Abstract
Mammalian ATP-gated nonselective cation channels (P2XRs) can be composed of seven possible subunits, denoted P2X1 to P2X7. Each subunit contains a large ectodomain, two transmembrane domains, and intracellular N and C termini. Functional P2XRs are organized as homomeric and heteromeric trimers. This review focuses on the binding sites involved in the activation (orthosteric) and regulation (allosteric) of P2XRs. The ectodomains contain three ATP binding sites, presumably located between neighboring subunits and formed by highly conserved residues. The detection and coordination of three ATP phosphate residues by positively charged amino acids are likely to play a dominant role in determining agonist potency, whereas an AsnPheArg motif may contribute to binding by coordinating the adenine ring. Nonconserved ectodomain histidines provide the binding sites for trace metals, divalent cations, and protons. The transmembrane domains account not only for the formation of the channel pore but also for the binding of ivermectin (a specific P2X4R allosteric regulator) and alcohols. The N- and C- domains provide the structures that determine the kinetics of receptor desensitization and/or pore dilation and are critical for the regulation of receptor functions by intracellular messengers, kinases, reactive oxygen species and mercury. The recent publication of the crystal structure of the zebrafish P2X4.1R in a closed state provides a major advance in the understanding of this family of receptor channels. We will discuss data obtained from numerous site-directed mutagenesis experiments accumulated during the last 15 years with reference to the crystal structure, allowing a structural interpretation of the molecular basis of orthosteric and allosteric ligand actions.
I. Introduction
The potential relevance of extracellular ATP in synaptic transmission was originally introduced in 1972 (Burnstock, 1972) but was received with skepticism until the first receptor was cloned in 1993 (Webb et al., 1993). It is now well established that there are two families of receptors activated by extracellular nucleotides: P2X receptors (P2XRs1) and P2Y receptors (P2YRs). P2XRs are a family of ligand-gated receptor channels. Seven mammalian purinergic receptor subunits, denoted P2X12 through P2X7, and several spliced forms of these subunits have been identified (North, 2002). P2YRs are G protein-coupled receptors. Eight mammalian P2YRs, denoted P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R, have been cloned (Fischer and Krügel, 2007). There are also four subtypes of nucleoside-activated G protein-coupled receptors known as P1 or adenosine receptors (ARs): A1R, A2AR, A2BR, and A3R (Ralevic and Burnstock, 1998). It has also been shown that nucleotides act not only as neurotransmitters but also as paracrine factors delivered by diffusion that requires several seconds, rather than a few milliseconds, to activate the receptors (Browne et al., 2010). The duration and distance of their actions are limited by several enzymes called ectonucleotidases (Yegutkin, 2008).
P2XRs are nonselective cation-conducting channels present in multiple species, from unicellular organisms to humans, but the phylogeny of these receptors remains to be established. The simplest organism that encodes a P2XR is the eukaryote green algae Ostreococcus tauri (Fountain et al., 2008). Although an ancestral prokaryotic P2XR has not been identified, these receptors are present in several invertebrate and vertebrate species (Fountain and Burnstock, 2009) and some of the properties of these channels (such as allosteric modulation) have been maintained evolutionarily (discussed in section IV). Some members of these channels provide not only a narrow conducting pathway for the passage of small ions but also a pathway for the passage of larger organic cations by dilation of the endogenous pore and/or integration of another channel or transporter. The native agonist for P2XRs is ATP, whereas both ATP and its metabolite ADP act as agonists for P2YRs in a receptor-specific manner. Other endogenous nucleotides, such as UTP, UDP, and UDP-glucose, are potent agonists for some P2YRs, but they have no activity at P2XRs (Jacobson et al., 2006). Diadenosine polyphosphates, known as dinucleotides, also act as agonists for P2XRs and P2YRs. These compounds are naturally occurring substances that are structurally related to ATP. They are composed of two adenosine moieties linked by their ribose 5′ ends to a variable number of phosphates (ApnA) (Pintor et al., 2000). Ectonucleotidase-derived AMP does not act as an agonist, but its degradation product, adenosine, is a natural agonist for ARs. Inosine, formed by the deamination of adenosine, has also been shown to have agonist activity at ARs (Guinzberg et al., 2006).
In this article, we review the current knowledge on orthosteric and allosteric regulation of P2XR function. Detailed literature on the expression, distribution, and function of P2XRs can be found elsewhere (see Burnstock and Knight, 2004; Burnstock, 2007; Surprenant and North, 2009). In contrast to G protein-coupled receptors, the wild-type P2XRs do not show obvious constitutive activity in the absence of agonist (North, 2002). The receptors probably have three classic agonist binding sites (Browne et al., 2010). Here we use the term “orthosteric sites” to describe all ATP binding sites on P2XRs, because they are the primary binding sites needed for the conformational changes that allow for the opening of the channels (gating).
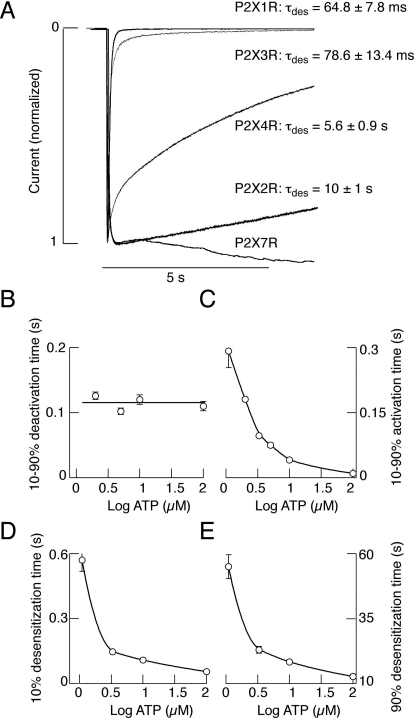
The gating of P2XRs usually consists of three phases: a rapid rising phase of inward current induced by the application of agonist (activation phase), a slowly developing decay phase in the presence of an agonist (desensitization phase), and a relatively rapid decay of current after ATP is removed (deactivation phase). The main difference among receptors is in their sensitivity for agonists and their activation and desensitization rates. Figure 1A shows the profile of P2XR currents in response to supramaximal concentrations of ATP (10 μM for P2X1R and P2X3R, 100 μM for P2X2R and P2X4R, and 10 mM for P2X7R). P2X1R and P2X3R rapidly activate and desensitize, whereas P2X2R and P2X4R slowly desensitize. Rat P2X5Rs generate low amplitude nondesensitizing currents, whereas human and chick P2X5R respond with larger currents. The P2X6R does not express well at the plasma membrane (Collo et al., 1996). On the other hand, the gating of P2X7R is more complex, as indicated by the secondary current growth during sustained agonist application (Fig. 1A).
Fig. 1.
Gating properties of P2XRs. A, profiles of P2XR currents induced by sustained agonist application. Recombinant rat receptors were expressed in HEK293 cells and stimulated with ATP (10 μM for P2X1R and P2X3R, 100 μM for P2X2R and P2X4R, and 3 mM for P2X7R). τdes indicates the desensitization time constant derived from monoexponential fitting (mean ± S.E.M.; values from at least five records per channel). B to E, characterization of P2X4R current. B, Deactivation time values (10–90%) are independent from the ATP concentration. C, inverse relationship between activation time and ATP concentration. D and E, inverse relationship between the values for 10% (D) and 90% (E) desensitization time and ATP concentrations.
The dependence of activation, desensitization, and deactivation kinetics on agonist concentration was studied in detail using P2X4R as a receptor model (Yan et al., 2006). The activation efficiency increases with elevation in ATP concentration, and the activation time inversely correlates with ATP concentration (Fig. 1C). There is also an inverse relationship between desensitization time and ATP concentration (Fig. 1, D and E). In contrast, deactivation kinetics is independent of agonist concentration (Fig. 1B). The P2X2R exhibits a similar dependence of gating properties on agonist concentrations (Zemkova et al., 2004). Experiments with outside-out patches containing recombinant rP2X2Rs revealed an 80-μs delay between the time when ATP arrives at the receptor and the opening of the channels. In addition, a brief pulse of saturating ATP leaves fully liganded channels without producing an opening at least 30% of the time (Moffatt and Hume, 2007). The deactivation properties of P2X1R and P2X3R are difficult to estimate because of the rapid receptor desensitization. The gating of P2X7R differs from that of other members of this family of channels (Yan et al., 2010). For details on the gating properties of P2XRs, see North (2002).
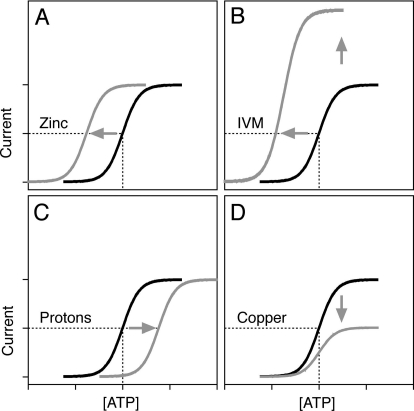
The behavior of P2XRs can be altered by allosteric interactions. These interactions occur at additional sites that are linked conformationally or by other mechanisms to the orthosteric site(s) in such way that binding to one site can change the nature and extent of binding or signaling via the other site (Christopoulos et al., 2004; Gao and Jacobson, 2006). Thus, allosteric ligands bind to sites that are topographically distinct from the orthosteric sites recognized by the receptor's endogenous agonist. Allosteric perturbation arises not only from the binding of small or large molecules extracellularly but also from changes in temperature, ionic strength, or concentration and from covalent modification tethering, glycosylation, phosphorylation, and ubiquitination, which could take place extracellularly in the transmembrane (TM) region or intracellularly (Tsai et al., 2009).
Allosteric systems are also much more versatile than orthosteric systems in that modulator ligands can augment, block, or potentiate the effects of the orthosteric agonist, and this effect can change with the nature of agonist (Kenakin, 2010). The term cooperativity is frequently used to describe the magnitude of an allosteric effect, which could be either positive or negative, leading to up- or down-regulation of receptor function. Thus, receptor allosterism can be defined as the effect on a receptor produced by simultaneous interactions with two distinct ligands: orthosteric and allosteric. The allosterically modified receptor exhibits different affinities and efficacies for all interactants. The term affinity is used to describe the ability of an agonist to bind to a receptor, whereas potency is a measure of agonist activity expressed in terms of the concentration required to generate an effect of a given intensity, usually expressed as the EC50 value. The term efficacy describes the degree to which different agonists (full and partial) produce maximal responses when occupying the same proportion of receptors, usually expressed as the Emax value.
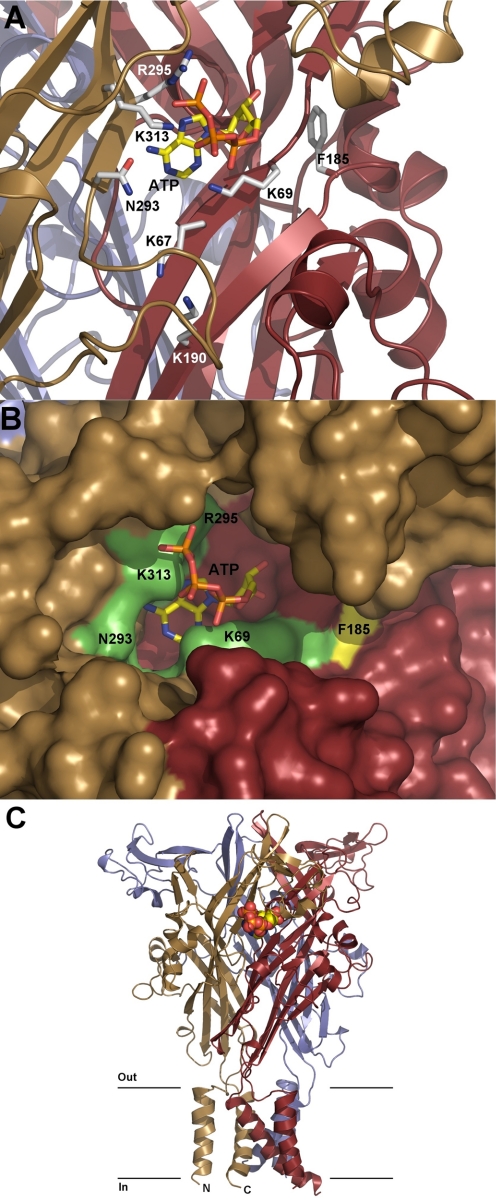
In this review, we begin by summarizing the work concerning the pharmacology and structure of the orthosteric binding sites. This will be followed by a summary of the effects of trace metals (zinc, copper, cobalt, and nickel), heavy metals (mercury and cadmium), and macro metals (calcium, magnesium, and sodium) on receptor function and the identification of residues responsible for the binding of these metals. We will also review the literature pertaining to the effects of protons, ivermectin (IVM), neurosteroids, alcohols, and related clinically relevant anesthetic drugs, as well as the signaling pathways involved in the regulation of these receptor channels, such as reactive oxygen species, calmodulin (CaM), kinases, and phosphoinositides. Based on the crystal structure of the zebrafish P2X4.1R (zP2X4.1R), solved at a 3.5-Å resolution (Kawate et al., 2009), we have also generated a homology model of rat P2X4R (rP2X4R) and reanalyzed data published by our own group and other groups that have been working on the structural and functional characterization of this receptor. Throughout the text, we will use the homology model of rP2X4R to discuss orthosteric and allosteric binding sites.
II. Molecular and Crystal Structure of P2X2 Receptors
All members of extracellular ligand-gated ion channels contain two functional domains: an extracellular domain that binds a native agonist and a TM domain that forms an ion channel. Among ligand-gated receptor channels, P2XRs are the simplest. Cloning of P2XRs revealed that all subunits have a large (∼280 amino acids) extracellular loop (hereafter referred to as the ectodomain), two TM domains (TM1 and TM2), and intracellularly located N and C termini of variable lengths, resembling the topology of structurally unrelated epithelial sodium channels and the mechanosensitive degenerin channels (Stojilkovic, 2008).
The precise biophysical characterization of native and recombinant P2XRs was instrumental in generating the hypothesis that these channels are organized as trimeric homomers or heteromers. The concentration-dependence of P2XR activation measured by whole-cell (Bean et al., 1990; Jiang et al., 2003) and single-cell (Ding and Sachs, 1999) recording suggested that three molecules of ATP were required for channel gating. Biochemical studies showed that under nondenaturizing conditions, P2XRs migrated as trimers in polyacrylamide gel electrophoresis, therefore suggesting that this was the conformation of native functional channels (Nicke et al., 1998). The same conclusion has been reached in a study with heteromeric P2XRs (Nicke et al., 2005). Other study showed that mutations that affect channel gating by methanethiosulfonate bromide block were effective only if they were contained in the three first subunits of concatenated P2XRs, further supporting a trimeric conformation (Stoop et al., 1999). Subsequently, other more sophisticated techniques, such as atomic force microscopy (Barrera et al., 2005; Nakazawa et al., 2005) and electron microscopy (Young et al., 2008), have corroborated the trimeric nature of P2XRs.
A structure for P2X2R at 15-Å resolution was also recently reported (Mio et al., 2009). Crystallization of the zP2X4.1R at 3.5-Å resolution showed that the receptor is indeed a trimer. As described by Kawate et al. (2009), each subunit rises from the plasma membrane, like a dolphin from the surface of the ocean, with its tail submerged within the lipid bilayer. The body regions of three subunits mutually intertwine, forming a central vertical cavity. The ectodomain projects 70 Å above the plasma membrane, and there are three vestibules in the center of the ectodomain (Kawate et al., 2009). Comparison of the zP2X4R with acid-sensing ion channel structure revealed similarity in pore architecture and aqueous vestibules (Gonzales et al., 2009).
Channels are organized as homotrimers or heterotrimers. Initial evidence for the formation of functional heteromers came from studies coexpressing the slowly desensitizing and αβ-meATP-insensitive P2X2R with the rapidly desensitizing and αβ-meATP-sensitive P2X3R, resulting in a slow desensitizing and αβ-meATP-sensitive P2X2/3R (Lewis et al., 1995). A series of 11 heteromers was predicted by immunoprecipitation studies (Torres et al., 1999a). So far, six functional P2XR heteromeric receptors have been characterized: P2X1/2R (Brown et al., 2002; Aschrafi et al., 2004), P2X1/4R (Nicke et al., 2005), P2X1/5R (Torres et al., 1998; Haines et al., 1999; Lê et al., 1999), P2X2/3R (Lewis et al., 1995; Liu et al., 2001a; Spelta et al., 2002; Jiang et al., 2003), P2X2/6R (King et al., 2000; Barrera et al., 2007), and P2X4/6R (Lê et al., 1998).
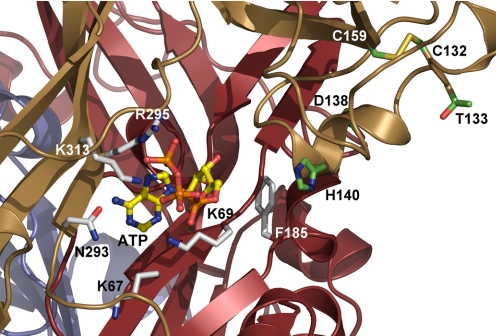
Studies using mutagenesis combined with functional expression have allowed for the initial designation of certain amino acids in specific receptor functions. The ecodomain contains 10 conserved cysteine residues that have been predicted to make bonds in the following order (P2X4R numbering): 116 to 165 (SS1), 126 to 149 (SS2), 132 to 159 (SS3), 217 to 227 (SS4), and 261 to 270 (SS5) (Clyne et al., 2002b; Ennion and Evans, 2002; Rokic et al., 2010). In P2X1R, the individual bonds are not essential for receptor function (Ennion and Evans, 2002). For P2X2R, however, the SS1 and SS4 bonds are individually required for proper receptor function (Clyne et al., 2002b). Disruption of the SS1, SS2, and SS4-P2X4R bonds by substituting both cysteines with threonine generated less sensitive receptors. Of these three bonds, mutation of the SS4 cysteine residues generated the most profound changes in receptor function (Rokic et al., 2010). The ectodomain also contains two to six asparagines that may contribute to N-linked glycosylation, an important process for the proper trafficking of P2XRs to the plasma membrane and receptor functions (North, 2002). As discussed in the following sections, SS bonds may participate in the formation of an allosteric regulatory domain, and other ectodomain residues may be important for the formation of ATP binding sites and several allosteric sites.
The crystal structure of zP2X4.1R provided direct evidence for the existence of these conserved disulfide bonds. The first three bonds are located in the head and beak regions of ectodomain, the fourth bond is located within the dorsal fin, and the fifth bond at the ends of two β-sheets in the low body region. The mammalian model of P2X4R, based on the crystal structure of zP2X4.1R, provides some rationale for the specific roles of the SS1 to -5 disulfide bonds in P2X4R function. The model shows that bonds SS1 to -3 are located within the head domain above the predicted ATP binding pocket, whereas SS4 is below the ATP binding pocket. The model also shows that the SS5 bond is located relatively far from the putative ATP binding site but close to the extracellular vestibule above the TM domains (Stojilkovic et al., 2010b). The SS4 bond is absent in the simple eukaryote O. tauri, and SS2, SS3, SS4 and SS5 bonds are absent in Dictyostelium discoideum (Surprenant and North, 2009). These receptors are functional, but a high concentration of agonist is required for their activation, suggesting that the formation of the SS bonds was an important step in the evolution of P2XR proteins. Together, these findings indicate that SS bonds provide the structural basis for the tridimensional organization of these receptors.
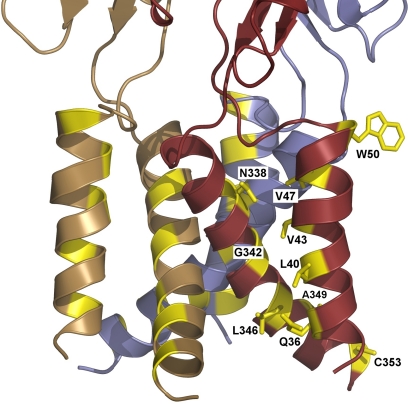
Perturbation of receptor function caused by scanning mutagenesis of the TM domains was useful in predicting that these regions adopt α-helical structures in activated P2X2Rs (Rassendren et al., 1997a; Egan et al., 1998; Haines et al., 2001a,b; Jiang et al., 2001; Khakh and Egan, 2005) and P2X4Rs (Silberberg et al., 2005; Jelínkova et al., 2008; Jindrichova et al., 2009). These experiments also helped identify the key regions involved in ion permeation. The helices of different subunits seem to move relative to each other during channel opening and closing (Egan et al., 1998), and TM2 seems to play a dominant role in receptor functions (Torres et al., 1999b; Duckwitz et al., 2006), channel assembly, gating, ion selectivity, and the permeability of divalent ions (Egan et al., 1998; Li et al., 2004; Khakh and Egan, 2005; Samways and Egan, 2007; Li et al., 2008). TM2 residues Thr336, Thr339, and Ser340 were suggested to contribute to the formation of the pore, gate, and selectivity filter of P2X2Rs (Migita et al., 2001; Egan and Khakh, 2004; Samways and Egan, 2007). It has been proposed that the external gate region Ile332 to Ile341 expands, and the pore-forming helices straighten, resulting in the opening of the channel pore (Li et al., 2010). None of the residues that seem to contribute to the formation of the P2X2R pore gate are conserved, clearly indicating that further structural information is required to understand the orientation of the TM2 residues in closed and open states.
Alanine and cysteine scanning of the P2X4R-TM1 domain indicated that Gly29, Met31, Tyr42, Gly45, and Val49 residues are mutation-sensitive (Jelínkova et al., 2008; Jindrichova et al., 2009). Among these residues, the conserved Tyr42 residue seemed to play the most important role in receptor function. At first, it was suggested that this residue affected the cation permeability of P2X2R only indirectly (Samways et al., 2008). The P2X4R-Y42 mutant showed an increased sensitivity to ATP and a decreased Emax (Silberberg et al., 2005; Jindrichova et al., 2009). In P2X1R, replacement of this residue with alanine resulted in nonfunctional channels, further supporting the importance of this residue in receptor function. The sensitivity of the P2X3R-Y37A mutant to ATP was also increased. In addition, αβ-MeATP was changed from a partial to full agonist for P2X2R and P2X4R with increased sensitivity. In contrast, mutation of the conserved TM1 tyrosine of P2X7R did not result in an increased sensitivity to ATP (Jindrichova et al., 2009).
Crystallization of zP2X4.1R (Kawate et al., 2009) confirmed the hypothesis that the TMs adopt α-helical structures. Consistent with the prediction that the TM2 region plays a dominant role in receptor function, channel assembly, gating, ion selectivity, and the permeability of divalent ions, the homology model of rat P2X4R shows that the TM2 domains form a triangle, which is the pore of the channel in a closed state, whereas the three TM1 domains are located more peripherally. The homology P2X4R model suggests that Tyr42 is located at the level of the membrane where the TM2 helices cross each other and the TM pore is the narrowest (Stojilkovic et al., 2010b). The model shows the position of Trp50 and Trp46 residues, which have been shown to influence the function of the Tyr42 residue. Furthermore, the model suggests that Met336 of the second subunit may interact with Tyr42 in the closed state, a possibility that should be tested experimentally. The zebrafish structure also provided important information about residues that could account for the cation selectivity of P2XRs. These include acidic residues in the central vestibule that could concentrate cations in the extracellular part of the channel (Asp59 and Asp61) and a residue that could directly interact with cations in the channel pore (Asn341). It also gave some clues about which residues are important for the channel gate: Leu340 and Asn341 in the extracellular part of the gate and Ala344, Leu346, and Ala347 as the hydrophobic residues that form the intracellular gate.
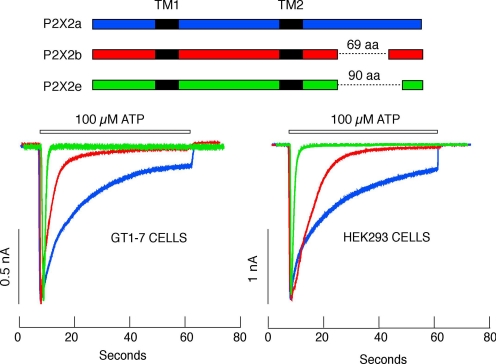
At present, we do not know the organization of the N and C termini in three dimensions. The N termini of all P2XRs is relatively short (around 25 amino acids), whereas the lengths of the C termini range from approximately 30 amino acids (P2X6R) to approximately 215 residues (P2X7R). Both the N and C termini serve as molecular targets for a series of post-transcriptional and post-translational modifications, including RNA splicing, phosphorylation, and protein-protein interactions (North, 2002). The physiological relevance of spliced channels has been well documented for P2X2R (Fig. 2). Two functional splice forms of these receptors, P2X2bR and P2X2eR, desensitize more rapidly than the full-sized receptor, P2X2aR (Brändle et al., 1997; Simon et al., 1997; Koshimizu et al., 1998b, 2006). In contrast, the C-terminal of P2X7R contains a unique Tyr358 to Glu375 sequence that contributes to the transition from the open to dilated state (Jiang et al., 2005a; Yan et al., 2008). The N and C termini also contain conserved phosphorylation sites for protein kinases A and C and other important regions responsible for the regulation of P2XRs, which is discussed in detail in the following sections.
Fig. 2.
Dependence of the mP2X2R current on the C-terminal structure. Top, schematic representation of the P2X2R splice forms. Bottom, typical patterns of ATP-induced current profiles for P2X2Rs expressed in GT1–7 (left) and HEK293 cells (right). Blue traces, P2X2aR; red traces, P2X2bR; green traces, P2X2eR.
III. Orthosteric Binding Sites and Receptor Function
A. P2X Receptor Agonism and Antagonism
All homomeric and heteromeric receptors are activated by ATP but in a receptor-specific manner, the EC50 values ranging from nanomolar to submillimolar concentrations. As stated under Introduction, P2XRs are more structurally restrictive than P2YRs with regard to agonist selectivity. P2XRs are also activated by naturally occurring diadenosine polyphosphates (ApnAs, n = 3–7) and closely related dinucleotides (ApnGs, n = 3–6), but with a lower potency and efficacy than ATP. Alone, these agonists cannot be used to distinguish P2XRs. Other nucleoside triphosphates, such as CTP and GTP, can also activate some P2XRs. In contrast, ADP, AMP, adenosine, UTP, UDP, and UMP activate these receptors either weakly or not at all. The chemical structure of most common P2X agonists and antagonists can be found elsewhere (Ralevic and Burnstock, 1998; Kim et al., 2001; Lambrecht et al., 2002).
It has been difficult to develop subunit-specific agonists for P2XRs. The agonists that currently exist are analogs of ATP and act at several P2XRs with different potencies and efficacies. Triphosphate modification resulted in the generation of several potent P2XR agonist analogs. α,β-Methylene-ATP (αβ-meATP) and β,γ-methylene-ATP (βγ-meATP) are phosphonic acid analogs of ATP in which the bridging oxygen atom between corresponding phosphates is replaced with the methylene group. These analogs are metabolically more stable than ATP, do not activate P2YRs, exhibit higher potencies at P2X1R and P2X3R homomeric and heteromeric receptors, and serve as radioligands for these receptors. The thio substitution at the terminal phosphates resulted in several analogs, including adenosine-5′-O-(3-thiotriphosphate) (ATPγS), which are relatively resistant to breakdown by ectonucleotidases. ATPγS activates all P2XRs except P2X7R, as well as several P2YRs. Substitution of the adenine ring resulted in the formation of 2-methylthio-ATP (2-meSATP), the most potent agonist for P2XRs and P2YRs, and several other analogs. Because it does not activate adenosine receptors, 2-meSATP was an important compound in establishing the existence of two P2 receptor families. 2′(3′)-O-4-benzoylbenzoyl)-ATP (BzATP), a modified ribose derivative, is a common agonist for P2XRs that, with the exception of P2Y11R and P2Y13R, does not activate P2YRs (Jacobson et al., 2002; Carrasquero et al., 2009; Jarvis and Khakh, 2009).
Several nucleotide derivatives also act as P2XR antagonists. Trinitrophenyl-ATP (TNP-ATP) and the corresponding di- and monophosphate derivatives inhibit P2X1R, P2X3R, and P2X2/3R at nanomolar concentrations (Virginio et al., 1998b). The oxidized form of ATP has been suggested to act as an mP2X7R-specific blocker (Jacobson et al., 2002). Ip5I, a diinosine polyphosphate, inhibits P2X1R currents at nanomolar concentrations, and Ip4I and Ip5I block P2X3R at micromolar concentrations (King et al., 1999).
Suramin is a large, complex, polysulfonated molecule and is one of the most widely used competitive P2R antagonists (Jacobson et al., 2002; Lambrecht et al., 2002). However, suramin is not specific for P2Rs and also antagonizes G proteins (Freissmuth et al., 1996; Hui and Nayak, 2002); inhibits several proteases, including HIV reverse transcriptase (Jentsch et al., 1987) and tyrosine phosphatase (Zhang et al., 1998); and stimulates ryanodine receptors (Hohenegger et al., 1996). Suramin inhibits ATP-induced currents in a receptor-specific manner (North, 2002). The replacement of Gln78 residue with Lys at rP2X4R was sufficient to increase the sensitivity of this receptor for suramin (Garcia-Guzman et al., 1997a) but not to the extent observed in cells expressing human receptor (hP2X1R) (Roberts and Evans, 2004), indicating that other receptor regions are important for the recognition of this drug. Human and mouse P2X1R also exhibit marked differences in sensitivity to suramin, and the nonconserved Lys138 residue may contribute to the antagonistic action of this drug and its derivatives (Braun et al., 2001; Sim et al., 2008).
A number of truncated forms of suramin, including 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulfonic acid (NF023), 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid (NF279), 4,4′,4″,4⁗-[carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))]tetrakis-1,3-benzenedisulfonic acid (NF449), and 8,8′,8″,8⁗-(carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino)))tetrakis-naphthalene-1,3,5-trisulfonic acid-dodecasodium salt (NF864) exhibit P2R antagonist activity. NF023 inhibits several homomeric and heteromeric P2XRs expressed in Xenopus laevis oocytes and is most potent at P2X1R (Soto et al., 1999). NF279 does not affect the activation of adenosine receptors, exhibits low inhibitory potency on P2YRs and ectonucleotidases, and antagonizes P2XRs (Damer et al., 1998), showing the greatest potency at P2X1R and P2X7R (Klapperstück et al., 2000; Rettinger et al., 2000; Donnelly-Roberts et al., 2009a). NF449 inhibits rP2X1R with subnanomolar potency (Braun et al., 2001; Kassack et al., 2004; Rettinger et al., 2005) but also inhibits Gs proteins (Hohenegger et al., 1998) and fibroblast growth factor receptor 3 signaling (Krejci et al., 2010). Three isomeric suramin analogs, 4,4′,4″,4⁗-[carbonylbis[imino-5,1,3-benzenetriylbis(carbonylimino)]]tetrakisbenzenesulfonic acid tetrasodium salt (NF110) and ortho-(2,2′,2″,2⁗-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetrakis-benzenesulfonic acid (MK3), are >200-fold less potent than NF449 in blocking P2X1R (Hausmann et al., 2006). NF864 selectively blocks hP2X1R with low nanomolar potency (Horner et al., 2005).
Pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS) is another compound that is commonly used as a P2XR inhibitor. In contrast to suramin, the P2R specificity of PPADS is very high. It acts noncompetitively and reduces the Emax value of ATP at high concentrations. PPADS blocks homomeric P2X1Rs, P2X2Rs, P2X3Rs, and P2X5Rs as well as heteromeric P2X2/3Rs, P2X1/5Rs, and P2Y1Rs, whereas it is weak or ineffective as an antagonist at rat P2X4Rs, P2X6Rs, P2X7Rs, and several P2YRs. Several attempts have been made to identify the ectodomain residues responsible for this receptor specificity. The sensitivity of P2X4R to PPADS was restored by replacing Glu249 with the lysine that occurs at the equivalent position in PPADS-sensitive P2X1R and P2X2R (Buell et al., 1996). However, the reverse mutation in the P2X2R did not remove inhibition, and this lysine is not present in PPADS-sensitive hP2X3Rs and hP2X7Rs. The human and mouse P2X4Rs have almost identical amino acid sequences around Glu294 but are sensitive to PPADS, indicating that other regions determine PPADS sensitivity (Garcia-Guzman et al., 1997a; Jones et al., 2000). Subsequent studies reveled that the 81-to-183 region of rP2X4R (Garcia-Guzman et al., 1997a), specifically Arg126 (Michel et al., 2008b), contributes to PPADS insensitivity.
A chemical cousin to PPADS, pyridoxalphosphate-6-azophenyl-2′,5′-disulfonic acid, has somewhat distinct pharmacological properties (Jacobson et al., 2002; Lambrecht et al., 2002). Pyridoxal-5′-phosphate-6-(2′-naphthylazo-6′-nitro-4′,8′-disulfonate (PPNDS) potently antagonized rP2XR-mediated responses in the vas deferens of rats and at recombinant rP2X1Rs expressed in X. laevis oocytes. PPNDS is approximately 50-fold less selective for P2Y1R and does not interact with α1A-adrenergic, adenosine A1 and A2B, histamine H1, or muscarinic M3 receptors (Lambrecht et al., 2000b). 1,5-dihydro-3-hydroxy-8-methyl[1,3,2]dioxaphosphepino[5,6-c]pyridin-9-ol-3-oxide (MRS2219) is a selective potentiator of ATP-evoked responses at rP2X1R, whereas the corresponding 6-azophenyl-2′,5′-disulfonate derivative, know as MRS2220, is a selective antagonist of this receptor (Jacobson et al., 1998). 4-[(4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl}-2-pyridinyl)azo]-benzoic acid (MRS2159) is a potent inhibitor of rP2X1R (Brown et al., 2001; Kim et al., 2001) and mouse, rat, and human P2X7R (Donnelly-Roberts et al., 2009a). Pyridoxal-5′-phosphate-6-azophenyl-3′,5′-bimethylenphosphonate (MRS2257) inhibits both P2X1R and P2X3R but with higher potency than PPADS (Brown et al., 2001). 6-((4,6,8-Trisulfo-1-naphthyl)iminocarbonyl-1, 3-(4-methylphenylene)iminocarbonyl-1,3-phenylene-azo)-pyridoxal-5′-phosphate (SB9) is a high-affinity P2Y1R antagonist with a 10-fold lower potency at P2X1R (Lambrecht et al., 2000a).
The anthraquinone class of compounds is also used to inhibit P2R. There has been some confusion concerning the identity and purity of commercially available reactive blue 2 (RB-2) and the corresponding pure isomer, Cibacron blue 3GA. Here we use term RB-2 to describe the antagonistic actions of the mixture of terminal ring meta- and para-sulfonate, and we discuss separately the allosteric actions of Cibacron blue 3GA, the purified ortho isomer, at recombinant P2XRs. The antagonistic actions of RB-2 were observed in several diverse ATP-mediated physiological responses, such as rat urinary bladder smooth muscle contraction (Hashimoto and Kokubun, 1995), rat cecum inhibitory junction potentials (Manzini et al., 1986), and calcium influx in rat parotid acinar cells (Soltoff et al., 1989). It has also been suggested that RB-2 inhibits ectonucleotidase activity in X. laevis oocytes (Ziganshin et al., 1996) and ATP-induced inflammation of the mouse hind paw (Ziganshina et al., 1996). The inhibitory effects of RB-2 were also observed in currents and calcium measurements in single PC12 cells (Nakazawa et al., 1991; Michel et al., 1996) as well as in experiments with cells expressing recombinant rP2X1R and P2X2R (Surprenant, 1996). For a more detailed description of the antagonistic actions of RB-2, see Ralevic and Burnstock (1998).
Several other related compounds, such as RB-4, RB-5, 1519, and acid blue-25, -41, -80, and -129, have also been tested for their antagonistic actions at P2Rs (Tuluc et al., 1998). A series of RB-2 type anthraquinone derivatives were also synthesized, re-evaluated, and tested for their potency at P2Rs. Among them, 1-amino-4-(4-(4-chloro-6-(2-sulfonatophenylamino)-(1,3,5)triazine-2-ylamino]-2-sulfonatophenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid trisodium salt (MG50-3-1) is the most potent antagonist for P2Y1R, and none of the compounds tested exhibited specificity for P2XRs (Glänzel et al., 2003, 2005).
1. Homomeric P2X1 Receptor.
One of the first pieces of evidence for the expression of P2X1R came from experiments indicating a role for ATP as a neurotransmitter involved in contractions of the guinea pig detrusor smooth muscle (Burnstock, 1972). Further studies found that αβ-meATP could be substituted for ATP in these neurogenic contractions (Burnstock et al., 1978; Kasakov and Burnstock, 1982). Electrophysiological studies showed transient concentration-dependent effects of ATP and αβ-meATP on inward currents causing membrane depolarization in isolated detrusor smooth muscle cells (Fujii, 1988; Inoue and Brading, 1990, 1991). Later, this receptor was cloned from the vas deferens of rats (Valera et al., 1994) and the human urinary bladder (Valera et al., 1995) and termed P2X1R.
In general, this receptor is densely localized in smooth muscle cells, including the urinary bladder, intestines, vas deferens, and arteries (Mulryan et al., 2000; Burnstock and Knight, 2004). Other tissues that express the P2X1R include the smooth muscle of small arteries, dorsal root, lung, central and peripheral nervous system, platelets, and megakaryocytes (Valera et al., 1994, 1995; Longhurst et al., 1996). In accordance with this, mice lacking the gene encoding P2X1R do not show rapidly desensitizing inward currents in the detrusor smooth muscle, vas deferens, and mesenteric arteries (Mulryan et al., 2000). Furthermore, platelets from P2X1R-deficient mice do not show normal aggregation, secretion, adhesion, and thrombus growth, and they have reduced mortality (Vial and Evans, 2000, 2002; Hechler et al., 2006). On the other hand, transgenic mice overexpressing human P2X1R exhibit hypersensitive platelet responses in vitro and increased mortality (Oury et al., 2003).
Recombinant receptors respond to agonist application with a rapid rise in current, followed by a decline in the current amplitude during the sustained agonist application (Fig. 1A). The rates of receptor desensitization are augmented by increases in the agonist concentration. Repeated agonist applications lead to progressively smaller currents when applied less than 10 min apart (Valera et al., 1994). A similar response pattern is also observed in cells expressing recombinant P2X3R (Fig. 1) and P2X2eR, the splice form of mP2X2R (Fig. 2). Run-down of current measured by the whole-cell recording was unaffected by changes in cytosolic calcium and was abolished when the amphotericin-perforated patch clamp was used for current recording (Lewis and Evans, 2000). Experiments using green fluorescent protein-attached P2X1Rs (Dutton et al., 2000) or the biotinylation of surface P2X1Rs (Ennion and Evans, 2001) suggest that P2X1R is internalized after agonist activation. This behavior could contribute to both long-term desensitization and the recovery from desensitization, although intrinsic channel properties could explain the fast desensitization. P2X1R has been shown to exhibit both constitutive and agonist-induced recycling after photo-bleaching, suggesting that this could be important in the recovery from desensitization (Lalo et al., 2010).
The pharmacological profile of P2X1R is shown in Table 1. Among P2XRs, this receptor has the highest affinity for ATP, with an EC50 in the submicromolar concentration range (Valera et al., 1994; Wildman et al., 2002; Rettinger and Schmalzing, 2003). The real EC50 value for ATP is probably lower, but it is masked by rapid receptor desensitization (Rettinger and Schmalzing, 2003). Other ATP analogs, including 2-meSATP, 2-chloro-ATP, ATPγS and BzATP, also activate these receptors (Evans et al., 1995). hP2X1R is activated by nanomolar concentrations of ATP, 2-meSATP, αβ-meATP, and BzATP, as estimated by using calcium measurements (Bianchi et al., 1999). Electrophysiological measurements found EC50 values for hP2X1R to be comparable with those observed in rP2X1R. In these experiments, ATP and 2-meSATP were full agonists at rP2X1R, whereas other ATP analogs were partial agonists (Evans et al., 1995). Ap6A is also a full agonist for rP2X1R but is less potent than ATP, whereas Ap5A, AP4A, Ap6G, and Ap5G act as partial agonists (Wildman et al., 1999a; Cinkilic et al., 2001). These diadenosine polyphosphates also activate hP2X1R at submicromolar concentrations (Bianchi et al., 1999). At first, it was believed that ADP also activates this channel at high concentrations, but subsequent studies have shown that purified ADP is unable to activate hP2X1R (Mahaut-Smith et al., 2000).
TABLE 1.
Pharmacological profile of P2X1R
EC50/IC50 values are micromolar unless otherwise specified.
(+), positive modulator; (−), negative modulator; PIPs, phosphoinositides; N.D. not determined.
P2X1R and P2X3R are distinct with regard to their activation by αβ-meATP at relatively low concentrations (Valera et al., 1994). This agonist can activate other P2XRs as well, but only at high concentrations (He et al., 2003c). In cells expressing recombinant human P2X1R, αβ-meATP is generally less potent than 2-meSATP and ATP (Valera et al., 1994; Evans et al., 1995; Torres et al., 1998; Bianchi et al., 1999). The defining pharmacological characteristic of P2X1R is the activation by βγ-meATP with a potency comparable with αβ-meATP. In contrast, βγ-meATP is approximately 30- to 50-fold less potent at P2X3R (Evans et al., 1995; Garcia-Guzman et al., 1997b).
TNP-ATP is a potent (IC50, 6 nM), but not selective, antagonist of hP2X1R. This compound also inhibits P2X3R (IC50,1 nM), as well as P2X2R, P2X4R, and P2X7R at micromolar concentrations (Virginio et al., 1998b). Native P2X1Rs expressed in rat mesenteric smooth muscle cells are also inhibited by TNP-ATP with an IC50 of 2 nM (Lewis et al., 1998). Ip5I is also a highly potent antagonist at recombinant rP2X1Rs (IC50 = 3 nM) (King et al., 1999). Both antagonists are subject to degradation by ectonucleotidases, which limits their in vivo use. This is not the case with PPADS, which inhibits rat and human P2X1Rs (Valera et al., 1994; Evans et al., 1995; Bianchi et al., 1999). The naphthylazo derivative of the PPADS family, PPNDS, inhibits rP2X1R at nanomolar concentrations, with an IC50 of 15 nM (Lambrecht et al., 2000b) but behaves as an agonist for the ATP receptor in Paramecium spp. (Wood and Hennessey, 2003). Two other PPADS derivatives, MRS2159 and MRS2220, are highly selective for ATP-induced responses in rP2X1R, and are ectonucleotidase resistant (Jacobson et al., 1998; Kim et al., 2001).
Suramin inhibits P2X1R (Valera et al., 1994; Evans et al., 1995), and suramin derivatives show great promise as selective antagonists of P2X1R. NF023 inhibits human and rat P2X1R with an IC50 value of ∼0.2 μM (Soto et al., 1999). NF279 is a highly potent rat and human P2X1R antagonist with IC50 values of 20 to 50 nM with preincubation before ATP application and 2 μM without preincubation. It also inhibits rP2X2R, hP2X7R, and rP2X3R, but with 40-, 60-, and 80-fold rightward shifts in the potency, respectively (Klapperstück et al., 2000; Rettinger et al., 2000). The more potent NF449 inhibits rP2X1R and hP2X1R with IC50 values of 0.03 and 0.5 nM (Braun et al., 2001; Hülsmann et al., 2003; Kassack et al., 2004). Both compounds have also been used to characterize the role of P2X1R in platelet activation (for review, see Hu and Hoylaerts, 2010). NF864 potently inhibits P2X1Rs expressed in platelets, as documented in a concentration-dependent study using calcium measurements (Horner et al., 2005). N-[(1R)-2-[[(1S,2R,3S)-1-(cyclohexylmethyl)-3-cyclopropyl-2,3-dihydroxypropyl]amino]-2-oxo-1-(4-thiazolylmethyl)ethyl]-1H-benzimidazole-2-carboxamide (Ro-0437626) is approximately 30-fold more selective for P2X1R over other purinergic receptor subtypes (Jaime-Figueroa et al., 2005).
2. Homomeric P2X2 Receptor and Heteromeric P2X1/2 Receptor.
The P2X2R was first cloned from rat pheochromocytoma PC12 cells (Brake et al., 1994). Subsequent localization studies showed broad tissue distribution of this receptor subtype. It is present in different central nervous system regions, including cortex, cerebellum, striatum, hippocampus, nucleus of the solitary tract, and the dorsal horn of the spinal cord (Kidd et al., 1995; Kanjhan et al., 1996; Vulchanova et al., 1996; Simon et al., 1997; Vulchanova et al., 1997; Pankratov et al., 1998; Kanjhan et al., 1999; Scheibler et al., 2004). It is also present in the hypothalamus (Xiang et al., 1998; Stojilkovic, 2009) and retina (Greenwood et al., 1997) as well as in the peripheral nervous system (Collo et al., 1996; Robertson et al., 1996; Simon et al., 1997; Vulchanova et al., 1997; Xiang et al., 1998; Zhong et al., 1998; Zhong et al., 2000, 2001; Calvert and Evans, 2004; Ma et al., 2004; Cockayne et al., 2005; Ma et al., 2005). These receptors are also expressed in non-neuronal cells, including pituitary cells (Stojilkovic and Koshimizu, 2001; Zemkova et al., 2006; Stojilkovic et al., 2010a), the adrenal medulla (Vulchanova et al., 1996), skeletal cells (Ryten et al., 2001; Jiang et al., 2005b), cardiac cells (Hansen et al., 1999a), smooth muscle cells (Lee et al., 2000), endothelial and epithelial cells (King et al., 1998; Hansen et al., 1999b; Birder et al., 2004), and lymphocytes (Di Virgilio et al., 2001).
P2X2R is unique among P2XRs, because multiple splice variants exist in humans, rats, mice, and guinea pigs and are able to generate homomeric and heteromeric channels with different functional properties. In the anterior pituitary, inner ear, and other brain regions, the primary P2X2 gene transcript undergoes extensive alternative splicing, resulting in modified mRNA sequences (Stojilkovic et al., 2000). The spliced subunit, P2X2b, lacks a series of 69 C-terminal amino acids and creates a functional homomeric channel that desensitizes more rapidly than the full-sized receptor, P2X2a (Brändle et al., 1997; Simon et al., 1997; Koshimizu et al., 1998b; Parker et al., 1998; Housley et al., 1999; Lynch et al., 1999). The electrostatic charges of six amino-acid side chains located near the proximal splicing site play a critical role in controlling the rate of receptor desensitization (Koshimizu et al., 1998a, 1999). In mouse pituitary cells, an additional functional splice form has been identified that lacks 90 amino acids in the C-terminal and is termed P2X2e (Koshimizu et al., 2006). This receptor has desensitization rates comparable with the rapidly desensitizing P2X1R and P2X3R (Fig. 2).
The pharmacology of P2X2R is listed in Table 2. ATP is a full agonist at this receptor, although the estimated EC50 value for this agonist at rP2X2Rs varies from lab to lab: 2 μM (Eickhorst et al., 2002), 3 μM (Zemkova et al., 2004), 5 μM (Li et al., 2004), 8 μM (Evans et al., 1995), 7 to 37 μM (Clyne et al., 2003), and 60 μM (Brake et al., 1994). Shorter forms of this receptor exhibit ATP sensitivities comparable with that of the full-sized receptor (Koshimizu et al., 1998b, 2006). Under the same experimental conditions, mP2X2R is less sensitive to ATP than rP2X2R (Eickhorst et al., 2002). hP2X2R seems to be the most sensitive to ATP, with comparable (∼1 μM) EC50 values for ATP, 2-meSATP, ATPγS, and BzATP (Lynch et al., 1999). The higher sensitivity of hP2X2R was also shown in current measurements performed under comparable experimental conditions (Tittle and Hume, 2008). ATP, 2-meSATP, ATPγS, and BzATP are roughly equipotent as agonists at the rP2X2R (Brake et al., 1994). A more detailed analysis revealed a somewhat lower potency of ATPγS and BzATP and smaller Emax values, suggesting that these compounds act as partial agonists (Evans et al., 1995). In all studies, ADP, αβ-meATP, and βγ-meATP had very weak effects on P2X2R activity. Ap4A is a full agonist for rP2X2R, and other members of this family of agonists are inactive (Wildman et al., 1999a). The splice forms of human, rat and mouse P2X2R show similar agonist sensitivities to the full-sized receptors (Koshimizu et al., 1998b, 2006; Lynch et al., 1999). Thus, none of the agonists is selective for this receptor subtype.
TABLE 2.
Pharmacological profile of P2X2R
EC50/IC50 values are micromolar unless otherwise specified.
(+), positive modulator; (−), negative modulator; DHEA, dehydroepiandrosterone; PIPs, phosphoinositides; N.D., not determined; VILIP1, visinin-like protein 1.
In addition, induces an increase in receptor desensitization/inactivation, also observed with Ba2+, Mn2+, and Mg2+.
Inhibition at human P2X2R (Tittle and Hume, 2008).
Biphasic effects, suggesting the existence of more than one allosteric site.
Through the oxidation of the intracellular Cys430.
Regulation of receptor desensitization by phosphatidylinositol 3-phosphate and phosphatidylinositol 3,5-bisphosphate.
For a long time, no P2X2R-specific antagonists were available. This has recently changed with the introduction of the anthraquinone derivatives sodium 1-amino-4-[3-(4,6-dichloro[1,3,5]triazine-2-ylamino)phenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate (PSB-10211) and disodium 1-amino-4-[3-(4,6-dichloro[1,3,5]triazine-2-ylamino)-4-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate (PSB-1011), which acts as P2X2R-selective antagonists in a nanomolar concentration range (Baqi et al., 2011). The P2X2R is also inhibited by suramin and PPADS at concentrations in the low micromolar range, showing less potency for these compounds than the homomeric P2X1R and P2X3R (Brake et al., 1994; Evans et al., 1995). Other compounds, such as TNP-ATP, NF279, and RB-2, are potent but nonspecific antagonists for this channel. TNT-ATP inhibits this receptor with an EC50 value of 2 μM, approximately 100-fold less potently than P2X1R and P2X3R (Virginio et al., 1998b). NF279 acts as a competitive antagonist at rP2X2R with an IC50 value of 0.76 μM (Rettinger et al., 2000), whereas NF023 shows low sensitivity at this receptor (IC50 > 50 μM) (Soto et al., 1999). The suramin derivates 7,7′-(carbonylbis(imino-3,1-phenylenecarbonylimino-3,1-(4-methyl-phenylene)carbonylimino))bis(1-methoxy-naphthalene-3,6-disulfonic acid) tetrasodium salt (NF770), 6,6′-(carbonylbis(imino-3,1-(4-methylphenylene)carbonylimino))bis(1-methoxynaphthalene-3,5-disulfonic acid) tetrasodium salt (NF776), and 6,6′-(carbonylbis(imino-3,1-phenylenecarbonylimino-3,1-(4-methyl-phenylene)carbonylimino))bis(1-methoxy-naphthalene-3,5-disulfonic acid) tetrasodium salt (NF778) have been shown to act as nanomolar P2X2R antagonists (Wolf et al., 2011). RB-2 inhibits P2X2R with an IC50 value of 0.4 μM (Liu et al., 2001a). When tested in the same experiment, RB-2 was more potent than TNP, which was more potent than suramin (Liu et al., 2001a). Native rP2X2Rs expressed in pituitary gonadotrophs are also inhibited by RB-2 (Zemkova et al., 2006).
P2X1 and P2X2 subunits can generate heteromeric receptors expressed at the plasma membrane (Aschrafi et al., 2004). Their biophysical and pharmacological profiles have been incompletely characterized. The profile of heteromers seems to resemble the current profile generated by homomeric P2X1R; however, the heteromers exhibit an acid sensitivity different from that of both P2X1R and P2X2R (Brown et al., 2002).
3. Homomeric P2X3 Receptor and Heteromeric P2X2/3 Receptor.
The gene encoding the P2X3R subunit was originally cloned from dorsal root ganglion (DRG) sensory neurons (Chen et al., 1995; Lewis et al., 1995). These neurons also express mRNA transcripts for the P2X2 subunit, and the native channels are probably P2X2/3R heteromers (Lewis et al., 1995). The distribution of homomeric rP2X3R and heteromeric P2X2/3R is highly restricted, occurring in the dorsal root, trigeminal, and nodus sensory ganglia (Vulchanova et al., 1997; Bradbury et al., 1998; Dunn et al., 2001). The receptor expressed in trigeminal sensory neurons is up-regulated by calcitonin gene-related peptide (Fabbretti et al., 2006). The hP2X3R was cloned from heart tissue, but transcripts for this receptor are also present in the spinal cord (Garcia-Guzman et al., 1997b).
Homomeric and heteromeric P2X3Rs play an important role in nociceptive transmission and mechanosensory transduction within visceral organs (Galligan, 2004; Ford et al., 2006). Pharmacological studies have shown that peripheral and spinal P2X3Rs and P2X2/3Rs are involved in transmitting persistent, chronic inflammatory, and neuropathic pain signals (Gever et al., 2006). Studies of P2X3R gene knockouts and transient gene disruption by antisense P2X3R have revealed similar findings (Cockayne et al., 2000; Souslova et al., 2000). The P2X2(−/−), P2X3(−/−), P2X2/P2X3Dbl(−/−) knockout mice were also used to investigate for the role of ATP signaling on the function of oxygen-sensitive chemoreceptor cells in the carotid body. These studies showed that a deficiency of the P2X2 subunit, but not P2X3, resulted in an attenuated ventilatory response to hypoxia and an impaired response of the afferent carotid sinus nerve to a oxygen decrease (Rong et al., 2003). Later, ATP was identified as the neurotransmitter linking taste buds to the gustatory nerves. This response is mediated through a combination of P2X2 and P2X3 subunits, because deleting either subunit alone did not result in the profound deficit in taste-mediated behaviors seen in the double deletion P2X2/P2X3Dbl(−/−) (Finger et al., 2005; Eddy et al., 2009). In other studies with knockout mice, heteromeric P2X2/3 receptors were shown to contribute to nociceptive responses and mechanosensory transduction within the urinary bladder (Cockayne et al., 2005), and P2X2 subunits were found to be important for fast synaptic excitation in myenteric neurons of the mouse small intestine (Ren et al., 2003).
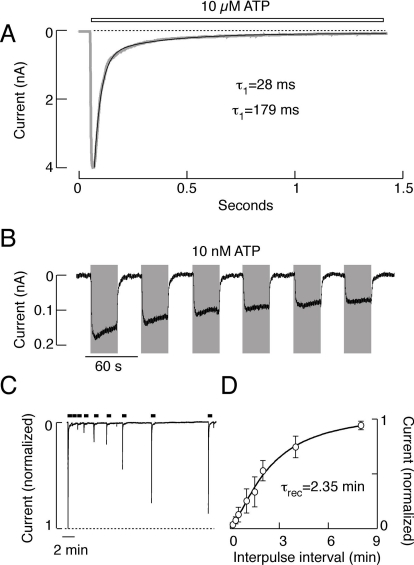
P2X3Rs generate fast, rapidly activating, and acutely desensitizing inward currents. In contrast to rP2X1R currents, rP2X3R currents do not desensitize completely during sustained agonist application (Fig. 3A). The rate of receptor desensitization is determined by the agonist concentration (Fig. 3, A and B), and at low agonist concentrations, the deactivation kinetics of the receptor can be analyzed (Fig. 3B). These experiments clearly show that the deactivation of rP2X3R is relatively rapid, occurring on a time scale of seconds (Zemkova et al., 2004), and does not determine the recovery kinetics of the receptor, which occur on a time scale of minutes (Fig. 3, C and D). Further studies revealed the presence of two types of receptor desensitization (Sokolova et al., 2006). As with rP2X4R (Yan et al., 2006), the fluorescently labeled rP2X3 subunits form fully functional channels that have biophysical and pharmacological properties highly comparable with those of the wild-type receptor (Grote et al., 2005).
Fig. 3.
Characterization of rP2X3R. A, the rates of receptor desensitization. Notice the presence of the residual current during sustained agonist application. B, patterns of P2X3R current responses during repetitive stimulation with 10 nM ATP. C, time course of recovery from desensitization.
Table 3 summarizes the pharmacological profile of homomeric P2X3Rs. ATP is a full agonist for rP2X3R with estimated EC50 values of 1.2 μM (Chen et al., 1995), 2.6 μM (Pratt et al., 2005), 4.1 μM (Asatryan et al., 2008), and 7.3 μM (Grote et al., 2005), but the precision of these estimates is limited by profound desensitization (Khmyz et al., 2008). Similar to the rP2X1R, αβ-meATP is a full agonist at rP2X3R, acting with a potency similar to (Pratt et al., 2005; Asatryan et al., 2008) or slightly lower than (Chen et al., 1995) that of ATP. 2-meSATP is also a full agonist for this receptor, whereas ATPγS acts as a partial agonist (Lewis et al., 1995; Liu et al., 2001a). Native (Jarvis et al., 2001) and recombinant (He et al., 2002) rP2X3Rs are also activated by BzATP. Native receptors present in neurons of the rat DRG show a similar agonistic profile (Rae et al., 1998). Ap4A, Ap5A, and Ap6A are also full agonists for rP2X3R with estimated EC50 values of 0.8, 1.3, and 1.6 μM, respectively (Wildman et al., 1999a). Ap5G and Ap6G also activate rP2X3R (Cinkilic et al., 2001). At hP2X3Rs, 2-meSATP is the most potent agonist (320 nM), followed by ATP (780 nM), αβ-meATP (2.55 μM), CTP (17.9 μM), and βγ-meATP (>100 μM) (Garcia-Guzman et al., 1997b).
TABLE 3.
Pharmacological Profile of P2X3R
EC50/IC50 values are micromolar unless otherwise specified.
| Compound | Method | EC50/IC50 | References |
|---|---|---|---|
| Full agonists | |||
| ATP | Current | 1 | Lewis et al., 1995; Chen et al., 1995 |
| αβ-meATP | Current | 1–2 | Lewis et al., 1995; Chen et al., 1995 |
| 2-meSATP | Current | 0.3 | Lewis et al., 1995; Chen et al., 1995; Garcia-Guzman et al., 1997 |
| Ap6A | Current | 1.5 | Wildman et al., 1999a |
| Ap5A | Current | 1 | Wildman et al., 1999a |
| Ap4A | Current | 1 | Wildman et al., 1999a |
| Partial agonists | |||
| ATPγS | Current | 10 | Liu et al., 2001 |
| BzATP | Calcium | N.D. | Jarvis et al., 2001; He et al., 2002 |
| βγ-meATP | Current | >300 | Chen et al., 1995 |
| Antagonists | |||
| Suramin | Current | 3 | Lewis et al., 1995 |
| PPADS | Current | 1.5 | Lewis et al., 1995 |
| TNP-ATP | Current | 1 nM | Virginio et al., 1998b |
| A-317491 | Current/Ca2+ | 20 nM | Jarvis et al., 2002 |
| NF023 | Current | 8.5 | Soto et al., 1999 |
| NF279 | Current | 2 | Rettinger et al., 2000 |
| NF449 | Current | 3 | Braun et al., 2001 |
| RO-85 | Calcium | 30 nM | Brotherton-Pleiss et al., 2010 |
| Ip4I | Current | 1 | King et al., 1999 |
| MRS2159 | Current | 150 nM | Kim et al., 2001 |
| MRS2257 | Current | 30 nM | Kim et al., 2001 |
| AF-353 | Current/Ca2+ | 10 nM | Gever et al., 2010 |
| RO-4 | Calcium | 13 nM | Carter et al., 2009 |
| RO-51 | Calcium | 10 nM | Jahangir et al., 2009 |
| Modulators | |||
| Protons (−) | Current | pKa 6.0 | Stoop et al., 1997; Gerevich et al., 2007 |
| Calcium (−) | Current | 90 mM | Virginio et al., 1998a |
| Zinc (+) a | Current | 10 | Wildman et al., 1999b |
| Cadmium (−) | Current | 100 | Nakazawa and Ohno, 1997 |
| Ethanol (+) | Current | 25 mM | Davies et al., 2005a |
| Cibracon Blue (+) | Current/Ca2+ | 1.5 | Alexander et al., 1999 |
| Toluene (−) | Current | 3 mM | Woodward et al., 2004 |
| Tetramethylpyrazine | Current | 1 mM | Gao et al., 2008 |
| Cdk-5 (−) | Current | Nair et al., 2010 | |
| Csk (−) | Current | D'Arco et al., 2009 |
N.D., not determined; (+), positive modulator; (−), negative modulator; Cdk-5, cyclin-dependent kinase 5; Src, C-terminal Src kinase.
Biphasic effects, suggesting the existence of more than one allosteric site.
Suramin and PPADS inhibit P2X3R (Lewis et al., 1995), but these antagonists have relatively low potencies for this receptor compared with other P2XRs (Jacobson et al., 2006). The suramin derivates NF023, NF279, and NF449 also antagonize P2X3R-mediated currents. P2X3Rs have an intermediate sensitivity to NF023, with IC50 values of 8 and 29 μM for rat and human subtypes, respectively (Soto et al., 1999). NF279 inhibits rP2X3R with IC50 values of 1.6 μM with preincubation and 85 μM when applied together with agonist (Rettinger et al., 2000). NF449 also inhibits rP2X3R with an IC50 value of approximately 3 μM, in contrast to the subnanomolar concentrations needed to inhibit P2X1R, indicating that it could be used to distinguish between these biophysically comparable channels in native tissues (Braun et al., 2001; Kassack et al., 2004; Horner et al., 2005). Several PPADS derivatives also inhibit P2X3R currents (Brown et al., 2001; Kim et al., 2001).
Because of the involvement of P2X3R in pain pathways, there have been numerous efforts to develop P2X3R-selective drugs. TNP-ATP strongly inhibits rP2X3R currents with an IC50 value of around 1 nM, and the effect of this compound was mimicked by TNP-ADP and TNP-AMP. TNP-ATP is approximately 1000 times less effective in cells expressing P2X2R, P2X4R, and P2X7R, indicating that it could be effectively used to inhibit P2X1R, P2X3R, and P2X2/3R-mediated responses (Virginio et al., 1998b). However, this compound is of limited use for in vivo experiments because of its rapid degradation by ectonucleotidases (Lewis et al., 1998). A compound named A317491 (5-[[[(3-phenoxyphenyl)methyl][(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbonyl]-1,2,4-benzenetricarboxylic acid sodium salt hydrate) seems to satisfy this requirement. The dissociation equilibrium constant for this compound is in the range of 10 to 100 nM. The compound also inhibits heteromeric P2X2/3Rs but is ineffective at a wide range of other receptors and channels; the R enantiomer, A317344, has no activity (Jarvis et al., 2002; North, 2004).
The heteromeric P2X2/3Rs exhibit pharmacological properties similar to those of P2X3R, including sensitivity to αβ-meATP and a similar rank order of agonist potencies, but they can be distinguished from homomeric P2X3Rs by the slow desensitization rate (Koshimizu et al., 2002). Selective agonists for P2X2/3R are Ap5A > αβ-meATP ≫ βγ-ATP > UTP (Liu et al., 2001a). ATPγS and 2-meSATP also activate these heteromers (Lewis et al., 1995). The probable composition of this trimeric channel is P2X2(P2X3)2 (Jiang et al., 2003), which probably explains why the heteromeric receptor is more tolerant of the αβ and βγ substitution (Spelta et al., 2003). The sensitivity of P2X2/3R to αβ-meATP provides an easy way to identify these channels, because this agonist does not activate P2X2Rs at low micromolar concentrations. In addition, P2X2/3Rs desensitize slowly in contrast to the rapid desensitization of P2X3R.
Selective antagonists for P2X2/3R are TNP-ATP ≫ suramin > RB-2 (Liu et al., 2001a). All inhibitors of P2X3R also inhibit P2X2/3R but are poor inhibitors of P2X2R. In most cases, the inhibition of P2X2/3 heteromers is slightly less effective than the inhibition of P2X3R homomers. rP2X2/3Rs have sensitivity to NF023 similar to that of homomeric P2X3Rs (Soto et al., 1999). In contrast to the homomeric rP2X2R, the activity of this heteromer is inhibited by TNP-ATP with an IC50 value of 7 nM (Virginio et al., 1998b). Concentrations of NF449 that are 3 to 4 orders of magnitude higher are required to block heteromeric P2X2/3Rs compared with homomeric and heteromeric P2X1Rs (Rettinger et al., 2005). However, the αβ-meATP-induced currents and calcium signals through recombinant and native P2X2/3Rs are slowly desensitizing, in contrast to homomeric P2X3Rs (Lewis et al., 1995; Burgard et al., 1999; Liu et al., 2001a; Koshimizu et al., 2002).
Several new inhibitors have recently been introduced. Spinorphin, an endogenous antinociceptive peptide (LVVYPWT), seems to be a potent and noncompetitive antagonist at hP2X3Rs (IC50 = 8.3 pM). The antagonistic properties are sustained when single alanine substitutions were made from the 1st to 4th amino acids and when the cyclic form of LVVYPWT was introduced (Jung et al., 2007). Two diaminopyrimidines, 5-[5-iodo-4-methoxy-2-(1-methylethyl)phenoxy]-2,4-pyrimidine diamine hydrochloride [RO-4, also known as AF-353 (Carter et al., 2009)] and 5-(5-ethynyl-2-isopropy-4-methoxy-phenoxy)-pyrimidine-2,4-diamine [RO-5, also known as AF-729) (Jahangir et al., 2009)], inhibit rP2X3Rs and hP2X2/3Rs at nanomolar concentrations. RO-5 also inhibits native presynaptic P2X3Rs and P2X2/3Rs (Kaan et al., 2010). 1-Methyl-3-phenyl-1H-thieno[2,3-c]pyrazole-5-carboxylic acid [(R)-2-(4-acetyl-piperazin-1-yl)-1-methyl-ethyl]-amide (RO-85) has demonstrated selectivity for P2X3Rs (IC50 = 30 nM) over heteromeric P2X2/3Rs (IC50 = 400 nM) and other P2XRs (IC50 > 10 μM), indicating the pharmacological possibility to distinguish between homomeric and heteromeric P2X3Rs (Brotherton-Pleiss et al., 2010). Finally, AF-353 inhibits hP2X3R (IC50, 10 nM), rP2X3R (IC50, 10 nM), and hP2X2/3R (IC50, 38 nM) at low nanomolar concentrations (Gever et al., 2010).
4. Homomeric P2X4 Receptor and Heteromeric P2X1/4 Receptor.
This receptor was identified as a distinct member of the P2XR family when it was shown that a single gene product was sufficient to generate to ion channels with distinct patterns and pharmacological properties (Bo et al., 1995; Buell et al., 1996; Soto et al., 1996). Homomeric rP2X4Rs bathed in physiological solutions activated rapidly, desensitized at a moderate rate, and displayed inwardly rectifying current-voltage relationships that reversed at 0 mV (Khakh et al., 1999a; Fountain and North, 2006). Figure 1 shows a typical pattern of ATP-induced current (A) and the dependence of receptor activation, desensitization, and deactivation kinetics on agonist concentration (B).
P2X4Rs are widely expressed in the brain, spinal cord, and autonomic and sensory ganglia (Rubio and Soto, 2001; Burnstock and Knight, 2004; Burnstock, 2007; Surprenant and North, 2009). They are also expressed in the anterior pituitary gland, specifically in lactotrophs, and their activation leads to the stimulation of electrical activity, promotion of voltage-gated and voltage-insensitive Ca2+ influx and prolactin release (He et al., 2003a; Zemkova et al., 2010). Recent studies have also shown that P2X4Rs are expressed in microglia and alveolar macrophages (Bowler et al., 2003) and that the up-regulation of these receptors in activated microglia located in the dorsal horn of the spinal cord contributes to neuropathic pain (Tsuda et al., 2003; Ulmann et al., 2008). Immortalized C8-B4 cells derived from cerebellar microglia also express P2X4Rs and were used to show that antidepressants indirectly inhibit the receptor-mediated responses by interfering with lysosomal trafficking (Toulme et al., 2010). In addition, P2X4Rs are present in human lung mast cells (Wareham et al., 2009) and PC12 cells (Sun et al., 2007). The P2X4R knockout mice have high blood pressure, probably reflecting the role of these channels in the regulation of the vascular tone on endothelial cells (Yamamoto et al., 2006) and show reduced amplitudes in long-term potentiation in the hippocampus (Sim et al., 2006). Experiments with the P2X4R-deficient mouse line also revealed the potential involvement of this receptor in ATP-mediated brain-derived neurotrophic factor microglial secretion and neuropathic pain (Ulmann et al., 2008). The native P2X4R and P2X7R currents are seen in recruited peritoneal macrophages of wild-type mice, and P2X4R inactivation in a mouse line eliminated the P2X4-like current, suggesting an immunologic role for this receptor (Brône et al., 2007).
Green fluorescent protein-tagged receptors were used to show that P2X4Rs, but not P2X2Rs, undergo rapid constitutive internalization and subsequent reinsertion into the plasma membrane in a dynamin-dependent manner. Internalization of P2X4/6 heterodimers was also observed, suggesting that one or two P2X4 subunits are sufficient to govern the trafficking properties of the receptor (Bobanovic et al., 2002). The C-terminal YXXGL motif serves as a noncanonical tyrosine-based sorting signal that is necessary for efficient endocytosis of this receptor (Royle et al., 2002). P2X4R rundown is evident after repetitive stimulation in a whole-cell configuration. This effect is completely prevented with the use of a perforated patch, indicating that small cytosolic factors that are lost during intracellular dialysis could be important in the trafficking of this receptor (Fountain and North, 2006). Endogenous P2X4Rs in cultured rat microglia, vascular endothelial cells, and freshly prepared peritoneal macrophages are localized predominantly to lysosomes, where the receptors can retain their function and subsequently travel out to the plasma membrane (Qureshi et al., 2007). Unstimulated macrophages express very low levels of functional P2X4Rs, but expression of these receptors at the plasma membrane was enhanced by the activation of phagocytosis (Stokes and Surprenant, 2009).
The pharmacological profile of P2X4R is shown in Table 4. This receptor is activated by ATP with estimated EC50 values of 3 μM (Yan et al., 2005; Yan et al., 2006), 4 to 5 μM (Zemkova et al., 2007; Jelínkova et al., 2008; Jindrichova et al., 2009), 7 μM (Soto et al., 1996), and 10 μM (Bo et al., 1995; Buell et al., 1996). Under similar experimental conditions, the potency of ATP at the mouse, rat, and human P2X4R was comparable, ranging between 1 and 8 μM (Garcia-Guzman et al., 1997a; Bianchi et al., 1999; Jones et al., 2000). 2-meSATP also activates rP2X4Rs with a potency similar to or lower than ATP (Buell et al., 1996; Soto et al., 1996). αβ-meATP and Ap4P are partial agonists for mouse, rat, and human receptors (Jones et al., 2000). The rP2X4R is activated by nucleotide analogs with the following order of efficacy: ATP > ATPγS > 2-meSATP > CTP > αβ-meATP. In calcium measurements, the potency order was ATP > BzATP > αβ-meATP (He et al., 2003c). The human receptor displays an agonist potency profile similar to that of rP2X4R (Garcia-Guzman et al., 1997a; Jones et al., 2000). Ap4A acts as a partial agonist for mouse, rat, and human P2X4Rs, whereas other members from this family are inactive (Wildman et al., 1999a; Jones et al., 2000). Experiments that measure the displacement of [35S]ATPγS from rP2X4Rs also indicated that ATP is the most potent agonist, followed by ATPγS, 2-meSATP, and αβ-meATP (Michel et al., 1997). A methanocarba derivative of AMP, 1′S,2R,3S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-1-[phosphoryloxymethyl] bicycle[3.1.0]hexane-2,3-diol) (MRS2339), has been found to act as a potent agonist of heart purinergic receptors; the authors suggested that this compound could be a P2X4R agonist (Zhou et al., 2010).
TABLE 4.
Pharmacological profile of P2X4R
EC50/IC50 values are micromolar unless otherwise specified.
(+), positive modulator; (−), negative modulator; N.D., not determined; ALP, allopregnanolone; THDOC, 3α,21-dihydroxy-5α-pregnan-20-one; PIPs, phosphoinositides; 5-BDBD, 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one.
In the absence of calcium, a secondary current is developed in P2X4Rs.
Biphasic effects, suggesting the existence of more than one allosteric site.
Mouse and rat P2X4Rs are insensitive to suramin. The rP2X4R is also insensitive to PPADS, whereas the mouse and human orthologs are inhibited by this compound (Garcia-Guzman et al., 1997a; Jones et al., 2000). TNT-ATP antagonizes the ATP-mediated currents with an IC50 of 15 μM (Virginio et al., 1998b). Rat and human P2X4Rs are not sensitive to NF023 in concentrations up to 100 μM (Soto et al., 1999), and BBG is a weak receptor antagonist (Jiang et al., 2000a). 5-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one has been suggested to act as a specific P2X4R inhibitor with an IC50 of 0.5 μM (Donnelly-Roberts et al., 2008). RB-2 and Coomassie blue are the most potent antagonists of this channel, producing an inhibition of [35S]ATPγS binding (Michel et al., 1997). Recent studies have also suggested the potential role of some serotoninergic antidepressants as P2X4R antagonists (Nagata et al., 2009; Sim and North, 2010). Paroxetine inhibits P2X4R-mediated currents and calcium increases with an IC50 of 2.5 and 1.9 μM at the rat and human receptor, respectively (Nagata et al., 2009). In contrast, amitriptyline modestly and noncompetitively inhibits rat and mouse P2X4Rs and has no effect on hP2X4Rs. However, this modest inhibition was not observed in P2X2R or P2X7R (Sim and North, 2010), suggesting an interesting possibility for the development of specific P2X4R antagonists.
Heteromeric assembly of P2X1 and P2X4 subunits results in a functional channel with kinetic properties resembling homomeric P2X4Rs and a pharmacological profile similar to homomeric P2X1R. Specifically, heteromers activated, desensitized, and deactivated slower than P2X1Rs and at rates comparable with those observed in cells expressing P2X4R. On the other hand, a leftward shift in the sensitivity of heteromers to αβ-meATP was observed compared with P2X4R. Furthermore, suramin and TNP-ATP blocked both P2X1R and P2X1/4R currents but not P2X4R currents (Nicke et al., 2005). It is currently unclear whether the native channels assemble as heteromers.
5. Homomeric P2X5 Receptor and Heteromeric P2X1/5 Receptor.
P2X5R was first cloned from rat celiac ganglia (Collo et al., 1996). The expression of P2X5R mRNA and protein transcripts is restricted to the trigeminal mesencephalic nucleus of the brainstem, sensory neurons, cervical spinal cord, and some blood vessels. A limited amount of the receptor was also detected in the heart, skeletal muscle, kidney, adrenal gland, and retina (Collo et al., 1996; Garcia-Guzman et al., 1996; Brändle et al., 1998; Phillips et al., 1998; Gröschel-Stewart et al., 1999; Phillips and Hill, 1999; Taylor et al., 1999; Gitterman and Evans, 2000; Ryten et al., 2001). The receptor was also detected in carcinomas of the skin and prostate (Greig et al., 2003; Calvert et al., 2004). In humans, the expression and function of this receptor is still unclear. The mRNA transcripts have been detected predominantly in tissues related to the immune system (Lê et al., 1997). The recombinant rat and zP2X5R generates a low-amplitude nondesensitizing current, whereas P2X5Rs from other species respond to ATP with large, rapidly activating and slowly desensitizing currents. The recovery from desensitization is also slow (Collo et al., 1996; Garcia-Guzman et al., 1996; Bo et al., 2000; Jensik et al., 2001; Diaz-Hernandez et al., 2002; Wildman et al., 2002; Bo et al., 2003). Like P2X7Rs (see section III.A.7), the pore of human, chick, and bullfrog P2X5Rs dilates during prolonged receptor activation (Bo et al., 2000, 2003; Jensik et al., 2001).
ATP is a full agonist for this receptor (Table 5) with estimated EC50 values of 0.4 μM (Wildman et al., 2002) and 8 μM (Garcia-Guzman et al., 1996) for rP2X5R, 0.3 μM (Kotnis et al., 2010) and 4 μM (Bo et al., 2003) for hP2X5R, and 2 μM for chicken P2X5R (Ruppelt et al., 2001). The potency order at the rP2X5R is ATP = 2-MeSATP = ATPγS > αβ-meATP = BzATP, with αβ-meATP and BzATP acting as partial agonists. Ap2A, Ap4A, Ap5A, and Ap6A are also partial agonists for this receptor (Wildman et al., 2002). At hP2X5R, concentration-response curves for ATP, BzATP, and αβ-meATP yield EC50 values of approximately 4, 6, and 161 μM, and BzATP, αβ-meATP, and 2-meSATP are partial agonists (Bo et al., 2003). Others observed a higher potency for αβ-meATP at hP2X5R with an EC50 value of approximately 12 μM (Kotnis et al., 2010). At the rP2X5R, the potency order for five antagonists was PPADS > TNP-ATP > suramin > RB-2 ≫ Ip5I (Wildman et al., 2002). At hP2X5R, PPADS, BBG, and suramin inhibited the ATP-evoked currents with IC50 values of 0.2, 0.5, and 2.9 μM, respectively (Bo et al., 2003). TNP-ATP and suramin also inhibited the ATP-evoked currents at hP2X5R (Kotnis et al., 2010).
TABLE 5.
Pharmacological profile of P2X5R
EC50/IC50 values are micromolar unless otherwise specified.
| Compound | Method | EC50/IC50 | References |
|---|---|---|---|
| Full agonists | |||
| ATP | Current | 0.5–4 | Wildman et al., 2002; Bo et al., 2003 |
| 2-meSATP | Current | 0.5 | Wildman et al., 2002 |
| ATPγS | Current | 0.5 | Wildman et al., 2002 |
| Partial agonists | |||
| αβ-meATP | Current | 1–12 | Wildman et al., 2002; Kotnis et al., 2010 |
| βγ-meATP | Current | 10 | Wildman et al., 2002 |
| BzATP | Current | 1–6 | Wildman et al., 2002; Bo et al., 2003 |
| Ap3A | Current | 5 | Wildman et al., 2002 |
| Ap4A | Current | 0.3 | Wildman et al., 2002 |
| Ap5A | Current | 0.7 | Wildman et al., 2002 |
| Ap6A | Current | 5 | Wildman et al., 2002 |
| Antagonists | |||
| Suramin | Current | 2–3 | Wildman et al., 2002; Bo et al., 2003 |
| PPADS | Current | 200–600 nM | Wildman et al., 2002; Bo et al., 2003; Kotnis et al., 2010 |
| TNP-ATP | Current | 600–700 nM | Wildman et al., 2002; Kotnis et al., 2010 |
| BBG | Current | 0.5 | Bo et al., 2003 |
| Modulators | |||
| Protons (−) | Current | Wildman et al., 2002 | |
| Calcium (−)a | Current | 10 mM | Wildman et al., 2002 |
| Zinc (+)b | Current | 10 | Wildman et al., 2002 |
(+), positive modulator; (−), negative modulator.
In addition, a sensitization of ATP-evoked currents is observed with calcium.
Biphasic effects, suggesting the existence of more than one allosteric site.
Many of the tissues expressing P2X5R also express other isoforms, including the P2X1 subunit, which raised the possibility of heteromeric expression. Consistent with this, several laboratories have found expression of heteromeric P2X1/5Rs (Torres et al., 1998; Haines et al., 1999; Lê et al., 1999; Surprenant et al., 2000). From a biophysical standpoint, this heteromer more closely resembles the P2X5R, because it generates a nondesensitizing plateau in current. Like P2X1R, however, the heteromers are activated by αβ-meATP (Torres et al., 1998; Lê et al., 1999). ATP activates these heteromers with an EC50 value of 0.7 μM (Haines et al., 1999). 2-meSATP is also the full and equipotent agonist for heteromeric P2X1/5R, whereas ATPγS and αβ-meATP are partial agonists. Suramin and PPADS are equipotent at P2X1R and P2X1/5R, but the heteromer is less sensitive to TNP-ATP (Haines et al., 1999). NF449 also potently inhibits rP2X1/5R with an IC50 value of 0.7 nM (Rettinger et al., 2005). The native channel in guinea pig submucosal arterioles is biophysically and pharmacologically similar to the recombinant P2X1/5R (Surprenant et al., 2000). Mouse cortical astrocytes express mRNAs for P2X1R and P2X5R subunits, and the high sensitivity to ATP, biphasic kinetics, and inhibition by PPADS were also comparable with the responses seen in recombinant P2X1/5Rs (Lalo et al., 2008).
6. Homomeric P2X6 Receptor and Heteromeric P2X2/6 and P2X4/6 Receptors.
The expression and immunoreactivity of P2X6R mRNA are seen throughout the central nervous system, including Purkinje cells in the cerebellum and pyramidal cells in the hippocampus (Collo et al., 1996; Rubio and Soto, 2001; Burnstock and Knight, 2004). Expression of this receptor has also been reported in sensory ganglia (Xiang et al., 1998), skeletal muscle (Meyer et al., 1999), uterus and granulose cells of the ovary (Collo et al., 1996), thymus (Glass et al., 2000), and the human salivary gland (Worthington et al., 1999). Up-regulation of this receptor has been detected in human heart tissue from patients with congestive heart failure (Banfi et al., 2005). The P2X6R subunit expressed alone generates functional membrane receptors very inefficiently (Collo et al., 1996; Lê et al., 1998; King et al., 2000; Koshimizu et al., 2000b; Jones et al., 2004).
This receptor seems to have higher sensitivity for ATP and 2-meSATP and is inhibited by TNP-ATP and PPADS (Jones et al., 2004). The current response was described as nondesensitizing and may be either sensitive (Jones et al., 2004) or insensitive (Collo et al., 1996) to αβ-meATP. This could reflect differences in heteromerization with endogenous P2XR subunits (Jones et al., 2004). At first, it was believed that the low expression in the plasma membrane was due primarily to a failure to form the proper homomers (Aschrafi et al., 2004). It has also been suggested that the protein could be expressed in the plasma membrane in a partially glycosylated state and that further glycosylation would yield a functional channel (Jones et al., 2004). Later studies indicated that an uncharged region of the P2X6R N terminus is responsible for receptor retention at the endoplasmic reticulum. When the charges were introduced by mutagenesis, the mutant receptors were able to form homotrimers and to glycosylate and were delivered to the membrane (Ormond et al., 2006).
Subsequent studies confirmed that rat P2X2 and P2X6 subunits form functional heteromers that have a somewhat different phenotype from homomeric P2XRs. This includes a reduction in ATP potency, a significant loss of Ap4A activity, and a distinct pattern of pH regulation. The rank order of agonist activation for these heteromers is highly comparable with that observed for P2X2Rs: ATP = ATPγS = 2-meSATP ≫ BzATP = αβ-meATP. The heteromer is also sensitive to inhibition by suramin, similar to the homomeric P2X2R (King et al., 2000). Both (P2X2)2P2X6 and P2X2(P2X6)2 compositions of heteromeric channels have been detected (Barrera et al., 2007). The rat P2X6 subunit also forms functional heteromers with the P2X4 subunit that are pharmacologically similar to P2X4R. These heteromers exhibit sensitivity to ATP and 2-meSATP similar to that of homomeric P2X4Rs, are activated by low micromolar αβ-meATP concentrations, and are blocked by suramin and RB-2 (Lê et al., 1998). Allosteric regulation of this heteromer resembles the regulation of P2X4Rs.
7. Homomeric P2X7 Receptor.
The P2X7R subunit, initially cloned from a rat brain cDNA library, shares an overall membrane topology with the other members of this family of receptors (Surprenant et al., 1996). The receptor is distinguished structurally from other members of P2XRs by its long intracellular C terminus tail containing multiple protein and lipid interaction motifs and a cysteine-rich 18 amino acid segment. These receptors are expressed on cells of the immune system, such as macrophages/monocytes, dendritic cells, lymphocytes, and mast cells, as well as in glia cells, including microglia, astrocytes, oligodendrocytes, and Schwann cells (Collo et al., 1997; Rassendren et al., 1997b; Di Virgilio et al., 2001, 2009; Franke et al., 2001; Chessell et al., 2005; Skaper et al., 2010). Epithelial cells, fibroblasts, osteoblasts, pituitary cells, and some neuronal populations also express P2X7Rs (Gröschel-Stewart et al., 1999; Koshimizu et al., 2000a; Deuchars et al., 2001; Gartland et al., 2001; Sim et al., 2004).
Two different genetically engineered P2X7-deficient mice from Pfizer (New York, NY) and GlaxoSmithKline (Brentford, Middlesex, UK) (Solle et al., 2001; Chessell et al., 2005) were used to study P2X7R physiology. However, a “P2X7-like” receptor that is similar, but not identical to, “native” P2X7Rs was found in the Pfizer knockout mice (Sánchez-Nogueiro et al., 2005; Marín-García et al., 2008), and a novel functional P2X7 splice variant with an alternative exon 1 and translation start was found in the GlaxoSmithKline knockout mice (Nicke et al., 2009). The Pfizer P2X7R knockout mice established the role of P2X7R in ATP-induced interleukin-1 post-translational processing, the inflammatory response (Solle et al., 2001; Labasi et al., 2002) and the regulation of bone formation and absorption (Ke et al., 2003). In the GlaxoSmithKline P2X7 knockout mice, inflammatory (in an adjuvant induced model) and neuropathic (in a partial nerve ligation model) hypersensitivity was abolished (Chessell et al., 2005). These findings and multiple subsequent studies became the basis for synthesizing and developing new P2X7 antagonists into therapeutic drugs to treat inflammatory and neuropathic pain (Perez-Medrano et al., 2009). Clinical studies combined with experiments using P2X7 knockout mice also highlighted the relevance of ATP-P2X7 cryopyrin/NALP3 caspase-1 inflammasome activation to the therapeutic efficacy for anthracycline-treated breast cancer patients (Ghiringhelli et al., 2009; Lamkanfi and Dixit, 2009).
Although numerous studies have been performed with the recombinant P2X7R since it was cloned, the gating properties of this receptor are not well understood. P2X7R operates as a nonselective cationic channel during initial agonist application, but with prolonged application, the receptor also provides a permeation pathway for molecules with molecular masses up to ∼800 Da, including the fluorescent dye YO-PRO-1, a process known as cell “permeabilization.” At first, it was suggested that the bifunctional permeation properties of P2X7R reflect a dilation of the integral pore of the channel (Surprenant et al., 1996; Chessell et al., 1997; Michel et al., 1999; Gudipaty et al., 2001). It was further suggested that a progressive dilation of the ion-conducting pathway during prolonged agonist application is not unique to the P2X7R, but also occurs in cells expressing P2X2R and P2X4R (Khakh et al., 1999a; Virginio et al., 1999).
However, this hypothesis has been questioned. One group noticed the uptake of fluorescent dyes was not affected in cells expressing P2X7R in which the receptor-specific C-terminal 18 amino acid sequence was deleted, although the mutant was not permeable to N-methyl-d-glucamine, suggesting that YO-PRO-1 entry occurs through a pathway separate from that of N-methyl-d-glucamine (Jiang et al., 2005a). Others observed that cells expressing a mutant P2X7R that was truncated at residue 581 had negligible ethidium uptake and normal current responses (Smart et al., 2003). It was also reported that the coupling of P2X7R to pannexin-1 channels accounts for cellular entry of fluorescent dyes (Pelegrin and Surprenant, 2006).
Recent studies have shown that naive rP2X7R activates and deactivates biphasically at higher agonist concentrations (Fig. 4A), and the slow secondary growth of current in the biphasic response coincides temporally with pore dilation (Fig. 4B). The secondary rise in current was observed under different ionic conditions as well as in cells with blocked pannexin channels and in cells not expressing these channels endogenously. Experiments with several mutants further indicated that the P2X7R dilates under physiological ion conditions, leading to generation of biphasic current, and that this process is controlled by residues near the intracellular side of the channel pore (Yan et al., 2008). It has also been shown recently that P2X2R display permeability dynamics, which are correlated with conformational changes in the cytosolic domain remote from the selectivity filter itself (Chaumont and Khakh, 2008). Several laboratories have also suggested that pannexins contribute to P2XR signaling by providing a pathway for release of intracellular ATP (Locovei et al., 2006; Iglesias et al., 2009; MacVicar and Thompson, 2010; Li et al., 2011).
Fig. 4.
Characterization of rP2X7R. A, concentration-dependent effects of BzATP on biphasic receptor activation. B, comparison of kinetics of the secondary current growth and changes in the reversal potentials. C, sensitization of receptors induced by repetitive application of agonist. D, sensitized receptors respond with monophasic currents with the peak amplitude of current determined by agonist concentration. E, abolition of rapid and sustained P2X7R currents by the P2X7R-specific antagonist AZ10606120 (Az). F, repetitive stimulation of receptors with 100 μM BzATP in the presence and absence of AZ10606120.
In contrast to other homomeric and heteromeric P2XRs, repetitive stimulation with the same agonist concentration causes sensitization of P2X7R, which manifests as a progressive increase in the current amplitude accompanied by a slower deactivation rate (Fig. 4C). Once a steady level of the secondary current is reached, responses at high agonist concentrations become monophasic (Fig. 4D). Both phases of response are abolished by the application of Az10606120, a P2X7R-specific antagonist (Fig. 4, E and F). These results further support the conclusion that pore dilation accounts for the secondary current growth (Yan et al., 2010). Sensitization of the receptors caused by repetitive agonist applications could be partially explained by calcium-CaM signaling (Roger et al., 2008).
The pharmacological profile of mammalian P2X7Rs is shown in Table 6. This is the least sensitive member of the P2XR family to activation by nucleotides. BzATP is the most potent agonist for this receptor with an EC50 value of 50 μM, compared with 3 to 4 mM for ATP. At the sensitized receptor, the EC50 values for BzATP and ATP are approximately 25 μM and 2 mM, respectively (Roger et al., 2008; Donnelly-Roberts et al., 2009a; Yan et al., 2010). Similar values have been suggested by others (Rassendren et al., 1997b; Hibell et al., 2000; Duan et al., 2003). The potency of ATP at the human receptor increases when calcium and magnesium are removed from the bath medium (Klapperstück et al., 2001). 2-meSATP and ATPγS are partial agonists of this receptor, whereas αβ-meATP and βγ-meATP have very little effect on activation.
TABLE 6.
Pharmacological profile of P2X7R
EC50/IC50 values are micromolar unless otherwise specified.
(+), positive modulator; (−), negative modulator; oATP, oxidized ATP; PIPs, phosphoinositides; N.D., not determined.
Positive modulator at rat and negative modulator at human P2X7R (Michel et al., 2008).
An interesting feature of P2X7R is its activation by ADP-ribosylation, a reaction that requires the enzyme ADP-ribosyltransferase and NAD as a substrate. In T-regulatory cells, both P2X7R and ADP-ribosyltransferase are expressed, and the activation of P2X7R by ADP-ribosylation observed in these cells was also mimicked in HEK293 cells cotransfected with both proteins (Schwarz et al., 2009). The same group also showed that the P2X7R-Arg125 residue is the target for ADP-ribosylation and that this chemical modification promotes channel gating (Adriouch et al., 2008; Schwarz et al., 2009). It has also been suggested that this mechanism could be relevant in regulating the functions of T-regulatory cells and in treatments of some tumors (Hubert et al., 2010).
As with rP2X4R, the rP2X7R also shows a resistance to the generally used P2 antagonist suramin (Surprenant et al., 1996; Bianchi et al., 1999; Duan et al., 2003). NF279, a suramin analog, inhibits hP2X7R with an IC50 of ∼3 μM, but concentrations above 100 μM are required to inhibit rP2X7R (Klapperstück et al., 2000; Donnelly-Roberts et al., 2009a). PPADS antagonizes the currents and calcium signals mediated by mammalian receptors with variable potencies ranging from 1 to 60 μM (Chessell et al., 1998; Bianchi et al., 1999; Gunosewoyo et al., 2007; Guile et al., 2009). MRS2159, a potent inhibitor of rP2X1Rs, also inhibits mouse, rat, and human P2X7Rs (Donnelly-Roberts et al., 2009a). Oxidized ATP is an irreversible antagonist that requires several hours of preincubation and concentrations of 100 to 300 μM to be effective (Murgia et al., 1993; Surprenant et al., 1996; Michel et al., 2000; Hibell et al., 2001). BBG also inhibits rat and human P2X7Rs with IC50 values of approximately 15 and 250 nM, respectively (Jiang et al., 2000a; Jacobson et al., 2002). Several blockers of calcium/CaM-dependent protein kinase II, including calmidazolium and 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), also inhibit the BzATP-induced hP2X7R current. This effect is probably kinase-independent, because the KN-04 compound is inactive toward kinases but inhibits P2X7R current (Gargett and Wiley, 1997; Chessell et al., 1998; Humphreys et al., 1998; Hibell et al., 2001; Jacobson et al., 2004).
More recently, several new compounds have been introduced as potent P2X7R inhibitors. N-(1-([(cyanoimino)(5-quinolinylamino) methyl] amino)-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide (A740003) is a selective and competitive antagonist of P2X7R-mediated calcium influx with IC50 values of 18 and 40 nM for the rat and human receptor and submicromolar potency at mP2X7R (Honore et al., 2006; Donnelly-Roberts et al., 2009a). The same group also introduced 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride (A438079). This compound inhibits mouse, rat, and human P2X7Rs at submicromolar concentrations (Nelson et al., 2006; Donnelly-Roberts et al., 2009a). A novel series of cyanoguanidine-piperazine P2X7R antagonists was designed based upon the structure of A740003 that focused on the piperazine moiety and the right-hand side substitution (Morytko et al., 2008). The compound 2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine (A804598) inhibits mouse, rat, and human P2X7Rs at low nanomolar concentrations (IC50 = 9–11 nM) (Donnelly-Roberts et al., 2009b).
Figure 4 shows that N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride (AZ10606120) also inhibits BzATP-induced currents at submicromolar concentrations (Yan et al., 2010). It has been suggested that AZ10606120 binds at separate, interacting sites on the P2X7R, which could suggest the allosteric nature of its action (Michel et al., 2007). N2-(3,4-difluorophenyl)-N1-((2-methyl-5-(1-piperazinylmethyl)phenyl)glycinamide dihydrochloride (GW791343) acts as a positive allosteric regulator of rP2X7R (Michel et al., 2008a). In contrast, 3-[1-[[(3′-nitro[1,1′-biphenyl]-4-yl)oxy]methyl]-3-(4-pyridinyl)propyl]-2,4-thiazolidinedione (AZ11645373) is a highly selective and potent (IC50 5–10 nM) allosteric antagonist at the human but not rat receptor (Stokes et al., 2006).
Initial coimmunoprecipitation assay suggested that the rP2X7R does not form heteromeric assemblies (Torres et al., 1999a). This conclusion was questioned by others, who suggested formation of functional P2X4/X7 heterotrimeric receptors (Guo et al., 2007). However, another group indicated that either heteromerization between P2X4R and P2X7 subunits results not in stable heteromeric complexes or P2X4/X7 heteromers do not represent a dominant subtype in native tissues (Nicke, 2008). In immune cells, expressing P2X4R and P2X7R, the preferred assembly pathway for both receptors is also the formation of homotrimers. Furthermore, the authors suggested that an interaction could occur between rather than within receptor complexes (Boumechache et al., 2009).
B. Ligand Binding Domain
1. Identification of Residues Contributing to ATP Binding.
The ectodomain contains ∼280 amino acids, the majority of which are conserved among the receptor subtypes, but does not contain the consensus sequences for agonist binding present in other ATP-sensitive proteins (Evans, 2009). In an attempt to identify the candidate residues responsible for binding sites in this domain, the secondary structure similarities between rP2X4R and class II aminoacyl-tRNA synthases were investigated (Freist et al., 1998). This study revealed that the receptor function was practically lost in the K190A, R278A, and D280A mutants. However, the model of the ATP binding site that emerged from this study also included nonconserved residues, arguing against a common sequence accounting for the ligand binding (Yan et al., 2005).
Experiments with variable chimeric receptors have provided useful information about the positions of the ATP binding site on P2XRs. The P2X2/X3R chimeras containing the Val60-Phe301 sequence of P2X3R instead of the Ile66-Tyr310 sequence of P2X2R not only preserved ATP binding but also developed two intrinsic functions of P2X3R: sensitivity to αβ-meATP and ecto-ATPase-dependent recovery from endogenous desensitization but not the rate of receptor desensitization (Koshimizu et al., 2002; Zemkova et al., 2004). The corresponding P2X2/X4R chimera showed the gain of function, as indicated by increased sensitivity to ligands and faster desensitization compared with both parental receptors (He et al., 2003c). The P2X2/X7R chimera exhibited a sensitivity to ATP that fell between the sensitivities observed in cells expressing the parental receptors (He et al., 2002). These experiments indicated that the Lys67-Lys313 ectodomain sequence not only contains an ATP binding domain but also accounts in part for the receptor agonist specificity of these sites (Stojilkovic et al., 2005).
The contribution of the majority of the conserved amino acids in this region has been studied using alanine scanning mutagenesis. The most systematic investigation in this field was performed by the Evans laboratory using the hP2X1R as a receptor model in which over 120 ectodomain amino acids were replaced with other residues. At first, they analyzed the relevance of positively charged ectodomain residues in ATP recognition (Ennion et al., 2000). Later, they also studied the relevance of aromatic (Roberts and Evans, 2004), structural (Ennion and Evans, 2002; Digby et al., 2005; Roberts and Evans, 2005), polar (Roberts and Evans, 2006), and negatively charged ectodomain residues (Ennion et al., 2001). The main conclusion reached in these experiments was that the majority of individual alanine substitutions have little or no effects on ATP potency at the receptor. They identified Lys67, Lys69, Arg295, and Lys313 (P2X4R numbering) as potential residues involved in coordinating the negatively charged phosphate group of ATP and that the adenine ring may be sandwiched between Phe185/Thr186 and Asn293/Phe294/Arg295 residues (Roberts and Evans, 2006). Alanine and arginine substitutions of the positively charged ectodoman residues in rP2X2R revealed that mutations at Lys67 (P2X4R numbering) were least tolerant, followed by Lys313, Arg295, Arg309, and Lys69 (Jiang et al., 2000b). For rP2X4R, the order was Lys67, Lys 313, Arg295, Lys190, and Phe185 (Zemkova et al., 2007). Replacement of Lys69 with alanine also affected the receptor function (Roberts et al., 2008). The relevance of Lys67, Lys69, Arg295, and Lys313 (P2X4R numbering) with regard to agonist potency was also identified in hP2X3R (Fischer et al., 2007).
The breakthrough in understanding the structure of the ATP binding site came with the finding that it is localized between two subunits. The first evidence supporting this view was provided by Wilkinson et al. (2006) using the wild-type and K67A and K313A mutants (P2X4R numbering). The work of Marquez-Klaka et al. (2007) further supported the hypothesis that the interface between the subunits is important for the formation of the ATP binding site. Specifically, this group showed that a disulfide bond could form between K67C and F294C from two adjacent subunits.
The pharmacology of the P2XRs discussed above provides additional insights into the structure of P2XRs. For example, the lack of ADP effects at P2XRs indicates that the coordination/detection of the three phosphate residues, but not the adenine ring or ribose, plays a dominant role in determining the affinity of drugs for the ATP binding site. Consistent with this view, longer chain phosphates, such as adenosine tetraphosphate and the diadenosine polyphosphates, also act as agonists (Lewis et al., 2000). The Lys138 residue of hP2X1R seems to account for the high potency of suramin and its analog NF449 at this receptor compared with rP2X1R (Sim et al., 2008). The exchange of one Lys246 to the equivalent position in the P2X2R (Glu249) transfers the PPADS sensitivity (Buell et al., 1996). In hP2X7R, Phe95 is required for the high sensitivity to the allosteric modulators GW791343 and SB203580 (Michel et al., 2009). The ADP-ribosylation of Arg125 is sufficient to activate this receptor (Adriouch et al., 2008).
Together, these extensive investigations suggest that P2XRs share a similar agonist binding site with some variations in the binding pocket or gating, which account for receptor specificity in agonist and antagonist binding/receptor gating. Eight residues have the potential to contribute to the formation of the ATP binding site: Lys67, Lys69, Phe185, Thr186, Asn293, Phe294, Arg295, and Lys313 (P2X4R numbering). All of these residues are also present in distantly related P2XRs (algae, slime mold amoebae, and choanoflagellates) (Surprenant and North, 2009). Positively charged lysines seem to coordinate the binding of the negatively charged phosphate tail of ATP, whereas aromatic phenylalanine residues could coordinate the binding of the ATP adenine ring directly or indirectly.
2. Number of Occupied Binding Sites Necessary to Channel Activation.
An interesting question about P2XRs is how many molecules of ATP are necessary to open a channel. Early studies of ATP-evoked currents in rat and bullfrog neurons suggested that three molecules of ATP were required for channel gating. This was based on the superlinearity of currents versus agonist concentration observed at low ATP concentrations (Bean et al., 1990). Single-channel experiments also suggested that the binding of three ATP molecules was required to activate the P2X2R (Ding and Sachs, 1999). However, other reports have suggested that the binding of two (Friel and Bean, 1988; Friel, 1988) or even one molecule of ATP (Krishtal et al., 1983) is sufficient to activate channels.
After the cloning of P2XRs, a great effort was made to determine the stoichiometry of the functional channel. Diverse experimental approaches suggested that the channel was composed of three P2X subunits (Nicke et al., 1998; Stoop et al., 1999; Jiang et al., 2003; Barrera et al., 2005, 2007; Young et al., 2008). The crystallization of the zP2X4.1R confirmed that the channel is composed of three subunits (Kawate et al., 2009), suggesting that it should contain three binding sites for ATP. Moreover, as discussed in the previous section, ATP binding sites are located at the interface of neighboring subunits, each contributing residues that are necessary for adenine and phosphate interactions (Marquez-Klaka et al., 2007; Roberts and Evans, 2007).
However, the heteromeric P2X2/3R requires only two functional binding sites. This has been derived from separate studies in which the Hill slope at low ATP concentrations indicates the binding of two ATP molecules (Jiang et al., 2003). In addition, the mutation of ATP-binding residues on the P2X2 subunit results in functional channels when they are coexpressed with P2X3 subunits (Wilkinson et al., 2006), suggesting that the disruption of only one binding site is not sufficient to shut down channel function (P2X2/3Rs are composed from two P2X3 and one P2X2 subunits). The same group also showed that mutations at Lys68 or Lys308 (P2X2R numbering) resulted in nonfunctional receptors. Surprisingly, when these two mutants were coexpressed, small but consistent ATP-mediated currents were observed, suggesting that channels containing subunits with different mutations can form at least one intersubunit binding site, and these channels account for the small currents (Wilkinson et al., 2006). We have characterized the gating of P2X7R, combining experimental data with a mathematical model that could explain the different activation states of this channel with the occupancy of two or three ATP binding sites (Yan et al., 2010).
3. Orthosteric Sites in the Context of the P2X4.1 Structure.
Although the reported crystal structure of zP2X4.1R was solved in the absence of ATP, it revealed a potential location for the ligand binding site (Kawate et al., 2009; Browne et al., 2010; Evans, 2010; Stojilkovic et al., 2010b; Young, 2010). This putative ATP binding site, shaped like an open jaw, is ∼42 Å from the extracellular limit of the plasma membrane on the interface between adjacent subunits. To discuss the location and the structure of this putative ligand binding site, we have generated a homology model of rat P2X4R with docked ATP molecule (Fig. 5). Homology models based on the crystal structure of zP2X4.1R suggest the position of three ATP binding sites embedded within the interfaces of the timer (Browne et al., 2010; Stojilkovic et al., 2010b; Young, 2010). The bottom of the predicted ATP binding site is formed by the upper part of the body domain of two neighboring subunits, whereas the head domain and the left flipper of one subunit and the dorsal fin of the other create its walls. Molecular docking suggested that ATP binds with its adenine ring and ribose moiety positioned at the bottom of the ligand binding pocket, making several van der Waals and polar contacts with both the main chain and side-chain atoms of the body and head domains, whereas the phosphate groups are oriented toward the surface of the receptor and surrounded by basic residues, including Lys69, Arg295, and Lys313 (in rat P2X4R numbering). The predicted ligand binding cleft also includes residues Lys67, Asn293, and Phe294 in good agreement with the site-directed mutagenesis experiments (Zemkova et al., 2007). Residues Phe185, Thr186, and Lys190 are located in close vicinity of the binding site. It is likely that upon the ATP-induced conformational change, these residues might be an integral part of the ligand-binding site.
Fig. 5.
Homology model of rat P2X4R with docked ATP. A, predicted structure of the ATP binding site. Residues suggested to be involved in ATP binding or located in the close vicinity of putative ATP-binding site are shown as sticks. Each subunit is shown in a different color. B, surface representation of the ATP binding pocket with a docked molecule of ATP. C, homology model of homotrimeric rat P2X4 viewed parallel to the membrane. Each subunit is depicted in a different color. The ATP molecule is shown as a Corey-Pauling-Koltun model. The black lines suggest the boundaries of the outer (out) and inner (in) layers of the plasma membrane. The homology model of rat P2X4R (sequence Arg33–Val355) was generated using the Modeler 9v7 package (Sali and Blundell, 1993) and the crystal structure of zP2X4.1R (Kawate et al., 2009) as a template. Missing side chains were built using the DeepView/Swiss-PdbViewer v4.0.1 program (Guex, 1997). Bad contacts were corrected manually and the model was energy-minimized using the DeepView/Swiss-PdbViewer with the GROMOS96 43B1 parameter set. The AutoDock v4.2 (Morris et al., 2009) was used to predict the position and the conformation of ATP within the putative ligand-binding site at the in1terface between two neighboring subunits. The figure was generated using PyMol v0.99 (http://www.pymol.org).
It has been speculated, based on the crystal structure of zP2X4.1R, that ATP binding induces movement of the head, right flipper, and dorsal fin domains, resulting in a conformational change within and between subunits and channel opening (Kawate et al., 2009). The bottom of the predicted ATP binding site is in the upper part of the rigid β-sandwich motif, where the residues Lys67, Lys69, Asn293, Arg295, and Lys313 are located. This transthyretin-like β-sandwich motif forms the skeleton of the extracellular body domain and seems to be very rigid because of an extensive number of hydrogen bonds between β-strands (Blake et al., 1978; Kawate et al., 2009). Therefore, it is reasonable to speculate that this rigid β-structure transmits the ATP-induced conformational change from the ligand-binding site to the base of the extracellular domain, resulting in the repositioning of the connected TM helices and channel opening.
The structure of zP2X4.1R does not provide a rationale for the differences in ligand sensitivity between receptors (e.g., the 200-fold difference in ATP potency at P2X1R and P2X7R, the selectivity of αβ-meATP for P2X1R and P2X3R, or the mechanism of action of the receptor-specific antagonists). However, mutagenesis studies have revealed receptor-specific effects of the replacement of some conserved residues. Mutations in Lys188, Asp259, and Arg304 residues decreased the potency of ATP for rP2X2R more than 300-fold (Jiang et al., 2000b) but did not significantly affect hP2X1R function (Ennion et al., 2000, 2001). Likewise, replacement of Lys190 and Phe230 with alanines generated practically nonresponsive rP2X4R receptors, whereas the function of equivalent mutants in hP2X1R was only slightly affected (Ennion et al., 2000; Roberts and Evans, 2004). Mutation of mP2X7-Arg206 resulted in increased agonist potency, but this mutation was ineffective at hP2X1R (Ennion et al., 2000). These differences could contribute to the heterogeneity of the pharmacological properties of these receptor subtypes. In the absence of a P2XR crystal structure with bound ATP; however, this evidence should be considered circumstantial and does not prove the precise localization of the ATP binding site or the specificity of these sites for various agonist analogs.
IV. Allosteric Binding Sites and Receptor Function
Allosteric modulators can change the affinity of receptors for native agonists and/or affect agonist efficacy in different ways. Allosteric antagonists may reduce agonist affinity without affecting agonist efficacy. Such a pattern of action resembles the effect of competitive antagonists at orthosteric binding sites, but in a limited way. The allosteric antagonistic effect is always saturable, because the allosteric effect will reach a maximum when all allosteric binding sites are occupied. In contrast, orthosteric ligands can have almost infinite competitive effects as long as the concentrations of the competitive ligands are changed in the correct manner. Allosteric agents may reduce both affinity and efficacy, producing a mixture of competitive/noncompetitive effects, or may act by reducing efficacy only, producing noncompetitive effects. Other combinations are also possible, such as increasing the affinity of an agonist and inhibiting its efficacy, potentiating the endogenous response through an increase in the receptor's affinity, or efficacy for its agonist (Kenakin and Miller, 2010). In this review, we talk about allosteric-induced changes in the potency and efficacy of ATP for particular receptor subtypes.
The majority of allosteric regulation of P2XRs occurs at extracellular allosteric binding sites. This includes binding sites for essential trace metals and other micro and macro metals, as well as binding sites for protons. Allosteric binding sites around the TM region include sites for IVM and alcohols. Regulation of P2XR function by calcium binding proteins, protein kinases, lipids, and reactive oxygen species also occurs through allosteric modulatory sites located at the N and C termini, whereas the sites affected by carbon monoxide, steroids, propofol, ketamine, and toluene, if they exist, have not been identified.
A. Metals as Allosteric Modulators
Numerous metals have profound effects on P2XR function, acting as potent allosteric modulators (Huidobro-Toro et al., 2008). The effects of essential trace metals, heavy metals, trivalent cations, and macro metals on P2XR functions are summarized in Tables 1 to 6 and are discussed in detail in the following sections.
1. Essential Trace Metals.
Nine metals (chromium, cobalt, copper, iron, manganese, molybdenum, nickel, selenium, and zinc) are now included in the group of essential trace elements. Some other metals, such as vanadium, may also be nutritionally important, but their essentiality has not been fully established. These elements occur in very small amounts (usually less than 1–10 parts per million) as constituents of living organisms. Their deficiency induces specific biochemical changes causing abnormalities in function, and these abnormalities can be corrected by supplementation with the elements (Reilly, 2004). Among the trace metals, zinc, copper, and iron have particular relevance (Mathie et al., 2006; Madsen and Gitlin, 2007). Zinc and copper are transported intracellularly and stored in synaptic vesicles together with transmitters through variable transporters (Kuo et al., 2001; Palmiter and Huang, 2004). After release to the synaptic cleft, these metals can reach concentrations as high as 250 to 300 μM (Assaf and Chung, 1984; Kardos et al., 1989). The released trace metals can bind to numerous receptors and channels, including voltage-gated sodium, calcium, or potassium channels, store-operated Ca2+ channels, transient receptor potential channels and ligand-gated channels, where they act as allosteric modulators (Mathie et al., 2006). Trace metals are also allosteric modulators of P2XRs.
a. P2X1, P2X3, and P2X5 receptors.
The function of rP2X1R is inhibited by zinc in a concentration-dependent manner without affecting Emax values, whereas rP2X3R function is potentiated by this metal, causing a leftward shift in the concentration-response to ATP application with a maximal effect at 10 μM. Higher zinc concentrations have been shown to reduce P2X3R current, suggesting the possible existence of two allosteric sites at this receptor, a high-affinity site with a potentiating effect and a low-affinity site with an inhibitory role (Wildman et al., 1999b). The rP2X5R is also modulated in a biphasic fashion by zinc, again suggesting the existence of at least two allosteric sites for this metal (Wildman et al., 2002). There is no information about the amino acid residues involved in the binding of trace metals at these receptors. In addition, effects of other trace metals on the function of these receptors have not been reported.
b. P2X2 receptor.
Zinc and copper both exhibit a biphasic effect on rP2X2R function: the ATP-induced responses are significantly potentiated at 10 to 100 μM and inhibition at millimolar concentrations (Xiong et al., 1999; Clyne et al., 2002a; He et al., 2003b; Lorca et al., 2005). Zinc also potentiates the ATP responses of native rP2X2Rs and rP2X2/3Rs expressed in neurons from parasympathetic ganglia (Ma et al., 2005), as well as in neurons from the rat hypothalamus (Vorobjev et al., 2003) and dorsal motor nucleus (Ueno et al., 2001). Cobalt and nickel also potentiate the activity of rP2X2R, but the potential inhibitory effect at high concentrations was not tested (Lorca et al., 2005). Site-directed mutagenesis revealed the critical role of extracellular His120 and His213 in the positive modulation by zinc and copper (Clyne et al., 2002a; Lorca et al., 2005). His192, His245, and His319 are also partially involved, probably contributing to the stabilization of the allosteric site (Lorca et al., 2005). Nagaya et al. (2005), using concatamers, showed that the allosteric sites for zinc and probably copper are formed by His120 and His213 of adjacent subunits. These results have been recently confirmed with the crystallization of P2X4.1R (Kawate et al., 2009). The flexibility of the zinc binding site of rP2X2R was also tested by shifting two essential histidines 13 residues upstream or downstream from their original position. The ability of zinc to potentiate the mutated channels followed the order His120 >His121 > His119 and His212 > His213 > His211 (Tittle et al., 2007). In contrast, it has been difficult to identify the residues that compose the zinc/copper inhibitory site (Clyne et al., 2002a; Friday and Hume, 2008). Tittle and Hume (2008) also showed that, unlike rat and mouse P2X2Rs, hP2X2R is inhibited by zinc and copper, revealing the species-specific modes of metal modulation. This group also found that none of the nine histidines in the extracellular domain of hP2XR were required for zinc inhibition, suggesting that the replacement of His213 with arginine in human receptors at least partially accounts for the change in the receptor behavior. Cobalt and nickel seem to act at the same allosteric site, as deduced from experiments using mutant receptors (Lorca et al., 2005).
c. P2X4 receptor.
The unique characteristic of P2X4R is its differential modulation by trace metals. Zinc and cobalt potentiate receptor activity and copper inhibits it. Figure 6 shows that zinc potentiation is due to an increase in ATP potency at P2X4R, whereas copper affects the Emax value without affecting the potency of the agonist. At higher concentrations (30 μM–1 mM), zinc inhibits P2X4R function via a reduction in ATP efficacy (Wildman et al., 1999b; Acuña-Castillo et al., 2000). Residues that are commonly present in copper binding sites include histidines, aspartic acids, glutamic acids, and cysteines, although other residues could also contribute (Aitken, 1999). Among the three ectodomain histidines, only the mutation of His140 abolished the inhibitory action of copper and modified the pattern of zinc action from a bell-shaped curve, typical of biphasic modulation, to a sigmoid curve, accompanied by an increase in the amplitude of response (Coddou et al., 2003). Subsequent studies focused on the Thr123-Thr146 ectodomain sequence in a search for other residues that contribute to the regulatory actions of copper and zinc. These experiments revealed that Asp138 is a second component of the P2X4R inhibitory site, whereas Cys132 is suggested to account for the positive zinc allosteric effect. These experiments also ruled out the involvement of Thr123, Ser124, Asp129, Asp131, Thr133, and Thr146 residues in the allosteric actions of copper and zinc (Coddou et al., 2007). Cobalt also potentiates P2X4R activity, but this action is long lasting, as indicated by the lack of recovery during a 45-min washout period (Coddou et al., 2005).
Fig. 6.
Patterns of rP2X4R allosteric modulations. A and C, zinc increases and protons decrease the sensitivity of receptors for agonist without affecting Emax. B, IVM treatment causes both a leftward shift in the sensitivity of receptors for agonist and an increase in the Emax value. D, copper decreases the Emax without affecting the sensitivity of receptors for agonist.
To visualize the location of these three residues, we built the homology model of rat P2X4R (sequence Arg33-Val355) using the Modeler 9v7 package (Sali and Blundell, 1993) and the crystal structure of zP2X4.1R (Kawate et al., 2009) as a template. Figure 7 shows that the His140 residue is located close to the proposed ATP binding site that includes Asn293, Arg295, and Lys313 from the same subunit and Lys67, Lys69, and Phe185 from the adjacent subunit. Thus, it is reasonable to speculate that Asp138 and His140 from the same subunit represent the P2X4R-specific region accounting for the inhibitory effects of copper and high concentrations of zinc. The finding that the replacement of Cys132 abolishes the potentiating effect of zinc also raises the possibility that the SS3 bond is important for zinc potentiation or that this bond is not permanent but instead breaks and reforms.
Fig. 7.
Residues of the rat P2X4R potentially involved in the modulatory effects of copper and zinc cations (Wildman et al., 1999; Acuña-Castillo et al., 2000; Coddou et al., 2003, 2007). Structural model of rat P2X4R shows the location of residues Asp138, His140, Thr133, Cys132, and Cys159 (shown as sticks) with respect to the predicted ATP-binding site. All these residues are present within the head domain of one subunit, which creates one wall of the predicted ATP-binding pocket and is probably involved in the ATP-induced conformational change. The figure was generated using PyMol v0.99 (http://www.pymol.org).
The zinc-induced potentiation of P2X4R does not depend on the preapplication time, whereas copper-inhibition is time dependent, and the inhibition persists when the metal is washed out before the channel is opened by ATP (Acuña-Castillo et al., 2000). This suggests that the zinc binding site appears on the receptor in the open state, whereas copper binds to the receptor in the closed state. This provides a rationale for the easy prediction of which residues participate in copper binding based on the crystal structure of the closed receptor (Kawate et al., 2009).
d. P2X7 receptor.
Our group examined effects of copper and zinc in X. laevis oocytes expressing rP2X7R (Acuña-Castillo et al., 2007). Copper noncompetitively inhibited the receptor-mediated current with an IC50 of 4 μM. Zinc also inhibited the ATP-gated current, but was 20-fold less potent. Other groups have also observed inhibition of rP2X7R currents when the receptor was expressed in HEK293 cells (Liu et al., 2008a). Differences in metal modulation across species have also been reported for this receptor. Unlike rP2X7R, copper, but not zinc, inhibited the mouse receptor (Moore and Mackenzie, 2008). Mutagenesis of six ectodomain histidines revealed that the H267A mutation resulted in a copper-resistant receptor, whereas the H201A and H130A mutants were less sensitive to copper than the wild-type receptor, and the H62A, H85A, and H219A mutants exhibited normal copper sensitivity. The zinc-induced inhibition of receptor function was reduced in H267A and H219A mutants (Acuña-Castillo et al., 2007). Other groups have reported some effects of replacing His201 and His267 on the copper- and zinc-mediated inhibition of P2X7R currents, but they also detected a dramatic reduction or almost a complete loss of inhibition by the two metals in cells expressing the H62A and D197A double mutant (Liu et al., 2008a). Further studies should clarify whether the differences are due to the expression system (oocytes versus HEK293 cells), the agonist used (ATP versus BzATP), or some other factors.
e. Other P2X receptors.
Native P2XRs expressed in bullfrog DRG neurons are also inhibited by zinc (Li et al., 1997). Five P2X-like genes are expressed in D. discoideum, and ATP-activated channels are expressed in the plasma membrane. The responses of these channels are inhibited by copper and zinc with IC50 values of 0.9 and 6.3 μM, respectively (Ludlow et al., 2008). A P2XR cloned from the parasitic platyhelminth Schistosoma mansoni is permeable to fluorescent dyes and is inhibited by extracellular zinc with an IC50 of 0.4 μM (Raouf et al., 2005). Zinc and copper also inhibited the ATP-induced current in cells expressing a P2XR cloned from the tardigrade Hypsibius dujardini with IC50 values of 63 and 20 μM, respectively. In contrast to vertebrate P2XRs, none of the ectodomain histidines plays a major role in coordinating metal binding at this receptor (Bavan et al., 2009).
f. Summary.
A common aspect of P2XRs is their regulation by essential trace metals. The nature of trace metal actions (positive or negative) depends on the subtype of receptor and species differences. This indicates that relatively small changes in the structure of receptors are sufficient to change the direction of modulatory action of trace metals. Furthermore, in contrast to the ATP binding sites, the amino acids involved in P2XR trace metal coordination do not seem to be aligned among P2X subunits. This may provide additional rationale for the opposing effects of allosteric regulators, such as zinc and copper, on receptor function. Two distinct and separate binding sites seem to exist for trace metals, and it seems that both sites can be occupied by different elements with variable affinities. In P2X4R, these sites provide opposite receptor modulation, positive for zinc and negative for copper. The three-dimensional structure of the P2XRs indicates that coordination sites of trace metals at the P2X4R are spatially close to the ATP binding site, allowing us to propose that these metals modify the ligand binding. For P2X2R, the ATP binding site and the zinc allosteric sites are not only close by but are also are formed at the interface between neighboring subunits.
2. Heavy Metals.
The term heavy metal refers to any metallic element that has a relatively high density and is toxic or poisonous at low concentrations. Examples of heavy metals include cadmium, lead, mercury, and thallium, as well as lighter metals, such as arsenic and chromium. These metals affect the function of numerous voltage- and ligand-gated channels and ionic pumps (Kiss and Osipenko, 1994). Mercury acts as a positive modulator of P2X2R. It causes a leftward shift in the potency of ATP and does not change the Emax value (Lorca et al., 2005). In contrast, mercury acts as a negative modulator of P2X4R (Coddou et al., 2005). In both cases, the actions of mercury resemble the actions of copper, suggesting that these metals may bind to the same allosteric site. However, this hypothesis was discarded when in was reported that the copper-resistant mutants were still modulated by mercury (Coddou et al., 2005; Lorca et al., 2005). Using chimeric P2X2/X4Rs, we recently found that the primary site of action for mercury is an intracellular cysteine at position 430 (Coddou et al., 2009). The residual action of mercury could be mediated by still unidentified ectodomain binding sites. Cadmium potentiates P2X2R and P2X4R (Coddou et al., 2005; Lorca et al., 2005) but inhibits P2X1R, P2X3R, and P2X7R (Nakazawa and Ohno, 1997; Virginio et al., 1997). This metal seems to bind to the allosteric sites identified for zinc in the P2X2R and P2X4R. This was inferred from competition studies using zinc and cadmium and from experiments with zinc-resistant mutants (Coddou et al., 2005; Lorca et al., 2005). Other metals, including lead, barium, palladium, silver, platinum, and gallium, had no effect on the ATP-evoked currents in P2X2R- and P2X4R-expressing cells, indicating that the allosteric sites have strict structural requirements (Coddou et al., 2005; Lorca et al., 2005).
3. Lanthanides.
These rare earth elements are commonly known for their ability to inhibit ion permeation by blocking the pore of various ion channels and causing toxicity (Kiss and Osipenko, 1994). These trivalent cations also inhibit P2XRs. Lanthanum, cerium, neodymium, and gadolinium inhibit P2X1R and P2X2R expressed in PC12 cells and X. laevis oocytes at 3 to 300 μM. This inhibition is due, at least in part, to allosteric mechanisms (Nakazawa et al., 1997). Gadolinium also inhibits D. discoideum P2XRs (Ludlow et al., 2008). The existence of allosteric binding sites for gadolinium was recently confirmed with the crystallization of zP2X4.1R. Using a gadolinium derivative, three sites located in the periphery of each subunit were identified, each site composed of Asp184 and Asn187 residues. The fourth site is formed by the Glu98 residues of each channel subunit (Kawate et al., 2009). At zP2X4.1R, Asn187 is adjacent to Phe188 and Thr189, two residues that were predicted to participate in the formation of the ATP binding pocket, suggesting that gadolinium binding reduces ATP affinity. At rP2X4R, an Asn residue is also present, but the effects of gadolinium on the function of this receptor have not been tested. The use of 1 mM gadolinium during receptor crystallization may explain the lack of a solved structure for an ATP-bound form. Other groups have shown that gadolinium increases recovery from desensitization in P2X3Rs (Cook et al., 1998).
4. Macro Metals.
Calcium, potassium, sodium, and magnesium belong to macro elements, which are present in human tissues in 14, 2, 1.4, and 0.27 g/kg body weight, respectively (Reilly, 2004). There is no information about the potential role of potassium in the regulation of P2XR function, and very limited information exists supporting a role for sodium in P2XR gating. Early evidence for the role of sodium comes from experiments in human lymphocytes showing that the ATP-evoked calcium influx is strongly inhibited by this cation (Wiley et al., 1992). A possible role of extracellular sodium in the regulation of P2X7R permeability to large molecules has been proposed by two groups (Jiang et al., 2005a; Li et al., 2005). In another study, it was demonstrated that sodium could regulate the motility of airway cilia by inhibiting native P2XRs, presumably P2X4R and/or P2X7R by binding to an extracellular allosteric site (Ma et al., 1999, 2006).
On the other hand, there is a large body of information concerning the effects of divalent calcium and magnesium ions on channel function. Calcium and magnesium inhibit recombinant P2XRs at millimolar concentrations (North, 2002). Native receptors are also sensitive to modulation by calcium (Nakazawa and Hess, 1993; Cook and McCleskey, 1997) and magnesium (Tomić et al., 1996; Miyoshi et al., 2010). It is well known that these cations make complexes with ATP, reducing the free acid form of ATP (or ATP4−) in solution. It has also been suggested that ATP4− acts as an agonist for histamine secretion by mast cells (Dahlquist and Diamant, 1974). This conclusion was further supported by experiments on the concentration dependence of ATP-induced histamine secretion in cells bathed in different calcium and magnesium concentrations (Cockcroft and Gomperts, 1979). More recently, the effect of ATP on P2X7R currents was studied in the presence and absence of calcium, and the same conclusion was reached (Klapperstück et al., 2001). Although it has never been supported by direct evidence, this hypothesis has prevailed in the literature during the last decade. However, numerous data strongly support alternative hypotheses.
Surprenant et al. (1996) reported that extracellular calcium inhibits both the cationic current and dye uptake. The inhibitory effect of this cation on rP2X7R function was mimicked by other divalent cations in the following order: Cu2+ > Cd2+ ∼Zn2+ > Ni2+ ≫Mg2+ ∼ Co2+ > Mn2+ > Ca2+ = Ba2+ ≫ Sr2+. For each of these metals, the IC50 values for current and dye uptake were similar. This inhibition was voltage-independent, a finding inconsistent with the hypothesis that divalent cations cause functional inhibition by blocking the channel pore. The same group also observed that the major inhibitory effect of divalent cations was observed in relatively low concentrations (1–3 mM), a pattern typical for allosteric modulation (Virginio et al., 1997). The rP2X7R-H130A mutant has been shown to be resistant to magnesium inhibition, reinforcing the hypothesis that this metal acts through an allosteric mechanism (Acuña-Castillo et al., 2007).
The inhibitory effects of calcium and magnesium were also observed in recombinant rP2X2Rs with single-channel recording. These effects manifest as a fast block, visible as a reduction in amplitude of the unitary currents (Ding and Sachs, 1999). In further work on this topic, this group showed that bath calcium concentrations also increase the rates of receptor desensitization, a finding inconsistent with ATP4− acting as an agonist. Furthermore, they showed a similar phenomenon with magnesium, barium, and manganese, but the calcium-induced desensitization exhibited a higher affinity and cooperativity, also not consistent with divalent cations determining ATP4− concentration as the sole mechanism of calcium modulation. The authors have not clarified whether this effect is due to a direct allosteric interaction of calcium with the receptor or occurs through the activation of an accessory calcium-dependent protein (Ding and Sachs, 2000).
Other groups have also observed a strong inhibitory effect of bath calcium on rP2X2Rs with a half-maximal block of the current at about 5 mM, but not on hP2X1Rs (Evans et al., 1996). rP2X3R was more than 10-fold less sensitive to blockade by bath calcium (IC50, 89 mM), whereas heteromeric rP2X2/3Rs were more sensitive (IC50 15 mM). Thus, the rank of order for inhibition by calcium at these receptors is P2X2R > P2X2/3R > P2X3R > P2X1R (Virginio et al., 1998a). A stimulatory role of calcium in P2X3R recovery from desensitization has also been proposed (Cook et al., 1998). A distinct role of calcium and magnesium in the modulation of native P2X3Rs from cultured rat DRG neurons was also reported. Calcium positively modulated these channels, whereas magnesium had an inhibitory effect (Giniatullin et al., 2003). Single-channel recordings of P2X4R further revealed that magnesium reversibly decreases the amplitude of ATP-evoked single channel currents in a concentration-dependent manner that is independent of the membrane potential. In addition, they found that this cation shortens the mean open time without affecting the mean closed time. The authors concluded that magnesium inhibits the function of human P2X4R by means of an open-channel block via a binding site located at the exterior surface of the pore (Negulyaev and Markwardt, 2000).
Calcium may also regulate P2XR function by acting as an intracellular messenger. All P2XRs conduct calcium through their pores, but these channels also produce cell depolarization and promote calcium influx indirectly by activating voltage-gated calcium channels. Elevated intracellular calcium concentrations can trigger many cellular functions, including the activation and inhibition of adenylyl and guanylyl cyclases, leading to up- and down-regulation of protein kinase A and G, as well as the activation of some protein kinase C isoforms. Calcium also binds to numerous signaling proteins, such as CaM, which can alter cellular functions either directly or through CaM-dependent kinases. A recent report describes the role of intracellular calcium through CaM on P2X7R function. In cells expressing this receptor, CaM contributes to current facilitation and membrane blebbing. The proposed CaM-binding site corresponds to the Ile541-Ser552 sequence located in the C-terminal tail of P2X7R (Roger et al., 2008). Single-nucleotide polymorphisms of hP2X7R, specifically Q521H, also affect receptor sensitivity to extracellular calcium inhibition (Roger et al., 2010). Calcium-CaM-dependent protein kinase II potentiates the ATP response in primary sensory DRG neurons, not in an allosteric manner but by promoting trafficking of P2XRs (Xu and Huang, 2004). A recent report also described a novel interaction of the P2X2R with the neuronal calcium sensor VILIP1. The authors found that VILIP1 constitutively binds to P2X2Rs, altering channel properties such as ATP-sensitivity and peak currents. This opens the field to study if such interactions occur in other P2XRs (Chaumont et al., 2008).
B. Protons as Allosteric Modulators
Extracellular and intracellular pH, determined by proton concentration, affects the gating properties of both voltage- and ligand-gated ion channels, including acid-sensing ion channels and the transient receptor potential vanilloid receptor channels (Caterina et al., 1997; Tominaga et al., 1998; Wemmie et al., 2006). With the cloning of P2XRs, it became obvious that these receptors can also sense hydrogen ions in the bath medium and respond by modulating the ATP-induced currents in a receptor-specific manner.
For rP2X2R, the potency of all agonists, but not antagonists, is enhanced 5- to 10-fold by acidification without affecting the maximal agonist response (King et al., 1996, 1997; Stoop et al., 1997; Clyne et al., 2002a). The receptor-specific sensitivity to pH is also obvious in single-cell calcium measurements (He et al., 2003b). This effect was also seen in cells expressing heteromeric rP2X2/3Rs (Stoop et al., 1997), whereas rP2X1/2Rs exhibit unique pH properties (Brown et al., 2002). The agonist-induced response of P2X2/3R is also extremely sensitive to small changes in extracellular pH (approximately 7.1–7.2) (Li et al., 1996a,b). The pH range was narrowed for the proton enhancement of the ATP response at the heteromeric rP2X2/6Rs (King et al., 2000). The basic His319 residue was identified as a putative pH sensor for rP2X2R (Clyne et al., 2002a). This residue is in a receptor region that has been suggested to operate as a linker region between the ligand binding domain and the channel pore in other P2XR subunits (Yan et al., 2006; Roberts and Evans, 2007).
All other homomeric P2XRs are inhibited by acidification. The ATP potency for rP2X1R was reduced 2-fold at pH 6.5 and 6-fold at pH 5.5 without altering the maximum current amplitude (Stoop et al., 1997; Wildman et al., 1999b). Effects of extracellular protons and zinc are additive for rP2X1R (Wildman et al., 1999b). Acidification also reduces the peak amplitude of the rP2X3R current at an EC50 concentration of agonists (Stoop et al., 1997), and it right-shifts the ATP concentration-response curve without altering Emax (Wildman et al., 1999). Another study performed with hP2X3R revealed a dual effect of acidification. This group found an inhibitory effect that shifts the concentration-response curves to the right and a stimulatory effect that increases the current peak amplitude and activation time constant and accelerates in the recovery from desensitization. The authors also identified the His206 residue as the responsible for these effects (Gerevich et al., 2007). It is interesting that only fully N-glycosylated hP2X3Rs recognize external protons (Wirkner et al., 2008). Acidification also decreased the potency of ATP for rP2X4R without a change in the maximum response (Stoop et al., 1997; He et al., 2003b), as summarized in Fig. 6. Alkalization, on the other hand, enhanced the current amplitude (Clarke et al., 2000). Mutation of His286 removes the extracellular pH sensitivity of this receptor subtype (Clarke et al., 2000; Yan et al., 2005). This residue is located far from the putative ATP binding site, suggesting that protons affect gating rather than ATP binding.
Acidification also inhibits the function of P2X5R and P2X7R, but with a pattern of inhibition that differs from other members of this family. In rP2X5R, acidification reduced both the potency and efficacy of ATP (Wildman et al., 2002), whereas in rat and human P2X7R, it causes a reduction in the peak amplitude of current without altering the agonist sensitivity (Liu et al., 2009). The inhibitory effect of protons on hP2X7R was also observed in single-channel recordings (Flittiger et al., 2010). Changes in the EC50 value of agonists caused by acidification of P2X7R could be masked by the complex gating properties of these receptors, including the generation of biphasic currents (Klapperstück et al., 2001; Yan et al., 2008) and the facilitation of responses during repetitive agonist application (Roger et al., 2008). Several residues seem able to contribute to the pH sensitivity of P2X7R, His130 and Asp197 playing major roles (Acuña-Castillo et al., 2007; Liu et al., 2009).
Native P2XRs are also sensitive to changes in pH. Acidic pH potentiates the ATP-gated currents in guinea pig cochlear outer hair cells (Kanjhan et al., 2003), mice DRG neurons (Light et al., 2008), and in neurons from different parasympathetic ganglia (Ma et al., 2005). The pH sensitivity of P2XRs seems to be conserved in evolution, in that D. discoideum P2XRs are also modified by acidification (Ludlow et al., 2008). These results are consistent with the hypothesis that acidic conditions in the synaptic cleft could modify purinergic transmission, which could be physiologically relevant in certain events, such as pain sensation during inflammation.
C. Ivermectin as the P2X4 Receptor-Specific Modulator
IVM, a semisynthetic derivative of the natural fermentation products of Streptomyces avermitilis, is a member of a class of lipophilic compounds known as avermectins. It is a relatively large molecule, spanning a distance of approximately 20 Å, as documented by nuclear magnetic resonance and X-ray crystallographic analysis (Springer et al., 1981; Hu et al., 1998). IVM is widely used in human and veterinary medicine as an antiparasitic drug, predominantly to treat river blindness caused by Onchocerca volvulus (Burkhart, 2000). The therapeutic effects of IVM are mediated by the action of this compound as an agonist and allosteric modulator of glutamate-gated chloride channels expressed in the nerves and muscles of the parasite, leading to muscle paralysis and starvation (Cully et al., 1994; Dent et al., 1997; Ikeda, 2003). IVM was also found to modulate other extracellular ligand-gated channels, including nicotinic and GABA receptors (Sigel and Baur, 1987; Krůsek and Zemková, 1994; Krause et al., 1998; Shan et al., 2001).
IVM also acts as a reversible allosteric modulator at mammalian homomeric and heteromeric P2X4Rs, but not at homomeric P2X2Rs, P2X3Rs, or P2X7Rs (Khakh et al., 1999b). Extracellularly applied IVM has multiple effects on the ATP-induced current. It increases both the current amplitude in response to supramaximal agonist concentration and the sensitivity of receptors to agonists (Fig. 6), reduces the desensitization rate, and greatly prolongs the deactivation of current after ATP removal (Khakh et al., 1999b; Priel and Silberberg, 2004; Jelínková et al., 2006). Single-channel analysis showed that IVM increases the channel conductance for approximately 20% and the probability of channel opening and prolongs the mean channel open time (Priel and Silberberg, 2004). In addition to human and rat P2X4R, S. mansoni P2XR is also potentiated by IVM (Agboh et al., 2004). More recently, a P2XR from the tardigrade species H. dujardini was cloned, and the gating properties of this receptor were also affected by IVM treatment (Bavan et al., 2009). Unlike rP2X4R, IVM does not affect the kinetics of desensitization for S. mansoni and H. dujardini P2XR currents. IVM alone does not open any of these channels. It has also been suggested that potentiation of P2X4R by IVM reflects an increase in the number of cell surface receptors, resulting from a mechanism dependent on the endocytosis of this receptor (Toulmé et al., 2006), but further work is needed to clarify this hypothesis.
Initial experiments also indicated that IVM is effective when applied extracellularly but not intracellularly, suggesting that the ectodomain contains the binding site for this compound (Priel and Silberberg, 2004). Experiments with chimeric receptors revealed that the TM domains and nearby residues of P2X4R are important for the effects of IVM on channel deactivation (Jelínková et al., 2006). This conclusion was confirmed by Silberberg et al. (2007) using TM domain chimeras between P2X4R and P2X2R. Their data also showed that there is widespread rearrangement of the TMs during the opening of P2X4 receptors. These conclusions were based on experiments with chimeric channels and tryptophan-scanning mutagenesis. To evaluate IVM effects in such mutants and to identify residues that alter the effects of IVM application, the authors used the EC50 concentrations of ATP, a saturating concentration of IVM, and between 2-fold and 5-fold increases in the amplitude of current in the presence of IVM. Their study revealed weakened effects of IVM at V28A, I39W, V43W, and V47W TM1 mutants as well as at G340W, G342W, L345W, and V348W TM2 mutants and enhanced effects of IVM at G29W, V35W, L37W, L40W, A41W, S341A, G347W, A349V, V351W, and C353A mutants. Helical net diagrams of the TM domains showed a random distribution of these residues, and none of these residues were P2X4R-specific (Silberberg et al., 2007).
In our hands, the IVM-sensitive residues were Gln36, Leu40, Val43, Val47, Trp50, Asn338, Gly342, Leu346, Ala349, and Ile356. Replacing these residues with cysteine and alanine attenuated the allosteric action of IVM, but none of these mutations alone accounted for all the effects of this compound on the receptor function (Jelínkova et al., 2008; Jindrichova et al., 2009). The pattern of these predominantly nonpolar residues, which are also present in the IVM-sensitive S. mansoni P2X subunit, was consistent with the helical topology of both TM domains. Every third or fourth amino acid was affected by substitution (Fig. 8). It is unlikely that these residues directly contribute to IVM binding, but they could reflect a change in the IVM binding pocket. The most probable location of the IVM allosteric site is at the interface of the TM1 and TM2 domains, and its mechanism of action could involve the rotation of TM1 relative to TM2, leading to an increased potency of ATP (Silberberg et al., 2007).
Fig. 8.
Cartoon representation of the TM domains of rat P2X4 viewed parallel to the plasma membrane plane. Residues sensitive to the presence of IVM are shown in yellow. Replacement of these residues with cysteine and alanine attenuated the allosteric action of IVM, but none of these mutations alone accounted for all of the effects of this compound on the receptor function (Jelínková et al, 2008; Jindrichova et al., 2009). For better clarity, the IVM-sensitive residues of only one subunit are shown as sticks. It is clear that the pattern of these predominantly nonpolar residues is consistent with the helical topology of both TM domains. The process of channel opening probably involves the tilting and/or rotation of TM helices (Doyle, 2004). Therefore, the mechanism of IVM action could involve facilitating the reorientation of the TM segments during the channel opening. The figure was generated using PyMol v0.99 (http://www.pymol.org).
The specificity of the allosteric actions of IVM was used to identify native P2X4Rs in rat pituitary lactotrophs (Zemkova et al., 2010), hepatocytes (Emmett et al., 2008), sensory neurons (De Roo et al., 2003), alveolar macrophages (Bowler et al., 2003), human lung mast cells (Wareham et al., 2009), porcine tracheal smooth muscle cells (Nagaoka et al., 2009), and rabbit airway ciliated cells (Ma et al., 2006). IVM-sensitive currents were also detected in numerous mouse cell types, including macrophages (Sim et al., 2007), cortical neurons (Lalo et al., 2007), glial cells (Raouf et al., 2007), ventricular myocytes (Shen et al., 2006), submandibular gland ductal cells (Pochet et al., 2007), and Leydig cells (Antonio et al., 2009). IVM was also used to study the functional interactions of P2X4R with other members of this family of channels (Alqallaf et al., 2009; Casas-Pruneda et al., 2009). We have also frequently used the allosteric control of rP2X4R in structural-functional characterization of these receptors (Zemkova et al., 2007; Jelínkova et al., 2008; Rokic et al., 2010).
D. Alcohols Influence P2X Receptor Gating
Alcohols can modulate the function of several voltage-gated and ligand-gated ionic channels, most likely through interactions with the TM domains or hydrophobic regions within the channel structure (Crews et al., 1996). Alcohols also affect the gating properties of both recombinant and native P2XRs. Experiments with recombinant rP2X4R expressed in X. laevis oocytes revealed a dose-dependent inhibitory effect of ethanol on receptor function, causing a 2-fold rightward shift in the sensitivity of this receptor to ATP without altering the Emax. This effect is not dependent on membrane potential, and ethanol does not change the reversal potential of ATP-activated currents (Xiong et al., 2000). Ethanol also inhibits rP2X2R function but does so less effectively than rP2X4R (Davies et al., 2002). In the case of rP2X3R, ethanol potentiates the ATP-gated currents in a concentration-dependent manner and increases the maximal response to zinc, indicating that ethanol and zinc act at different sites (Davies et al., 2005a). In contrast, the function of hP2X3R was unaffected by 100 mM ethanol (Köles et al., 2000).
In the native P2XRs of amphibian DRG neurons, ethanol also inhibits P2XR function by shifting the agonist concentration-response curve to the right in a parallel manner, increasing the EC50 without affecting Emax. To distinguish whether this inhibition involves competitive antagonism or a decrease in the affinity of the agonist binding site, the authors also studied the kinetics of activation and deactivation of the P2XR current. These studies revealed that ethanol decreases the time-constant of P2XR deactivation without affecting the time-constant of activation, indicating that ethanol acts as an allosteric regulator, causing a decrease in the affinity of receptors for ATP. They also compared the potency of different alcohols for inhibiting ATP-activated current. The following rank order was reported: 1-propanol = trifluoroethanol > monochloroethanol > ethanol > methanol (Li et al., 1993, 1998; Weight et al., 1999). The effect of ethanol was not specific for this type of neuron but has also been observed in freshly isolated rat hippocampal CA1 neurons (Li et al., 2000) and ventral tegmental dopaminergic neurons (Xiao et al., 2008). The relevance of these findings to the pharmacology of ethanol and alcoholism remains elusive.
Several studies have focused on the identification of the binding site responsible for the allosteric actions of ethanol. The intracellular application of ethanol does not enhance the inhibition of current induced by extracellular ethanol, suggesting that the alcohol inhibition of ATP-gated ion channel function involves the extracellular domain of the receptor (Weight et al., 1999). Conserved extracellular cysteines in the P2X4R differentially regulate the inhibitory effect of ethanol in this receptor (Yi et al., 2009). The effects of mutating the conserved cysteine residues on ethanol modulation are probably indirect because of changes in the structure of the receptor, which in turn affects agonist binding and the molecular changes associated gating. In another study, His241 was identified as a residue responsible for the ethanol sensitivity of P2X4Rs (Xiong et al., 2005). Experiments with chimeric P2X2R and P2X3R revealed that ectodomain segments at the TM interfaces play key roles in determining the qualitative and quantitative responses to ethanol (Asatryan et al., 2008). Further experiments by the same group identified Asp331 and Met336 as critical TM residues for the ethanol sensitivity of P2X4R (Popova et al., 2010). It has recently been suggested that IVM could antagonize the ethanol-dependent inhibition of P2X4R current and that mutation of the Met336 residue affects both IVM and ethanol effects. A homology model built from the crystallized zP2X4.1R revealed that a pocket formed by Asp331, Met336, Trp46, and Trp50 could play a role in the allosteric action of ethanol and IVM (Asatryan et al., 2010).
E. Roles of Protein Kinases in Regulation of P2X Receptor Function
Protein kinase A (PKA) has been suggested to play a role in the prostaglandin E2-induced potentiation of P2X3R currents in DRG neurons (Wang et al., 2007). A role for PKA in the glucocorticoid-induced inhibition of P2XR current in the HT4 neuroblastoma cells of mice has also been proposed (Han et al., 2005). Both 8-bromo-cAMP and the PKA catalytic subunit cause a reduction in the amplitude of rP2X2R current, presumably through the phosphorylation of the C-terminal residue Ser431 (Chow and Wang, 1998). More recently, the potentiation of P2X4R function by this kinase was also reported and involves the phosphorylation of an accessory protein that interacts with the YXXGL endocytosis motif located in the C-terminal, resulting in an augmentation of P2X4Rs in the plasma membrane (Brown and Yule, 2010).
The ATP-induced P2X1R and P2X3R currents (Paukert et al., 2001; Vial et al., 2004; Brown and Yule, 2007), but not P2X4R and P2X7R currents (Brown and Yule, 2007), are potentiated by the phorbol ester phorbol 12-myristate 13-acetate, an activator of protein kinase C (PKC). Potentiation of P2X1R currents by 5-hydroxytryptamine 2A receptors is mediated by diacylglycerol- and calcium-dependent kinases (Ase et al., 2005). Other groups have suggested that P2X7R function is potentiated by PKCγ in the type-2 astrocyte cell line RBA-2 (Hung et al., 2005). The inhibitory effects of phorbol esters on agonist-induced P2XR currents have been observed in isolated DRG neurons from adult rats (Bie et al., 2009). A critical role of the cAMP sensor Epac in the sensitization of native P2X3R was also reported (Wang et al., 2007).
P2XRs share a putative PKC binding motif (TXR/K) at the receptor N-terminal that has been proposed as a site for modulation by phosphorylation. At first, mutation of the N-terminal Thr18 residue of rP2X2R was found to dramatically affect receptor desensitization, and the addition of the PKC activator phorbol 12-myristate 13-acetate recovers the desensitization profile in chimeric receptors (Boué-Grabot et al., 2000). Mutation of the respective threonine residues resulted in a receptor with either significantly smaller amplitudes or an absence of P2X1R- and P2X3R-gated currents (Liu et al., 2003; Franklin et al., 2007). However, there is no biochemical evidence of direct phosphorylation of P2X2R and at this residue (Franklin et al., 2007; Vial et al., 2004 Brown and Yule, 2007). The function of P2X7R is also affected by replacing Thr15 with other residues in a PKC-independent manner (Yan et al., 2008). These observations do not exclude the potential phosphorylation of other residues by PKC. In hP2X3R, the substitution of Ser/Thr residues situated within the PKC consensus phosphorylation sites with Ala either abolished (T134A, S178A) or did not alter (T196A, S269A) the UTP-induced potentiation of current (Wirkner et al., 2005; Stanchev et al., 2006).
It has also been suggested that the P2X3R current is down-regulated by the C-terminal SRC kinase and cyclin-dependent kinase-5-dependent receptor phosphorylation. For the C-terminal SRC kinase-dependent regulation, the C-terminal Tyr393 residue has been proposed as a target for phosphorylation (D'Arco et al., 2009; Nair et al., 2010). In mouse trigeminal sensory neurons, the role of this kinase is controlled by nerve growth factors (D'Arco et al., 2007).
F. Regulation of P2X Receptors by Lipids
Although phosphoinositides constitute only a small fraction of cellular phospholipids, their importance in the regulation of cellular functions is enormous. Among others, phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidylinositol-3,4,5-trisphosphate control the function of numerous membrane transport proteins, including Kv and Cav channels, ion channels that mediate sensory and nociceptive responses, epithelial transport proteins, and ionic exchangers (Gamper and Shapiro, 2007). Phosphoinositides also play a role in the regulation of P2XRs. At first, it was shown that PI3K inhibitors accelerate the P2X2R desensitization and that two positively charged residues, Lys365 and Lys369, located in the C-domain, are critical for the interaction of membrane phosphoinositides with the P2X2R (Fujiwara and Kubo, 2006). Immediately thereafter, it was demonstrated that PIP2 directly interacts with Lys364 of the P2X1R and that the inhibition of this interaction, by either the mutagenesis of Lys364 or reducing the abundance of membrane phosphoinositides by PI3K/PI4K blockade, resulted in a decrease of current amplitude and the recovery from desensitization (Bernier et al., 2008b). In heteromeric P2X1/5Rs, PIP2 modulates the receptor function through the P2X1 lipid-binding domain (Ase et al., 2010). The same group also showed similar effects of PIP2 and PIP3 on P2X4R activity. Using an in vitro binding assay, they found that phosphoinositides interact with the Cys360-Val375 sequence located in the C terminus of the receptor (Bernier et al., 2008a). Likewise, P2X3R and P2X7R are inhibited by decreases in PIP2 induced either by PI4K inhibition or phospholipase C activation by coexpressed G protein coupled receptors (Zhao et al., 2007a,b). Native and recombinant P2X2/3Rs are also regulated by phosphoinositides (Mo et al., 2009).
A lipopolysaccharide (LPS) is a large molecule consisting of a lipid and a polysaccharide joined by a covalent bond. LPSs are found in the outer membrane of Gram-negative bacteria act as endotoxins and elicit strong immune responses in animals. P2X7R has been shown to modulate the LPS-induced macrophage production of numerous inflammatory mediators. The P2X7 C-terminal domain contains several apparent protein-protein and protein-lipid interaction motifs that are potentially important for macrophage signaling and LPS action, including a conserved LPS-binding domain. Peptides derived from this P2X7 sequence bind LPS in vitro and neutralize the ability of LPS to activate the extracellular signal-regulated kinases and to promote the degradation of inhibitor of nuclear factor κB, α isoform, in vivo. Taken together, these data suggest that P2X7R, through its C terminus, may directly coordinate several signal transduction events related to macrophage function and LPS action (Denlinger et al., 2001). Other groups have reported effects of LPS on native P2X4Rs in microglia (Raouf et al., 2007).
Lysophosphatidylcholine (LPC), an inflammatory phospholipid that promotes microglial activation, may also contribute to P2X7R signaling. LPC was found to facilitate agonist-induced Ca2+ signaling in P2X7R-expressing microglial cell lines and to enhance the P2X7R-associated formation of membrane pores and the activation of the p44/42 mitogen-activated protein kinase. These results suggest that LPC may regulate microglial functions in the brain by enhancing the sensitivity of P2X7R (Takenouchi et al., 2007). Serum constituents can also affect P2X7R function (Michel et al., 2001). Polymyxin B, specifically its hydrophobic tail, positively modulates P2X7R-mediated ethidium uptake (Ferrari et al., 2006). In further support of the role of lipids in P2X7R activity, it has been shown that a diverse range of lipids increase agonist potency at the P2X7R in functional and binding studies (Michel and Fonfria, 2007).
Cholesterol-rich lipid rafts (Pike, 2003, 2004) may also play a role in P2XR function. It has been suggested that P2X3R localizes into lipid rafts in primary cultures of cerebellar granule neurons as well as in brain and DRG extracts (Vacca et al., 2004) and that the receptor undergoes rapid constitutive and cholesterol-dependent endocytosis (Vacca et al., 2009). Some reports have suggested that P2X3R does not require cholesterol for its function (Liu et al., 2006). P2X7R is also localized in lipid rafts (Gonnord et al., 2009), interacts with caveolin-1 (Barth et al., 2007), and controls the cellular levels of several lipid messengers through the modulation of phospholipases A2, C, and D (Garcia-Marcos et al., 2006). P2X1R is also expressed in lipid rafts (Vial and Evans, 2005; Vial et al., 2006). Cholesterol-depleting agents reduce P2X1R current by approximately 90%, but do not change P2X2R, P2X3R, and P2X4R currents. The N-terminal 20 to 23 and 27 to 19 residues seem to play a role in the cholesterol-dependent gating of this receptor (Allsopp et al., 2010).
G. Multiple Roles of Steroid Hormones on P2X Receptor Function
In addition to their genomic effects, which require a longer exposure time, steroids can also exhibit rapid effects, either by activating cell membrane G protein-coupled steroid receptors or by their allosteric actions on signaling molecules. The effect of neurosteroids on the GABAA receptor channel is well established (Harrison and Simmonds, 1984). There are also reports of allosteric actions of neurosteroids on nicotinic α4β2, N-methyl-d-aspartate, and glycine receptors (Paradiso et al., 2001; Ahrens et al., 2008; Johansson et al., 2008). Sex and adrenal steroid hormones influence P2XR expression by activating cytosolic receptors, leading to changes in transcriptional regulation (Holbird et al., 2001; Wang et al., 2008; Fan et al., 2009; Urabe et al., 2009). As discussed in detail below, steroids also exhibit rapid, nongenomic effects on P2XR function. In the absence of information about the binding site(s) for steroids, however, we cannot rule out the possibility that neuroactive steroids might interact with a cell membrane G protein-coupled steroid receptor that directly or indirectly modifies the ATP-gated currents.
The first report concerning the nongenomic effects of 17β-estradiol on the activity of native P2XRs expressed in rabbit detrusor was reported in 1999 (Ratz et al., 1999). 17β-estradiol also inhibits P2X2Rs expressed in PC12 cells in a nongenomic manner (Kim et al., 2000). Dehydroepiandrosterone sulfate and estrone also inhibit P2XRs expressed in PC12 cells (Liu et al., 2001b). A fast potentiating effect of dehydroepiandrosterone on native P2XRs expressed in rat sensory neurons, but only at submaximal concentrations of ATP, has also been reported (De Roo et al., 2003). In the same cells, progesterone also rapidly and reversibly potentiates submaximal, but not saturating, responses evoked by ATP. A similar effect was observed in cells expressing recombinant P2X2Rs, but not P2X1Rs, P2X3Rs, or P2X4Rs, indicating the receptor specificity of steroid action (De Roo et al., 2010). A nongenomic inhibitory effect of testosterone on P2XR function in isolated rat urinary bladder has also been observed (Hall et al., 2002).
It has also been reported that the neurosteroid alfaxalone potentiates the ATP-evoked P2X4R currents within 60 s, independent of the expression system used (HEK293 or oocytes). Likewise, allopregnanolone and 3α,21-dihydroxy-5α-pregnan-20-one potentiates the ATP-gated currents, but less effectively. In contrast, 0.3 to 10 μM pregnanolone, but not its sulfated derivative, inhibits the ATP-gated currents by approximately 40% in both cell types. Gonadal steroids 17β-estradiol and progesterone were inactive, revealing explicit structural requirements for steroid action on receptor function. Alfaxalone or 3α,21-dihydroxy-5α-pregnan-20-one gated P2X4R at concentrations 30- to 100-fold larger than those required to modulate the receptor, eliciting suramin and brilliant blue-sensitive currents, and potentiated the P2X4R more than 10-fold over the effect of 10 μM zinc (Codocedo et al., 2009).
Adrenal steroid hormones also rapidly influence P2XR function. In mouse HT4 neuroblastoma cells, the glucocorticoids corticosterone and cortisol and the synthetic glucocorticoid hormone dexamethasone inhibit P2XR-mediated calcium influx through a membrane-initiated, nongenomic pathway (Han et al., 2005). A rapid nongenomic effect of dexamethasone on the ATP-induced changes in cytosolic calcium was also observed in cochlear spiral ganglion neurons (Yukawa et al., 2005). Rapid inhibition of the ATP-induced currents and calcium signals by corticosterone was also observed in rat DRG neurons (Liu et al., 2008b, 2010).
H. Other Modulators
1. Reactive Oxygen Species.
Reactive oxygen species (ROS), including superoxide and other oxygen ions, free radicals and peroxides are chemically unstable, highly reactive molecules known to induce cell damage via oxidative stress. Hydrogen peroxide is a prominent endogenous ROS that can significantly increase as a result of several pathophysiological conditions. In a recent report, the modulation of P2X2R function by hydrogen peroxide, mercury, and the mitochondrial stress inducers myxothiazol and rotenone was described. Intracellular Cys430 was identified as a redox sensor for this receptor. It is noteworthy that the ATP-evoked currents in P2X4R were slightly inhibited by hydrogen peroxide, suggesting an opposite redox modulation that remains to be identified (Coddou et al., 2009). These novel findings indicate that P2XRs can modify their activity depending on the redox state of the cell. Future experiments are required to determine whether other P2XRs are also modulated by ROS.
2. Carbon Monoxide.
Carbon monoxide (CO), the metabolic product of heme oxygenases, is a potent modulator of a wide variety of physiological processes. It has been reported that CO also potentiates ATP-evoked currents in cells that express the P2X2R but was ineffective in cells expressing either the P2X3R or the heteromeric P2X2/3R. Moreover, these authors found a small but significant inhibition of the ATP-evoked currents in P2X4R-expressing cells (Wilkinson et al., 2009). These results suggest that P2X2R and P2X4R may have an allosteric site for CO or that this gas exerts its actions through indirect mechanisms. In this regard, the effects of CO in P2X2R and P2X4R are similar to those of ROS and mitochondrial stress inducers (Coddou et al., 2009). Therefore, it is plausible that CO can bind to mitochondrial complexes and stimulate ROS production, a mechanism that has been shown to modulate L-type Cav channels (Peers et al., 2009).
3. Cibacron Blue 3GA.
The putative nonselective P2R antagonist Cibacron blue (the purified ortho-isomer) (Ralevic and Burnstock, 1998) acts as an allosteric regulator of P2XRs at concentrations lower than that required for P2R antagonism. One study described its potentiating activity on rP2X4R. Pretreatment with Cibacron blue mediated a 4-fold increase in the potency of ATP (EC50, 3.3 μM) without affecting the maximum response (Miller et al., 1998). Electrophysiological and calcium influx data also suggest that Cibacron blue functions as a positive allosteric modulator of hP2X3R activity and may also contribute to the increasing the rate of receptor recovery from agonist-induced desensitization. These effects were independent of the agonist used to activate receptor and were concentration-dependent with an EC50 of 1.4 μM (Alexander et al., 1999). Cibacron blue also potentiates BzATP-induced nociception, probably acting through P2X3R and P2X2/3R (Jarvis et al., 2001). However, the effects of Cibacron blue on recombinant P2X2/3R have not been tested.
4. Propofol, Ketamine, and Toluene.
Propofol, an intravenous anesthetic that is widely used clinically, potentiates the activity of P2X4Rs expressed in HEK293 cells and has no effect on P2X2Rs or P2X2/3Rs (Tomioka et al., 2000; Davies et al., 2005b). Propofol also potentiates native P2X7Rs expressed in microglia, an effect that was mimicked by two other clinically relevant intravenous anesthetics, thiopental and ketamine (Nakanishi et al., 2007). In another study, neither ketamine nor propofol affected P2X2Rs (Furuya et al., 1999). Toluene, an abused organic solvent, potentiates the ATP-evoked P2X2R and P2X4R currents, as well as P2X4/6R and P2X2/3R currents, and inhibits P2X3R currents (Woodward et al., 2004). There is no information regarding the residues involved in the actions of these compounds.
5. Tetramethylpyrasine.
This is an alkaloid used in traditional Chinese medicine as an analgesic for injury and dysmenorrhea (Liang et al., 2005). It inhibits the effects of nucleotides at native P2X3Rs responsible for the primary afferent transmission of neuropathic pain states, presumably acting as an allosteric modulator by binding at an unidentified extracellular domain (Gao et al., 2008).
V. Future Directions
Although it took 7 years to get the zP2X4.1R crystal (Silberberg and Swartz, 2009), it is still reasonable to suggest that one of the P2XRs will be crystallized in the presence of agonist, which will provide a major advance in understanding the binding domain and gating mechanisms of these receptors. In the absence of such data, additional structural based modeling could provide a better understanding of the possible organization of orthosteric binding sites and development of subunit specific antagonists to be used in further studies and therapeutics and the gating of the channel pore. Further studies should also help in understanding how changes in intracellular receptor domains are transmitted to the rest of the channel, including changes caused by the association of P2XRs with numerous interacting proteins. Future studies could also clarify how activation of numerous signaling pathways by calcium influx and the cross-talk with G protein-coupled receptors affects P2XR function. In addition to structural and mathematical modeling, voltage-clamp fluorometry and other techniques used to examine the changes in accessibility of the protein domains could provide some temporal resolution of the conformational changes caused by the binding of an agonist in the presence and absence of allosteric modulators.
Allosteric modulators constitute a valuable alternative for future drug design, especially for receptor-specific drugs. The close topological location of the trace metal coordination amino acid residues to the orthosteric ATP sites also provides clues for further structural modeling and investigation into the actions of metals in the receptor ectodomain. Trace metals can be released to the synaptic cleft and exert effects on signaling, altering neuronal excitability, but further studies are needed to clarify the physiological conditions for the action of these metals on P2XR function in vivo. The finding that other endogenous compounds, such as protons, ROS, or neurosteroids, modulate P2XR activity also places allosteric modulation in a more physiological/pathophysiological context. For example, changes in cellular pH caused by ischemia could affect P2X3R activity. Furthermore, an intracellular redox sensor for the P2X2R that modulates channel activity could play an important role in oxidative stress or the response to abundant ROS production by either pathophysiological conditions or drugs. Likewise, the discovery that P2XRs are modulated by neurosteroids in a positive and negative manner raises the possibility that P2XR signaling and brain excitability could change with variations in steroid levels caused by physiological and pathophysiological conditions as reproductive cycles and emotional and/or endocrine state of the body. These observations provide a basis for further studies regarding the dependence of the activity of a particular receptor on the metabolic state of cells, tissues, and organs.
VI. Conclusions
The recent 3.5-Å crystallography of zP2X4.1R in a closed state is a remarkable breakthrough in the purinergic field. Not only has it provided a detailed description of P2XR tertiary and quaternary structure but also it confirmed the assumption of the trimeric nature of P2XRs raised from pioneer biochemical, functional and microscopy studies. The identity of the 10 highly conserved ectodomain cysteines, plus the correct assignment of the five possible sulfhydryl bonds and their role in the tertiary receptor folding, was established beyond a doubt. The structure of zP2X4.1R was solved in the absence of ATP, which limits our understanding of the residues involved in the formation of orthosteric binding sites and the positive and negative cooperativity with allosteric binding sites. However, the cloning of P2X sequences that are distantly related through phylogeny has demonstrated that relatively few ectodomain residues are conserved across all subunits, and their importance in receptor functions has been shown in mutagenesis studies. As with other ligand-gated channels, P2XRs have numerous regulatory sites in both the extra- and intracellular domains. Therefore, channel activity is multiregulated by a complex set of synapse- and/or paracrine-derived native agents, as well as intracellular metabolites. In contrast to the ATP binding sites, the nonconserved residues seem to play a critical role in allosteric modulation of these channels by trace metals, divalent cations, and protons, which explains the receptor specificity of the actions of these molecules. P2XRs are also modulated by membrane lipids, kinases, and ROS acting intracellularly. Drugs also modify ATP signaling. A prominent example is the positive and potent modulator role of IVM on P2X4R that probably occurs within the TM receptor domain and is currently used as a pharmacological criterion to characterize the involvement of this particular receptor in cellular functions. Thus, the P2XR affinity for ATP and/or the changes in the maximal currents is not only determined by the ligand concentration but also depends on the availability of these regulators in the receptor milieu. The current information regarding the residues involved in orthosteric and allosteric modulation combined with the channel structure provided by crystallization will lead to a better understanding of the structure-activity relationships that govern these processes.
Acknowledgments
This research was funded in part by the Intramural Research Program of the National Institutes of Health National Institute of Child Health and Human Development (to C.C., Z.Y., S.S.S.); the Internal Grant Agency of the Academy of Sciences (to T.O.); the Fondo de Financiamiento de Centros de Excelencia en Investigación [Grant 13980001], and the Instituto Milenio de Biología Fundamental y Aplicada and PFB 12/2007 (to J.P.H.-T.).
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Coddou, Yan, Obsil, Huidobro-Toro, and Stojilkovic.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.110.003129.
According to the IUPHAR website (http://www.iuphar-db.org), the P2XR numbers should not be subscript as was commonly used in the past.
- A317491
- 5-[[[(3-phenoxyphenyl)methyl][(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbonyl]-1,2,4-benzenetricarboxylic acid sodium salt hydrate
- A438079
- 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride
- A740003
- N-(1-([(cyanoimino)(5-quinolinylamino) methyl] amino)-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide
- A804598
- 2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine
- ApnA
- diadenosine polyphosphate (where n = the number of phosphates)
- AR
- adenosine receptor
- ATPγS
- adenosine-5′-O-(3-thiotriphosphate)
- AZ10606120
- N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride
- AZ11645373
- 3-[1-[[(3′-nitro[1,1′-biphenyl]-4-yl)oxy]methyl]-3-(4-pyridinyl)propyl]-2,4-thiazolidinedione
- BBG
- Coomassie brilliant blue G
- BzATP
- 2′(3′)-O-4-benzoylbenzoyl)-ATP
- CaM
- calmodulin
- DRG
- dorsal root ganglion
- GW791343
- N2-(3,4-difluorophenyl)-N1-((2-methyl-5-(1-piperazinylmethyl)phenyl)glycinamide dihydrochloride
- h
- human
- HEK
- human embryonic kidney
- IpnI
- diinosine polyphosphate (where n = the number of phosphates)
- IVM
- ivermectin
- KN-62
- 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine
- LPC
- lysophosphatidylcholine
- LPS
- lipopolysaccharide
- m
- mouse
- meATP
- methylene-ATP
- 2-meSATP
- 2-methylthio-ATP
- MG50-3-1
- 1-amino-4-(4-(4-chloro-6-(2-sulfonatophenylamino)-(1,3,5)triazine-2-ylamino]-2-sulfonatophenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid trisodium salt
- MK3
- ortho-(2,2′,2″,2⁗-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetrakis-benzenesulfonic acid
- MRS2159
- 4-[(4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl}-2-pyridinyl)azo]-benzoic acid
- MRS2219
- 1,5-dihydro-3-hydroxy-8-methyl[1,3,2]dioxaphosphepino[5,6-c]pyridin-9-ol-3-oxide
- MRS2220
- 6-azophenyl-2′,5′-disulfonate derivative
- MRS2257
- pyridoxal-5′-phosphate-6-azophenyl-3′,5′-bimethylenphosphonate
- MRS2339
- 1′S,2R,3S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-1-[phosphoryloxymethyl] bicycle[3.1.0]hexane-2,3-diol
- NF023
- 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulfonic acid
- NF110
- 4,4′,4″,4⁗-[carbonylbis[imino-5,1,3-benzenetriylbis(carbonylimino)]]tetrakisbenzenesulfonic acid tetrasodium salt
- NF279
- 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid
- NF449
- 4,4′,4″,4⁗-[carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))]tetrakis-1,3-benzenedisulfonic acid
- NF770
- 7,7′-(carbonylbis(imino-3,1-phenylenecarbonylimino-3,1-(4-methyl-phenylene)carbonylimino))bis(1-methoxy-naphthalene-3,6-disulfonic acid) tetrasodium salt
- NF776
- 6,6′-(carbonylbis(imino-3,1-(4-methylphenylene)carbonylimino))bis(1-methoxynaphthalene-3,5-disulfonic acid) tetrasodium salt
- NF778
- 6,6′-(carbonylbis(imino-3,1-phenylenecarbonylimino-3,1-(4-methyl-phenylene)carbonylimino))bis(1-methoxy-naphthalene-3,5-disulfonic acid) tetrasodium salt
- NF864
- 8,8′,8″,8⁗-(carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino)))tetrakis-naphthalene-1,3,5-trisulfonic acid-dodecasodium salt
- P2XRs
- purinergic P2X cation-conducting receptor channels
- P2YRs
- G protein-coupled P2Y receptors
- PIP2
- phosphatidylinositol-4,5-bisphosphate
- PKA
- protein kinase A
- PKC
- protein kinase C
- PPADS
- pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulfonic acid
- PPNDS
- pyridoxal-5′-phosphate-6-(2′-naphthylazo-6′-nitro-4′,8′-disulfonate
- PSB-1011
- disodium 1-amino-4-[3-(4,6-dichloro[1,3,5]triazine-2-ylamino)-4-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate
- PSB-10211
- 1-amino-4-[3–4,6-dichloro[1,3,(5]triazine-2-ylamino)phenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate
- r
- rat
- RB-2
- reactive blue 2
- Ro-0437626
- N-[(1R)-2-[[(1S,2R,3S)-1-(cyclohexylmethyl)-3-cyclopropyl-2,3-dihydroxypropyl]amino]-2-oxo-1-(4-thiazolylmethyl)ethyl]-1H-benzimidazole-2-carboxamide
- RO-4
- 5-[5-iodo-4-methoxy-2-(1-methylethyl)phenoxy]-2,4-pyrimidine diamine hydrochloride
- RO-5
- 5-(5-ethynyl-2-isopropyl-4-methoxy-phenoxy)-pyrimidine-2,4-diamine
- RO-85
- 1-methyl-3-phenyl-1H-thieno[2,3-c]pyrazole-5-carboxylic acid [(R)-2-(4-acetyl-piperazin-1-yl)-1-methyl-ethyl]-amide
- ROS
- reactive oxygen species
- SB9
- 6-((4,6,8-trisulfo-1-naphthyl)iminocarbonyl-1, 3-(4-methylphenylene)iminocarbonyl-1,3-phenylene-azo)-pyridoxal-5′-phosphate
- SS
- disulfide bridges
- TM
- transmembrane
- TNP-ATP
- trinitrophenyl-ATP
- z
- zebrafish.
References
- Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. (2007) Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem 101:17–26 [DOI] [PubMed] [Google Scholar]
- Acuña-Castillo C, Morales B, Huidobro-Toro JP. (2000) Zinc and copper modulate differentially the P2X4 receptor. J Neurochem 74:1529–1537 [DOI] [PubMed] [Google Scholar]
- Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F. (2008) ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J 22:861–869 [DOI] [PubMed] [Google Scholar]
- Agboh KC, Webb TE, Evans RJ, Ennion SJ. (2004) Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem 279:41650–41657 [DOI] [PubMed] [Google Scholar]
- Ahrens J, Leuwer M, Demir R, Krampfl K, Foadi N, Haeseler G. (2008) The anaesthetic steroid alphaxalone positively modulates alpha1-glycine receptor function. Pharmacology 82:228–232 [DOI] [PubMed] [Google Scholar]
- Aitken A. (1999) Protein consensus sequence motifs. Mol Biotechnol 12:241–253 [DOI] [PubMed] [Google Scholar]
- Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, van Biesen T. (1999) Allosteric modulation and accelerated resensitization of human P2X(3) receptors by cibacron blue. J Pharmacol Exp Ther 291:1135–1142 [PubMed] [Google Scholar]
- Allsopp RC, Lalo U, Evans RJ. (2010) Lipid raft association and cholesterol sensitivity of P2X1–4 receptors for ATP: chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J Biol Chem 285:32770–32777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqallaf SM, Evans BA, Kidd EJ. (2009) Atypical P2X receptor pharmacology in two human osteoblast-like cell lines. Br J Pharmacol 156:1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio LS, Costa RR, Gomes MD, Varanda WA. (2009) Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal 5:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins D, Trudell JR, Alkana RL, Davies DL. (2010) Ivermectin antagonizes ethanol inhibition in P2X4 receptors. J Pharmacol Exp Ther 334:720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. (2008) Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology 55:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. (2004) Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol 342:333–343 [DOI] [PubMed] [Google Scholar]
- Ase AR, Bernier LP, Blais D, Pankratov Y, Séguéla P. (2010) Modulation of heteromeric P2X1/5 receptors by phosphoinositides in astrocytes depends on the P2X1 subunit. J Neurochem 113:1676–1684 [DOI] [PubMed] [Google Scholar]
- Ase AR, Raouf R, Bélanger D, Hamel E, Séguéla P. (2005) Potentiation of P2X1 ATP-gated currents by 5-hydroxytryptamine 2A receptors involves diacylglycerol-dependent kinases and intracellular calcium. J Pharmacol Exp Ther 315:144–154 [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. (1984) Release of endogenous Zn2+ from brain tissue during activity. Nature 308:734–736 [DOI] [PubMed] [Google Scholar]
- Banfi C, Ferrario S, De Vincenti O, Ceruti S, Fumagalli M, Mazzola A, D' Ambrosi N, Volontè C, Fratto P, Vitali E, et al. (2005) P2 receptors in human heart: upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFalpha and potential role in myocardial cell death. J Mol Cell Cardiol 39:929–939 [DOI] [PubMed] [Google Scholar]
- Baqi Y, Hausmann R, Rosefort C, Rettinger J, Schmalzing G, Muller CE. (2011) Discovery of potent competitive antagonists and positive modulators of the P2X2 receptor. J Med Chem 54:817–830 [DOI] [PubMed] [Google Scholar]
- Barrera NP, Henderson RM, Murrell-Lagnado RD, Edwardson JM. (2007) The stoichiometry of P2X2/6 receptor heteromers depends on relative subunit expression levels. Biophys J 93:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. (2005) Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280:10759–10765 [DOI] [PubMed] [Google Scholar]
- Barth K, Weinhold K, Guenther A, Young MT, Schnittler H, Kasper M. (2007) Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J 274:3021–3033 [DOI] [PubMed] [Google Scholar]
- Bavan S, Straub VA, Blaxter ML, Ennion SJ. (2009) A P2X receptor from the tardigrade species Hypsibius dujardini with fast kinetics and sensitivity to zinc and copper. BMC Evol Biol 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Williams CA, Ceelen PW. (1990) ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci 10:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boué-Grabot E, Logothetis D, Séguéla P. (2008a) Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci 28:12938–12945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Tong X, Hamel E, Blais D, Zhao Q, Logothetis DE, Séguéla P. (2008b) Direct modulation of P2X1 receptor-channels by the lipid phosphatidylinositol 4,5-bisphosphate. Mol Pharmacol 74:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, et al. (1999) Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 376:127–138 [DOI] [PubMed] [Google Scholar]
- Bie BH, Zhang YH, Zhao ZQ. (2009) Inhibition of P2X receptor-mediated inward current by protein kinase C in small-diameter dorsal root ganglion neurons of adult rats. Neurosci Bull 25:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287:F1084–F1091 [DOI] [PubMed] [Google Scholar]
- Blake CC, Geisow MJ, Oatley SJ, Rérat B, Rérat C. (1978) Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol 121:339–356 [DOI] [PubMed] [Google Scholar]
- Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. (2003) Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol 63:1407–1416 [DOI] [PubMed] [Google Scholar]
- Bo X, Schoepfer R, Burnstock G. (2000) Molecular cloning and characterization of a novel ATP P2X receptor subtype from embryonic chick skeletal muscle. J Biol Chem 275:14401–14407 [DOI] [PubMed] [Google Scholar]
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. (1995) A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett 375:129–133 [DOI] [PubMed] [Google Scholar]
- Bobanovic LK, Royle SJ, Murrell-Lagnado RD. (2002) P2X receptor trafficking in neurons is subunit specific. J Neurosci 22:4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boué-Grabot E, Archambault V, Séguéla P. (2000) A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X(2) ATP-gated channels. J Biol Chem 275:10190–10195 [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Górecki DC, Murrell-Lagnado R. (2009) Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 284:13446–13454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler JW, Bailey RJ, North RA, Surprenant A. (2003) P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol 140:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. (1998) The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12:256–268 [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. (1994) New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371:519–523 [DOI] [PubMed] [Google Scholar]
- Brändle U, Guenther E, Irrle C, Wheeler-Schilling TH. (1998) Gene expression of the P2X receptors in the rat retina. Brain Res Mol Brain Res 59:269–272 [DOI] [PubMed] [Google Scholar]
- Brändle U, Spielmanns P, Osteroth R, Sim J, Surprenant A, Buell G, Ruppersberg JP, Plinkert PK, Zenner HP, Glowatzki E. (1997) Desensitization of the P2X(2) receptor controlled by alternative splicing. FEBS Lett 404:294–298 [DOI] [PubMed] [Google Scholar]
- Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. (2001) NF449: a subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn Schmiedebergs Arch Pharmacol 364:285–290 [DOI] [PubMed] [Google Scholar]
- Brône B, Moechars D, Marrannes R, Mercken M, Meert T. (2007) P2X currents in peritoneal macrophages of wild type and P2X4−/− mice. Immunol Lett 113: 83–89 [DOI] [PubMed] [Google Scholar]
- Brotherton-Pleiss CE, Dillon MP, Ford AP, Gever JR, Carter DS, Gleason SK, Lin CJ, Moore AG, Thompson AW, Villa M, et al. (2010) Discovery and optimization of RO-85, a novel drug-like, potent, and selective P2X3 receptor antagonist. Bioorg Med Chem Lett 20:1031–1036 [DOI] [PubMed] [Google Scholar]
- Brown DA, Yule DI. (2007) Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta 1773:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Yule DI. (2010) Protein kinase A regulation of P2X(4) receptors: Requirement for a specific motif in the C-terminus. Biochim Biophys Acta 1803:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SG, Kim YC, Kim SA, Jacobson KA, Burnstock G, King BF. (2001) Actions of a series of PPADS analogs at P2X1 and P2X3 receptors. Drug Dev Res 53:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SG, Townsend-Nicholson A, Jacobson KA, Burnstock G, King BF. (2002) Heteromultimeric P2X(1/2) receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther 300:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LE, Jiang LH, North RA. (2010) New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. (1996) An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J 15:55–62 [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, van Biesen T, Lynch KJ, Touma E, Metzger RE, Kowaluk EA, Jarvis MF. (1999) P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol 82:1590–1598 [DOI] [PubMed] [Google Scholar]
- Burkhart CN. (2000) Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet Hum Toxicol 42:30–35 [PubMed] [Google Scholar]
- Burnstock G. (1972) Purinergic nerves. Pharmacol Rev 24:509–581 [PubMed] [Google Scholar]
- Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Cocks T, Crowe R, Kasakov L. (1978) Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol 63:125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 [DOI] [PubMed] [Google Scholar]
- Calvert JA, Evans RJ. (2004) Heterogeneity of P2X receptors in sympathetic neurons: contribution of neuronal P2X1 receptors revealed using knockout mice. Mol Pharmacol 65:139–148 [DOI] [PubMed] [Google Scholar]
- Calvert RC, Shabbir M, Thompson CS, Mikhailidis DP, Morgan RJ, Burnstock G. (2004) Immunocytochemical and pharmacological characterisation of P2-purinoceptor-mediated cell growth and death in PC-3 hormone refractory prostate cancer cells. Anticancer Res 24:2853–2859 [PubMed] [Google Scholar]
- Carrasquero LM, Delicado EG, Bustillo D, Gutiérrez-Martín Y, Artalejo AR, Miras-Portugal MT. (2009) P2X7 and P2Y13 purinergic receptors mediate intracellular calcium responses to BzATP in rat cerebellar astrocytes. J Neurochem 110:879–889 [DOI] [PubMed] [Google Scholar]
- Carter DS, Alam M, Cai H, Dillon MP, Ford AP, Gever JR, Jahangir A, Lin C, Moore AG, Wagner PJ, et al. (2009) Identification and SAR of novel diaminopyrimidines. Part 1: The discovery of RO-4, a dual P2X(3)/P2X(2/3) antagonist for the treatment of pain. Bioorg Med Chem Lett 19:1628–1631 [DOI] [PubMed] [Google Scholar]
- Casas-Pruneda G, Reyes JP, Pérez-Flores G, Pérez-Cornejo P, Arreola J. (2009) Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J Physiol 587:2887–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824 [DOI] [PubMed] [Google Scholar]
- Chaumont S, Compan V, Toulme E, Richler E, Housley GD, Rassendren F, Khakh BS. (2008) Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal 1:ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont S, Khakh BS. (2008) Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA 105:12063–12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377:428–431 [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, et al. (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114:386–396 [DOI] [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey PP. (1997) Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol 121:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey PP. (1998) Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol 124:1314–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow YW, Wang HL. (1998) Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem 70:2606–2612 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, May LT, Avlani VA, Sexton PM. (2004) G-protein-coupled receptor allosterism: the promise and the problem(s). Biochem Soc Trans 32:873–877 [DOI] [PubMed] [Google Scholar]
- Cinkilic O, King BF, van der Giet M, Schlüter H, Zidek W, Burnstock G. (2001) Selective agonism of group I P2X receptors by dinucleotides dependent on a single adenine moiety. J Pharmacol Exp Ther 299:131–136 [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR, Meadows HJ. (2000) Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 523:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Brown TC, Hume RI. (2003) Expression level dependent changes in the properties of P2X2 receptors. Neuropharmacology 44:403–412 [DOI] [PubMed] [Google Scholar]
- Clyne JD, LaPointe LD, Hume RI. (2002a) The role of histidine residues in modulation of the rat P2X(2) purinoceptor by zinc and pH. J Physiol 539:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Wang LF, Hume RI. (2002b) Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci 22:3873–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, et al. (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, et al. (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015 [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Gomperts BD. (1979) Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol 296:229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Acuña-Castillo C, Bull P, Huidobro-Toro JP. (2007) Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J Biol Chem 282:36879–36886 [DOI] [PubMed] [Google Scholar]
- Coddou C, Codocedo JF, Li S, Lillo JG, Acuña-Castillo C, Bull P, Stojilkovic SS, Huidobro-Toro JP. (2009) Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. J Neurosci 29:12284–12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Lorca RA, Acuña-Castillo C, Grauso M, Rassendren F, Huidobro-Toro JP. (2005) Heavy metals modulate the activity of the purinergic P2X4 receptor. Toxicol Appl Pharmacol 202:121–131 [DOI] [PubMed] [Google Scholar]
- Coddou C, Morales B, González J, Grauso M, Gordillo F, Bull P, Rassendren F, Huidobro-Toro JP. (2003) Histidine 140 plays a key role in the inhibitory modulation of the P2X4 nucleotide receptor by copper but not zinc. J Biol Chem 278:36777–36785 [DOI] [PubMed] [Google Scholar]
- Codocedo JF, Rodríguez FE, Huidobro-Toro JP. (2009) Neurosteroids differentially modulate P2X ATP-gated channels through non-genomic interactions. J Neurochem 110:734–744 [DOI] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology 36:1277–1283 [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. (1996) Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. (1997) Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology 36:1303–1308 [DOI] [PubMed] [Google Scholar]
- Cook SP, Rodland KD, McCleskey EW. (1998) A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci 18:9238–9244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. (1996) Effects of ethanol on ion channels. Int Rev Neurobiol 39:283–367 [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371:707–711 [DOI] [PubMed] [Google Scholar]
- D'Arco M, Giniatullin R, Leone V, Carloni P, Birsa N, Nair A, Nistri A, Fabbretti E. (2009) The C-terminal Src inhibitory kinase (Csk)-mediated tyrosine phosphorylation is a novel molecular mechanism to limit P2X3 receptor function in mouse sensory neurons. J Biol Chem 284:21393–21401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, Fabbretti E. (2007) Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci 27:8190–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist R, Diamant B. (1974) Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 34:368–384 [DOI] [PubMed] [Google Scholar]
- Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, Rettinger J, Mutschler E, Lambrecht G. (1998) NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. Eur J Pharmacol 350:R5–R6 [DOI] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. (2005a) Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology 49:243–253 [DOI] [PubMed] [Google Scholar]
- Davies DL, Kuo ST, Alkana RL. (2005b) Differential effects of propofol and ethanol on P2X4 receptors expressed in Xenopus oocytes. Int Congr Ser 1283:285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. (2002) Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res 26:773–778 [PubMed] [Google Scholar]
- De Roo M, Boué-Grabot E, Schlichter R. (2010) Selective potentiation of homomeric P2X2 ionotropic ATP receptors by a fast non-genomic action of progesterone. Neuropharmacology 58:569–577 [DOI] [PubMed] [Google Scholar]
- De Roo M, Rodeau JL, Schlichter R. (2003) Dehydroepiandrosterone potentiates native ionotropic ATP receptors containing the P2X2 subunit in rat sensory neurones. J Physiol 552:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, Proctor RA, Bertics PJ. (2001) Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol 167:1871–1876 [DOI] [PubMed] [Google Scholar]
- Dent JA, Davis MW, Avery L. (1997) avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J 16:5867–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. (2001) Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 21:7143–7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 32:79–87 [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97:587–600 [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Cox JA, Migita K, Haines W, Egan TM, Voigt MM. (2002) Cloning and characterization of two novel zebrafish P2X receptor subunits. Biochem Biophys Res Commun 295:849–853 [DOI] [PubMed] [Google Scholar]
- Digby HR, Roberts JA, Sutcliffe MJ, Evans RJ. (2005) Contribution of conserved glycine residues to ATP action at human P2X1 receptors: mutagenesis indicates that the glycine at position 250 is important for channel function. J Neurochem 95:1746–1754 [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. (1999) Ion permeation and block of P2X(2) purinoceptors: single channel recordings. J Membr Biol 172:215–223 [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. (2000) Inactivation of P2X2 purinoceptors by divalent cations. J Physiol 522:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. (2008) Painful purinergic receptors. J Pharmacol Exp Ther 324:409–415 [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. (2009a) Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol 157:1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, Carroll WA, Jarvis MF. (2009b) [3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology 56:223–229 [DOI] [PubMed] [Google Scholar]
- Doyle DA. (2004) Structural changes during ion channel gating. Trends Neurosci 27:298–302 [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. (2003) P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 23:1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckwitz W, Hausmann R, Aschrafi A, Schmalzing G. (2006) P2X5 subunit assembly requires scaffolding by the second transmembrane domain and a conserved aspartate. J Biol Chem 281:39561–39572 [DOI] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. (2001) P2X receptors in peripheral neurons. Prog Neurobiol 65:107–134 [DOI] [PubMed] [Google Scholar]
- Dutton JL, Poronnik P, Li GH, Holding CA, Worthington RA, Vandenberg RJ, Cook DI, Barden JA, Bennett MR. (2000) P2X(1) receptor membrane redistribution and down-regulation visualized by using receptor-coupled green fluorescent protein chimeras. Neuropharmacology 39:2054–2066 [DOI] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. (2009) Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses 34:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Haines WR, Voigt MM. (1998) A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci 18:2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Khakh BS. (2004) Contribution of calcium ions to P2X channel responses. J Neurosci 24:3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhorst AN, Berson A, Cockayne D, Lester HA, Khakh BS. (2002) Control of P2X(2) channel permeability by the cytosolic domain. J Gen Physiol 120:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, McWilliams R, Doctor RB, Fitz JG. (2008) Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology 47:698–705 [DOI] [PubMed] [Google Scholar]
- Ennion S, Hagan S, Evans RJ. (2000) The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem 275:35656. [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. (2001) Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett 489:154–158 [DOI] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. (2002) Conserved cysteine residues in the extracellular loop of the human P2X(1) receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol Pharmacol 61:303–311 [DOI] [PubMed] [Google Scholar]
- Ennion SJ, Ritson J, Evans RJ. (2001) Conserved negatively charged residues are not required for ATP action at P2X(1) receptors. Biochem Biophys Res Commun 289:700–704 [DOI] [PubMed] [Google Scholar]
- Evans RJ. (2009) Orthosteric and allosteric binding sites of P2X receptors. Eur Biophys J 38:319–327 [DOI] [PubMed] [Google Scholar]
- Evans RJ. (2010) Structural interpretation of P2X receptor mutagenesis studies on drug action. Br J Pharmacol 161:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. (1995) Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol 48:178–183 [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. (1996) Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol 497:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti E, D'Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. (2006) Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26:6163–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yu LH, Zhang Y, Ni X, Ma B, Burnstock G. (2009) Estrogen altered visceromotor reflex and P2X(3) mRNA expression in a rat model of colitis. Steroids 74:956–962 [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883 [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310:1495–1499 [DOI] [PubMed] [Google Scholar]
- Fischer W, Krügel U. (2007) P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem 14:2429–2455 [DOI] [PubMed] [Google Scholar]
- Fischer W, Zadori Z, Kullnick Y, Gröger-Arndt H, Franke H, Wirkner K, Illes P, Mager PP. (2007) Conserved lysin and arginin residues in the extracellular loop of P2X(3) receptors are involved in agonist binding. Eur J Pharmacol 576:7–17 [DOI] [PubMed] [Google Scholar]
- Flittiger B, Klapperstück M, Schmalzing G, Markwardt F. (2010) Effects of protons on macroscopic and single-channel currents mediated by the human P2X7 receptor. Biochim Biophys Acta 1798:947–957 [DOI] [PubMed] [Google Scholar]
- Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. (2006) Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147 (Suppl 2):S132–S143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Burnstock G. (2009) An evolutionary history of P2X receptors. Purinergic Signal 5:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cao L, Young MT, North RA. (2008) Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem 283:15122–15126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, North RA. (2006) A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem 281:15044–15049 [DOI] [PubMed] [Google Scholar]
- Franke H, Grosche J, Schädlich H, Krügel U, Allgaier C, Illes P. (2001) P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 108:421–429 [DOI] [PubMed] [Google Scholar]
- Franklin C, Braam U, Eisele T, Schmalzing G, Hausmann R. (2007) Lack of evidence for direct phosphorylation of recombinantly expressed P2X(2) and P2X (3) receptors by protein kinase C. Purinergic Signal 3:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Boehm S, Beindl W, Nickel P, Ijzerman AP, Hohenegger M, Nanoff C. (1996) Suramin analogues as subtype-selective G protein inhibitors. Mol Pharmacol 49:602–611 [PubMed] [Google Scholar]
- Freist W, Verhey JF, Stühmer W, Gauss DH. (1998) ATP binding site of P2X channel proteins: structural similarities with class II aminoacyl-tRNA synthetases. FEBS Lett 434:61–65 [DOI] [PubMed] [Google Scholar]
- Friday SC, Hume RI. (2008) Contribution of extracellular negatively charged residues to ATP action and zinc modulation of rat P2X2 receptors. J Neurochem 105:1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD. (1988) An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol 401:361–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Bean BP. (1988) Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol 91:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. (1988) Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol 404:39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. (2006) Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol 576:135–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya R, Oka K, Watanabe I, Kamiya Y, Itoh H, Andoh T. (1999) The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P2x purinoceptors in PC12 cells. Anesth Analg 88:174–180 [DOI] [PubMed] [Google Scholar]
- Galligan JJ. (2004) Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol 141:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. (2007) Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8:921–934 [DOI] [PubMed] [Google Scholar]
- Gao Y, Xu C, Liang S, Zhang A, Mu S, Wang Y, Wan F. (2008) Effect of tetramethylpyrazine on primary afferent transmission mediated by P2X3 receptor in neuropathic pain states. Brain Res Bull 77:27–32 [DOI] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA. (2006) Keynote review: allosterism in membrane receptors. Drug Discov Today 11:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stühmer W. (1997a) Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol 51:109–118 [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Laube B, Stühmer W. (1996) Molecular cloning and functional expression of a novel rat heart P2X purinoceptor. FEBS Lett 388:123–127 [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Stühmer W, Soto F. (1997b) Molecular characterization and pharmacological properties of the human P2X3 purinoceptor. Brain Res Mol Brain Res 47:59–66 [DOI] [PubMed] [Google Scholar]
- Garcia-Marcos M, Pochet S, Marino A, Dehaye JP. (2006) P2X7 and phospholipid signalling: the search of the “missing link” in epithelial cells. Cell Signal 18:2098–2104 [DOI] [PubMed] [Google Scholar]
- Gargett CE, Wiley JS. (1997) The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol 120:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland A, Hipskind RA, Gallagher JA, Bowler WB. (2001) Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res 16:846–856 [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Zadori ZS, Köles L, Kopp L, Milius D, Wirkner K, Gyires K, Illes P. (2007) Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem 282:33949–33957 [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537 [DOI] [PubMed] [Google Scholar]
- Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, et al. (2010) AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol 160:1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15:1170–1178 [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Sokolova E, Nistri A. (2003) Modulation of P2X3 receptors by Mg2+ on rat DRG neurons in culture. Neuropharmacology 44:132–140 [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. (2000) Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol 131:1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glänzel M, Bültmann R, Starke K, Frahm AW. (2003) Constitutional isomers of Reactive Blue 2 - selective P2Y-receptor antagonists? Eur J Med Chem 38:303–312 [DOI] [PubMed] [Google Scholar]
- Glänzel M, Bültmann R, Starke K, Frahm AW. (2005) Structure-activity relationships of novel P2-receptor antagonists structurally related to Reactive Blue 2. Eur J Med Chem 40:1262–1276 [DOI] [PubMed] [Google Scholar]
- Glass R, Townsend-Nicholson A, Burnstock G. (2000) P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res 300:295–306 [DOI] [PubMed] [Google Scholar]
- Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. (2009) Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J 23:795–805 [DOI] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. (2009) Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D, Yao WP, Housley GD. (1997) Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the retina. Neuroreport 8:1083–1088 [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Healy V, Lim P, Clayton E, Rustin MH, McGrouther DA, Burnstock G. (2003) Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol 121:315–327 [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. (1999) Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res 296:599–605 [DOI] [PubMed] [Google Scholar]
- Grote A, Boldogkoi Z, Zimmer A, Steinhäuser C, Jabs R. (2005) Functional characterization of P2X3 receptors fused with fluorescent proteins. Mol Membr Biol 22:497–506 [DOI] [PubMed] [Google Scholar]
- Gudipaty L, Humphreys BD, Buell G, Dubyak GR. (2001) Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol 280:C943–C953 [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723 [DOI] [PubMed] [Google Scholar]
- Guile SD, Alcaraz L, Birkinshaw TN, Bowers KC, Ebden MR, Furber M, Stocks MJ. (2009) Antagonists of the P2X(7) receptor. From lead identification to drug development. J Med Chem 52:3123–3141 [DOI] [PubMed] [Google Scholar]
- Guinzberg R, Cortés D, Díaz-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Piña E. (2006) Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab 290:E940–E951 [DOI] [PubMed] [Google Scholar]
- Gunosewoyo H, Coster MJ, Kassiou M. (2007) Molecular probes for P2X7 receptor studies. Curr Med Chem 14:1505–1523 [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. (2007) Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72:1447–1456 [DOI] [PubMed] [Google Scholar]
- Haines WR, Migita K, Cox JA, Egan TM, Voigt MM. (2001a) The first transmembrane domain of the P2X receptor subunit participates in the agonist-induced gating of the channel. J Biol Chem 276:32793–32798 [DOI] [PubMed] [Google Scholar]
- Haines WR, Torres GE, Voigt MM, Egan TM. (1999) Properties of the novel ATP-gated ionotropic receptor composed of the P2X(1) and P2X(5) isoforms. Mol Pharmacol 56:720–727 [PubMed] [Google Scholar]
- Haines WR, Voigt MM, Migita K, Torres GE, Egan TM. (2001b) On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J Neurosci 21:5885–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R, Andrews PL, Hoyle CH. (2002) Effects of testosterone on neuromuscular transmission in rat isolated urinary bladder. Eur J Pharmacol 449:301–309 [DOI] [PubMed] [Google Scholar]
- Han JZ, Lin W, Chen YZ. (2005) Inhibition of ATP-induced calcium influx in HT4 cells by glucocorticoids: involvement of protein kinase A. Acta Pharmacol Sin 26:199–204 [DOI] [PubMed] [Google Scholar]
- Hansen MA, Bennett MR, Barden JA. (1999a) Distribution of purinergic P2X receptors in the rat heart. J Auton Nerv Syst 78:1–9 [DOI] [PubMed] [Google Scholar]
- Hansen MA, Dutton JL, Balcar VJ, Barden JA, Bennett MR. (1999b) P2X (purinergic) receptor distributions in rat blood vessels. J Auton Nerv Syst 75:147–155 [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. (1984) Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res 323:287–292 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kokubun S. (1995) Contribution of P2-purinoceptors to neurogenic contraction of rat urinary bladder smooth muscle. Br J Pharmacol 115:636–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R, Rettinger J, Gerevich Z, Meis S, Kassack MU, Illes P, Lambrecht G, Schmalzing G. (2006) The suramin analog 4,4′,4″,4‴-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol 69:2058–2067 [DOI] [PubMed] [Google Scholar]
- He ML, Gonzalez-Iglesias AE, Stojilkovic SS. (2003a) Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem 278:46270–46277 [DOI] [PubMed] [Google Scholar]
- He ML, Koshimizu TA, Tomić M, Stojilkovic SS. (2002) Purinergic P2X(2) receptor desensitization depends on coupling between ectodomain and C-terminal domain. Mol Pharmacol 62:1187–1197 [DOI] [PubMed] [Google Scholar]
- He ML, Zemkova H, Koshimizu TA, Tomić M, Stojilkovic SS. (2003b) Intracellular calcium measurements as a method in studies on activity of purinergic P2X receptor channels. Am J Physiol Cell Physiol 285:C467–C479 [DOI] [PubMed] [Google Scholar]
- He ML, Zemkova H, Stojilkovic SS. (2003c) Dependence of purinergic P2X receptor activity on ectodomain structure. J Biol Chem 278:10182–10188 [DOI] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. (2006) MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther 316:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibell AD, Kidd EJ, Chessell IP, Humphrey PP, Michel AD. (2000) Apparent species differences in the kinetic properties of P2X(7) receptors. Br J Pharmacol 130:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. (2001) Complexities of measuring antagonist potency at P2X(7) receptor orthologs. J Pharmacol Exp Ther 296:947–957 [PubMed] [Google Scholar]
- Hohenegger M, Matyash M, Poussu K, Herrmann-Frank A, Sarközi S, Lehmann-Horn F, Freissmuth M. (1996) Activation of the skeletal muscle ryanodine receptor by suramin and suramin analogs. Mol Pharmacol 50:1443–1453 [PubMed] [Google Scholar]
- Hohenegger M, Waldhoer M, Beindl W, Böing B, Kreimeyer A, Nickel P, Nanoff C, Freissmuth M. (1998) Gsalpha-selective G protein antagonists. Proc Natl Acad Sci USA 95:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbird D, Jensik P, Cox T. (2001) Aldosterone upregulates purinergic responses in larval amphibian skin epithelium. J Comp Physiol B 171:413–420 [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, et al. (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319:1376–1385 [DOI] [PubMed] [Google Scholar]
- Horner S, Menke K, Hildebrandt C, Kassack MU, Nickel P, Ullmann H, Mahaut-Smith MP, Lambrecht G. (2005) The novel suramin analogue NF864 selectively blocks P2X1 receptors in human platelets with potency in the low nanomolar range. Naunyn Schmiedebergs Arch Pharmacol 372:1–13 [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Järlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, et al. (1999) Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci 19:8377–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Hoylaerts MF. (2010) The P2X1 ion channel in platelet function. Platelets 21:153–166 [DOI] [PubMed] [Google Scholar]
- Hu X, Gu J, Chen L, HuangPu Y. (1998) [Studies on the crystal structure of ivermectin (H2B1a)]. Yao Xue Xue Bao 33:449–452 [PubMed] [Google Scholar]
- Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, Koch-Nolte F, Boyer O, Seman M, Adriouch S. (2010) Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J Exp Med 207:2561–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EK, Nayak DP. (2002) Role of G protein and protein kinase signalling in influenza virus budding in MDCK cells. J Gen Virol 83:3055–3066 [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Lorca RA, Coddou C. (2008) Trace metals in the brain: allosteric modulators of ligand-gated receptor channels, the case of ATP-gated P2X receptors. Eur Biophys J 37:301–314 [DOI] [PubMed] [Google Scholar]
- Hülsmann M, Nickel P, Kassack M, Schmalzing G, Lambrecht G, Markwardt F. (2003) NF449, a novel picomolar potency antagonist at human P2X1 receptors. Eur J Pharmacol 470:1–7 [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. (1998) Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol 54:22–32 [DOI] [PubMed] [Google Scholar]
- Hung AC, Chu YJ, Lin YH, Weng JY, Chen HB, Au YC, Sun SH. (2005) Roles of protein kinase C in regulation of P2X7 receptor-mediated calcium signalling of cultured type-2 astrocyte cell line, RBA-2. Cell Signal 17:1384–1396 [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T. (2003) Pharmacological effects of ivermectin, an antiparasitic agent for intestinal strongyloidiasis: its mode of action and clinical efficacy. Nippon Yakurigaku Zasshi 122:527–538 [DOI] [PubMed] [Google Scholar]
- Inoue R, Brading AF. (1990) The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol 100:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Brading AF. (1991) Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purinoceptor activation. Br J Pharmacol 103:1840–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Costanzi S, Joshi BV, Besada P, Shin DH, Ko H, Ivanov AA, Mamedova L. (2006) Agonists and antagonists for P2 receptors. Novartis Found Symp 276:58–68; discussion 68–72, 107–112, 275–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Costanzi S, Ohno M, Joshi BV, Besada P, Xu B, Tchilibon S. (2004) Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Current topics in medicinal chemistry 4:805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. (2002) Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45:4057–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Kim YC, Wildman SS, Mohanram A, Harden TK, Boyer JL, King BF, Burnstock G. (1998) A pyridoxine cyclic phosphate and its 6-azoaryl derivative selectively potentiate and antagonize activation of P2X1 receptors. J Med Chem 41:2201–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangir A, Alam M, Carter DS, Dillon MP, Bois DJ, Ford AP, Gever JR, Lin C, Wagner PJ, Zhai Y, et al. (2009) Identification and SAR of novel diaminopyrimidines. Part 2: The discovery of RO-51, a potent and selective, dual P2X(3)/P2X(2/3) antagonist for the treatment of pain. Bioorg Med Chem Lett 19:1632–1635 [DOI] [PubMed] [Google Scholar]
- Jaime-Figueroa S, Greenhouse R, Padilla F, Dillon MP, Gever JR, Ford AP. (2005) Discovery and synthesis of a novel and selective drug-like P2X(1) antagonist. Bioorg Med Chem Lett 15:3292–3295 [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, et al. (2002) A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA 99:17179–17184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS. (2009) ATP-gated P2X cation-channels. Neuropharmacology 56:208–215 [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Wismer CT, Schweitzer E, Yu H, van Biesen T, Lynch KJ, Burgard EC, Kowaluk EA. (2001) Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X(3) allosteric modulator, cibacron blue. Br J Pharmacol 132:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelínkova I, Vávra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. (2008) Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch 456:939–950 [DOI] [PubMed] [Google Scholar]
- Jelínková I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemková H. (2006) Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun 349:619–625 [DOI] [PubMed] [Google Scholar]
- Jensik PJ, Holbird D, Collard MW, Cox TC. (2001) Cloning and characterization of a functional P2X receptor from larval bullfrog skin. Am J Physiol Cell Physiol 281:C954–C962 [DOI] [PubMed] [Google Scholar]
- Jentsch KD, Hunsmann G, Hartmann H, Nickel P. (1987) Inhibition of human immunodeficiency virus type I reverse transcriptase by suramin-related compounds. J Gen Virol 68:2183–2192 [DOI] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. (2003) Subunit arrangement in P2X receptors. J Neurosci 23:8903–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Surprenant A. (2000a) Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol 58:82–88 [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. (2005a) N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol 289:C1295–C1302 [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Spelta V, Surprenant A, North RA. (2001) Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X(2) receptor. J Biol Chem 276:14902–14908 [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA. (2000b) Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem 275:34190–34196 [DOI] [PubMed] [Google Scholar]
- Jiang T, Yeung D, Lien CF, Górecki DC. (2005b) Localized expression of specific P2X receptors in dystrophin-deficient DMD and mdx muscle. Neuromuscul Disord 15:225–236 [DOI] [PubMed] [Google Scholar]
- Jindrichova M, Vavra V, Obsil T, Stojilkovic SS, Zemkova H. (2009) Functional relevance of aromatic residues in the first transmembrane domain of P2X receptors. J Neurochem 109:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson T, Frändberg PA, Nyberg F, Le Grevès P. (2008) Molecular mechanisms for nanomolar concentrations of neurosteroids at NR1/NR2B receptors. J Pharmacol Exp Ther 324:759–768 [DOI] [PubMed] [Google Scholar]
- Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP. (2000) Functional characterization of the P2X(4) receptor orthologues. Br J Pharmacol 129:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Vial C, Sellers LA, Humphrey PP, Evans RJ, Chessell IP. (2004) Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol 65:979–985 [DOI] [PubMed] [Google Scholar]
- Jung KY, Moon HD, Lee GE, Lim HH, Park CS, Kim YC. (2007) Structure-activity relationship studies of spinorphin as a potent and selective human P2X(3) receptor antagonist. J Med Chem 50:4543–4547 [DOI] [PubMed] [Google Scholar]
- Kaan TK, Yip PK, Grist J, Cefalu JS, Nunn PA, Ford AP, Zhong Y, McMahon SB. (2010) Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci 30:4503–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. (1999) Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407:11–32 [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Thorne PR, Christie DL, Palmer DJ, Luo L, Ryan AF. (1996) Localization of ATP-gated ion channels in cerebellum using P2x2R subunit-specific antisera. Neuroreport 7:2665–2669 [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Raybould NP, Jagger DJ, Greenwood D, Housley GD. (2003) Allosteric modulation of native cochlear P2X receptors: insights from comparison with recombinant P2X2 receptors. Audiol Neurootol 8:115–128 [DOI] [PubMed] [Google Scholar]
- Kardos J, Kovács I, Hajós F, Kálmán M, Simonyi M. (1989) Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett 103:139–144 [DOI] [PubMed] [Google Scholar]
- Kasakov L, Burnstock G. (1982) The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol 86:291–294 [DOI] [PubMed] [Google Scholar]
- Kassack MU, Braun K, Ganso M, Ullmann H, Nickel P, Böing B, Müller G, Lambrecht G. (2004) Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist. Eur J Med Chem 39:345–357 [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, et al. (2003) Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17:1356–1367 [DOI] [PubMed] [Google Scholar]
- Kenakin T, Miller LJ. (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. (2010) Ligand detection in the allosteric world. J Biomol Screen 15:119–130 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. (1999a) Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci 2:322–330 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Egan TM. (2005) Contribution of transmembrane regions to ATP-gated P2X2 channel permeability dynamics. J Biol Chem 280:6118–6129 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. (1999b) Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci 19:7289–7299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O. (2008) P2X3 receptor gating near normal body temperature. Pflugers Arch 456:339–347 [DOI] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. (1995) Localization of P2X purinoceptor transcripts in the rat nervous system. Mol Pharmacol 48:569–573 [PubMed] [Google Scholar]
- Kim YC, Brown SG, Harden TK, Boyer JL, Dubyak G, King BF, Burnstock G, Jacobson KA. (2001) Structure-activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J Med Chem 44:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hur EM, Park TJ, Kim KT. (2000) Nongenomic inhibition of catecholamine secretion by 17beta-estradiol in PC12 cells. J Neurochem 74:2490–2496 [DOI] [PubMed] [Google Scholar]
- King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. (1999) Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol 128:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, Burnstock G. (2000) Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci 20:4871–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. (1997) Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol 121:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshina LE, Pintor J, Burnstock G. (1996) Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br J Pharmacol 117:1371–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Housley GD, Raybould NP, Greenwood D, Salih SG. (1998) Expression of ATP-gated ion channels by Reissner's membrane epithelial cells. Neuroreport 9:2467–2474 [DOI] [PubMed] [Google Scholar]
- Kiss T, Osipenko ON. (1994) Toxic effects of heavy metals on ionic channels. Pharmacol Rev 46:245–267 [PubMed] [Google Scholar]
- Klapperstück M, Büttner C, Nickel P, Schmalzing G, Lambrecht G, Markwardt F. (2000) Antagonism by the suramin analogue NF279 on human P2X(1) and P2X(7) receptors. Eur J Pharmacol 387:245–252 [DOI] [PubMed] [Google Scholar]
- Klapperstück M, Büttner C, Schmalzing G, Markwardt F. (2001) Functional evidence of distinct ATP activation sites at the human P2X(7) receptor. J Physiol 534:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köles L, Wirkner K, Fürst S, Wnendt S, Illes P. (2000) Trichloroethanol inhibits ATP-induced membrane currents in cultured HEK 293-hP2X3 cells. Eur J Pharmacol 409:R3–R5 [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Koshimizu M, Stojilkovic SS. (1999) Contributions of the C-terminal domain to the control of P2X receptor desensitization. J Biol Chem 274:37651–37657 [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Tomić M, Koshimizu M, Stojilkovic SS. (1998a) Identification of amino acid residues contributing to desensitization of the P2X2 receptor channel. J Biol Chem 273:12853–12857 [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Tomić M, Van Goor F, Stojilkovic SS. (1998b) Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol 12:901–913 [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Kretschmannova K, He ML, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. (2006) Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol 69:1588–1598 [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Tomić M, Wong AO, Zivadinovic D, Stojilkovic SS. (2000a) Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology 141:4091–4099 [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. (2002) Heteromultimerization modulates P2X receptor functions through participating extracellular and C-terminal subdomains. J Biol Chem 277:46891–46899 [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Van Goor F, Tomić M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. (2000b) Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol 58:936–945 [DOI] [PubMed] [Google Scholar]
- Kotnis S, Bingham B, Vasilyev DV, Miller SW, Bai Y, Yeola S, Chanda PK, Bowlby MR, Kaftan EJ, Samad TA, et al. (2010) Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol Pharmacol 77:953–960 [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. (1998) Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 53:283–294 [DOI] [PubMed] [Google Scholar]
- Krejci P, Murakami S, Prochazkova J, Trantirek L, Chlebova K, Ouyang Z, Aklian A, Smutny J, Bryja V, Kozubik A, et al. (2010) NF449 is a novel inhibitor of fibroblast growth factor receptor 3 (FGFR3) signaling active in chondrocytes and multiple myeloma cells. J Biol Chem 285:20644–20653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Pidoplichko VI. (1983) Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett 35:41–45 [DOI] [PubMed] [Google Scholar]
- Krůsek J, Zemková H. (1994) Effect of ivermectin on gamma-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. Eur J Pharmacol 259:121–128 [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98:6836–6841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168:6436–6445 [DOI] [PubMed] [Google Scholar]
- Lalo U, Allsopp RC, Mahaut-Smith MP, Evans RJ. (2010) P2X1 receptor mobility and trafficking; regulation by receptor insertion and activation. J Neurochem 113:1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. (2008) P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci 28:5473–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Verkhratsky A, Pankratov Y. (2007) Ivermectin potentiates ATP-induced ion currents in cortical neurones: evidence for functional expression of P2X4 receptors? Neurosci Lett 421:158–162 [DOI] [PubMed] [Google Scholar]
- Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. (2002) Structure-activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des 8:2371–2399 [DOI] [PubMed] [Google Scholar]
- Lambrecht G, Ganso M, Bäumert HG, Spatz-Kümbel G, Hildebrandt C, Braun K, Mutschler E. (2000a) The novel heteromeric bivalent ligand SB9 potently antagonizes P2Y(1) receptor-mediated responses. J Auton Nerv Syst 81:171–177 [DOI] [PubMed] [Google Scholar]
- Lambrecht G, Rettinger J, Bäumert HG, Czeche S, Damer S, Ganso M, Hildebrandt C, Niebel B, Spatz-Kümbel G, Schmalzing G, et al. (2000b) The novel pyridoxal-5′-phosphate derivative PPNDS potently antagonizes activation of P2X(1) receptors. Eur J Pharmacol 387:R19–R21 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. (2009) Inflammasomes: guardians of cytosolic sanctity. Immunol Rev 227:95–105 [DOI] [PubMed] [Google Scholar]
- Lê KT, Babinski K, Séguéla P. (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18:7152–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê KT, Boué-Grabot E, Archambault V, Séguéla P. (1999) Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J Biol Chem 274:15415–15419 [DOI] [PubMed] [Google Scholar]
- Lê KT, Paquet M, Nouel D, Babinski K, Séguéla P. (1997) Primary structure and expression of a naturally truncated human P2X ATP receptor subunit from brain and immune system. FEBS Lett 418:195–199 [DOI] [PubMed] [Google Scholar]
- Lee HY, Bardini M, Burnstock G. (2000) Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163:2002–2007 [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377:432–435 [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. (2000) Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br J Pharmacol 131:1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Gitterman DP, Schlüter H, Evans RJ. (2000) Effects of diadenosine polyphosphates (Ap(n)As) and adenosine polyphospho guanosines (Ap(n)Gs) on rat mesenteric artery P2X receptor ion channels. Br J Pharmacol 129:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Surprenant A, Evans RJ. (1998) 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP)–a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br J Pharmacol 124:1463–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Aguayo L, Peoples RW, Weight FF. (1993) Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol 44:871–875 [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1996a) Acid pH augments excitatory action of ATP on a dissociated mammalian sensory neuron. Neuroreport 7:2151–2154 [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1996b) Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. J Neurophysiol 76:3048–3058 [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1997) Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. J Physiol 505:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1998) Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol 123:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiong K, Weight FF. (2000) Ethanol inhibition of adenosine 5′-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci Lett 295:77–80 [DOI] [PubMed] [Google Scholar]
- Li M, Chang TH, Silberberg SD, Swartz KJ. (2008) Gating the pore of P2X receptor channels. Nat Neurosci 11:883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kawate T, Silberberg SD, Swartz KJ. (2010) Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun 1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Luo X, Muallem S. (2005) Regulation of the P2X7 receptor permeability to large molecules by extracellular Cl- and Na+. J Biol Chem 280:26922–26927 [DOI] [PubMed] [Google Scholar]
- Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. (2011) Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology 152:2342–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Migita K, Samways DS, Voigt MM, Egan TM. (2004) Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J Neurosci 24:7378–7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SD, Xu CS, Zhou T, Liu HQ, Gao Y, Li GL. (2005) Tetramethylpyrazine inhibits ATP-activated currents in rat dorsal root ganglion neurons. Brain Res 1040:92–97 [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. (2008) Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100:1184–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GJ, Brockhausen J, Bennett MR. (2003) P2X1 receptor currents after disruption of the PKC site and its surroundings by dominant negative mutations in HEK293 cells. Auton Neurosci 108:12–16 [DOI] [PubMed] [Google Scholar]
- Liu M, Huang W, Wu D, Priestley JV. (2006) TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 24:1–6 [DOI] [PubMed] [Google Scholar]
- Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A, Burnstock G. (2001a) Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther 296:1043–1050 [PubMed] [Google Scholar]
- Liu PS, Hsieh HL, Lin CM. (2001b) Dehydroepiandrosterone sulfate (DHEAS) suppresses P2X purinoceptor-coupled responses in PC12 cells. Neurochem Int 39:193–198 [DOI] [PubMed] [Google Scholar]
- Liu X, Ma W, Surprenant A, Jiang LH. (2009) Identification of the amino acid residues in the extracellular domain of rat P2X(7) receptor involved in functional inhibition by acidic pH. Br J Pharmacol 156:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Surprenant A, Mao HJ, Roger S, Xia R, Bradley H, Jiang LH. (2008a) Identification of key residues coordinating functional inhibition of P2X7 receptors by zinc and copper. Mol Pharmacol 73:252–259 [DOI] [PubMed] [Google Scholar]
- Liu X, Zeng J, Zhao Y, Xiao Z, Fang C, Ruan H. (2010) Inhibition of ATP-induced Ca2+ influx by corticosterone in dorsal root ganglion neurons. Neurochem Res 35:804–810 [DOI] [PubMed] [Google Scholar]
- Liu XH, Zeng JW, Zhao YD, Chen PH, Xiao Z, Ruan HZ. (2008b) Rapid inhibition of ATP-induced currents by corticosterone in rat dorsal root ganglion neurons. Pharmacology 82:164–170 [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. (2006) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103:7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhurst PA, Schwegel T, Folander K, Swanson R. (1996) The human P2x1 receptor: molecular cloning, tissue distribution, and localization to chromosome 17. Biochim Biophys Acta 1308:185–188 [DOI] [PubMed] [Google Scholar]
- Lorca RA, Coddou C, Gazitúa MC, Bull P, Arredondo C, Huidobro-Toro JP. (2005) Extracellular histidine residues identify common structural determinants in the copper/zinc P2X2 receptor modulation. J Neurochem 95:499–512 [DOI] [PubMed] [Google Scholar]
- Ludlow MJ, Traynor D, Fisher PR, Ennion SJ. (2008) Purinergic-mediated Ca2+ influx in Dictyostelium discoideum. Cell Calcium 44:567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KJ, Touma E, Niforatos W, Kage KL, Burgard EC, van Biesen T, Kowaluk EA, Jarvis MF. (1999) Molecular and functional characterization of human P2X(2) receptors. Mol Pharmacol 56:1171–1181 [DOI] [PubMed] [Google Scholar]
- Ma B, Ruan HZ, Burnstock G, Dunn PM. (2005) Differential expression of P2X receptors on neurons from different parasympathetic ganglia. Neuropharmacology 48:766–777 [DOI] [PubMed] [Google Scholar]
- Ma B, Ruan HZ, Cockayne DA, Ford AP, Burnstock G, Dunn PM. (2004) Identification of P2X receptors in cultured mouse and rat parasympathetic otic ganglion neurones including P2X knockout studies. Neuropharmacology 46:1039–1048 [DOI] [PubMed] [Google Scholar]
- Ma W, Korngreen A, Uzlaner N, Priel Z, Silberberg SD. (1999) Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature 400:894–897 [DOI] [PubMed] [Google Scholar]
- Ma W, Korngreen A, Weil S, Cohen EB, Priel A, Kuzin L, Silberberg SD. (2006) Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 571:503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. (2010) Non-junction functions of pannexin-1 channels. Trends Neurosci 33:93–102 [DOI] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. (2007) Copper and iron disorders of the brain. Annu Rev Neurosci 30:317–337 [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Ennion SJ, Rolf MG, Evans RJ. (2000) ADP is not an agonist at P2X(1) receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol 131:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini S, Hoyle CH, Burnstock G. (1986) An electrophysiological analysis of the effect of reactive blue 2, a putative P2-purinoceptor antagonist, on inhibitory junction potentials of rat caecum. Eur J Pharmacol 127:197–204 [DOI] [PubMed] [Google Scholar]
- Marín-García P, Sánchez-Nogueiro J, Gómez-Villafuertes R, León D, Miras-Portugal MT. (2008) Synaptic terminals from mice midbrain exhibit functional P2X7 receptor. Neuroscience 151:361–373 [DOI] [PubMed] [Google Scholar]
- Marquez-Klaka B, Rettinger J, Bhargava Y, Eisele T, Nicke A. (2007) Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J Neurosci 27:1456–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. (2006) Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther 111:567–583 [DOI] [PubMed] [Google Scholar]
- Meyer MP, Gröschel-Stewart U, Robson T, Burnstock G. (1999) Expression of two ATP-gated ion channels, P2X5 and P2X6, in developing chick skeletal muscle. Dev Dyn 216:442–449 [DOI] [PubMed] [Google Scholar]
- Michel AD, Chambers LJ, Clay WC, Condreay JP, Walter DS, Chessell IP. (2007) Direct labelling of the human P2X7 receptor and identification of positive and negative cooperativity of binding. Br J Pharmacol 151:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Chambers LJ, Walter DS. (2008a) Negative and positive allosteric modulators of the P2X(7) receptor. Br J Pharmacol 153:737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Chessell IP, Humphrey PP. (1999) Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol 359:102–109 [DOI] [PubMed] [Google Scholar]
- Michel AD, Clay WC, Ng SW, Roman S, Thompson K, Condreay JP, Hall M, Holbrook J, Livermore D, Senger S. (2008b) Identification of regions of the P2X(7) receptor that contribute to human and rat species differences in antagonist effects. Br J Pharmacol 155:738–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Fonfria E. (2007) Agonist potency at P2X7 receptors is modulated by structurally diverse lipids. Br J Pharmacol 152:523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Grahames CB, Humphrey PP. (1996) Functional characterisation of P2 purinoceptors in PC12 cells by measurement of radiolabelled calcium influx. Naunyn Schmiedebergs Arch Pharmacol 354:562–571 [DOI] [PubMed] [Google Scholar]
- Michel AD, Kaur R, Chessell IP, Humphrey PP. (2000) Antagonist effects on human P2X(7) receptor-mediated cellular accumulation of YO-PRO-1. Br J Pharmacol 130:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Miller KJ, Lundström K, Buell GN, Humphrey PP. (1997) Radiolabeling of the rat P2X4 purinoceptor: evidence for allosteric interactions of purinoceptor antagonists and monovalent cations with P2X purinoceptors. Mol Pharmacol 51:524–532 [PubMed] [Google Scholar]
- Michel AD, Ng SW, Roman S, Clay WC, Dean DK, Walter DS. (2009) Mechanism of action of species-selective P2X(7) receptor antagonists. Br J Pharmacol 156:1312–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Xing M, Humphrey PP. (2001) Serum constituents can affect 2′- & 3′-O-(4-benzoylbenzoyl)-ATP potency at P2X(7) receptors. Br J Pharmacol 132:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Haines WR, Voigt MM, Egan TM. (2001) Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X(2) receptor. J Biol Chem 276:30934–30941 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Michel AD, Chessell IP, Humphrey PP. (1998) Cibacron blue allosterically modulates the rat P2X4 receptor. Neuropharmacology 37:1579–1586 [DOI] [PubMed] [Google Scholar]
- Mio K, Ogura T, Yamamoto T, Hiroaki Y, Fujiyoshi Y, Kubo Y, Sato C. (2009) Reconstruction of the P2X(2) receptor reveals a vase-shaped structure with lateral tunnels above the membrane. Structure 17:266–275 [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Yamaoka K, Urabe S, Kodama M, Kudo Y. (2010) Functional expression of purinergic P2X7 receptors in pregnant rat myometrium. Am J Physiol Regul Integr Comp Physiol 298:R1117–R1124 [DOI] [PubMed] [Google Scholar]
- Mo G, Bernier LP, Zhao Q, Chabot-Doré AJ, Ase AR, Logothetis D, Cao CQ, Séguéla P. (2009) Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt L, Hume RI. (2007) Responses of rat P2X2 receptors to ultrashort pulses of ATP provide insights into ATP binding and channel gating. J Gen Physiol 130:183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SF, Mackenzie AB. (2008) Species and agonist dependent zinc modulation of endogenous and recombinant ATP-gated P2X7 receptors. Biochem Pharmacol 76:1740–1747 [DOI] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. (2009) AutoDock4 and AutoDockTooIs4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morytko MJ, Betschmann P, Woller K, Ericsson A, Chen H, Donnelly-Roberts DL, Namovic MT, Jarvis MF, Carroll WA, Rafferty P. (2008) Synthesis and in vitro activity of N′-cyano-4-(2-phenylacetyl)-N-o-tolylpiperazine-1-carboximidamide P2X7 antagonists. Bioorg Med Chem Lett 18:2093–2096 [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, et al. (2000) Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403:86–89 [DOI] [PubMed] [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. (1993) Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem 268:8199–8203 [PubMed] [Google Scholar]
- Nagaoka M, Nara M, Tamada T, Kume H, Oguma T, Kikuchi T, Zaini J, Moriya T, Ichinose M, Tamura G, et al. (2009) Regulation of adenosine 5′-triphosphate (ATP)-gated P2X(4) receptors on tracheal smooth muscle cells. Respir Physiol Neurobiol 166:61–67 [DOI] [PubMed] [Google Scholar]
- Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K. (2009) Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. (2005) An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem 280:25982–25993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Simonetti M, Fabbretti E, Nistri A. (2010) The Cdk5 kinase downregulates ATP-gated ionotropic P2X3 receptor function via serine phosphorylation. Cell Mol Neurobiol 30:505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Mori T, Nishikawa K, Sawada M, Kuno M, Asada A. (2007) The effects of general anesthetics on P2X7 and P2Y receptors in a rat microglial cell line. Anesth Analg 104:1136–1144 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Hess P. (1993) Block by calcium of ATP-activated channels in pheochromocytoma cells. J Gen Physiol 101:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Fujimori K, Takanaka A. (1991) Effects of ATP antagonists on purinoceptor-operated inward currents in rat phaeochromocytoma cells. Pflugers Arch 418:214–219 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Liu M, Inoue K, Ohno Y. (1997) Potent inhibition by trivalent cations of ATP-gated channels. Eur J Pharmacol 325:237–243 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Ohno Y. (1997) Effects of neuroamines and divalent cations on cloned and mutated ATP-gated channels. Eur J Pharmacol 325:101–108 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Yamakoshi Y, Tsuchiya T, Ohno Y. (2005) Purification and aqueous phase atomic force microscopic observation of recombinant P2X2 receptor. Eur J Pharmacol 518:107–110 [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Markwardt F. (2000) Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci Lett 279:165–168 [DOI] [PubMed] [Google Scholar]
- Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, et al. (2006) Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem 49:3659–3666 [DOI] [PubMed] [Google Scholar]
- Nicke A. (2008) Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun 377:803–808 [DOI] [PubMed] [Google Scholar]
- Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17:3016–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem 92:925–933 [DOI] [PubMed] [Google Scholar]
- Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, Górecki DC, Murrell-Lagnado RD, Soto F. (2009) A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem 284:25813–25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed] [Google Scholar]
- North RA. (2004) P2X3 receptors and peripheral pain mechanisms. J Physiol 554:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. (2000) Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40:563–580 [DOI] [PubMed] [Google Scholar]
- Ormond SJ, Barrera NP, Qureshi OS, Henderson RM, Edwardson JM, Murrell-Lagnado RD. (2006) An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the endoplasmic reticulum. Mol Pharmacol 69:1692–1700 [DOI] [PubMed] [Google Scholar]
- Oury C, Kuijpers MJ, Toth-Zsamboki E, Bonnefoy A, Danloy S, Vreys I, Feijge MA, De Vos R, Vermylen J, Heemskerk JW, et al. (2003) Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood 101:3969–3976 [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Huang L. (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447:744–751 [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. (1998) A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci 10:3898–3902 [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. (2001) The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci 21:6561–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MS, Larroque ML, Campbell JM, Bobbin RP, Deininger PL. (1998) Novel variant of the P2X2 ATP receptor from the guinea pig organ of Corti. Hear Res 121:62–70 [DOI] [PubMed] [Google Scholar]
- Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Gründer S. (2001) Inflammatory mediators potentiate ATP-gated channels through the P2X(3) subunit. J Biol Chem 276:21077–21082 [DOI] [PubMed] [Google Scholar]
- Peers C, Dallas ML, Scragg JL. (2009) Ion channels as effectors in carbon monoxide signaling. Commun Integr Biol 2:241–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Medrano A, Donnelly-Roberts DL, Honore P, Hsieh GC, Namovic MT, Peddi S, Shuai Q, Wang Y, Faltynek CR, Jarvis MF, et al. (2009) Discovery and biological evaluation of novel cyanoguanidine P2X(7) antagonists with analgesic activity in a rat model of neuropathic pain. J Med Chem 52:3366–3376 [DOI] [PubMed] [Google Scholar]
- Phillips JK, Hill CE. (1999) Neuroreceptor mRNA expression in the rat mesenteric artery develops independently of innervation. Int J Dev Neurosci 17:377–386 [DOI] [PubMed] [Google Scholar]
- Phillips JK, McLean AJ, Hill CE. (1998) Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol 124:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. (2003) Lipid rafts: bringing order to chaos. J Lipid Res 44:655–667 [DOI] [PubMed] [Google Scholar]
- Pike LJ. (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Díaz-Hernández M, Gualix J, Gómez-Villafuertes R, Hernando F, Miras-Portugal MT. (2000) Diadenosine polyphosphate receptors. from rat and guinea-pig brain to human nervous system. Pharmacol Ther 87:103–115 [DOI] [PubMed] [Google Scholar]
- Pochet S, Garcia-Marcos M, Seil M, Otto A, Marino A, Dehaye JP. (2007) Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells. Cell Signal 19:2155–2164 [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, Davies DL. (2010) A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem 112:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP. (2005) Use-dependent inhibition of P2X3 receptors by nanomolar agonist. J Neurosci 25:7359–7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. (2004) Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol 123:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. (2007) Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci 120:3838–3849 [DOI] [PubMed] [Google Scholar]
- Rae MG, Rowan EG, Kennedy C. (1998) Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br J Pharmacol 124:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492 [PubMed] [Google Scholar]
- Raouf R, Blais D, Séguéla P. (2005) High zinc sensitivity and pore formation in an invertebrate P2X receptor. Biochim Biophys Acta 1669:135–141 [DOI] [PubMed] [Google Scholar]
- Raouf R, Chabot-Doré AJ, Ase AR, Blais D, Séguéla P. (2007) Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology 53:496–504 [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. (1997a) Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J 16:3446–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. (1997b) The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem 272:5482–5486 [DOI] [PubMed] [Google Scholar]
- Ratz PH, McCammon KA, Altstatt D, Blackmore PF, Shenfeld OZ, Schlossberg SM. (1999) Differential effects of sex hormones and phytoestrogens on peak and steady state contractions in isolated rabbit detrusor. J Urol 162:1821–1828 [PubMed] [Google Scholar]
- Reilly C. (2004) The Nutritional Trace Metals. Wiley-Blackwell; Oxford, UK [Google Scholar]
- Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. (2003) P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol 552:809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Braun K, Hochmann H, Kassack MU, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. (2005) Profiling at recombinant homomeric and heteromeric rat P2X receptors identifies the suramin analogue NF449 as a highly potent P2X1 receptor antagonist. Neuropharmacology 48:461–468 [DOI] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. (2003) Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol 121:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G, Damer S, Müller G, Nickel P, Lambrecht G. (2000) The suramin analogue NF279 is a novel and potent antagonist selective for the P2X(1) receptor. Neuropharmacology 39:2044–2053 [DOI] [PubMed] [Google Scholar]
- Roberts JA, Digby HR, Kara M, El Ajouz S, Sutcliffe MJ, Evans RJ. (2008) Cysteine substitution mutagenesis and the effects of methanethiosulfonate reagents at P2X2 and P2X4 receptors support a core common mode of ATP action at P2X receptors. J Biol Chem 283:20126–20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. (2004) ATP binding at human P2X1 receptors. Contribution of aromatic and basic amino acids revealed using mutagenesis and partial agonists. J Biol Chem 279:9043–9055 [DOI] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. (2005) Mutagenesis studies of conserved proline residues of human P2X receptors for ATP indicate that proline 272 contributes to channel function. J Neurochem 92:1256–1264 [DOI] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. (2006) Contribution of conserved polar glutamine, asparagine and threonine residues and glycosylation to agonist action at human P2X1 receptors for ATP. J Neurochem 96:843–852 [DOI] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. (2007) Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2X receptors. J Neurosci 27:4072–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Rae MG, Rowan EG, Kennedy C. (1996) Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. Br J Pharmacol 118:951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H, Baldwin SA, Surprenant A, Jiang LH. (2010) Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J Psychiatr Res 44:347–355 [DOI] [PubMed] [Google Scholar]
- Roger S, Pelegrin P, Surprenant A. (2008) Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28:6393–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokic MB, Tvrdoňová V, Vávra V, Jindřichová M, Obšil T, Stojilkovic SS, Zemková H. (2010) Roles of conserved ectodomain cysteines of the rat P2X4 purinoreceptor in agonist binding and channel gating. Physiol Res 59:927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. (2003) Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23:11315–11321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Bobanović LK, Murrell-Lagnado RD. (2002) Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem 277:35378–35385 [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. (2001) Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppelt A, Ma W, Borchardt K, Silberberg SD, Soto F. (2001) Genomic structure, developmental distribution and functional properties of the chicken P2X(5) receptor. J Neurochem 77:1256–1265 [DOI] [PubMed] [Google Scholar]
- Ryten M, Hoebertz A, Burnstock G. (2001) Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev Dyn 221:331–341 [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815 [DOI] [PubMed] [Google Scholar]
- Samways DS, Egan TM. (2007) Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2X receptor family. J Gen Physiol 129:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways DS, Migita K, Li Z, Egan TM. (2008) On the role of the first transmembrane domain in cation permeability and flux of the ATP-gated P2X2 receptor. J Biol Chem 283:5110–5117 [DOI] [PubMed] [Google Scholar]
- Sánchez-Nogueiro J, Marín-García P, Miras-Portugal MT. (2005) Characterization of a functional P2X(7)-like receptor in cerebellar granule neurons from P2X(7) knockout mice. FEBS Lett 579:3783–3788 [DOI] [PubMed] [Google Scholar]
- Scheibler P, Pesic M, Franke H, Reinhardt R, Wirkner K, Illes P, Nörenberg W. (2004) P2X2 and P2Y1 immunofluorescence in rat neostriatal medium-spiny projection neurones and cholinergic interneurones is not linked to respective purinergic receptor function. Br J Pharmacol 143:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N, Fliegert R, Adriouch S, Seman M, Guse AH, Haag F, Koch-Nolte F. (2009) Activation of the P2X7 ion channel by soluble and covalently bound ligands. Purinergic Signal 5:139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. (2001) Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem 276:12556–12564 [DOI] [PubMed] [Google Scholar]
- Shen JB, Pappano AJ, Liang BT. (2006) Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. FASEB J 20:277–284 [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. (1987) Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oocytes. Mol Pharmacol 32:749–752 [PubMed] [Google Scholar]
- Silberberg SD, Chang TH, Swartz KJ. (2005) Secondary structure and gating rearrangements of transmembrane segments in rat P2X4 receptor channels. J Gen Physiol 125:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. (2007) Ivermectin Interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron 54:263–274 [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Swartz KJ. (2009) Structural biology: Trimeric ion-channel design. Nature 460:580–581 [DOI] [PubMed] [Google Scholar]
- Sim JA, Broomhead HE, North RA. (2008) Ectodomain lysines and suramin block of P2X1 receptors. J Biol Chem 283:29841–29846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. (2006) Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci 26:9006–9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, North RA. (2010) Amitriptyline does not block the action of ATP at human P2X4 receptor. Br J Pharmacol 160:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Park CK, Oh SB, Evans RJ, North RA. (2007) P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol 152:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24:6307–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Kidd EJ, Smith FM, Chessell IP, Murrell-Lagnado R, Humphrey PP, Barnard EA. (1997) Localization and functional expression of splice variants of the P2X2 receptor. Mol Pharmacol 52:237–248 [DOI] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. (2010) The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24:337–345 [DOI] [PubMed] [Google Scholar]
- Smart ML, Gu B, Panchal RG, Wiley J, Cromer B, Williams DA, Petrou S. (2003) P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem 278:8853–8860 [DOI] [PubMed] [Google Scholar]
- Sokolova E, Skorinkin A, Moiseev I, Agrachev A, Nistri A, Giniatullin R. (2006) Experimental and modeling studies of desensitization of P2X3 receptors. Mol Pharmacol 70:373–382 [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. (2001) Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276:125–132 [DOI] [PubMed] [Google Scholar]
- Soltoff SP, McMillian MK, Talamo BR. (1989) Coomassie Brilliant Blue G is a more potent antagonist of P2 purinergic responses than Reactive Blue 2 (Cibacron Blue 3GA) in rat parotid acinar cells. Biochem Biophys Res Commun 165:1279–1285 [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stühmer W. (1996) P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA 93:3684–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stühmer W, Busch AE. (1999) Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology 38:141–149 [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, et al. (2000) Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407:1015–1017 [DOI] [PubMed] [Google Scholar]
- Spelta V, Jiang LH, Surprenant A, North RA. (2002) Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol 135:1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelta V, Mekhalfia A, Rejman D, Thompson M, Blackburn GM, North RA. (2003) ATP analogues with modified phosphate chains and their selectivity for rat P2X2 and P2X2/3 receptors. Br J Pharmacol 140:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JP, Arison BH, Hirshfield JM, Hoogsteen K. (1981) The absolute stereochemistry and conformation of avermectin B2a. J Am Chem Soc 203:4221–4224 [Google Scholar]
- Stanchev D, Flehmig G, Gerevich Z, Nörenberg W, Dihazi H, Fürst S, Eschrich K, Illes P, Wirkner K. (2006) Decrease of current responses at human recombinant P2X3 receptors after substitution by Asp of Ser/Thr residues in protein kinase C phosphorylation sites of their ecto-domains. Neurosci Lett 393:78–83 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS. (2008) Ion channels, transporters, and electrical signaling, in Neuroscience in Medicine (Coon PM. ed), Humana Press, Totowa, NJ [Google Scholar]
- Stojilkovic SS. (2009) Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab 20:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, He ML, Koshimizu TA, Balik A, Zemkova H. (2010a) Signaling by purinergic receptors and channels in the pituitary gland. Mol Cell Endocrinol 314:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Koshimizu T. (2001) Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab 12:218–225 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tomic M, He ML, Yan Z, Koshimizu TA, Zemkova H. (2005) Molecular dissection of purinergic P2X receptor channels. Ann NY Acad Sci 1048:116–130 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tomic M, Van Goor F, Koshimizu T. (2000) Expression of purinergic P2X2 receptor-channels and their role in calcium signaling in pituitary cells. Biochem Cell Biol 78:393–404 [PubMed] [Google Scholar]
- Stojilkovic SS, Yan Z, Obsil T, Zemkova H. (2010b) Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells. Cell Mol Neurobiol 30:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, Furber M, Mortimore M, Lawson M, Theaker J, et al. (2006) Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br J Pharmacol 149:880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L, Surprenant A. (2009) Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol 39:986–995 [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North RA. (1997) Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol 78:1837–1840 [DOI] [PubMed] [Google Scholar]
- Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North RA. (1999) Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol 56:973–981 [DOI] [PubMed] [Google Scholar]
- Sun JH, Cai GJ, Xiang ZH. (2007) Expression of P2X purinoceptors in PC12 phaeochromocytoma cells. Clin Exp Pharmacol Physiol 34:1282–1286 [DOI] [PubMed] [Google Scholar]
- Surprenant A. (1996) Functional properties of native and cloned P2X receptors. Ciba Found Symp 198:208–219; discussion 219–222 [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. (2009) Signaling at purinergic P2X receptors. Annu Rev Physiol 71:333–359 [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738 [DOI] [PubMed] [Google Scholar]
- Surprenant A, Schneider DA, Wilson HL, Galligan JJ, North RA. (2000) Functional properties of heteromeric P2X(1/5) receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J Auton Nerv Syst 81:249–263 [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sato M, Kitani H. (2007) Lysophosphatidylcholine potentiates Ca2+ influx, pore formation and p44/42 MAP kinase phosphorylation mediated by P2X7 receptor activation in mouse microglial cells. J Neurochem 102:1518–1532 [DOI] [PubMed] [Google Scholar]
- Taylor AL, Schwiebert LM, Smith JJ, King C, Jones JR, Sorscher EJ, Schwiebert EM. (1999) Epithelial P2X purinergic receptor channel expression and function. J Clin Invest 104:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittle RK, Hume RI. (2008) Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci 28:11131–11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittle RK, Power JM, Hume RI. (2007) A histidine scan to probe the flexibility of the rat P2X2 receptor zinc-binding site. J Biol Chem 282:19526–19533 [DOI] [PubMed] [Google Scholar]
- Tomić M, Jobin RM, Vergara LA, Stojilkovic SS. (1996) Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem 271:21200–21208 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543 [DOI] [PubMed] [Google Scholar]
- Tomioka A, Ueno S, Kohama K, Goto F, Inoue K. (2000) Propofol potentiates ATP-activated currents of recombinant P2X(4) receptor channels expressed in human embryonic kidney 293 cells. Neurosci Lett 284:167–170 [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. (1999a) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274:6653–6659 [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. (1999b) Identification of a domain involved in ATP-gated ionotropic receptor subunit assembly. J Biol Chem 274:22359–22365 [DOI] [PubMed] [Google Scholar]
- Torres GE, Haines WR, Egan TM, Voigt MM. (1998) Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol 54:989–993 [DOI] [PubMed] [Google Scholar]
- Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. (2010) P2X4 receptors in activated C8–B4 cells of cerebellar microglial origin. J Gen Physiol 135:333–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé E, Soto F, Garret M, Boué-Grabot E. (2006) Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol 69:576–587 [DOI] [PubMed] [Google Scholar]
- Tsai CJ, Del Sol A, Nussinov R. (2009) Protein allostery, signal transmission and dynamics: a classification scheme of allosteric mechanisms. Mol Biosyst 5:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783 [DOI] [PubMed] [Google Scholar]
- Tuluc F, Bültmann R, Glänzel M, Frahm AW, Starke K. (1998) P2-receptor antagonists: IV. Blockade of P2-receptor subtypes and ecto-nucleotidases by compounds related to reactive blue 2. Naunyn Schmiedebergs Arch Pharmacol 357:111–120 [DOI] [PubMed] [Google Scholar]
- Ueno T, Ueno S, Kakazu Y, Akaike N, Nabekura J. (2001) Bidirectional modulation of P2X receptor-mediated response by divalent cations in rat dorsal motor nucleus of the vagus neurons. J Neurochem 78:1009–1018 [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28:11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe S, Miyoshi H, Fujiwara H, Yamaoka K, Kudo Y. (2009) Enhanced expression of P2X4 and P2X7 purinergic receptors in the myometrium of pregnant rats in preterm delivery models. Reprod Sci 16:1186–1192 [DOI] [PubMed] [Google Scholar]
- Vacca F, Amadio S, Sancesario G, Bernardi G, Volonté C. (2004) P2X3 receptor localizes into lipid rafts in neuronal cells. J Neurosci Res 76:653–661 [DOI] [PubMed] [Google Scholar]
- Vacca F, Giustizieri M, Ciotti MT, Mercuri NB, Volonté C. (2009) Rapid constitutive and ligand-activated endocytic trafficking of P2X receptor. J Neurochem 109:1031–1041 [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. (1994) A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371:516–519 [DOI] [PubMed] [Google Scholar]
- Valera S, Talabot F, Evans RJ, Gos A, Antonarakis SE, Morris MA, Buell GN. (1995) Characterization and chromosomal localization of a human P2X receptor from the urinary bladder. Receptors Channels 3:283–289 [PubMed] [Google Scholar]
- Vial C, Evans RJ. (2000) P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Evans RJ. (2002) P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 62:1438–1445 [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. (2005) Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem 280:30705–30711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Fung CY, Goodall AH, Mahaut-Smith MP, Evans RJ. (2006) Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem Biophys Res Commun 343:415–419 [DOI] [PubMed] [Google Scholar]
- Vial C, Tobin AB, Evans RJ. (2004) G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem J 382:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. (1997) Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 36:1285–1294 [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–321 [DOI] [PubMed] [Google Scholar]
- Virginio C, North RA, Surprenant A. (1998a) Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. J Physiol 510:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. (1998b) Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol Pharmacol 53:969–973 [PubMed] [Google Scholar]
- Vorobjev VS, Sharonova IN, Sergeeva OA, Haas HL. (2003) Modulation of ATP-induced currents by zinc in acutely isolated hypothalamic neurons of the rat. Br J Pharmacol 139:919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. (1996) Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA 93:8063–8067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. (1997) Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229–1242 [DOI] [PubMed] [Google Scholar]
- Wang C, Gu Y, Li GW, Huang LY. (2007) A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol 584:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Cong B, He P, Xu M, Ni X, Ma B. (2008) Transient changes in P2X3 but not TRPV1 mRNA expression in rat after prenatal exposure to glucocorticoids. Auton Neurosci 141:112–116 [DOI] [PubMed] [Google Scholar]
- Wareham K, Vial C, Wykes RC, Bradding P, Seward EP. (2009) Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br J Pharmacol 157:1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. (1993) Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett 324:219–225 [DOI] [PubMed] [Google Scholar]
- Weight FF, Li C, Peoples RW. (1999) Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors). Neurochem Int 35:143–152 [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29:578–586 [DOI] [PubMed] [Google Scholar]
- Wildman SS, Brown SG, King BF, Burnstock G. (1999a) Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur J Pharmacol 367:119–123 [DOI] [PubMed] [Google Scholar]
- Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, King BF. (2002) Sensitization by extracellular Ca(2+) of rat P2X(5) receptor and its pharmacological properties compared with rat P2X(1). Mol Pharmacol 62:957–966 [DOI] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. (1999b) Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol 128:486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JS, Chen R, Wiley MJ, Jamieson GP. (1992) The ATP4- receptor-operated ion channel of human lymphocytes: inhibition of ion fluxes by amiloride analogs and by extracellular sodium ions. Arch Biochem Biophys 292:411–418 [DOI] [PubMed] [Google Scholar]
- Wilkinson WJ, Gadeberg HC, Harrison AW, Allen ND, Riccardi D, Kemp PJ. (2009) Carbon monoxide is a rapid modulator of recombinant and native P2X(2) ligand-gated ion channels. Br J Pharmacol 158:862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson WJ, Jiang LH, Surprenant A, North RA. (2006) Role of ectodomain lysines in the subunits of the heteromeric P2X2/3 receptor. Mol Pharmacol 70:1159–1163 [DOI] [PubMed] [Google Scholar]
- Wirkner K, Stanchev D, Köles L, Klebingat M, Dihazi H, Flehmig G, Vial C, Evans RJ, Fürst S, Mager PP, et al. (2005) Regulation of human recombinant P2X3 receptors by ecto-protein kinase C. J Neurosci 25:7734–7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Stanchev D, Milius D, Hartmann L, Kato E, Zadori ZS, Mager PP, Rubini P, Nörenberg W, Illes P. (2008) Regulation of the pH sensitivity of human P2X receptors by N-linked glycosylation. J Neurochem 107:1216–1224 [DOI] [PubMed] [Google Scholar]
- Wolf C, Rosefort C, Fallah G, Kassack MU, Hamacher A, Bodnar M, Wang H, Illes P, Kless A, Bahrenberg G, et al. (2011) Molecular determinants of potent P2X2 antagonism identified by functional analysis, mutagenesis, and homology docking. Mol Pharmacol 79:649–661 [DOI] [PubMed] [Google Scholar]
- Wood CR, Hennessey TM. (2003) PPNDS is an agonist, not an antagonist, for the ATP receptor of Paramecium. J Exp Biol 206:627–636 [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Nowak M, Davies DL. (2004) Effects of the abused solvent toluene on recombinant P2X receptors expressed in HEK293 cells. Brain Res Mol Brain Res 125:86–95 [DOI] [PubMed] [Google Scholar]
- Worthington RA, Dutton JL, Poronnik P, Bennett MR, Barden JA. (1999) Localisation of P2X receptors in human salivary gland epithelial cells and human embryonic kidney cells by sodium dodecyl sulfate-polyacrylamide gel electrophoresis/Western blotting and immunofluorescence. Electrophoresis 20:2065–2070 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. (1998) Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res 813:390–397 [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. (2008) Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther 327:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. (2005) The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol 145:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. (2000) Inhibition by ethanol of rat P2X(4) receptors expressed in Xenopus oocytes. Br J Pharmacol 130:1394–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. (1999) Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol 81:2088–2094 [DOI] [PubMed] [Google Scholar]
- Xu GY, Huang LY. (2004) Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA 101:11868–11873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, et al. (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–137 [DOI] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. (2010) Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30:14213–14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Li S, Liang Z, Tomić M, Stojilkovic SS. (2008) The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Liang Z, Obsil T, Stojilkovic SS. (2006) Participation of the Lys313-Ile333 sequence of the purinergic P2X4 receptor in agonist binding and transduction of signals to the channel gate. J Biol Chem 281:32649–32659 [DOI] [PubMed] [Google Scholar]
- Yan Z, Liang Z, Tomic M, Obsil T, Stojilkovic SS. (2005) Molecular determinants of the agonist binding domain of a P2X receptor channel. Mol Pharmacol 67:1078–1088 [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694 [DOI] [PubMed] [Google Scholar]
- Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW, et al. (2009) Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun 381:102–106 [DOI] [PubMed] [Google Scholar]
- Young MT. (2010) P2X receptors: dawn of the post-structure era. Trends Biochem Sci 35:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT, Fisher JA, Fountain SJ, Ford RC, North RA, Khakh BS. (2008) Molecular shape, architecture, and size of P2X4 receptors determined using fluorescence resonance energy transfer and electron microscopy. J Biol Chem 283:26241–26251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa H, Shen J, Harada N, Cho-Tamaoka H, Yamashita T. (2005) Acute effects of glucocorticoids on ATP-induced Ca2+ mobilization and nitric oxide production in cochlear spiral ganglion neurons. Neuroscience 130:485–496 [DOI] [PubMed] [Google Scholar]
- Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. (2006) Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol 20:1423–1436 [DOI] [PubMed] [Google Scholar]
- Zemkova H, He ML, Koshimizu TA, Stojilkovic SS. (2004) Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J Neurosci 24:6968–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. (2010) Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab 298:E644–E651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, Yan Z, Liang Z, Jelinkova I, Tomic M, Stojilkovic SS. (2007) Role of aromatic and charged ectodomain residues in the P2X(4) receptor functions. J Neurochem 102:1139–1150 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Keng YF, Zhao Y, Wu L, Zhang ZY. (1998) Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases. J Biol Chem 273:12281–12287 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Logothetis DE, Séguéla P. (2007a) Regulation of ATP-gated P2X receptors by phosphoinositides. Pflugers Arch 455:181–185 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Yang M, Ting AT, Logothetis DE. (2007b) PIP(2) regulates the ionic current of P2X receptors and P2X(7) receptor-mediated cell death. Channels (Austin) 1:46–55 [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Burnstock G. (2000) Guinea-pig sympathetic neurons express varying proportions of two distinct P2X receptors. J Physiol 523:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Burnstock G. (2001) Multiple P2X receptors on guinea-pig pelvic ganglion neurons exhibit novel pharmacological properties. Br J Pharmacol 132:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Xiang Z, Bo X, Burnstock G. (1998) Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglion neurons. Br J Pharmacol 125:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Mamdani M, Qanud K, Shen JB, Pappano AJ, Kumar TS, Jacobson KA, Hintze T, Recchia FA, Liang BT. (2010) Treatment of heart failure by a methanocarba derivative of adenosine monophosphate: implication for a role of cardiac purinergic P2X receptors. J Pharmacol Exp Ther 333:920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshin AU, Ziganshina LE, King BF, Pintor J, Burnstock G. (1996) Effects of P2-purinoceptor antagonists on degradation of adenine nucleotides by ecto-nucleotidases in folliculated oocytes of Xenopus laevis. Biochem Pharmacol 51:897–901 [DOI] [PubMed] [Google Scholar]
- Ziganshina LE, Ziganshin AU, Hoyle CH, Burnstock G. (1996) Acute paw oedema formation induced by ATP: re-evaluation of the mechanisms involved. Inflamm Res 45:96–102 [DOI] [PubMed] [Google Scholar]