Repeating units distribution in multimers.
Presence of compounds with low molecular weight.
Ionic groups present.
Unexpected units or chain defects present.
Sterilization process.
Site of implantation.
Storage history.
Effects of process parameters on particle size
The characteristics of the particle produced in the SAS method for drug encapsulation are influenced by various parameters such as type of supercritical fluid and its properties, properties of the solute including the drug, biodegradable polymer, and organic solvent, and operating conditions such as temperature, pressure, concentration, nozzle geometry, feed flowrate, the rate of antisolvent, and the degree of mixing.13,22,70 Therefore, optimization of these parameters to produce the smaller mean particle size with narrower distribution becomes crucial.
Effects of pressure and temperature
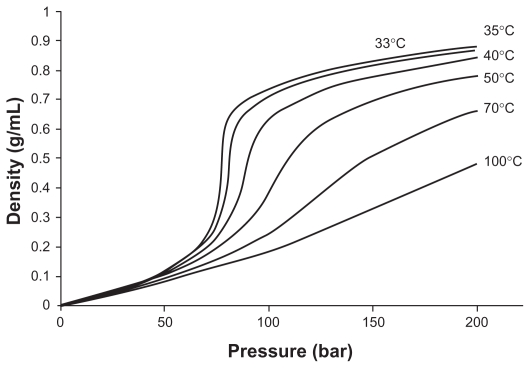
The density of supercritical fluid affects mass transfer between organic solvent and supercritical fluid during precipitation. The density of supercritical fluid depends on the temperature and pressure parameters of the fluid, as shown in Figure 3.
Figure 3.
Density dependence of CO2 at various temperatures.17
Note: Reproduced from Gupta and Kompella with permission from the publisher.
Near the critical point, a small change in the pressure causes considerable density changes, as shown in Figure 3.5,16,17,63
Hydrodynamic theory, such as Weber numbers, have been applied to the supercritical antisolvent process. Weber number is the proportion of the deforming external pressure and reforming surface tension forces, such as:
| (5) |
where, ρA is the density of antisolvent, UR is the relative velocity, D is the initial droplet diameter, and σ is the interface tension.
Increasing the ratio of deforming external pressure forces with respect to the reforming surface tension forces the drops to break up into smaller droplets. During the SAS process, the Weber number is very high compared with that in other techniques.12,28,40,56,63 The solubility of high-molecular-weight drugs in the supercritical fluid is related to the density. The solubility increases at higher densities and the effect of density on particle solubility is rapidly enhanced at higher densities. Increase in density enhances the molecular interaction, and, thus, the solubility.16,17,21 The effect of pressure on particle size produces various results according to these experiments. At higher pressure with higher density of supercritical fluid (antisolvent), the deforming pressure forces must be increased to break the droplets into smaller particles, according to the explanation given above.63 Moreover, particle nucleation and its growth are other important factors affecting particle size. Rapid mass transfer of antisolvent and solvent causes high supersaturations for the solute.56 High supersaturation results in rapid nucleation and growth of more than one particle per primary droplet.71 The solubility of supercritical CO2 will vary with pressure. The variation of solubility shows a linear relation with pressure at low pressures, and with pressure enhancement the ScCO2 solubility will be increased linearly. At the pressure of the polymer saturation with supercritical fluid, the pressure variations do not have a prolonged effect on the solubility, because the free intermolecular volume of the polymer will be occupied at the saturation pressure.3 In some cases, the particle size declines with reduction of pressure during precipitation. In a situation above the critical condition, reduction in pressure is observed to decrease the solubility, which then results in higher maximum supersaturation being achieved in the reactor; therefore, smaller particles are produced.5,28,32,45 In a subcritical condition, increasing the pressure produces smaller particles.32,37,72 Other authors found that pressure variations do not exert a great effect on the mean particle size in pressures higher than asymptotic volume expansion.8,41
Increasing the temperature reduces the solubility and thus enhances the maximum supersaturation, so that smaller particles are obtained.5,32 Also, higher temperature reduces the drying time and thus there is rapid removal of the residual solvent; therefore, more spherical particles are formed.71 Properties of the polymers, such as viscosity, change rapidly with changes in the reactor conditions during spraying, and lead to precipitation of the polymer. If the biodegradable polymer is precipitated at a higher rate than it is completely atomized, both the size and morphology of the particles will be undesirable. Conversely, if the biodegradable polymer is not precipitated during the spraying, polymeric droplets are produced, and they fuse together due to their semi-fluid nature; thus, separate particles are not formed. Therefore, the best condition lies somewhere between the two scenarios.74 The temperature needs to be lower than the glass transition temperature to avoid plasticizing of the polymer particles.75,76 In amorphous polymers, CO2 molecules slip into the interstitial spaces of the polymers acting as lubricants and the polymers are plasticized.8,76 Plasticizing causes particle coalescence and increases the particle size.75,76 In some polymers, especially amorphous polymers, the glass transition temperature may be decreased (4–30°C/MPa) after coming in contact with the supercritical fluid due to CO2 activity within a very short time span because of the intermolecular interaction between the biodegradable polymer and dissolved supercritical fluid.3,8,24,30 In a low-pressure region, the melting point of the biodegradable polymer during the SAS process decreases linearly due to the increased pressure. The melting point is minimal at the saturation state of the polymer, with CO2. Later, increasing the pressure raises the melting point due to the hydrostatic pressure effect. However, the temperature must also be sufficient to evaporate the solvent rapidly.3,75 Solubility increases with density, and the effect of density (pressure and temperature) is observed to be greater at higher densities. With increase in the density, molecular interaction is enhanced, the solubility is increased, and smaller particle size is obtained. Therefore, raising the temperature has two opposite effects on the process; namely decreasing the density reduces the solubility,21,32 and increasing the volatility of the solvent enhances the solubility.21 Therefore, a proper selection of sufficient temperature and pressure optimizes the process.
Effects of concentration
The initial concentration of the solution significantly affects particle size. Different results are reported in various experiments. In some cases, reducing the concentration produces smaller particles with narrower particle size distribution. At lower concentrations, supersaturation of the drug occurs very late and, therefore, the precipitation delays and nucleation dominate growth, producing smaller particles. By enhancing the concentration, supersaturation occurs sooner, with growth dominating over the nucleation process, and crystals will be formed, thus increasing the particle size.22,33,34,36,38,63,77 Besides, increasing the concentration enhances the viscosity and surface tension of the solution, producing larger droplets; therefore, particles of larger diameter will be formed.11,27,33,36,52,75,77 Conversely, in some cases, by increasing the concentration, the particle size decreases because the increased initial concentration enhances the maximum supersaturation and, therefore, smaller particle size will be formed.32,33 Actually, when the initial solution is of a higher concentration, the concentration of the solvent is reduced and then the solvent is removed more easily. In addition, more uniform particle size distribution is obtained as more supersaturation causes homogeneous nucleation.8,33 According to the following explanation, the initial concentration of the solution has two opposite effects on particle size. On the one hand, increased concentration produces higher supersaturation and faster nucleation; therefore particle size and its distribution will be reduced. On the other hand, the higher concentration will cause higher condensation and increase the particle size and widen particle size distribution. These results show that the particle size is influenced by the degree of supersaturation and initial concentration, simultaneously. Therefore, it becomes crucial to balance the rate of crystallization (nucleation) and rate of growth. As a result, by adjusting a lower initial concentration and higher degree of supersaturation of the solution, smaller particles with narrower particle size distribution would be obtained.18,33
Effects of chemical composition of the organic solvent
The chemical composition of the solvent is another important factor that affects particle size and its distribution. Increasing the volatility of the solvent will decrease particle size.27,41 Solvents with higher volatility force the system to reach the supersaturation state much faster, resulting in reduced particle size.63 The solubility of both the biodegradable polymer and drug in the organic solvent must be considered. For effective encapsulation of the drug, it is essential that the solubility of the biodegradable polymer in the organic solvent is higher than the solubility of its drug content. This function results in first precipitating the drug, then coating it with the biodegrading polymer, and finally nanoencapsulation will occur.27 Also, the strength of the solvent is very important, too. The stronger solvents increase the interaction between the solvent and solute which prevents crystal growth, thus producing smaller particle size.63
Effects of chemical composition of the solute (drug and biodegradable polymer)
Properties of drugs, such as partitioning in the supercritical fluid and solubility, are influenced by chemical composition and greatly affect final particle size. In the SAS process, the supercritical fluid acts as the antisolvent. If the drug dissolves in the supercritical fluid under the operating conditions, it will be removed into the gas phase and no precipitation will occur and no particles will be produced. Thus, the lower the solubility of the drug in the supercritical fluid, the more rapid will the precipitation be.59 Besides, the properties of the drug influence the drug loading during nanoencapsulation of the drug in a biodegradable polymer.60 Some research has shown that enhancement of the liophilicity of the drug reduces the loading drug efficiency in the SAS process. This phenomenon explains that lipophilic drugs are entrained by supercritical fluid during the precipitation. The efficiency of the encapsulation and morphology of the particles are influenced by nucleation and growth mechanisms. A rapid initial nucleation and growth rate of the drug coupled with the slow rate of polymer precipitation produces the drug needles encapsulated in the coated biodegradable polymer.23,60
Another important factor is the structure of biodegradable polymer. CO2 supercritical fluid diffusivity and solubility in the biodegradable polymers are influenced by two variants:
Molecular structure influences the interaction between the supercritical fluid and molecular chains of the biodegradable polymer.
Morphology of polymer could be crystalline, semicrystalline, or amorphous and related to the free volume of the polymer.
For the first variant, the polymer chain flexibility must be considered and the availability of the reaction groups can enhance the dissolution of the supercritical fluid more easily. For example, ether groups or carbonyl groups which are available on side chains or in the backbone can particularly interact with CO2 supercritical fluid.3,15 But the most important factor is the morphology and free volume of the biodegradable polymer.15,64 In the SAS process, the diffusivity of the antisolvent CO2 gas in crystalline biodegradable polymers is higher than in amorphous polymers. Conversely, the solubility of the antisolvent CO2 gas in amorphous polymers is higher than in the crystalline biodegradable polymers. Both are because of the greater free volume in the amorphous polymers. Therefore, the rate of mass transfer and the resulting rate of precipitation are higher in the crystalline polymer, and there is a higher supersaturation ratio in the crystalline polymer than in the amorphous polymer, which results in smaller particles and narrower particle size distribution.15,35,67 However, the solidification rate will be decreased by the presence of amorphous polymers; and the microparticles tend to aggregate due to plasticizing effects of the residual carbon dioxide.7,15,62,68 Particle morphology is found to be strongly related to the inherent characteristic of the biodegradable polymer molecules. The semicrystalline polymer produces the spherical shape, whereas the highly crystalline polymer probably forms fibrous or spherulitic morphology. Besides, the morphology of the particle produced is influenced by the molecular weight of the polymer that controls the dimension of chain polymer.24 Reducing the polymer molecular weight decreases the glass transition temperature and increases the glassy and rubbery state of the polymer.69
Effects of the nozzle geometry
The diameter of the nozzle and its geometry are other factors that significantly affect particle size in the SAS process. The smaller diameter of the nozzle produces a higher spray velocity and reduces the droplet size. However, as the pressure drop increases, the surface tension increases resulting in an enhanced mass transfer rate, and in the higher supersaturation, smaller particles will be formed.16,45 Also, the nozzle diameter influences particle morphology. Some research has shown that when a lower mass of solute is sprayed from a small-bore nozzle, it produces less cooling on the environment surrounding the nozzle in the reactor and negates the reduced temperature in the body of nozzle and its closed region. Therefore, the droplets sprayed through a smaller-bore nozzle precipitate more slowly and more spherical particles will be formed.74 Other research has shown that the effect of the nozzle diameter is not very significant. Its effect was explained according to the Weber number, which is the proportion of the internal and surface forces and related to the fluid velocities and surface tension. During the SAS process, under supercritical conditions, the surface tension is approximately equal to zero and the Weber number is no longer applicable. Therefore, the variation in solution velocity does not significantly affect the break-up behavior.26
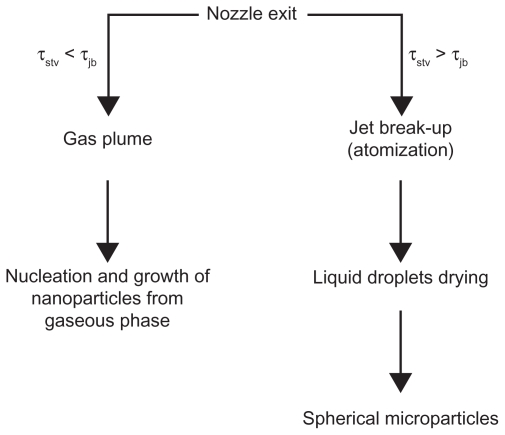
Reverchon et al explained that the smaller droplets were obtained with faster jet break-up than surface tension. He proposed two different mechanisms for particle production according to these characteristic times: namely, time of the surface tension vanishing, which is the required time to decrease the surface tension near to zero (τstv), and time of the jet break up, which is the required time to break the liquid jet at the exit of the nozzle [(τ)jb]. If τstv < τjb, then the gas plume process will occur. In contrast, if τstv > τjb, jet break-up will occur to produce the droplets. These two mechanisms are shown in Figure 4.22 With formation of the droplets, the supercritical antisolvent diffuses toward the liquid interface. Because the surface tension tends to vanish after the droplets’ production and during their drying, the original spherical shape of particles is maintained. 22 The process can be improved by spraying the drug and biodegradable polymers through two different co-axial nozzles to generate smaller particle size.15,18,34 The co-axial nozzle is specially designed to improve the particle morphology. The solution is sprayed through the core of the nozzle and the supercritical fluid through the annulus. The schematic of the co-axial nozzle is shown in Figure 5. By decreasing the Weber number, atomization is reduced and larger droplets are produced in the jet. For high-viscosity supercritical fluids and therefore higher Reynolds number, the mass transfer outside of the jet is faster, which results in less agglomeration.58
Figure 4.
The two mechanisms in competition for particles formation during the supercritical antisolvent process at P > PC and XCO2 ≥ XMCP.22
Note: Reprinted from The Journal of Supercritical Fluids, vol 47, Reverchon E, Adami R, Caputo G, De Marco I, Spherical microparticles production by supercritical antisolvent precipitation: interpretation of results, pages 70–84, copyright 2008, with permission from Elsevier.
Figure 5.
Coaxial nozzle employed for the simultaneous introduction of the organic solution and the supercritical antisolvent process.34
Note: Reprinted with permission from Kalogiannis CG, Pavlidou E, Panayiotou CG. Production of amoxicillin microparticles by supercritical antisolvent precipitation. Ind Eng Chem Res. 2005;44:9339–9346. Copyright 2005 American Chemical Society.
Abbreviation: SF, supercritical fluid.
Effects of flow rates of CO2 and liquid phase
Increasing the ratio of CO2 flow rate to the organic solution flow rate reduces the particle size. Enhancing the solution flow rate increases the system turbulence, thus improving the mixing of agents. Therefore, higher supersaturation occurs in the system, forming smaller particle sizes. Hence, the composition of the bulk fluid is reduced by CO2 flow rate which affects CO2 dissolving in the organic solvent solution. If the CO2 flow rate decreases, the amount of bulk fluid declines to less than the amount of organic solvent. Therefore, the solubility of the solute will be reduced and smaller particles will be produced.72,77
Larger particles with broader particle size distribution could be obtained by reduction in the CO2 molar fraction. By decreasing the CO2 molar fraction, the fluid phase produced in the reactor contained larger quantities of the solvent and therefore solubilization and solute precipitation processes occur more slowly. Thus, the microparticles production process shifts toward the growth process and therefore larger particles would be produced.11,52
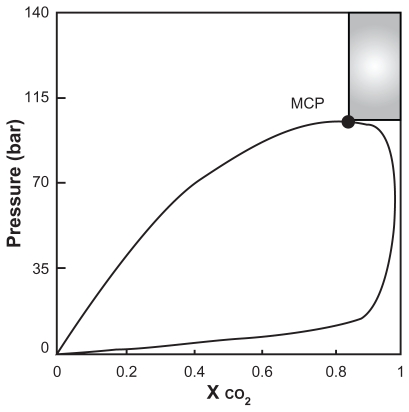
For production of the spherical microparticles, mole fraction of CO2 must be larger than the mole fraction at which the binary mixture CO2–liquid solvent shows the mixture critical point (MCP). Mixture critical point depends on the temperature and the nature of the liquid phase. The best conditions for production of spherical microparticles are at the pressure above the critical pressure of the mixture; the CO2 mole fraction above the MCP is shown as the shaded region in Figure 6.22 The observations are summarized in Table 2.
Figure 6.
Qualitative diagram pressure versus CO2 molar reaction.22
Note: Reprinted from The Journal of Supercritical Fluids, vol 47, Reverchon E, Adami R, Caputo G, De Marco I, Spherical microparticles production by supercritical antisolvent precipitation: interpretation of results, pages 70–84, copyright 2008, with permission from Elsevier.
Abbreviation: MCP, melting critical point.
Table 2.
Effects of the process parameters on the particle size in the supercritical antisolvent (SAS) process
| Effects | Ref |
|---|---|
| Effects of pressure | |
| At higher pressure, obtained smaller particle size. At higher pressure, the deforming pressure forces must be increased to break the droplets into smaller particles. Moreover, particle nucleation and its growth are other important factors affecting particle size. Rapid mass transfer of antisolvent and solvent causes high supersaturations for the solute. High supersaturation results in rapid nucleation and growth of more than one particle per primary droplet. | 56,63,72–73 |
| At lower pressure, obtained smaller particle size. In a situation above the critical condition, reduction in pressure is observed to decrease the solubility which then results in higher maximum supersaturation in the reactor; therefore, smaller particles are produced. | 32,42,45 |
| Pressure variations have no significant effect on particle size because the free intermolecular volume of the polymer will be occupied at the saturation pressure. | 8,41,63 |
| Effects of temperature | |
| At higher temperature, smaller size and more spherical particles obtained. But the temperature must be lower than the Tg of the polymer. | 32,42,63,72,78 |
| At lower temperature, smaller particle size, obtained due to higher volatility. | 32,73 |
| Effects of concentration | |
| At higher concentration, smaller particle size obtained because the increased initial concentration enhances the maximum supersaturation and, therefore, smaller particles will be formed. | 8,32–33 |
| At lower concentration, smaller particle size obtained because supersaturation of the drug occurs very late and therefore, the precipitation delay and nucleation dominate growth, producing smaller particles. | 27,33,36,42,63,72,73,75 |
| Effects of chemical composition of the organic solvent | |
| Particle size decreases with increase in volatility of the solvent. | 27,41 |
| Particle size decreased by using a stronger solvent. | 41 |
| Solubility of the biodegradable polymer in the organic solvent must be higher than the solubility of its drug contents. | 27 |
| Effects of chemical composition of the drug | |
| Lower solubility of the drug in a supercritical fluid enhances rapid precipitation. | 29,60 |
| Enhancement of drug lipophilicity reduces the loading drug efficiency in the SAS process. | 29,59 |
| Effects of chemical composition of the biodegradable polymer | |
| The crystalline polymer forms smaller particle size with narrower particle size distribution. | 7,67–68 |
| Drug stability in amorphous polymers is higher than in crystalline polymers. | 67–68 |
| Effects of the nozzle geometry | |
| A smaller nozzle diameter reduces the particle size and produces more spherical-shaped particles. | 45,63 |
| The effect of the nozzle diameter is not highly significant. | 63 |
| Co-axial nozzle, is especially designed for improvement of the morphology. | 45,63 |
| Effects of flow rates of CO2and liquid phase | |
| Increasing the ratio of CO2 flow rate over the organic solution flow rate reduces particle size. | 72,77 |
Abbreviation: Tg, glass transition temperature.
Drug release mechanisms
The encapsulation of pharmaceutical ingredients using a suitable polymer is an interesting method for controlled drug delivery. The drug release from the polymer occurs in a sustained manner and the dose of drug is controlled at the optimal therapeutic effects. The polymer can also protect fragile drugs such as peptides and proteins. It can reduce drug administration frequency and improve patient compliance. In controlled drug delivery, polymer-based microspheres can have two different structures, namely a matrix structure or an encapsulated structure. In a matrix structure, a solid phase disperses inside another solid phase, while in an encapsulated structure or reservoir structure a core of material is coated by another solid phase. These structures are shown in Figure 7.
Figure 7.
A schematic of a matrix structure (A), and encapsulated structure (B).
The drug can be released from a polymer by either diffusion mechanism or degradation mechanisms. During diffusion, the drug can pass through the polymer pores or chains. In this release mechanism, smart polymers have to be chosen which a permeability related to the environmental conditions. During the degradation mechanism, the biodegradable polymer degrades in the body due to the natural biological reactions. The degradation depends on the chemical structure and molecular weight of polymer. Therefore, the selection of a suitable polymer is critical for controlled drug delivery.79–81
Conclusion
Recently, processing of pharmaceutical compounds with supercritical fluid has received increased attention. Conventional methods cannot usually encapsulate drugs with a rate-controlled release or that target a specific site. Conventional drugs provide almost a sharp drug release and thus provide potentially toxic levels. New methods using biodegradable polymers can control the drug release rate. Encapsulation by means of supercritical fluid is of great interest in the pharmaceutical industries because of its ability to produce uniform particle size and controlled morphology. Due to the nonuniform temperature in conventional encapsulation methods, nonuniform supersaturation occurs, and thus nonuniform crystallization takes place and results in a broad particle size distribution. Conversely, in SAS fluid encapsulation, the mass transfer is so fast that it produces fine particles with a narrower size distribution. In the SAS process, the solvent and supercritical CO2 interaction plays the key role, while temperature and CO2 dissolution parameters control the process. Encapsulation of pharmaceutical products in biodegradable polymers is useful to control the rate of drug release within the body. Supercritical CO2 fluid is a relatively poor solvent for most biodegradable polymers and pharmaceutical products. Therefore, the SAS process is a suitable technique to produce fine spherical particles. Because the process conditions influence particle size and morphology, it is crucial to optimize the process parameters to produce smaller particle size with narrower size distribution.
Footnotes
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- 1.Thote AJ, Gupta RB. Formation of nanoparticles of a hydrophilic drug using supercritical carbon dioxide and microencapsulation for sustained release. Nanomedicine. 2005;1:85–90. doi: 10.1016/j.nano.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Peppas B. Polymers in controlled drug delivery. Medical Plastics and Biomaterials Magazine. 1997 [Google Scholar]
- 3.Cocero MMJ, Mattea F, Varona S. Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications. J Supercrit Fluids. 2009;47:546–555. [Google Scholar]
- 4.Yulu W, Dave RN, Pfeffer R. Polymer coating/encapsulation of nanoparticles using a supercritical anti-solvent process. J Supercrit Fluids. 2004;28:85–99. [Google Scholar]
- 5.Spasic AM, Hsu J-P, editors. Finely Dispersed Particles: micro-, nano-, and atto-engineering. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 6.Fages J, et al. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technology. 2004;141:219–226. [Google Scholar]
- 7.Ginty PJ, Whitaker MJ, Shakesheff KM, Howdle SM. Drug delivery goes supercritical. Materials Today. 2005;8(8 Suppl 1):42–48. [Google Scholar]
- 8.Ke W, Jing L. Precipitation of a biodegradable polymer using compressed carbon dioxide as anti-solvent. J Supercrit Fluids. 2008;46:211–216. [Google Scholar]
- 9.Yunqing Kang JW, et al. Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA micro-particles using supercritical CO2 technique. Eur J Pharm Biopharm. 2008;70:85–97. doi: 10.1016/j.ejpb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Chang AAL. PhD thesis. North California State University; 2006. Study of particle formation using supercritical CO2 as an antisolvent. [Google Scholar]
- 11.Patomchaiviwat V, Paeratakul O, Kulvanich P. Formation of inhalable rifampicin–poly(L-lactide) microparticles by supercritical anti-solvent process. AAPS Pharm Sci Tech. 2008;9:1119–1129. doi: 10.1208/s12249-008-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens AP, et al. High-resolution imaging of the supercritical antisolvent process. Exp Fluids. 2005;38:708–719. [Google Scholar]
- 13.Meziani MJ, Pathak P, Sun YP. Supercritical fluid technology for nanotechnology. In: de Villiers MM, Aramwit P, Kwon GS, editors. Nanotechnology in Drug Delivery. New York, NY: Springer; 2009. pp. 69–104. [Google Scholar]
- 14.Pasquali RBI. Are pharmaceutics really going supercritical. Int J Pharm. 2008;364:176–187. doi: 10.1016/j.ijpharm.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Davies OR, et al. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliv Rev. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Shekunov BY, Baldyga J, York P. Particle formation by mixing with supercritical antisolvent at high Reynolds numbers. Chem Eng Sci. 2001;56:2421–2433. [Google Scholar]
- 17.Gupta RB, Kompella UB, editors. Nanoparticle Technology for Drug Delivery. New York, NY: Taylor and Francis; 2006. [Google Scholar]
- 18.Bristow S, et al. Analysis of the supersaturation and precipitation process with supercritical CO2. J Supercrit Fluids. 2001;21:257–271. [Google Scholar]
- 19.Letcher TM, editor. Chemical thermodynamics for industry. London: Royal Society of Chemistry (Great Britain); 2004. [Google Scholar]
- 20.Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology – towards biomedical applications. Adv Drug Deliv Rev. 2008;60:299–327. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.York P, Kompella UB, Shekunov BY, editors; Swarbrick J, editor. Supercritical Fluid Technology for Drug Product Development. New York, NY: Marcel Dekker; 2004. [Google Scholar]
- 22.Reverchon E, Adami R, Caputo G, De Marco I. Spherical microparticles production by supercritical antisolvent precipitation: interpretation of results. J Supercrit Fluids. 2008;47:70–84. [Google Scholar]
- 23.Reverchon E, Adamia R. Nanomaterials and supercritical fluids. J Supercrit Fluids. 2006;37:1–22. [Google Scholar]
- 24.Yeo SD, Kiran E. Formation of polymer particles with supercritical fluids: a review. J Supercrit Fluids. 2005;34:287–308. [Google Scholar]
- 25.Jung J, Perrut M. Particle design using supercritical fluids: literature and patent survey. J Supercrit Fluids. 2001;20:179–219. [Google Scholar]
- 26.Reverchon E, Caputo G, De Marco I. Role of phase behavior and atomization in the supercritical antisolvent precipitation. Ind Eng Chem Res. 2003;42:6406–6414. [Google Scholar]
- 27.Taki EBS, Charbit G. Controlled release system formed by supercritical anti-solvent coprecipitation of a herbicide and a biodegradable polymer. J Supercrit Fluids. 2001;21:61–70. [Google Scholar]
- 28.Rantakyl M, et al. The effect of initial drop size on particle size in the supercritical antisolvent precipitation (SAS) technique. J Supercrit Fluids. 2002;24:251–263. [Google Scholar]
- 29.Reverchon E, Marco ID. Supercritical antisolvent micronization of Cefonicid: thermodynamic interpretation of results. J Supercrit Fluids. 2004;31:207–215. [Google Scholar]
- 30.Kikic I, Vecchione F. Supercritical impregnation of polymers. Current Opinion in Solid State and Materials Science. 2003;7:399–405. [Google Scholar]
- 31.Lee S, et al. Controlled delivery of a hydrophilic drug from a biodegradable microsphere system by supercritical anti-solvent precipitation technique. J Microencapsul. 2006;23:741–749. doi: 10.1080/09687860600945552. [DOI] [PubMed] [Google Scholar]
- 32.Miguel F, et al. Supercritical anti solvent precipitation of lycopene: effect of the operating parameters. J Supercrit Fluids. 2006;36:225–235. [Google Scholar]
- 33.Ai-Zheng C, et al. Application of organic nonsolvent in the process of solution-enhanced dispersion by supercritical CO2 to prepare puerarin fine particles. J Supercrit Fluids. 2009;49:394–402. [Google Scholar]
- 34.Kalogiannis CG, Pavlidou E, Panayiotou CG. Production of amoxicillin microparticles by supercritical antisolvent precipitation. Ind Eng Chem Res. 2005;44:9339–9346. [Google Scholar]
- 35.Majerik V, et al. Bioavailability enhancement of an active substance by supercritical antisolvent precipitation. J Supercrit Fluids. 2007;40:101–110. [Google Scholar]
- 36.Reverchon E, Marco De, Porta GD. Tailoring of nano- and microparticles of some superconductor precursors by supercritical antisolvent precipitation. J Supercrit Fluids. 2002;23:81–87. [Google Scholar]
- 37.Kim MS, et al. Micronization of cilostazol using supercritical antisolvent (SAS) process: effect of process parameters. Powder Technology. 2007;177:64–70. [Google Scholar]
- 38.Reverchon E, Porta GD, Falivene MG. Process parameters and morphology in amoxicillin micro and submicro particles generation by supercritical antisolvent precipitation. J Supercrit Fluids. 2000;17:239–248. [Google Scholar]
- 39.Park HJ, et al. Recrystallization of fluconazole using the supercritical antisolvent (SAS) process. Int J Pharm. 2007;328:152–160. doi: 10.1016/j.ijpharm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Adami RS. PhD thesis. University of Turku; 2007. Micronization of pharmaceuticals and food ingredients using supercritical fluid teqniques. [Google Scholar]
- 41.Reverchon E, et al. Supercritical antisolvent precipitation of nanoparticles of a zinc oxide precursor. Powder Technology. 1999;102:127–134. [Google Scholar]
- 42.Wang Y, Pfeffer R, Dave R, Enick R. Polymer encapsulation of fine particles by Supercritical antisolvent process. AIChE Journal. 2005;51:440–455. [Google Scholar]
- 43.Reverchon E, Porta GD, Pallado P. Supercritical antisolvent precipitation of salbutamol microparticles. Powder Technology. 2001;114:17–22. [Google Scholar]
- 44.Reverchon E, Porta GD. Production of antibiotic micro- and nanoparticles by supercritical antisolvent precipitation. Powder Technology. 1999;106:23–29. [Google Scholar]
- 45.Gregory WJS, Sacha A, Nail SL. Identification of critical process variables affecting particle size following precipitation using a supercritical fluid. Pharm Dev Technol. 2006;11:187–194. doi: 10.1080/10837450600691237. [DOI] [PubMed] [Google Scholar]
- 46.Yeo SD, Kim MS, Lee JC. Recrystallization of sulfathiazole and chlorpropamide using the supercritical fluid antisolvent process. J Supercrit Fluids. 2003;25:143–154. [Google Scholar]
- 47.Tenorio A, et al. Controlled submicro particle formation of ampicillin by supercritical antisolvent precipitation. J Supercrit Fluids. 2007;40:308–316. [Google Scholar]
- 48.Park C, Shin MS, Kim H. Micronization of arbutine using supercritical anti-solvent. Korean Journal of Chemical Engineering. 2008;25:581–584. [Google Scholar]
- 49.Yeo SD, Lee JC. Crystallization of sulfamethizole using the supercritical and liquid antisolvent processes. J Supercrit Fluids. 2004;30:315–323. [Google Scholar]
- 50.Chong GH. Thesis. University Putra Malaysia; 2009. Co-precipitation of acetaminophen and eudragit RL 100 using supercritical anti-solvent in controlled drug delivery. [Google Scholar]
- 51.Lee BM, et al. Supercritical antisolvent micronization of cyclotrimethylenetrinitramin: influence of the organic solvent. Ind Eng Chem Res. 2009;48:11162–11167. [Google Scholar]
- 52.Reverchon E, Marco ID. Supercritical antisolvent precipitation of cephalosporins. Powder Technology. 2006;164:139–146. [Google Scholar]
- 53.Winters BLK, et al. Precipitation of proteins in supercritical carbon dioxide. J Pharm Sci. 1996;85:586–594. doi: 10.1021/js950482q. [DOI] [PubMed] [Google Scholar]
- 54.Pascale S, et al. Precipitation and phase behavior of theophylline in solvent- supercritical CO2 mixtures. J Supercrit Fluids. 2005;35:95–105. [Google Scholar]
- 55.Duarte MSC, et al. Preparation of controlled release microspheres using supercritical fluid technology for delivery of anti-inflammatory drugs. Int J Pharm. 2006;308:168–174. doi: 10.1016/j.ijpharm.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Randolph TW, et al. Sub-micrometer-sized biodegradable particles of poly(L-lactic acid) via the gas antisolvent spray precipitation process. Biotechnol Prog. 1993;9:429–435. doi: 10.1021/bp00022a010. [DOI] [PubMed] [Google Scholar]
- 57.Moneghini MPB, et al. Supercritical antisolvent precipitation of nime-sulide: preliminary experiments. Curr Drug Deliv. 2007;4:241–248. doi: 10.2174/156720107781023901. [DOI] [PubMed] [Google Scholar]
- 58.Mishima K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv Drug Deliv Rev. 2008;60:411–432. doi: 10.1016/j.addr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Steckel HTJ, Muller BW. Micronizing of steroids for pulmonary delivery by supercritical carbon dioxide. Int J Pharm. 1997;152:99–110. [Google Scholar]
- 60.Tom JW, Debendetti PG, Prod’homme RK. Applications of supercritical fluids in controlled release of drugs Supercritical Fluid Engineering Science, ACS Symposium Series 514. Washington (DC): American Chemical Society; 1993. p. 238. [Google Scholar]
- 61.Bodmeier RWH, et al. Polymeric microspheres prepared by spraying into compressed carbon dixoide. Pharm Res. 1995;12:1211–1217. doi: 10.1023/a:1016276329672. [DOI] [PubMed] [Google Scholar]
- 62.Kompella UB, Koushik K. Preparation of large porous deslorelin–PLGA microparticles with reduced residual solvent and cellular uptake using a supercritical carbon dioxide process. Pharm Res. 2004;21:524–535. doi: 10.1023/B:PHAM.0000019308.25479.a4. [DOI] [PubMed] [Google Scholar]
- 63.Tu LS, Dehghani F, Foster NR. Micronisation and microencapsulation of pharmaceuticals using a carbon dioxide antisolvent. Powder Technology. 2002;126:134–149. [Google Scholar]
- 64.Peppas B. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm. 1995;116:1–9. [Google Scholar]
- 65.Visova VZ, et al. Encapsulation of indomethacin in magnetic biodegradable polymer nanoparticles. J Magn Magn Mater. 2007;311:379–382. [Google Scholar]
- 66.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as medical devices. Medical Plastics and Biomaterials Magazine. 1998 doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 67.Kawashima Y, York P. Drug delivery applications of supercritical fluid technology. Adv Drug Deliv Rev. 2008;60(3):297–298. doi: 10.1016/j.addr.2007.10.011. Epub 2007 Nov 6. [DOI] [PubMed] [Google Scholar]
- 68.Raouf Ghaderi PA, Carlfors J. A new method for preparing biodegradable microparticles and entrapment of hydrocortisone in DL-PLG microparticles using supercritical fluids. Eur J Pharm Sci. 2000;10:1–9. doi: 10.1016/s0928-0987(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 69.Park TG. Degradation of poly(o,L-lactic acid) microspheres: effect of molecular weight. J Control Release. 1994;30:161–173. [Google Scholar]
- 70.Kompella UB, Koushik K. Preparation of drug delivery systems using supercritical fluid technology. Crit Rev Ther Drug Carrier Syst. 2001;18(2):173–199. [PubMed] [Google Scholar]
- 71.Lengsfeld CS, et al. Mechanism governing microparticle morphology during precipitation by a compressed antisolvent: atomization vs nucleation and growth. J Phys Chem B. 2000;104:2725–2735. [Google Scholar]
- 72.Xiuhua Z, Yuangang Z, Qingyong L, et al. Application of supercritical fluid to preparation of powders of high-molecular weight drugs for inhalation. Adv Drug Deliv Rev. 2010;60:433–446. doi: 10.1016/j.addr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Okamoto H, Danjo K. Preparation and characterization of camptothecin powder micronized by a supercritical antisolvent (SAS) process. J Supercrit Fluids. 2008;51:412–419. [Google Scholar]
- 74.Hao J, et al. Supercritical fluid assisted melting of poly(ethylene glycol): a new solvent-free route to microparticles. J Mater Chem. 2005;15:1148–1153. [Google Scholar]
- 75.Reverchon E, Antonacci A. Polymer microparticles production by supercritical assisted atomization. J Supercrit Fluids. 2007;39:444–452. [Google Scholar]
- 76.Uzun I, Sipahigil O, Dincer S. Coprecipitation of cefuroxim axetil-PVP microparticles by batch supercritical antisolvent process. J Supercrit Fluids. 2011;55:1059–1069. [Google Scholar]
- 77.Martin A, Cocero MJ. Numerical modeling of jet hydrodynamics, mass transfer, and crystallization kinetics in the supercritical antisolvent (SAS) process. J Supercrit Fluids. 2004;32:203–219. [Google Scholar]
- 78.Sosa M, Rodriguez-Rojo S, Mattea F, Cismondi M, Cocero M. Green tea encapsulation by means of high pressure antisolvent coprecipitation. J Supercrit Fluids. 2010;56:304–311. [Google Scholar]
- 79.Yulu W, Yiping W, Jun Y, Pfeffer R, Dave R, Michniak B. The application of a supercritical antisolvent process for sustained drug delivery. Powder Technology. 2006;164:94–102. [Google Scholar]
- 80.Bahrami M, Ranjbarian S. Production of micro- and nano-composite particles by supercritical carbon dioxide. J Supercrit Fluids. 2007;40:263–283. [Google Scholar]
- 81.Priamo W, Cezaro A, Benetti S, Oliveira J, Ferreira S. In vitro release profiles of β-carotene encapsulated in PHBV by means of supercritical carbon dioxide micronization. J Supercrit Fluids. 2010;56:137–143. [Google Scholar]