Abstract
6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine (compound C) is a cell-permeable pyrrazolopyrimidine derivative that acts as a potent inhibitor of AMP-activated protein kinase (AMPK). Although compound C is often used to determine the role of AMPK in various physiological processes, it also evokes AMPK-independent actions. In the present study, we investigated whether compound C influences vascular smooth muscle cell (SMC) function through the AMPK pathway. Treatment of rat aortic SMCs with compound C (0.02–10 μM) inhibited vascular SMC proliferation and migration in a concentration-dependent fashion. These actions of compound C were not mimicked or affected by silencing AMPKα expression or infecting SMCs with an adenovirus expressing a dominant-negative mutant of AMPK. In contrast, the pharmacological activator of AMPK 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside inhibited the proliferation and migration of SMCs in a manner that was strictly dependent on AMPK activity. Flow cytometry experiments revealed that compound C arrested SMCs in the G0/G1 phase of the cell cycle, and this was associated with a decrease in cyclin D1 and cyclin A protein expression and retinoblastoma protein phosphorylation and an increase in p21 protein expression. Finally, local perivascular delivery of compound C immediately after balloon injury of rat carotid arteries markedly attenuated neointima formation. These studies identify compound C as a novel AMPK-independent regulator of vascular SMC function that exerts inhibitory effects on SMC proliferation and migration and neointima formation after arterial injury. Compound C represents a potentially new therapeutic agent in treating and preventing occlusive vascular disease.
Introduction
The proliferation and migration of vascular smooth muscle cells (SMCs) plays a fundamental role in the development of atherosclerosis and contributes to the failure of interventional therapeutic approaches to ameliorate vascular occlusion after vein bypass grafting, organ transplantation, and angioplasty (Gallo et al., 1999; Orford et al., 2000; Dzau et al., 2002). In particular, the long-term outcome of revascularization therapy of stenotic arteries by angioplasty is compromised by restenosis, requiring multiple reinterventions in a substantial percentage of patients. Neointima formation after invasive revascularization procedures is not restricted to coronary arteries but has also been reported for peripheral vessels, including carotid arteries (de Borst et al., 2007). Although the use of drug-eluting stents releasing cytotoxic agents, such as rapamycin and paclitaxel, has significantly reduced the incidence of restenosis after coronary angioplasty (Moses et al., 2003; Gershlick et al., 2004), concerns persist regarding the safety of these drug-eluting stents, given the potential for fatal coronary in-stent thrombosis (Jeremias and Kirtane, 2008). Moreover, the long-term efficacy of these drug-eluting stents has not been fully established in the revascularization of cerebrovascular or peripheral arteries (Gupta et al., 2006; Zeller, 2007). These findings collectively underscore the need to develop alternative pharmacological approaches that target the proliferation and migration of vascular SMCs to prevent neointima formation in injured arteries.
6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine (compound C) is a cell-permeable pyrrazolopyrimidine derivative that acts as a potent ATP-competitive inhibitor of AMP-activated protein kinase (AMPK) (Zhou et al., 2001). In vitro assays indicate that compound C blocks AMPK activity with reported IC50 ranging from 0.1 to 0.2 μM (Bain et al., 2007). Although compound C is commonly used to ascertain the role for AMPK in various physiological processes, an array of AMPK-independent actions of compound C have been reported. In particular, compound C has been found to inhibit the activation of hypoxia-inducible factor-1, bone morphogenetic protein type I receptors, and several kinases in an AMPK-independent fashion (Bain et al., 2007; Emerling et al., 2007; Yu et al., 2008). Of interest, studies indicate that compound C exerts a potent antiproliferative effect in both rodent and human tumor cell lines via AMPK-dependent and -independent mechanisms (Vucicevic et al., 2009). In addition, compound C has been demonstrated to block the clonal expansion of preadipocytes irrespective of AMPK inhibition (Nam et al., 2008).
Given the reported antiproliferative action of compound C in different cell lines, the present study investigated whether this pyrrazolopyrimidine derivative regulates vascular SMC function. In particular, this study determined whether compound C modulates vascular SMC proliferation and migration and examined the role of AMPK in mediating the actions of this agent. In addition, it explored whether compound C influences neointima formation after arterial injury.
Materials and Methods
Materials.
Minimum essential medium, M199 medium, and bovine serum were from Invitrogen (Carlsbad, CA). Penicillin, trichloroacetic acid, streptomycin, SDS, propidium iodide, RNase A, collagenase, elastase, compound C, and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) were from Sigma-Aldrich (St. Louis, MO). Antibodies against cyclin D1, cyclin E, cyclin A, p21, p27, p53, cyclin-dependent kinase 2, cyclin-dependent kinase 4, cyclin-dependent kinase 6, and β-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A pan-specific antibody against AMPKα and phosphorylation-specific antibodies against AMPKα, acetyl-CoA-carboxylate (ACC), and retinoblastoma protein were from Cell Signaling Technologies (Beverley, MA). [3H]Thymidine (20 Ci/mmol) was from PerkinElmer Life and Analytical Sciences (Waltham, MA). α-[32P]dCTP (3000 Ci/mmol) was from GE Healthcare (Little Chalfont, Buckinghamshire, UK).
Cell Culture.
Male Sprague-Dawley rats (450–500 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and maintained on standard rat chow and water ad libitum with 12-h light/dark cycles. Animals were injected intraperitoneally with ketamine (100 mg/kg) and xylazine (7.5 mg/kg) (Butler Schein Animal Health Corporation, Dublin, OH) and euthanized by pneumothorax and exsanguination. Thoracic aorta were rapidly removed, washed in phosphate-buffered saline (4°C), opened longitudinally, and stripped of adventitia and endothelium. Vessels were cut into fine pieces and incubated sequentially with elastase (0.05%) and collagenase (0.3%) at 37°C. Cell suspensions were pelleted, resuspended in complete minimum essential medium containing 10% bovine serum, Earle's salts, and 2 mM l-glutamine, and characterized by morphological and immunological criteria, as described previously (Durante et al., 1993). The culture medium was also supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml), and cells were propagated at 37°C in an atmosphere of 95% air and 5% CO2.
Small Interfering RNA and Infection with Adenoviral Vectors.
Gene expression was silenced using specific small interfering (si) RNAs targeting rat AMPKα1/2. The experimental and control nontargeting siRNAs were obtained from QIAGEN (Valencia, CA) and delivered to cells (50 nM) using the HiPerFect transfection reagent (QIAGEN). A replication-defective adenovirus expressing a dominant-negative AMPK mutant (AdAMPK-DN) was generously provided by Dr. Ming-Hui Zou (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Cells were infected with AdAMPK-DN or an adenovirus expressing green fluorescent protein (AdGFP) at a multiplicity of infection of 50.
Cell Proliferation.
Vascular SMCs were seeded (2–5 × 104 cells/well) onto six-well plates in serum-containing media. After 24 h, culture media were exchanged for serum-free media containing bovine serum albumin (0.1%) for an additional 48 h. Cells were then treated with serum in the presence or absence of compound C. Cell number determinations were performed at various times by dissociating cells with trypsin and counting cells in a Beckman Z1 Coulter Counter (Beckman Coulter, Fullerton, CA). Vascular SMC proliferation was also monitored by measuring DNA synthesis, as described previously (Peyton et al., 2002). Quiescent SMCs were incubated in the presence of serum for 20 h, [3H]thymidine was then added, and cells were incubated for another 4 h. SMCs were then washed three times with ice-cold PBS and fixed with 10% trichloroacetic acid for 30 min at 4°C, and DNA was extracted with 0.2% SDS-0.2 N NaOH. Radioactivity was determined by scintillation spectroscopy.
Cell Viability.
Cell viability was assessed by measuring the uptake of the membrane-impermeable stain trypan blue. Cells were treated with trypsin (0.25%), collected, diluted (1:4) with trypan blue, and examined by microscopy. Viability was determined by the percentage of cells that excluded trypan blue, as described previously (Liu et al., 2003).
Cell Cycle Analysis.
Cell cycle progression was assessed by flow-activated cell sorting, as reported previously (Peyton et al., 2009). Vascular SMCs were serum-starved for 2 days and then treated with serum in the presence or absence of compound C for 24 h. After treatment, cells were harvested, suspended in phosphate-buffered saline, and pelleted by centrifugation at 1000g for 5 min. Pellets were washed twice with phosphate-buffered saline and recentrifuged. Pellets were suspended in 70% ethanol and fixed overnight at 4°C. Fixed cells were briefly vortexed and centrifuged at 13,000g for 15 min. Pellets were then incubated with propidium iodide (50 μg/ml) and RNase A (100 μg/ml) for 1 h at room temperature. DNA fluorescence was measured in a Beckman Coulter CyAN ADP Cytometer.
Cell Migration.
Cell migration was assessed using a scratch wound migration assay. Confluent, quiescent cells were scraped with a pipette tip to generate a scratch wound, rinsed with PBS, and then incubated in serum-containing culture media in the presence and absence of compound C. Cells were photographed immediately and 24 h after the scratch with a digital camera (QImaging QICAM; Hitschfel Instruments, Inc., St. Louis, MO). The wound area was then measured to determine cell migration.
Protein Analysis.
Vascular SMCs were lysed in electrophoresis buffer [125 mM Tris (pH 6.8), 12.5% glycerol, 2% SDS, and trace bromphenol blue] and proteins were separated by SDS-polyacrylamide gel electrophoresis. After transfer to nitrocellulose membranes, membranes were blocked with PBS and nonfat milk (5%) and then incubated with antibodies against cyclin D1 (1:500), cyclin E (1:500), cyclin A (1:500), p27 (1:300), p21 (1:500), cyclin-dependent kinase (1:500), phospho-retinoblastoma protein (1:200), AMPKα1/2 (1:500), phospho-AMPKα 1/2 (1:100), phospho-ACC (1:300), or β-actin (1:200). Membranes were washed in PBS, incubated with horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-goat antibodies, and developed with commercial chemoluminescence reagents. Protein expression was quantified by scanning densitometry and normalized with respect to β-actin.
AMPK Activation.
AMPK activity was determined by Western blotting using phospho-specific antibodies directed against AMPKα or ACC (Liu et al., 2011).
Carotid Artery Injury.
Male Sprague-Dawley rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (7.5 mg/kg), and experimental balloon injury was performed on the left common carotid artery, as described previously (Tulis et al., 2001; Granada et al., 2005; Peyton et al., 2009). Depth of anesthesia was monitored by the absence of a withdrawal reflex to toe and tail pinch and the absence of a blink reflex. In brief, a Fogarty 2F embolectomy catheter (Baxter Healthcare Corporation, Deerfield, IL) was introduced through an external carotid arteriotomy site and advanced through the left common carotid artery to the level of the aortic arch. The balloon catheter was then inflated and withdrawn with rotation to the level of the carotid bifurcation. This was repeated three times, and then the catheter was removed and the incision was closed. Immediately after injury, a local polymer-based delivery system was used to administer compound C to the injured vessel wall. The delivery system consisted of 200 μl of a 25% copolymer gel solution (Pluronic F-127; BASF, Chicago, IL) containing compound C (1 mg) that was applied in a circumferential manner to the exposed adventitia of the carotid arteries. A separate cohort of animals received an empty gel, which has previously been demonstrated to have no effect on vascular remodeling (Hu et al., 1999; Tulis et al., 2001). After 2 weeks, rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (7.5 mg/kg) and euthanized by pneumothorax and exsanguination, and carotid arteries were collected for analysis. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the institutional animal care and use committee.
Histology.
Carotid arteries were perfusion-fixed, excised, and embedded in paraffin. Sections (5 μm) were stained in Verhoeff-Van Gieson for measurement of vessel dimensions. Microscopic determination of vessel dimensions was performed using Image-Pro Plus (Media Cybernetics, Inc., Bethesda, MD) and Adobe Photoshop software (Adobe Systems, Mountain View, CA) linked through a digital camera (QICAM Fast 1394; Hitschfel Instruments, Inc.) to an Olympus model BX41TF light microscope (Olympus America Inc., Center Valley, PA), as described previously (Tulis et al., 2001; Granada et al., 2005; Peyton et al., 2009).
Statistics.
Results are expressed as means ± S.E.M. Statistical analyses were performed with the use of a Student's two-tailed t test and an analysis of variance with the Bonferroni post hoc test when more than two treatment regimens were compared. P < 0.05 was considered statistically significant.
Results
Compound C Inhibits Vascular SMC Proliferation and Migration in an AMPK-Independent Manner.
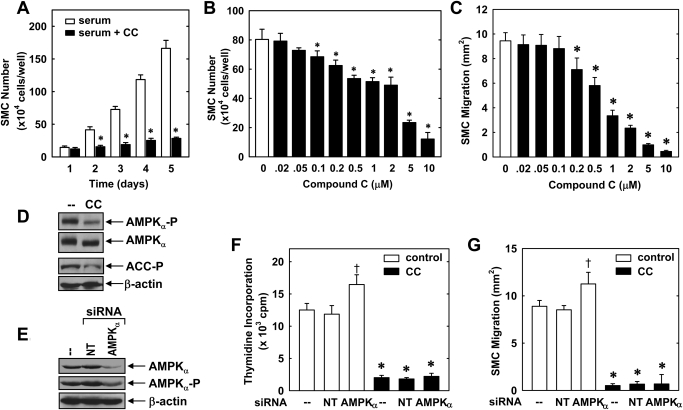
Treatment of vascular SMCs with serum stimulated a time-dependent increase in cell number that was blocked by compound C (10 μM) (Fig. 1A). The antiproliferative effect of compound C (0.02–10 μM) was concentration-dependent (Fig. 1B). A significant inhibition of cell growth by compound C was noted at a concentration of 0.1 μM and near-total abolition of proliferation was observed with 10 μM. Compound C also inhibited the migration of SMCs after scratch wounding (Fig. 1C). Treatment of SMCs with compound C (0.02–10 μM) resulted in a concentration-dependent inhibition of SMC migration beginning at a concentration of 0.2 μM. In contrast, compound C had no significant effect on cell viability, as determined by trypan blue exclusion [control 96.3 ± 3.3% versus compound C (10 μM) 95.2 ± 3.6%, n = 4].
Fig. 1.
Compound C inhibits the proliferation and migration of vascular SMCs in an AMPK-independent manner. A, serum (5%) stimulates a time-dependent increase in cell number that is blocked by compound C (CC; 10 μM). B, compound C inhibits the proliferation of SMCs in a concentration-dependent manner. Cells were treated with serum (5%) in the absence and presence of compound C (0.02–10 μM) for 4 days. C, compound C (0.02–10 μM) inhibits the migration of serum (5%)-stimulated SMCs in a concentration-dependent manner. D, compound C (10 μM) inhibits AMPK activity in SMCs, as reflected by the inhibition of the phosphorylation of AMPKα and the AMPK substrate, ACC. E, silencing AMPKα expression inhibits AMPK activity. Cells were transfected with AMPKα siRNA (50 nM) or NT siRNA (50 nM) and AMPK activity determined by monitoring the phosphorylation of AMPKα. F, effect of silencing AMPKα expression on SMC DNA synthesis. Cells were transfected with AMPKα siRNA (50 nM) or NT siRNA (50 nM) for 48 h and then treated with serum (5%) in the absence and presence of compound C (10 μM) for an additional 24 h. G, effect of silencing AMPKα expression on SMC migration. Cells were transfected with AMPKα siRNA (50 nM) or NT siRNA (50 nM) for 48 h and then treated with serum (5%) in the absence and presence of compound C (10 μM) for an additional 24 h. Results are means ± S.E.M. (n = 6). †, statistically significant effect of compound C. †, statistically significant effect of AMPKα siRNA.
Because compound C is an established inhibitor of AMPK, we examined whether the effects of compound C on vascular SMC function were dependent on the inhibition of AMPK activity. Consistent with previous reports (Zhou et al., 2001), we confirmed that compound C inhibits AMPK activity by demonstrating that compound C (10 μM) blocked the phosphorylation of AMPKα and ACC, a downstream target of AMPK (Fig. 1D). Silencing AMPKα expression by transfecting SMCs with AMPKα siRNA also inhibited AMPK activity (Fig. 1E). However, silencing of AMPKα expression failed to inhibit SMC proliferation (Fig. 1F). In fact, a modest, but significant, increase in SMC DNA synthesis was observed when AMPKα expression/activity was suppressed in cells transfected with AMPKα siRNA (Fig. 1F). In contrast, the nontargeting (NT) siRNA failed to modulate AMPKα expression/activity or SMC DNA synthesis (Fig. 1, E and F). Of significance, AMPKα knockdown had no effect on the antiproliferative action of compound C (Fig. 1F). Likewise, down-regulation of AMPKα expression did not mimic the antimigratory action of compound C but rather promoted SMC migration (Fig. 1G). Silencing AMPKα expression also failed to modify the inhibitory effect of compound C on SMC migration. Transfection of SMCs with the NT siRNA had no effect on cell migration (Fig. 1G).
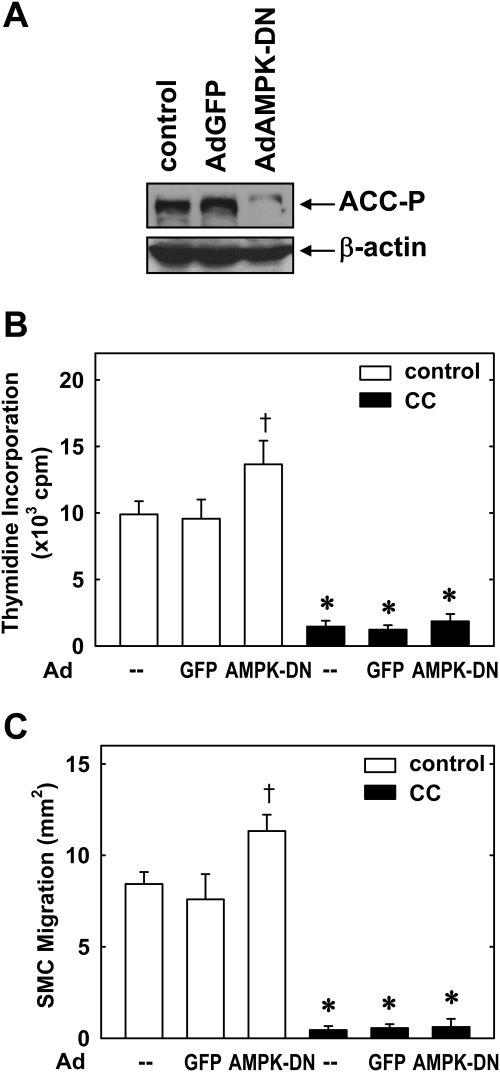
The role of AMPK in mediating the actions of compound C was also assessed using an adenovirus expressing AdAMPK-DN. Infection of SMCs with AdAMPK-DN nearly abolished AMPK activity and resulted in an increase in serum-mediated SMC proliferation and migration (Fig. 2). However, AdAMPK-DN had no effect on the ability of compound C to block SMC proliferation or migration. Infection of SMCs with AdGFP had no effect on AMPK activity, SMC proliferation or migration, or the ability of compound C to modulate SMC function. Taken together, these results indicate that compound C inhibits vascular SMC proliferation and migration in an AMPK-independent manner.
Fig. 2.
A dominant-negative AMPK mutant fails to modify the effect of compound C on vascular SMC proliferation and migration. A, infection of SMCs with AdAMPK-DN but not AdGFP inhibits AMPK activity, as reflected by the phosphorylation of ACC. B, effect of AdAMPK-DN on SMC DNA synthesis. Cells were infected with AdAMPK-DN or AdGFP for 48 h and then treated with serum (5%) in the absence and presence of compound C (CC; 10 μM) for an additional 24 h. C, effect of AdAMPK-DN on SMC migration. Cells were infected with AdAMPK-DN or AdGFP for 48 h and then treated with serum (5%) in the absence and presence of compound C (10 μM) for an additional 24 h. Results are means ± S.E.M. (n = 6). *, statistically significant effect of compound C. †, statistically significant effect of AdAMPK-DN.
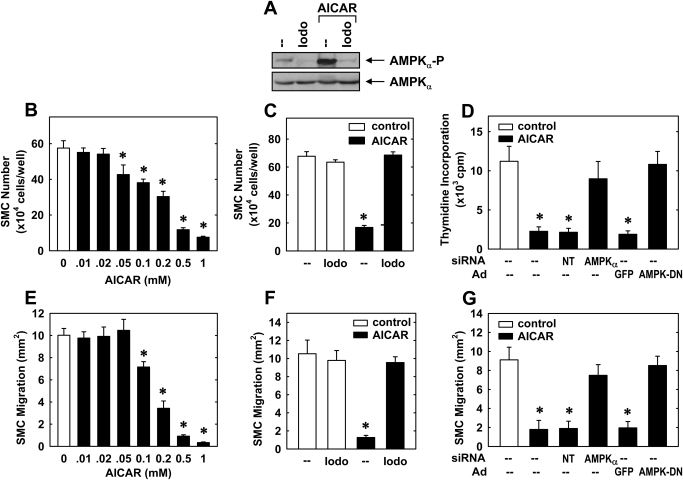
AMPK Activator AICAR Inhibits Vascular SMC Proliferation and Migration in an AMPK-Dependent Manner.
To examine the effect of AMPK activation on vascular SMC function, cells were treated with the potent, cell-permeable AMPK activator AICAR. AICAR stimulated a significant increase in AMPK activity in vascular SMCs (Fig. 3A), and this was associated with a concentration-dependent decrease in serum-mediated SMC proliferation (Fig. 3B). The adenosine kinase inhibitor 5′-iodotubercidin (Henderson et al., 1972), which blocks the intracellular conversion of AICAR to 5-aminoimidazole-4-carboxamide that is required for AMPK activation, abrogated the activation of AMPK by AICAR (Fig. 3A) and the antiproliferative action of AICAR (Fig. 3C). Furthermore, the inhibition of SMC DNA synthesis by AICAR was reversed by transfecting cells with AMPKα siRNA or by infecting cells with AdAMPK-DN (Fig. 3D). AICAR also attenuated SMC migration in a concentration-dependent fashion (Fig. 3E), and this was prevented by 5′-iodotubercidin (Fig. 3F) or by treating cells with AMPKα siRNA or AdAMPK-DN (Fig. 3G). In contrast, NT siRNA or AdGFP had no effect on AICAR-mediated SMC proliferation or migration.
Fig. 3.
Activation of AMPK inhibits vascular SMC proliferation and migration. A, effect of AICAR (0.5 mM for 4 h) on AMPK activity, as reflected by the phosphorylation of AMPKα in the absence and presence of 5′-iodotubercidin (Iodo; 0.3 μM). B, AICAR inhibits the proliferation of SMCs in a concentration-dependent manner. Cells were treated with serum (5%) in the absence and presence of AICAR (0.01–1.0 mM) for 4 days. C, effect of AICAR (0.5 mM) on serum (5%)-stimulated SMC proliferation in the absence and presence of 5′-iodotubercidin (0.3 μM). D, effect of silencing AMPKα expression or infecting cells with AMPK-DN on AICAR-stimulated SMC DNA synthesis. Cells were transfected with AMPKα siRNA (50 nM) or NT siRNA (50 nM) or infected with AdAMPK-DN or AdGFP for 48 h and then treated with serum (5%) in the presence of AICAR (0.5 mM) for an additional 24 h. E, AICAR (0.01–1.0 mM) inhibits the migration of serum (5%)-stimulated SMCs in a concentration-dependent manner. F, effect of AICAR (0.5 mM) on serum (5%)-stimulated SMC migration in the absence and presence of 5′-iodotubercidin (0.3 μM). G, effect of silencing AMPKα expression or infecting cells with AMPK-DN on AICAR-stimulated SMC migration. Cells were transfected with AMPKα siRNA (50 nM) or NT siRNA (50 nM) or infected with AdAMPK-DN or AdGFP for 48 h and then treated with serum (5%) in the presence of AICAR (0.5 mM) for an additional 24 h. Results are means ± S.E.M. (n = 3–5). *, statistically significant effect of AICAR.
Compound C Arrests SMCs in the G0/G1 Phase of the Cell Cycle and Regulates the Expression of Cell Cycle Regulatory Proteins.
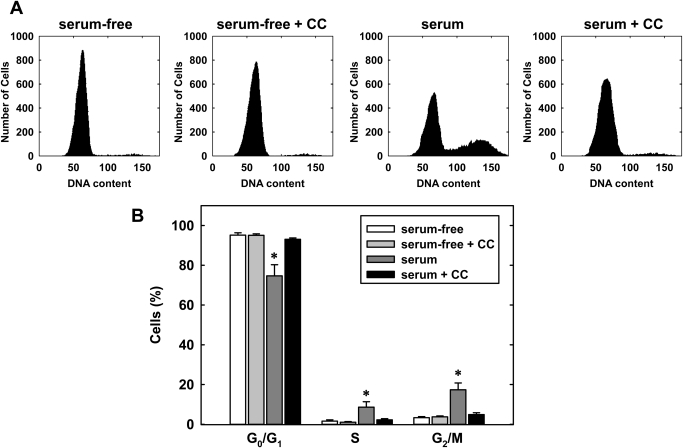
Subsequently, we determined the effect of compound C on cell cycle progression. Quiescent vascular SMCs accumulated in the G0/G1 phase of the cell cycle after serum deprivation, but the administration of serum for 24 h stimulated cell cycle progression and the population of cells in the G0/G1 phase diminished, whereas the fraction of cells in the S and G2M phases increased (Fig. 4, A and B). However, the coadministration of compound C (10 μM) for 24 h blocked the effect of serum and increased the percentage of cells in the G0/G1 phase of the cell cycle, and this was paralleled by a decrease in the fraction of cells in the S phase. In the absence of serum, compound C had no effect on the distribution of cells in the cell cycle. In addition, no apparent toxicity was noted by compound C as reflected by the lack of a sub-G0/G1 fraction (Fig. 4A).
Fig. 4.
Compound C inhibits cell cycle progression by vascular SMCs. A, representative histograms of vascular SMCs treated with serum (5%) for 24 h in the absence or presence of compound C (CC; 10 μM). B, effect of compound C (10 μM) on the distribution of serum (5%)-stimulated SMCs in the cell cycle. Results are means ± S.E.M. (n = 3). *, statistically significant effect of compound C.
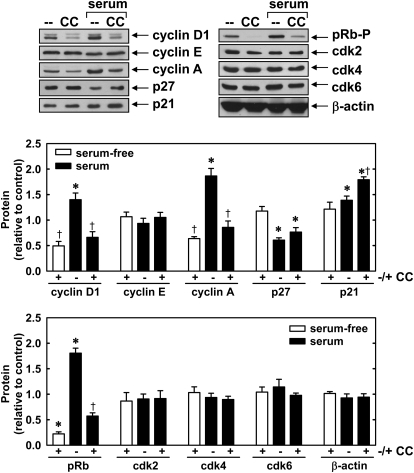
To determine the mechanism by which compound C disrupts cell cycle progression, we examined the effect of this agent on the expression of cell cycle regulatory proteins. Compound C inhibited both basal and serum-mediated increases in cyclin D1 and cyclin A protein expression as well as the phosphorylation of retinoblastoma protein (Fig. 5). In addition, compound C elevated the expression of the cyclin-dependent kinase inhibitor, p21, in serum-treated cells. In contrast, compound C had no effect on the expression of cyclin E, p27, and cyclin-dependent kinase 2, 4, and 6.
Fig. 5.
Effect of compound C on the expression of cell cycle regulatory proteins by vascular SMCs. A, effect of compound C on the expression of cell cycle regulatory proteins and the phosphorylation of retinoblastoma protein (pRb-P). Cells were treated with serum (5%) for 24 h in the absence or presence of compound C (CC; 10 μM). B, quantification of protein expression or phosphorylation relative to serum-free controls. Results are means ± S.E.M. (n = 5). *, statistically significant effect of serum. †, statistically significant effect of compound C.
Compound C Inhibits Neointima Formation in Injured Rat Carotid Arteries.
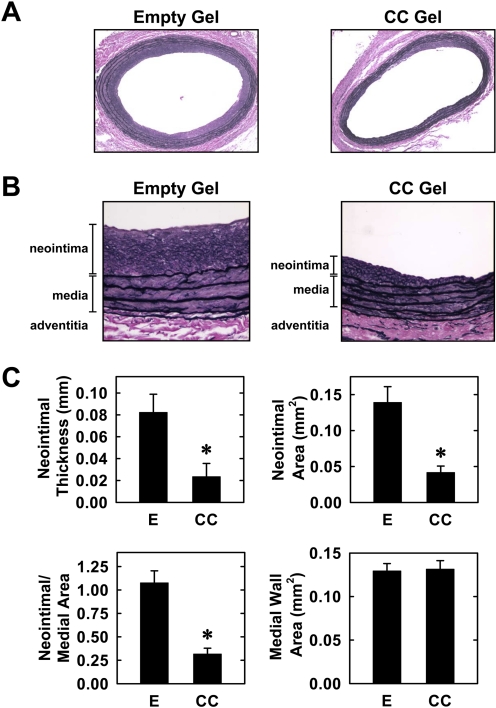
Finally, the effect of compound C on neointima formation was investigated in an in vivo rat model of carotid artery injury. Figure 6A depicts cross-sections of perfusion-fixed, Verhoeff-Van Gieson-stained tissues 2 weeks after injury. Animals treated with an empty gel exhibited a concentric and significant neointima with a clearly defined medial wall and elastic lamina. However, vessels exposed to compound C display a markedly diminished neointima, appearing sporadically as a thin nonconcentric layer adjacent to the internal elastic lamina (Fig. 6, A and B). Morphometric measurements revealed that neointimal area, neointimal thickness, and neointimal/medial wall area ratio were all significantly reduced by compound C (Fig. 6C). However, the medial wall area was not significantly affected by compound C.
Fig. 6.
Compound C inhibits neointima formation in injured rat carotid arteries. A, representative cross-sections of injured carotid arteries treated with an empty gel (E) or a gel containing compound C (CC; 1 mg) 2 weeks after injury (original magnification, 100×). B, higher magnification images showing the neointima and medial layers of injured carotid arteries treated with an empty gel or a compound C gel 2 weeks after injury (original magnification, 400×). C, morphometric analysis of neointima formation 2 weeks after arterial injury. Results are means ± S.E.M. (n = 8). *, statistically significant effect of compound C.
Discussion
The present study identifies compound C as a novel regulator of vascular SMC function. We found that compound C is a potent inhibitor of vascular SMC proliferation and migration and that it exerts these actions in a concentration-dependent but AMPK-independent fashion. In addition, compound C arrests SMCs in the G0/G1 phase of the cell cycle, and this is associated with alterations in the expression and phosphorylation of several cell cycle regulatory proteins. Moreover, local perivascular administration of compound C attenuates neointima formation after injury of rat carotid arteries. These findings suggest a potentially important therapeutic role for compound C in treating occlusive vascular disorders.
Although studies indicate that compound C blocks the proliferation of nonvascular cells, its direct action on vascular SMC growth was not known (Nam et al., 2008; Vucicevic et al., 2009). In the current study, we found that compound C is a robust inhibitor of vascular SMC proliferation. The antiproliferative effect of compound C is concentration-dependent: a threshold effect of compound C on cell growth is observed at 0.2 μM and is almost complete at 10 μM. Of note, compound C inhibits cell proliferation in the absence of cell death, as revealed by trypan blue staining and propidium iodide binding, indicating that compound C functions as a cytostatic rather cytotoxic agent. The failure of these concentrations of compound C to evoke apoptosis is consistent with reports in astrocytes and melanoma cells but contrast with findings in glioma cells, renal cancer cells, and breast carcinoma cells in which similar concentrations of compound C stimulate apoptosis (Jin et al., 2009; Vucicevic et al., 2009; Jang et al., 2010). Thus, compound C appears to exert cytostatic effects in a cell-specific manner.
The antiproliferative action of compound C does not involve the inhibition of AMPK. Silencing AMPKα expression or infecting cells with AdAMPK-DN failed to duplicate the arrest in SMC growth observed with compound C and had no effect on the antiproliferative property of compound C. Our current study also reveals that compound C inhibits SMC migration. Here again, the antimigratory action of compound C is independent of its inhibitory effect on AMPK, because blocking AMPK activity using either an siRNA or dominant-negative approach does not interfere with the capacity of compound C to repress SMC migration. Taken together, these findings add to a growing list of AMPK-independent actions of compound C and suggest caution in the use of this agent as a pharmacological probe in identifying vascular functions of AMPK.
In agreement with previous studies (Nagata et al., 2004; Igata et al., 2005), we found that AMPK inhibits vascular SMC proliferation. Suppression of AMPK activity by treating cells with AMPKα siRNA or AdAMPK-DN augments SMC proliferation whereas activation of AMPK by AICAR blocks SMC growth. The antiproliferative action of AICAR is AMPK-dependent because pharmacological or molecular inhibition of AMPK negates the growth inhibitory effect of AICAR. Of interest, our study also discovered that AMPK functions as a key regulator of SMC migration. Knockdown of AMPKα or infection with AdAMPK-DN promotes SMC migration, whereas AICAR blocks SMC migration. In addition, AICAR-mediated inhibition of SMC migration is prevented by pharmacological or molecular inhibition of AMPK, providing further support for the antimigratory action of AMPK. The ability of AMPK to inhibit vascular SMC migration provides a novel mechanism by which this kinase blocks vascular lesion formation (Igata et al., 2005).
Vascular SMC proliferation requires the entry of quiescent cells into the cell cycle where they progress through distinctive phases of the cell cycle. Transition through the G1 phase of the cell cycle is dependent on the induction of the G1 cyclins, which bind and activate their respective cyclin-dependent kinases (Sherr, 1994; Weinberg, 1995). However, cyclin-dependent kinase activity is a tightly regulated process that is influenced by the expression of cyclin-dependent kinase inhibitors such as p21 and p27, which bind to the cyclin/cyclin-dependent kinase complex to inhibit its activity. Activation of G1 cyclin-dependent kinases results in the phosphorylation and activation of retinoblastoma protein, which triggers entry of cells in the S phase. Analysis of cell cycle distribution indicates that compound C blocks cell cycle progression by arresting SMCs in the G0/G1 phase of the cell cycle. The inhibition of cell cycle progression by compound C is associated with the suppression of cyclin D1 and cyclin A expression as well as the phosphorylation of retinoblastoma protein, key events that are required for S phase entry. In addition, compound C elevates the level of p21, a cyclin-dependent kinase inhibitor known to block G1 to S phase progression (Sherr, 1994; Weinberg, 1995). Thus, compound C prevents G1 to S phase transition by disrupting the expression of multiple cell cycle regulatory proteins. Our finding that compound C increases p21 expression is in agreement with another study in preadipocytes and probably reflects the ability of compound C to stabilize the protein (Nam et al., 2008).
The capacity of compound C to regulate the expression of cell cycle regulatory proteins may also contribute to its antimigratory effect because cell cycle progression and cell migration are tightly coupled processes. Migration of vascular SMCs requires entrance into the cell cycle, and many cytostatic agents that prevent cell cycle entry block cell migration (Fukui et al., 2000). Of significance, overexpression of p21 in vascular SMCs has been shown to inhibit their migration, whereas overexpression of cyclin D1 stimulates SMC migration both in vitro and in vivo (Fukui et al., 1997; Karpurapu et al., 2010). Thus, the ability of compound C to divergently regulate the expression of these two cell cycle regulatory proteins may participate in its ability to regulate SMC locomotion.
Because the proliferation and migration of vascular SMCs play a critical role in promoting vascular lesion formation, we also investigated the effect of compound C on vascular remodeling using the rat carotid artery balloon injury model. This model is characterized by its high degree of reproducibility and is associated with the development of a SMC-rich neointima. Compound C was applied topically to the adventitia of the blood vessel using a specific local delivery copolymer. This drug-targeting approach has been used successfully by us and others and permits the sustained release of drugs while avoiding possible nonspecific effects associated with the systemic administration of drugs (Hu et al., 1999; Tulis et al., 2001; Granada et al., 2005; Peyton et al., 2009). We found that local application of compound C markedly attenuates neointima formation after arterial injury, demonstrating that this pyrrazolopyrimidine derivative dampens the vascular response to injury. The attenuation of lesion formation by compound C probably involves the direct antiproliferative and antimigratory actions of this agent on SMCs; however, the ability of compound C to inhibit AMPK activity may also influence the remodeling response. In this respect, we recently demonstrated that AMPK blocks endothelial cell apoptosis in response to inflammatory mediators by inducing the expression of heme oxygenase-1 (Liu et al., 2011). This finding raises the possibility that compound C-mediated inhibition of AMPK activity may sensitize vascular cells to apoptotic stimuli found at sites of arterial damage, leading to the loss of vascular SMCs and endothelial cells. Thus, compound C may regulate the accumulation of vascular cells after arterial injury via both AMPK-independent and -dependent mechanisms. Although the perivascular application of compound C is not a technically feasible approach for treating coronary restenosis after percutaneous coronary intervention in humans, it may be used via drug-eluting stents. Furthermore, compound C may be beneficial in preventing stenosis after other surgical interventions when the extravascular surface is accessible for application of the drug, including coronary artery bypass surgery and the creation of graft anastomosis in hemodialysis patients with arteriovenous fistulas.
In summary, we identified compound C as a novel inhibitor of vascular SMC proliferation and migration that markedly attenuates neointima formation after arterial injury in rat carotid arteries. The antiproliferative and antimigratory actions of compound C are independent of its ability to inhibit AMPK activity and are associated with the arrest of SMCs in the G0/G1 phase of the cell cycle. Compound C represents an attractive therapeutic agent in the treatment of occlusive vascular disease.
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [Grants R01-HL74966, R01-HL59976, R01-HL77288]; and a University of Missouri Summer Research Internship in Cell and Molecular Biology (to B.Y.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.181784.
- SMC
- smooth muscle cell
- compound C
- 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine
- AMPK
- AMP-activated protein kinase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- ACC
- acetyl-CoA-carboxylate
- siRNA
- small interfering RNA
- AdAMPK-DN
- adenovirus expressing a dominant-negative AMPK mutant
- AdGFP
- adenovirus expressing green fluorescent protein
- PBS
- phosphate-buffered saline
- NT
- nontargeting.
Authorship Contributions
Participated in research design: Peyton, Shebib, Wang, and Durante.
Conducted experiments: Peyton, Yu, Yates, Shebib, Liu, and Durante.
Performed data analysis: Peyton, Yu, Yates, and Durante.
Wrote or contributed to the writing of the manuscript: Peyton, Wang, and Durante.
References
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Borst GJ, Ackerstaff RG, de Vries JP, vd Pavoordt ED, Vos JA, Overtoom TT, Moll FL. (2007) Carotid angioplasty and stenting for postendarterectomy stenosis: long-term follow-up. J Vasc Surg 45:118–123 [DOI] [PubMed] [Google Scholar]
- Durante W, Schini VB, Catovsky S, Kroll MH, Vanhoutte PM, Schafer AI. (1993) Plasmin potentiates the induction of nitric oxide synthesis by interleukin-1β in vascular smooth muscle cells. Am J Physiol 264:H617–H623 [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. (2002) Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 8:1249–1256 [DOI] [PubMed] [Google Scholar]
- Emerling BM, Viollet B, Tormos KV, Chandel NS. (2007) Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 581:5727–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, Amakawa M, Hoshiga M, Shibata N, Kohbayashi E, Seto M, Sasaki Y, Ueno T, Negoro N, Nakakoji T, et al. (2000) Increased migration in late G1 phase in cultured smooth muscle cells. Am J Physiol Cell Physiol 279:C999–C1007 [DOI] [PubMed] [Google Scholar]
- Fukui R, Shibata N, Kohbayashi E, Amakawa M, Furutama D, Hoshiga M, Negoro N, Nakakouji T, Ii M, Ishihara T, et al. (1997) Inhibition of smooth muscle cell migration by the p21 cyclin-dependent kinase inhibitor (Cip1). Atherosclerosis 132:53–59 [DOI] [PubMed] [Google Scholar]
- Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, et al. (1999) Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 99:2164–2170 [DOI] [PubMed] [Google Scholar]
- Gershlick A, De Scheerder I, Chevalier B, Stephens-Lloyd A, Camenzind E, Vrints C, Reifart N, Missault L, Goy JJ, Brinker JA, et al. (2004) Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent: the European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation 109:487–493 [DOI] [PubMed] [Google Scholar]
- Granada JF, Ensenat D, Keswani AN, Kaluza GL, Raizner AE, Liu XM, Peyton KJ, Azam MA, Wang H, Durante W. (2005) Single perivascular delivery of mitomycin C stimulates p21 expression and inhibits neointima formation in rat arteries. Arterioscler Thromb Vasc Biol 25:2343–2348 [DOI] [PubMed] [Google Scholar]
- Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, Uchino K, Hammer MD, Wechsler LR, Jovin TG. (2006) Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke 37:2562–2566 [DOI] [PubMed] [Google Scholar]
- Henderson JF, Paterson AR, Caldwell IC, Paul B, Chan MC, Lau KF. (1972) Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2 3:71–85 [PubMed] [Google Scholar]
- Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. (1999) Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation 100:861–868 [DOI] [PubMed] [Google Scholar]
- Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, et al. (2005) Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97:837–844 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jang JH, Lee TJ, Yang ES, Min do S, Kim YH, Kim SH, Choi YH, Park JW, Choi KS, Kwon TK. (2010) Compound C sensitizes Caki renal cancer cells to TRAIL-induced apoptosis through reactive oxygen species-mediated down-regulation of c-FLIPL and Mcl-1. Exp Cell Res 316:2194–2203 [DOI] [PubMed] [Google Scholar]
- Jeremias A, Kirtane A. (2008) Balancing efficacy and safety of drug-eluting stents in patients undergoing percutaneous coronary intervention. Ann Intern Med 148:234–238 [DOI] [PubMed] [Google Scholar]
- Jin J, Mullen TD, Hou Q, Bielawski J, Bielawska A, Zhang X, Obeid LM, Hannun YA, Hsu YT. (2009) AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res 50:2389–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S, Rao GN. (2010) Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem 285:3510–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Ensenat D, Wang H, Schafer AI, Durante W. (2003) Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett 541:52–56 [DOI] [PubMed] [Google Scholar]
- Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. (2011) Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol 300:H84–H93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323 [DOI] [PubMed] [Google Scholar]
- Nagata D, Takeda R, Sata M, Satonaka SM, Suzuki E, Nagano T, Hirata Y. (2004) AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation 110:444–451 [DOI] [PubMed] [Google Scholar]
- Nam M, Lee WH, Bae EJ, Kim SG. (2008) Compound C inhibits clonal expansion of preadipocytes by increasing p21 level irrespectively of AMPK inhibition. Arch Biochem Biophys 479:74–81 [DOI] [PubMed] [Google Scholar]
- Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. (2000) The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol 86:6H–11H [DOI] [PubMed] [Google Scholar]
- Peyton KJ, Ensenat D, Azam MA, Keswani AN, Kannan S, Liu XM, Wang H, Tulis DA, Durante W. (2009) Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol 29:488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Schafer AI, Durante W. (2002) Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood 99:4443–4448 [DOI] [PubMed] [Google Scholar]
- Sherr CJ. (1994) G1 phase progression: cycling on cue. Cell 79:551–555 [DOI] [PubMed] [Google Scholar]
- Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. (2001) Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 104:2710–2715 [DOI] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E, Sumarac-Dumanovic M, Micic D, et al. (2009) AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol 77:1684–1693 [DOI] [PubMed] [Google Scholar]
- Weinberg RA. (1995) The retinoblastoma protein and cell cycle control. Cell 81:323–330 [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller T. (2007) Current state of endovascular treatment of femoro-popliteal artery disease. Vasc Med 12:223–234 [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]