Abstract
The thyrotropin [thyroid-stimulating hormone (TSH)] receptor (TSHR) is known to acutely and persistently stimulate cAMP signaling and at higher TSH concentrations to acutely stimulate phosphoinositide signaling. We measured persistent signaling by stimulating TSHR-expressing human embryonic kidney-EM293 cells with TSH and measuring cAMP or inositol monophosphate (IP1) production, a measure of phosphoinositide signaling, 60 min or longer after TSH removal. In contrast to persistent cAMP production, persistent IP1 production increased progressively when TSH exposure was increased from 1 to 30 min, whereas the rates of decay of persistent signaling were similar. A small-molecule agonist and a thyroid-stimulating antibody also caused persistent IP1 and cAMP signaling. A small-molecule inverse agonist and a neutral antagonist inhibited TSH-stimulated persistent IP1 production, whereas the inverse agonist but not the neutral antagonist inhibited persistent cAMP production. As with persistent cAMP production, persistent IP1 production was not affected when TSHR internalization was inhibited or enhanced. Moreover, Alexa546-TSH-activated TSHR internalization was not accompanied by Gαq coupling protein internalization. Thus, transient exposure to high concentrations of TSH causes persistent phosphoinositide and cAMP signaling that is not dependent on internalization. To our knowledge, this is the first demonstration of persistent activation by any G protein-coupled receptor (GPCR) via the Gαq pathway and of two G protein-mediated pathways by any GPCR.
Introduction
Although G protein-coupled receptors (GPCRs) are able to signal independently of G proteins, the major pathways for GPCR signaling involve coupling of the activated receptor to one or more G proteins (Woehler and Ponimaskin, 2009). The human thyrotropin [thyroid-stimulating hormone (TSH)] receptor (TSHR) has been shown to couple to several G proteins (Laugwitz et al., 1996), including the stimulatory G protein (Gs), which activates adenylyl cyclase to produce cAMP (cAMP pathway), and Gq/11, which activates phospholipase C to produce inositol-1,4,5-trisphosphate (I-1,4,5-P3) (phosphoinositide pathway). The Gs-mediated stimulation of cAMP formation has been regarded as the principal intracellular signaling mechanism mediating the action of TSH. However, recently Kero et al. (2007) demonstrated that the Gq/G11-mediated signaling pathway is required for TSH-induced thyroid hormone synthesis and release in the adult and that the lack of Gq/G11 leads to hypothyroidism. Additional support for an essential physiological role of Gq/G11 proteins in mediating the regulation of thyroid function was demonstrated by a TSHR germline mutation that preferentially affected the phosphoinositide pathway (Grasberger et al., 2007).
Until recently, it was believed that GPCRs with dissociable agonists signal transiently and that the signaling pathway was rapidly desensitized by several mechanisms including receptor internalization (Hausdorff et al., 1990). However, over the last 2 years, it was shown that three GPCRs—TSHR (Calebiro et al., 2009; Neumann et al., 2010a); the parathyroid hormone receptor (Ferrandon et al., 2009), which also couples to Gs; and the sphingosine-1-phosphate receptor (Mullershausen et al., 2009), which couples to Gi to decrease cAMP production—exhibit persistent signaling even after the agonist has been removed. With these receptors, persistent signaling has been found to last for more than several hours.
In this study, we sought to determine whether the TSHR signaled persistently via the phosphoinositide pathway. It was shown that higher concentrations of TSH are required to stimulate I-1,4,5-P3 production than cAMP production (Van Sande et al., 1990). By using high concentrations of TSH, we were able to stimulate cAMP and phosphoinositide signaling simultaneously. We found that TSHR exhibits persistent activation of the phosphoinositide pathway as it does the cAMP pathway and that persistent phosphoinositide signaling occurs independently of internalization. We also show that persistent signaling can be caused by a small-molecule agonist of TSHR and by a thyroid-stimulating antibody.
Materials and Methods
Cell Culture and Transfection.
The generation of a HEK-EM293 cell lines stably expressing human TSHR (HEK-TSHR cells) or human TRH receptor were described previously (Engel et al., 2006; Neumann et al., 2009). Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 10 μg/ml streptomycin and 250 μg/ml hygromycin B (both from Invitrogen, Carlsbad, CA) at 37°C in a humidified 5% CO2 incubator.
Cells were transiently transfected with β-arrestin-2 (βArr2) or K44A (both kindly provided by Dr. Marc Caron, Duke University Medical Center, Durham, NC), Gαq-YFP chimera (Hughes et al., 2001) (kindly provided by Dr. Catherine Berlot, Weis Center for Research, Geisinger Clinic, Danville, PA) in 24-well plates (7.5 × 104 cells/well) with 0.2 μg of DNA/well or on poly(d-lysine)-coated coverglass culture dishes (MatTek, Ashland, MA) (104 cells/cm2) with 0.3 μg of DNA using FuGENE6 reagent (Roche Diagnostics, Indianapolis, IN). Experiments were performed 48 or 72 h after transfection.
cAMP Production.
Cells seeded into 24-well plates at a density of 2.2 × 105 cell/well were cultured for 24 h. Then they were washed three times with 0.5 ml of 37°C HBSS and incubated in HBSS/10 mM HEPES, pH 7.4, for 30 min. Thereafter, short-term TSH stimulation was measured as cAMP production in cells incubated in a humidified incubator at 37C for 30 min in HBSS/HEPES containing increasing concentrations (0–300 mU/ml) of bovine TSH (bTSH; Sigma-Aldrich, St. Louis, MO) and 1 mM 3-isobutyl-1-methylxanthine (IBMX).
To determine the persistent effect of TSH, the small-molecule ligand C2, and the thyroid-stimulating antibody M22 on cAMP production, cells were incubated with 100 mU/ml bTSH, 15 or 30 μM C2, or 100 or 200 ng/ml M22 for 30 min (“pretreatment”). Thereafter, the cells were washed three times with 0.5 ml of ice-cold HBSS followed by one wash at room temperature and incubated in 0.25 ml of HBSS/HEPES at 37°C. To determine the time course of loss of persistent signaling, after 1 h (standard “washout”) or at designated times (1, 2, 4, and 6 h, and overnight) after the washes, the media were aspirated and the cells were incubated in 0.2 ml of HBSS/HEPES containing 1 mM IBMX at 37°C for 30 to 60 min (“production”) (Fig. 2). Total cAMP production was measured by adding 0.2 ml of lysis buffer (cAMP-Screen Direct System; Applied Biosystems, Foster City, CA) to the wells. cAMP content in the samples was determined according to the manufacturer's protocol. Chemiluminescence was measured in VICTOR3 V 1420 Multilabel Counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). To determine the time of acquisition of persistent signaling, the TSH “pretreatment” was performed for 0, 1, 2.5, 10, or 30 min.
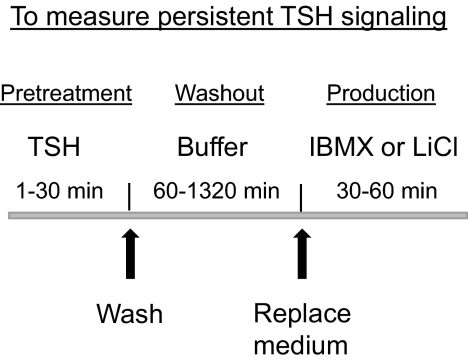
Fig. 2.
Schematic of the protocol to measure persistent TSH signaling. Cells, which were seeded and cultured for 24 h, were incubated with 100 mU/ml bTSH for 1 to 30 min (pretreatment). Thereafter, the cells were washed and incubated in HBSS/HEPES at 37°C for 60 to 1320 min (washout). Thereafter the mediums were aspirated and the cells were incubated in HBSS/HEPES containing IBMX (cAMP) or LiCl (IP1) for 30 to 60 min and total cAMP or IP1 levels were measured (production).
IP1 Production.
To determine the effects of short-term and persistent TSH stimulation, total IP1 production was measured under the same conditions as described above for cAMP with the exception of using 50 mM LiCl instead of IBMX. The incubations were terminated by adding 0.05 ml of lysis buffer (IP-One enzyme-linked immunosorbent assay kit; Cis Bio International, Gif-sur-Yvette, France) to the wells. IP1 content in the samples was determined according to the manufacturer's protocol. Optical density was measured in SpectraMax Plus384 (Molecular Devices, Sunnyvale, CA).
Acid-Resistant Binding.
To measure acid resistant binding, cells were incubated in binding buffer (HBSS/HEPES containing 2.5% milk powder and 0.2% bovine serum albumin) with 60,000 cpm bovine 125I-TSH (Brahms Aktiengesellschaft, Hennigsdorf, Germany) for 30 min at 37°C and then washed three times with 0.5 ml of ice-cold HBSS or with ice-cold acetic acid/sodium acetate buffer, pH 2.8. Total binding was measured in the absence of unlabeled TSH and nonspecific binding in the presence of 100 mU/ml unlabeled bovine TSH. The percentage of acid-resistant binding was calculated as 100 times specific binding (total minus nonspecific binding) after acid wash divided by specific binding after HBSS wash. Although it has traditionally been assumed that all acid-resistant binding of ligands to receptors monitors internalized receptors, it has been shown that this is not the case because some acid-resistant binding is present even when internalization is completely inhibited (Jones and Hinkle, 2008; Neumann et al., 2010a).
Fluorescent Microscopy.
Alexa Fluor 546-modified bovine TSH (Alexa-TSH) was synthesized as described previously (Neumann et al., 2010a). Monolayers were washed twice with HEPES/HBSS and then blocked with HBSS containing 4% bovine serum albumin for 15 min at 37°C. For binding, Alexa-TSH (10 μl) was diluted with 500 μl of blocking buffer, added to monolayer cultures, and incubated for 30 min at 37°C. Confocal (<0.5 μm) images were acquired on a Zeiss 510 NLO/Meta system (Carl Zeiss Inc., Thornwood, NY), using a Plan-Apochromat 40× 1.3 numerical aperture objective and are representative of slices at midpoint cell thickness. Detector gains and microscope parameters remained unchanged throughout all experimental conditions. Quantitative colocalization analysis was performed using Zeiss Zen software. Each region of interest within a confocal acquisition was assigned to include an entire cell. At least 5 unique images and 13 regions of interest were analyzed for each experimental condition. Pearson's correlation coefficient (Rr) and Manders' overlap coefficient (R) were calculated (Zinchuk and Zinchuk, 2008).
Statistical Analysis.
The data were analyzed by Student's t test or one-way analysis of variance; P < 0.05 was considered significant.
Results
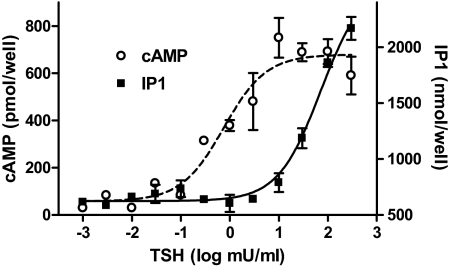
We used IP1 production to quantify activation of the phosphoinositide pathway because I-1,4,5-P3 is rapidly metabolized to IP1, and IP1 can be trapped in the presence of LiCl, which inhibits the IP1 mono phosphatase. This is similar to quantifying the cAMP pathway by measuring cAMP in the presence of IBMX, which inhibits the cAMP phosphodiesterase. Figure 1 illustrates that TSHR activation quickly leads to stimulation of the production of cAMP and IP1 in HEK-TSHR cells and confirms the observation reported previously that much lower concentrations of TSH stimulate cAMP production compared with those required for stimulation of IP1 production (Van Sande et al., 1990). TSH rapidly stimulated cAMP production with an EC50 = 0.75 mU/ml and IP1 production with an estimated EC50 ≥ 71 mU/ml. Indeed, the estimated EC50 for IP1 production is probably an underestimate because maximal stimulation may not have been achieved. We, therefore, needed to use high concentrations of TSH to stimulate both pathways simultaneously.
Fig. 1.
Short-term stimulation of cAMP and IP1 production by TSH. HEK-TSHR cells were immediately exposed to the noted concentrations of TSH in HBSS/HEPES with 1 mM IBMX (cAMP) or 50 mM LiCl (IP1) at 37°C. After 60 min, the incubation was stopped, and total cAMP or IP1 levels were measured by enzyme-linked immunosorbent assay as described under Materials and Methods. The apparent EC50 values for cAMP and IP1 production were 0.75 and >71 mU/ml, respectively (p < 0.001). The data are from one of three experiments with duplicate samples and are presented as mean ± range.
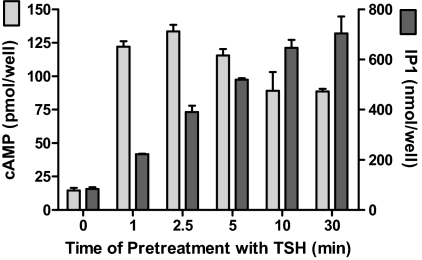
Figure 2 illustrates the experimental protocol we used to measure persistent TSH signaling. Cells were exposed to TSH (pretreatment), washed four times, and incubated in buffer alone (washout) and then incubated in buffer with IBMX or LiCl for measurement of cAMP or IP1 production, respectively. We had found previously that the level of activation of persistent cAMP signaling in HEK-TSHR cells by 10 mU/ml TSH increased between 1 and 5 min and then remained constant for up to 30 min (Neumann et al., 2010a). Figure 3 illustrates the time courses of acquisition of persistent signaling by the cAMP and IP1 pathways stimulated by 100 mU/ml TSH. This high concentration of TSH caused maximal persistent cAMP production after 1 min of stimulation. In contrast, the level of persistent IP1 production increased progressively with increasing time of exposure to 100 mU/ml TSH over the 30-min period. We showed previously that HEK-293EM cells expressing human receptors for luteinizing hormone/chorionic gonadotropin or follicle-stimulating hormone do not exhibit persistent cAMP production (Neumann et al., 2010a). We found that HEK-293EM cells expressing human receptors for thyrotropin-releasing hormone do not exhibit persistent IP1 production (data not shown). Thus, not all GPCRs exhibit persistent signaling in this cell model.
Fig. 3.
Time course of acquisition of persistent cAMP and IP1 production caused by TSH. HEK-TSHR cells were incubated with 100 mU/ml TSH for the pretreatment times indicated at 37°C. After 0 (control), 1, 2.5, 5, 10, or 30 min of exposure to TSH (pretreatment), the cells were washed and then incubated in HBSS/HEPES for 60 min (washout). Thereafter, the cells were incubated in HBSS/HEPES with IBMX (cAMP) or LiCl (IP1). After an additional 30 min (production), the incubations were stopped and total cAMP and IP1 levels were measured. cAMP and IP1 production increased persistently after 1 min of exposure (p < 0.01) and the levels of IP1 production increased progressively up to 10 min of exposure (p < 0.01). The bars are the mean ± range of duplicate measurements in one of three experiments.
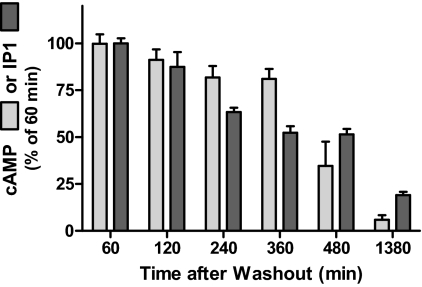
Figure 4 illustrates the time courses of loss of persistent cAMP and IP1 production stimulated by 100 mU/ml TSH. Both cAMP and IP1 production fell progressively at rates of approximately 3% per hour and had returned to pretreatment basal levels after 23 h.
Fig. 4.
Time course of decay of persistent cAMP and IP1 production caused by TSH. HEK-TSHR cells were incubated without or with 100 mU/ml TSH at 37C. After 30 min (pretreatment), the cells were washed and then incubated in HBSS/HEPES for 60 min (washout). At the times indicated after washout, the buffers were aspirated and the cells were incubated in HBSS/HEPES with IBMX (cAMP) or LiCl (IP1) for 30 min (production). The incubations were stopped and total cAMP or IP1 levels were measured. The individual levels of production in the three experiments were: for cAMP 130, 105, and 10 pmol/well and for IP1 were 460, 102, and 345 nmol/well. The decreases in IP1 production were progressive beginning after 120 min (p < 0.01). The data are from three experiments with duplicate or triplicate samples and are presented as mean ± S.E.
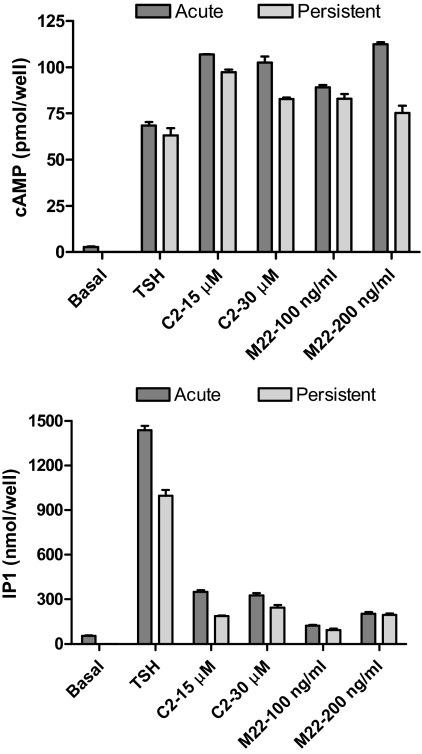
We determined whether other TSHR agonists—a small-molecule agonist C2 [NCGC00161870; N-[4-]]5-]3-(furan-2-ylmethyl)-4-oxo-1,2-dihydroquinazolin-2-yl]-2-methoxyphenyl]methoxy]phenyl]acetamide] (Neumann et al., 2009) and a thyroid-stimulating antibody M22 (Sanders et al., 2004)—would cause persistent signaling. We used maximally effective doses of C2 and M22 as determined previously (Sanders et al., 2004; Neumann et al., 2009) and showed that they were maximally effective because the two doses gave similar levels of stimulation. We found qualitatively similar effects of C2 and M22 on persistent cAMP and IP1 signaling. Figure 5 illustrates that C2 and M22 are full agonists for cAMP production when added immediately and stimulated persistent cAMP signaling to similar levels as TSH. In contrast, C2 and M22 are partial agonists for short-term IP1 production and stimulated persistent IP1 signaling to proportionately lower levels than TSH. Thus, C2 and M22 are functionally selective agonists (Strange, 2008) at TSHR and stimulate persistent signaling to levels concordant with their efficacies for short-term stimulation.
Fig. 5.
Comparison of persistent signaling caused by TSH, C2, and M22. HEK-TSHR cells were incubated without or with 100 mU/ml TSH, 15 or 30 μM C2, or 100 or 200 ng/ml M22 at 37C. After 30 min (pretreatment), the cells were washed and then incubated in HBSS/HEPES for 60 min (washout). At the times indicated after washout, the buffers were aspirated and the cells were incubated in HBSS/HEPES with IBMX (cAMP) or LiCl (IP1) for 60 min (production). The incubations were stopped and total cAMP or IP1 levels were measured. TSH, C2, and M22 caused persistent cAMP and IP1 production (p < 0.001). The data are from one of three experiments with duplicate samples and are presented as mean ± S.D.
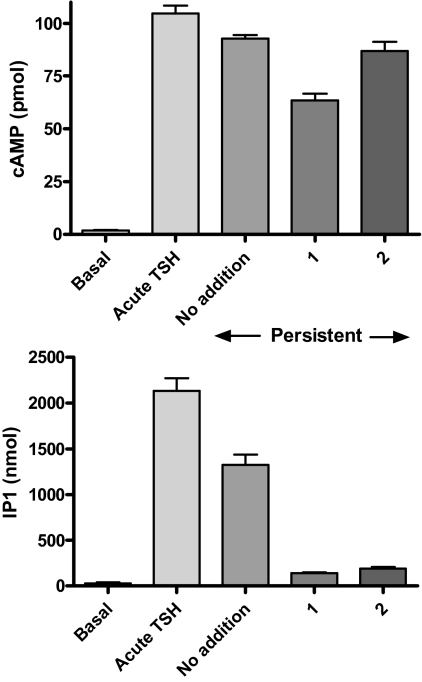
We showed previously that a small-molecule inverse agonist of TSHR, 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(pyridin-3-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (NCGC00229600; compound 1), partially inhibited persistent cAMP signaling (Neumann et al., 2010b). We have developed a small-molecule neutral antagonist for TSHR, NCGC00242595 (compound 2). Supplemental Fig. 1 illustrates the structure and synthetic scheme of 2, and Supplemental Fig. 2 illustrates that 2 does not inhibit basal signaling by TSHR but inhibits TSH-stimulated signaling and is therefore a neutral antagonist. 2 inhibited cAMP production immediately stimulated by an EC50 dose of TSH by 55% with an EC50 of 2.7 μM. Figure 6 illustrates that 1 inhibited persistent cAMP production by 34% and persistent IP1 production by 89%, whereas 2 had no effect on persistent cAMP production but inhibited persistent IP1 production by 84%. Thus, both 1 and 2 inhibited persistent IP1 production, but only 1 inhibited persistent cAMP production.
Fig. 6.
Inhibition of persistent signaling by 1 and 2. HEK-TSHR cells were incubated without or with 100 mU/ml TSH at 37°C. After 30 min (pretreatment), the cells were washed and then incubated in HBSS/HEPES for 45 min (first washout period). After 45 min, the cells were incubated in HBSS/HEPES with no addition or with 30 μM 1 or 30 μM 2 for 15 min (second washout phase). After both washout phases, the buffers were aspirated, and the cells were incubated in HBSS/HEPES with IBMX (cAMP) or LiCl (IP1) without (no addition) or with 1 or 2 for 60 min (productions phase). The incubations were stopped and total cAMP or IP1 levels were measured. 1 but not 2 inhibited persistent cAMP production (p < 0.001). 1 and 2 inhibited persistent IP1 production (p < 0.001). The data are from one of three experiments with duplicate or triplicate samples and are presented as mean ± S.D.
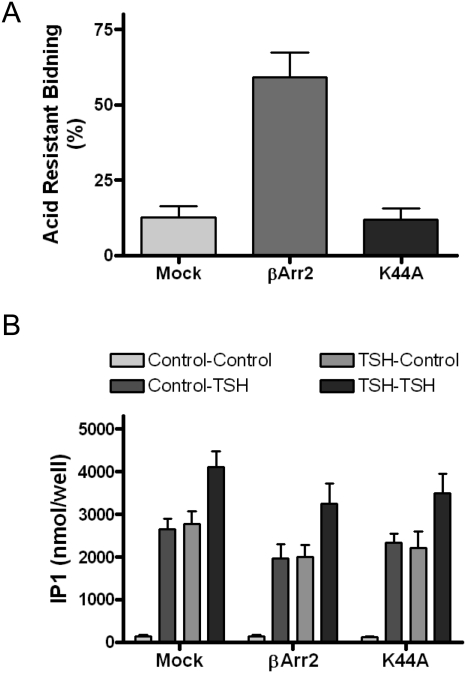
Because we had provided evidence previously that persistent cAMP production stimulated by TSH did not depend on TSHR internalization (Neumann et al., 2010a; but see Calebiro et al., 2009), we determined whether persistent IP1 production was affected by TSHR internalization. We measured IP1 production in cells in which internalization was inhibited by expressing the dominant-negative dynamin mutant protein (K44A) or was enhanced by the expression of βArr2 in these cells (Neumann et al., 2010a). Figure 7 illustrates that both expression of βArr2, which increased acid-resistant 125I-bTSH binding from 12 (control) to 59%, and of K44A, which had no effect on acid-resistant 125I-bTSH binding, had no effect on basal, on short-term TSH stimulation of, on TSH-activated persistent stimulation of, or on restimulation by TSH of IP1 production. [The lack of effect of K44A expression on acid resistant binding in these cells confirms our previous findings (Neumann et al., 2010a) and the conclusion that not all acid-resistant binding is caused by internalization (Jones and Hinkle, 2008)]. Most importantly, inducing robust TSHR internalization with βArr2 (see below) did not affect TSH stimulation of the phosphoinositide pathway.
Fig. 7.
Dominant-negative dynamin mutant protein (K44A) or βArr2 had no effect on persistent IP1 production. HEK-TSHR cells were transfected with empty plasmid (Mock), plasmid to express βArr2 or plasmid to express K44A. A, acid-resistant 125I-TSH binding. After 48 h, specific 125I-TSH binding (HBSS wash) and 125I-TSH binding after acid wash were measured and acid-resistant binding calculated as described under Materials and Methods. βArr2 increased acid-resistant binding (p < 0.001) whereas K44A had no effect (p > 0.1). The bars are the mean ± S.E. of duplicate measurements in three experiments. B, IP1 production. After 48 h, the cells were incubated without (Control) or with 100 mU/ml TSH at 37°C. After 30 min (pretreatment), the cells were washed and incubated in HBSS/HEPES at 37°C (washout). At 60 min after the washes, the cells were incubated in HBSS/HEPES with LiCl alone or LiCl and 100 mU/ml TSH at 37°C for 30 min (production). The cells were lysed and IP1 content was measured in the cell lysates. Control-Control, no pretreatment/no treatment during production phase; Control, TSH; no pretreatment, TSH added during production phase; TSH-Control, TSH pretreatment/no treatment during production phase (persistent signaling); TSH-TSH, TSH pretreatment/TSH treatment during production phase. There was no increase in IP1 production in cells expressing βArr2 (p > 0.1) nor a decrease in IP1 production in cells expressing K44A (p > 0.1). The bars are the mean ± S.E. of duplicate measurements in three experiments.
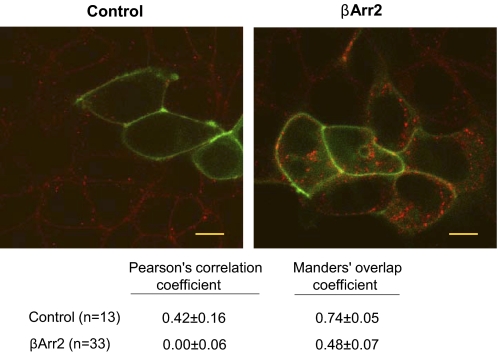
To further address the issue of whether TSHR internalization is necessary for persistent phosphoinositide pathway activation, we visualized TSH-bound TSHRs using Alexa Fluor 546-tagged TSH (Alexa-TSH) in HEKTSHR cells expressing Gαq-YFP (Hughes et al., 2001) (Fig. 8). There was no apparent TSHR internalization in HEK-TSHR cells or in HEK-TSHR cells expressing K44A (data not shown) but robust internalization in HEK-TSHR cells expressing βArr2, as has been reported previously (Frenzel et al., 2006; Neumann et al., 2010a). However, Gαq-YFP remained at the surface of all cells including cells expressing βArr2 (Fig. 8). Quantitative analysis confirmed that there was a high level of colocalization of Alexa-TSH/TSHR and Gαq-YFP on the surface of control cells not expressing βArr2 but no significant colocalization (Pearson's correlation coefficient) or a marked loss in colocalization (Manders's overlap coefficient) in cells expressing βArr2. Thus, HEK-TSHR cells do not internalize TSH-bound TSHRs but can be made to internalize TSHRs after expression of βArr2, but Gαq-YFP does not cointernalize with TSHRs. These findings, together with those in Fig. 7, show that persistent IP1 production stimulated by TSHR does not require TSHR (or Gαq) internalization.
Fig. 8.
Gαq-YFP does not internalize with TSHRs. HEK-TSHR cells were transfected to transiently express βArr2 and Gαq-YFP. After 48 h, the cells were incubated with Alexa-TSH for 30 min at 37°C. Images were acquired as described under Materials and Methods. The micrographs shown are representative of slices at the midpoint of the cell thickness. Left, control. Right, βArr2. Alexa-TSH, red. Gαq-YFP, green. Scale bar, 10 μm. Below are the values from the quantitative colocalization analysis. Both coefficients were different in cells expressing βArr2 (p < 0.001); n = number of cells.
Discussion
We show herein that TSHR activation by TSH leads to persistent signaling by the phosphoinositide pathway and by the cAMP pathway, which was reported previously (Calebiro et al., 2009; Neumann et al., 2010a). As shown previously (Van Sande et al., 1990) and confirmed herein, the potency of TSH to activate the phosphoinositide pathway was approximately 100-fold lower than that needed to activate the cAMP pathway. Therefore, it was necessary to use high TSH concentrations to activate the cAMP and phosphoinositide pathways simultaneously. We measured persistent activation of the phosphoinositide pathway using a protocol similar to the one we used for measurement of persistent cAMP signaling (Neumann et al., 2010a). For the cAMP pathway, we pretreated HEK-TSHR cells with TSH, washed the cells to remove TSH, and incubated the cells in medium without TSH for 1 h. After incubation without TSH (washout), we measured cAMP production as the amount of cAMP accumulated during 30- to 60-min incubations in the presence of IBMX, which inhibits cAMP degradation (Fig. 2). For the phosphoinositide pathway, we performed the same pretreatment, washes, and washout incubation and then added LiCl to inhibit IP1 degradation by the IP1 phosphatase because I-1,4,5-P3 is rapidly metabolized to IP1, and there is no effective inhibitor of I-1,4,5-P3 metabolism. We measured IP1 production as the amount of IP1 accumulated during a 30- to 60-min incubation in the presence of LiCl. We are, therefore, able to make direct quantitative comparisons between persistent signaling in these two pathways.
We showed that the time courses of acquisition of persistent signaling activated by TSH were different for the cAMP and phosphoinositide pathways. Acquisition of persistent cAMP production was very rapid, whereas persistent IP1 production was progressive over 30-min exposure to TSH. We believe that these different findings were most likely caused by the need to occupy a smaller fraction of TSHRs to generate maximal cAMP production and a higher fraction of TSHRs to generate maximal IP1 production. Indeed, our finding that persistent cAMP production was not increased (Neumann et al., 2010a) but IP1 production was increased further upon re-exposure to TSH (Fig. 7) may have been because not all TSHR binding sites were occupied during the pretreatment exposure to TSH. By contrast, decay of persistent cAMP and IP1 production were similar (3% per hour) and may reflect an intrinsic mechanism of inactivation of TSH/TSHR complexes.
In the previous reports of persistent cAMP signaling by activated TSHRs, the only agonist used was TSH (Calebiro et al., 2009; Neumann et al., 2010a). In this study, we activated TSHR with TSH; a small-molecule agonist, C2 (Neumann et al., 2009); and a thyroid-stimulating antibody, M22 (Sanders et al., 2004). We found qualitatively similar effects of C2 and M22 on persistent cAMP and IP1 signaling. Both C2 and M22 are full agonists for cAMP production when added immediately and stimulated persistent cAMP signaling to levels similar to those of TSH, whereas C2 and M22 are partial agonists for short-term IP1 production and stimulated persistent IP1 signaling to proportionately lower levels than TSH. Thus, both C2 and M22 exhibit functionally selective short-term signaling (Strange, 2008). It had been shown previously that some thyroid-stimulating antibodies exhibit functionally selective signaling at TSHR (Morshed et al., 2009, 2010). It is noteworthy that there was a clear parallelism between the efficacies of these agonists to immediately and persistently stimulate cAMP and IP1 production. These findings are consistent with the idea that the activatory conformation achieved after binding a given agonist to stimulate second-messenger formation immediately is retained during persistent signaling. One hypothesis that is compatible with this idea is that the persistently signaling complex is composed of agonist/activated TSHR/Gα subunit. Another possibility is that the persistently signaling complex is activated TSHR/Gα subunit but does not contain agonist.
To attempt to distinguish between these possibilities, we took advantage of our recent discovery of a small-molecule neutral antagonist of TSHR, 2 (Supplemental Figs. 1 and 2). In our previous report (Neumann et al., 2010a), we used another structurally related small-molecule inverse agonist [NCGC00161856, 2-[3-[(2,6-dimethylphenoxy)methyl]-4-methoxyphenyl]-3-(furan-2-ylmethyl)-1,2-dihydroquinazolin-4-one] and showed that it inhibited persistent cAMP production. These previous data allowed us to conclude that activated TSHR was necessary for persistent cAMP production but could not allow the determination of whether TSH was still present in the complex because an inverse agonist may inhibit signaling by the competition of agonist binding to receptors and by unoccupied receptors. Herein, we showed that both 1 and 2 markedly inhibited persistent IP1 production. Therefore, if 2 is a neutral antagonist at TSHR for IP1 production as it is for cAMP production, which is difficult to determine because basal IP1 production stimulated by TSHR is very low, then we may conclude that the complex that persistently signals includes the agonist-occupied, activated TSHR. We confirmed that 1 inhibits persistent cAMP production but found that 2 did not. The explanation for the finding that 2 inhibits persistent IP1 signaling but not persistent cAMP signaling may be because a lower fraction of TSHRs needs be bound by TSH to cause persistent cAMP signaling and a higher fraction for IP1 signaling. Another possibility is that there may be “spare receptors” for cAMP but not for IP1 signaling. We are currently performing experiments that may distinguish between these possibilities.
In the initial reports (Calebiro et al., 2009; Ferrandon et al., 2009; Mullershausen et al., 2009), persistent signaling by GPCRs with dissociable agonists was shown to correlate with their internalization. Indeed, for the parathyroid hormone receptor (Ferrandon et al., 2009), parathyroid hormone, which stimulated internalization, caused persistent cAMP signaling, whereas parathyroid hormone-related peptide, which did not cause internalization, did not. Evidence was also presented that the GPCRs colocalized with Gs and adenylyl cyclase intracellularly within an early endosomal compartment. These authors, therefore, hypothesized that persistent cAMP signaling was mediated by activated receptors within intracellular compartments. This mechanism was likened to the well established findings that some tyrosine kinase receptors signal intracellularly (Barbieri et al., 2004) and that some GPCRs signal via interactions with arrestin proteins that may be in intracellular vesicles (Hanyaloglu and von Zastrow, 2008). However, no direct evidence showing that persistent GPCR signaling was dependent on internalization was presented. We have reported that the TSHR could cause persistent cAMP signaling in HEK-TSHR cells, in which internalization did not occur or was inhibited, and that persistent cAMP signaling was not increased in HEK-TSHR cells in which internalization was induced by exogenously expressing βArr2 (Neumann et al., 2010a). We concluded, therefore, that persistent cAMP signaling was not dependent on internalization. Indeed, we showed that a small-molecule antagonist of TSHR (Neumann et al., 2010a) could inhibit persistent signaling and allow readded TSH to activate the receptor. We, therefore, hypothesized that persistently signaling TSHRs were not in endosomal vesicles but were in a compartment that was accessible to the TSHR antagonist and TSH. Herein, we present evidence that persistent signaling via the phosphoinositide pathway is independent of internalization also. We show that inhibiting or increasing internalization does not affect persistent IP1 production stimulated by TSH and that in cells in which TSHR internalization is increased, there is no detectable internalization of Gαq-YFP.
Even though the concentrations of TSH needed to stimulate the phosphoinositide pathway are higher than the levels found in the blood of healthy subjects, these levels may be found in tissues in which TSH-producing cells and TSHR-expressing cells are found together, such as the pituitary gland (Prummel et al., 2000) and epidermis (Bodó et al., 2010; Cianfarani et al., 2010). Moreover, thyrostimulin, another heterodimeric glycoprotein activator of TSHR that is believed to act in a paracrine fashion (Nakabayashi et al., 2002; Sun et al., 2010), may achieve concentrations sufficient to stimulate TSHR-mediated phosphoinositide signaling locally. Thyrostimulin has been shown to activate cAMP and calcium signaling, which is most likely initiated by coupling of TSHR to Gq (Nagasaki et al., 2006). Strong support for the idea that TSHR signaling via the phosphoinositide pathway is physiologically relevant was provided by the recent demonstrations that the Gq/G11-mediated signaling pathway is required for TSH-induced thyroid hormone synthesis and release in the adult and that the lack of Gq/G11 leads to hypothyroidism (Kero et al., 2007) and the finding that a TSHR germline mutation that preferentially affected the phosphoinositide pathway affected thyroid gland function (Grasberger et al., 2007). Thus, we think that phosphoinositide signaling mediated by activation of TSHR is physiologically relevant.
In conclusion, we showed that TSHR activation by TSH leads to persistent signaling by both the cAMP (Calebiro et al., 2009; Neumann et al., 2010a) and phosphoinositide pathways. To our knowledge, this is the first demonstration of persistent activation by any GPCR via the Gq pathway and of persistent signaling of two G protein-mediated pathways by any GPCR.
Supplementary Material
Acknowledgments
We thank Dr. Marc Caron (Duke University Medical Center, Durham, NC) for the plasmids expressing βArr2 and K44A and Dr. Catherine Berlot (Weis Center for Research, Geisinger Clinic, Danville, PA) for the plasmid expressing Gαq-YFP chimera (Hughes et al., 2001). We thank the National Institutes of Health Chemical Genomics Center analytical group for analytical support.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases [Grants Z01-DK011006, Z01-DK047045]; the National Human Genome Research Institute; and the Molecular Libraries Initiative of the Roadmap for Medical Research, National Institutes of Health.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.072157.
- GPCR
- G protein-coupled receptor
- TSH
- thyroid-stimulating hormone
- TSHR
- thyroid-stimulating hormone receptor
- IP1
- inositol monophosphate
- I-1,4,5-P3
- inositol-1,4,5-trisphosphate
- YFP
- yellow fluorescent protein
- HBSS
- Hanks' balanced salt solution
- IBMX
- 3-isobutyl-1-methylxanthine
- HEK
- human embryonic kidney
- βArr2
- β-arrestin-2
- NCGC00161870
- N-[4-]]5-]3-(furan-2-ylmethyl)-4-oxo-1,2-dihydroquinazolin-2-yl]-2-methoxyphenyl]methoxy]phenyl]acetamide
- NCGC00161856
- 2-[3-[(2,6-dimethylphenoxy)methyl]-4-methoxyphenyl]-3-(furan-2-ylmethyl)-1,2-dihydroquinazolin-4-one
- NCGC00229600
- 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(pyridin-3-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one.
Authorship Contributions
Participated in research design: Boutin, Allen, Geras-Raaka, Neumann, and Gershengorn.
Conducted experiments: Boutin, Geras-Raaka, and Gershengorn.
Contributed new reagents or analytic tools: Allen, Geras-Raaka, Huang, and Gershengorn.
Performed data analysis: Boutin, Geras-Raaka, and Gershengorn.
Wrote or contributed to the writing of the manuscript: Boutin, Geras-Raaka, Neumann, and Gershengorn.
References
- Barbieri MA, Ramkumar TP, Fernadez-Pol S, Chen PI, Stahl PD. (2004) Receptor tyrosine kinase signaling and trafficking—paradigms revisited. Curr Top Microbiol Immunol 286:1–20 [PubMed] [Google Scholar]
- Bodó E, Kany B, Gáspár E, Knüver J, Kromminga A, Ramot Y, Bíró T, Tiede S, van Beek N, Poeggeler B, et al. (2010) Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology 151:1633–1642 [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors.PLoS Biol 7:e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfarani F, Baldini E, Cavalli A, Marchioni E, Lembo L, Teson M, Persechino S, Zambruno G, Ulisse S, Odorisio T, et al. (2010) TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol 130:93–101 [DOI] [PubMed] [Google Scholar]
- Engel S, Neumann S, Kaur N, Monga V, Jain R, Northup J, Gershengorn MC. (2006) Low affinity analogs of thyrotropin-releasing hormone are super-agonists. J Biol Chem 281:13103–13109 [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel R, Voigt C, Paschke R. (2006) The human thyrotropin receptor is predominantly internalized by beta-arrestin 2. Endocrinology 147:3114–3122 [DOI] [PubMed] [Google Scholar]
- Grasberger H, Van Sande J, Hag-Dahood Mahameed A, Tenenbaum-Rakover Y, Refetoff S. (2007) A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab 92:2816–2820 [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48:537–568 [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ. (1990) Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J 4:2881–2889 [PubMed] [Google Scholar]
- Hughes TE, Zhang H, Logothetis DE, Berlot CH. (2001) Visualization of a functional Gαq-green fluorescent protein fusion in living cells. Association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not be activation mediated by receptors or AlF4−. J Biol Chem 276:4227–4235 [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM. (2008) Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol Pharmacol 74:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kero J, Ahmed K, Wettschureck N, Tunaru S, Wintermantel T, Greiner E, Schütz G, Offermanns S. (2007) Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest 117:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. (1996) The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed SA, Ando T, Latif R, Davies TF. (2010) Neutral antibodies to the TSH receptor are present in Graves' disease and regulate selective signaling cascades. Endocrinology 151:5537–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed SA, Latif R, Davies TF. (2009) Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology 150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 5:428–434 [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Wang Z, Jackson VR, Lin S, Nothacker HP, Civelli O. (2006) Differential expression of the thyrostimulin subunits, glycoprotein alpha2 and beta5 in the rat pituitary. J Mol Endocrinol 37:39–50 [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Matsumi H, Bhalla A, Bae J, Mosselman S, Hsu SY, Hsueh AJ. (2002) Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J Clin Invest 109:1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Geras-Raaka E, Marcus-Samuels B, Gershengorn MC. (2010a) Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization. FASEB J 24:3992–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, Gershengorn MC. (2010b) A small molecule inverse agonist for the human thyroid-stimulating hormone receptor. Endocrinology 151:3454–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, et al. (2009) Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA 106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prummel MF, Brokken LJ, Meduri G, Misrahi M, Bakker O, Wiersinga WM. (2000) Expression of the thyroid-stimulating hormone receptor in the folliculo-stellate cells of the human anterior pituitary. J Clin Endocrinol Metab 85:4347–4353 [DOI] [PubMed] [Google Scholar]
- Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Núñez Miguel R, et al. (2004) Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14:560–570 [DOI] [PubMed] [Google Scholar]
- Strange PG. (2008) Signaling mechanisms of GPCR ligands. Curr Opin Drug Discov Devel 11:196–202 [PubMed] [Google Scholar]
- Sun SC, Hsu PJ, Wu FJ, Li SH, Lu CH, Luo CW. (2010) Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J Biol Chem 285:3758–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sande J, Raspé E, Perret J, Lejeune C, Maenhaut C, Vassart G, Dumont JE. (1990) Thyrotropin activates both the cyclic AMP and the PIP2 cascades in CHO cells expressing the human cDNA of TSH receptor. Mol Cell Endocrinol 74:R1–R6 [DOI] [PubMed] [Google Scholar]
- Woehler A, Ponimaskin EG. (2009) G protein–mediated signaling: same receptor, multiple effectors. Curr Mol Pharmacol 2:237–248 [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O. (2008) Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr Protoc Cell Biol Chapter 4:Unit 4.19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.