Abstract
We describe a modification of receptor theory for the estimation of observed affinities (Kobs) and relative efficacies of orthosteric ligands in functional assays that exhibit constitutive activity. Our theory includes parameters for the fractions of the occupied receptor population in the active (intrinsic efficacy, ε) and inactive (εi) states and analogous parameters for the fractions of the free receptor population in the active (εsys) and inactive (εi-sys) states. The total stimulus represents the summation of the active states of the free and occupied receptor populations. A modified operational model is developed that expresses the response as a logistic function of the total stimulus. This function includes the standard parameters related to affinity and efficacy (Kobs and τ) as well as a parameter proportional to the activity of the free receptor complex, τsys. Two related parameters are proportional to the fraction of the free (τi-sys) and occupied (τi) receptor populations in the inactive state. We show that the estimates of the affinity constants of orthosteric ligands for the active (Kb) and inactive (Ka) states of the receptor are equivalent to τKobs/τsys and τiKobs/τi-sys, respectively. We verify our method with computer simulation techniques and apply it to the analysis of M2 and M3 muscarinic receptors. Our method is applicable in the analysis of ligand bias in drug discovery programs.
Introduction
Constitutive activity is an inevitable property of any ligand-sensitive molecular switch that evolved to be off (R) in the absence of endogenous ligand and on (R*) in its presence. For whether by conformational selection,
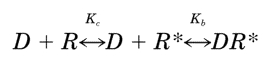 |
or conformational induction,
activation of the receptor by the ligand (D) cannot occur unless there is some spontaneous activation of the receptor in the absence of ligand (Kc > 0; Fig. 1a). It is now clear that many G protein-coupled receptors exhibit constitutive activity, particularly when overexpressed or when the appropriate G protein is overexpressed (Seifert and Wenzel-Seifert, 2002).
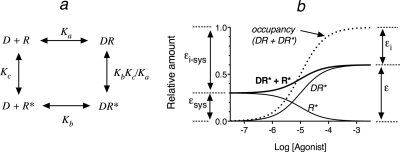
Fig. 1.
The simple two-state model for receptor activation and the components of the total stimulus for an orthosteric agonist. a, the equilibrium of an orthosteric ligand (D) with a receptor having active (R*) and inactive states (R) is shown. Kb and Ka denote the affinity constants of D for the active and inactive states, respectively. The equilibrium constant for the relative amounts of active and inactive states in the absence of D is denoted by Kc. b, The relative amounts of receptor occupancy (DR* + DR), the stimulus (DR*), the constitutive stimulus (R*), and the total stimulus (DR* + R*) are shown as a function of the agonist concentration. The fraction of the orthosteric ligand-receptor complex in the active and inactive states is denoted by intrinsic efficacy (ε) and intrinsic inactivity (εi), and the fraction of the free receptor in the active and inactive states is denoted by the intrinsic efficacy of the system (εsys) and the intrinsic inactivity of the system (εi-sys), respectively.
Different mechanisms have evolved to minimize receptor activation in the absence of endogenous ligand. Ligand-gated ion channels, for example, often incorporate at least two ligand-binding sites per channel (Hille, 2001), which increases ligand induction of the active state by the nth power of the ratio of affinity constants for active and inactive states (Kb/Ka)n, where n denotes the number of linked binding sites (Monod et al., 1965; Ehlert, 2008). This greater capacity of the ligand to induce the open state allows the channel to exhibit a lower probability of opening in the absence of ligand. One of the most unique solutions to the problem minimizing constitutive activity is exhibited by opsin. Its covalently attached ligand, 11-cis-retinal, behaves as an inverse agonist, whereas the light-induced all-trans form behaves as an agonist (Surya et al., 1995).
Current methods for deducing the receptor-activation function of a population of G protein-coupled receptors involve analyzing a downstream response using a null equation (Stephenson, 1956; Furchgott, 1966) or the operational model (Black and Leff, 1983). These innovative techniques enable the estimation of the observed affinity constant (Kobs) of a ligand and the intrinsic efficacy (ε) of one agonist expressed relative to that of another, provided that there is little constitutive response in the absence of orthosteric ligand. It is also possible to obtain relative estimates of the affinity constants of agonists for the active and inactive states of the receptor (Tran et al., 2009; Ehlert et al., 2011) using modifications of these methods.
Although constitutive activity may often seem like a novelty of overexpressed recombinant receptors, this type of experimental system is often used in pharmacological research and drug development (Chen et al., 1999), and appropriate methods for analyzing drug action in these systems would be useful. In the present article, therefore, we describe an extension of receptor theory that enables the estimation of the observed affinities and relative efficacies of orthosteric ligands, including agonists, neutral antagonists, and inverse agonists, in receptor systems exhibiting constitutive activity. We also show how to estimate the affinity constants of orthosteric ligands for active and inactive states of the receptor, in units of inverse molarity (M−1), whenever there is constitutive activity for a simple two-state receptor system. The method enables the estimation of Kb for all orthosteric ligands except full inverse agonists and the Ka of all ligands having an efficacy less than one-third that of the most efficacious agonist in a series. Finally, we describe how these calculations relate to a more complicated system having multiple active receptor states.
Materials and Methods
Cell Culture.
Human embryonic kidney 293 cells stably expressing Gα15 (HEK 293 Gα15; provided by Dr. Olivier Civelli, University of California, Irvine, CA) were cultured in Dulbecco's modified Eagle medium with high glucose plus l-glutamine supplemented with 10% fetal calf serum, 3.7 g/l sodium bicarbonate, penicillin-streptomycin (100 units/ml and 100 μg/ml, respectively), puromycin (0.625 μg/ml), and (2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol (G418) (0.4 mg/ml) at 37°C in a humidified atmosphere of 5% CO2/95% air. The plasmid encoding the human M3 muscarinic receptor (provided by Dr. Tom Bonner, National Institutes of Health, Bethesda, MD) was transfected into HEK 293 Gα15 cells grown in 100-mm culture plates using GeneJammer (Agilent Technologies, Santa Clara, CA) following the manufacturer's protocols. After 24 h, the cells were seeded into 24-well culture plates coated with poly-d-lysine (BD Biosciences, San Jose, CA) and incubated an additional 24 h before labeling with [3H]inositol for the phosphoinositide assay described next.
Agonist-Stimulated Phosphoinositide Hydrolysis.
Agonist-stimulated [3H]inositolphosphate accumulation was measured in adherent HEK Gα15 cells prelabeled with [3H]inositol using a modification of the methods described by Griffin et al. (2007). Cells were grown in 24-well plates and incubated with [3H]inositol (2 μCi) for approximately 18 h. The cells were washed, and agonist-stimulated phosphoinositide hydrolysis was measured in the presence of lithium in Dulbecco's modified Eagle's medium. The incubation with agonist lasted 2 h, and the reaction was stopped by the addition of perchlorate. Other details of the assay are described by Griffin et al. (2007).
Simulation of Agonist Concentration-Response Curves.
We generated theoretical ligand concentration-response curves exhibiting constitutive activity to verify that our methods of analysis were accurate in estimating the theoretical microscopic parameters that were used to simulate the data in the first place. Our approach has been described previously (Ehlert, 2000, 2011; Tran et al., 2009). It is based on the assumption that the active state of the ligand-receptor complex bound with G protein and guanine nucleotide (DRs*GX) is proportional to ligand-induced receptor activation and the amount of free active receptor complex bound with G protein and guanine nucleotide (Rs*GX) is proportional to constitutive receptor activity. We substituted an equation for the summation of these two components of receptor activation into the operational model to simulate a functional response downstream from receptor activation:
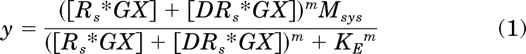 |
In this equation, m represents the transducer slope factor, Msys is the maximum response of the system, and KE is the sensitivity constant of the transduction mechanism. The model is essentially equivalent to that described by Black and Leff (1983), but with receptor occupancy replaced with the summation of [Rs*GX] and [DRs*GX]. A complete description of the model and the equations used to generate the active states of the RGX and DRGX complexes is described in Ehlert et al. (2011).
Analysis of Ligand Concentration-Response Curves.
Both simulated and experimental concentration-response curves were analyzed by global nonlinear regression analysis using Prism (GraphPad Software, Inc., San Diego, CA) and eqs. 28, 43, 44, and 51 as described under Results. A description of how to estimate the initial parameter estimates for these regression equations is given in Appendix.
Results
Receptor Theory
The thesis of this article is that it is possible to estimate the affinity constants of an orthosteric ligand for active (Kb) and inactive (Ka) states of the receptor through analysis of its concentration-response curve whenever a receptor exhibits a detectable level of constitutive activity. In this section, we develop a model for constitutive receptor activity and illustrate how the Kb and Ka values of an orthosteric ligand are related to the receptor activation function. We also apply this theory to develop an operational model that can be used to estimate Kb and Ka from the concentration-response curve of an orthosteric ligand for eliciting a downstream response at a G protein-coupled receptor. Finally, we also develop the requisite theory for estimating the observed affinity constant and relative efficacy of an orthosteric ligand when there is constitutive receptor activity.
Model for Constitutive Activity.
Using the model shown in Fig. 1a, it is possible to develop a mathematical expression for receptor activation as a function of the orthosteric ligand concentration when there is constitutive activity. The level of constitutive receptor activation is determined by the constant Kc, which is equivalent to the ratio of active (R*) to inactive (R) states of the unoccupied receptor. Normally, the value of Kc is very low, but for the example shown in Fig. 1, it has been set to an arbitrarily high value (0.43) so that it is easy to illustrate the various receptor species as a function of the agonist concentration (Fig. 1b). The affinity constants of the orthosteric ligand (D) for the active (R*) and inactive (R) states of the receptor are denoted by Kb and Ka. The concentration of agonist required for half-maximal formation of the active (DR*) and inactive (DR) receptor complexes is equivalent to the reciprocal of the observed dissociation constant (Kobs), which is defined by eq. 8.
Fig. 1b tracks the various receptor species as a function of the agonist concentration. The fraction of the unoccupied receptor population in the active state (R*) is denoted by the intrinsic efficacy of the system (εsys). The value of εsys is constant, and in the absence of agonist, it is equivalent to the fraction of the total receptor population in the active state (0.3). As the concentration of agonist increases, receptor occupancy of the active (DR*) and inactive states (DR) of the receptor increases. The fraction of the agonist-receptor population in the active state is denoted by intrinsic efficacy (ε), and at 100% receptor occupancy, it is equivalent to the fraction of the total receptor population in the active state.
There are two useful terms that describe the fraction of receptor complex in the inactive state. The intrinsic inactivity of the system (εi-sys) denotes the fraction of the free receptor population in the inactive state, and intrinsic inactivity (εi) denotes the fraction of the ligand-receptor population in the inactive state. The relationship between these latter terms and the intrinsic efficacy terms are:
As receptor occupancy increases, the amount of constitutive activity (R*) decreases because the latter is defined as the fraction of the free receptor in the active state, and the free receptor population decreases with an increase in receptor occupancy. The total amount of receptor in the active state is denoted by the summation of R* and DR* in Fig. 1b.
Using the model in Fig. 1a it is possible to express the affinity constants of the active and inactive states of the receptor in terms of the parameters of the receptor population:
 |
 |
 |
These equations can be proved by deriving equations for ε, εi, εsys, εi-sys, and Kobs and then substituting these equations into eqs. 4 to 6 to determine whether the latter are equalities. It has been shown that ε and Kobs are defined by Tran et al. (2009):
 |
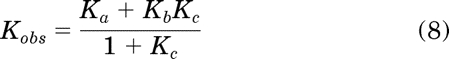 |
Solving eq. 2 for ε and substituting the result into eq. 7 and simplifying yields:
 |
The efficacy of the system (εsys) can be derived from Kc:
 |
Solving eq. 3 for εsys, substituting the result into eq. 10 and simplifying yields:
 |
Substituting in eqs. 7 to 11 for the corresponding parameters in eqs. 4 to 6 yields:
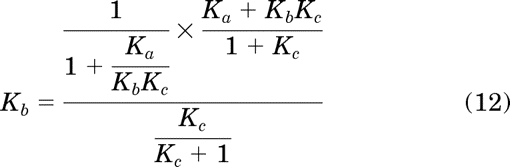 |
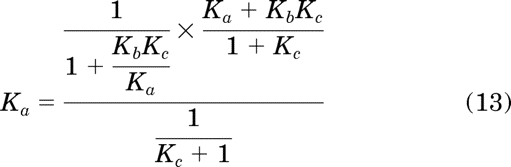 |
 |
Simplification of eqs. 12 to 14 yields the equalities Kb = Kb, Ka = Ka, and Kc = Kc, respectively, which proves the original equations (4–6) from which they were derived.
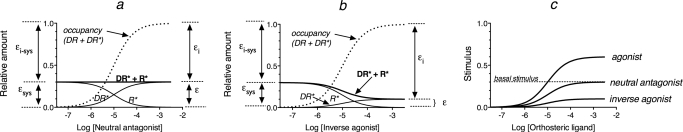
The implications of this theory for neutral antagonists and inverse agonists are shown in Fig. 2, a and b, respectively. Because ligand receptor occupancy decreases the amount of unoccupied receptor in the active state (R*), neutral antagonists inhibit constitutive activity and replace it with an equivalent amount of ligand-receptor complex in the active state (DR*), so that there is no change in the total level of receptor activation (DR* + R*). Thus, neutral antagonists have intrinsic efficacy, but its value (ε) is equivalent to that of the system (εsys). Likewise, the intrinsic inactivity of a neutral antagonist (εi) is equivalent to that of the system (εi-sys).
Fig. 2.
The components of the total stimulus for a neutral antagonist (a) and an inverse agonist (b) and the stimulus component for the different types of orthosteric ligands (c). The various receptor species and efficacy components in a and b are defined in the legend to Fig. 1. In c, the stimulus represents the fraction of the orthosteric ligand-receptor complex in the active state (DR*).
It can also be shown that an inverse agonist has intrinsic efficacy, but its value is less than that of the system, and its intrinsic inactivity is greater than that of the system (Fig. 2b).
The amounts of active ligand-receptor complex (DR*) generated by an agonist, neutral antagonist, and inverse agonist are illustrated in Fig. 2c. Although the concept that a neutral antagonist and an inverse agonist have intrinsic efficacy may seem counterintuitive, these properties follow directly from the definition of efficacy established by Stephenson (1956) and Furchgott (1966). These concepts also yield the somewhat more intuitive conclusions that the ε value of a neutral antagonist is equivalent to that of the system and that the εi value of an inverse agonist is greater than that of the system. Regardless, eqs. 4 and 5 also apply to the estimation of the Kb and Ka values of neutral antagonists and inverse agonists.
Operational Model for Constitutive Activity.
The theory described above applies to the activation of an isolated receptor. G protein-coupled receptors, however, interact with G proteins and other effector proteins to elicit a response, and the response that is usually measured is downstream from receptor activation. To analyze such data, the theory can be incorporated into the operational model of Black and Leff (1983) so that Kb and Ka can be estimated through the analysis of functional responses downstream from receptor activation.
Our strategy involves 1) developing an equation that describes total receptor activation (DR* + R*) as a function of the orthosteric ligand concentration (total stimulus) and 2) substituting this function for the stimulus in the logistic-transducer function described by Black and Leff (1983). This transducer function is given by:
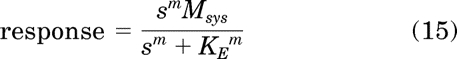 |
in which s denotes the stimulus (agonist-receptor activation), Msys is the maximum response of the system, m is the transducer-slope factor, and KE is a constant reflecting the sensitivity of the system. Msys is equivalent to the parameter Em of Black and Leff (1983). Below we describe how to develop an equation for the total stimulus, which can be substituted for s in eq. 15.
The total stimulus (T) of a receptor system generated in the presence of an orthosteric ligand is defined as:
in which ca and s denote constitutive activity (R*) and the amount of orthosteric ligand receptor complex in the active state (DR*), respectively.
Constitutive receptor activity represents a constant fraction (εsys) of the unoccupied receptor population. The relative amount of unoccupied receptors (R/RT) decreases as the concentration of the orthosteric ligand (D) increases:
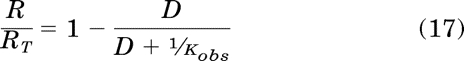 |
This equation shows that the fractional amount of unoccupied receptors decreases proportionally with receptor occupancy by the orthosteric ligand. The fractional amount of constitutively active receptor (ca) is equivalent to the product of the right side of eq. 17 and εsys, which represents the fraction of active, unoccupied receptors.
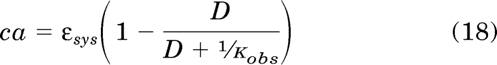 |
The parameter εsys can be expressed in terms of measurable parameters of the operational model by substituting in εsys for s in eq. 15:
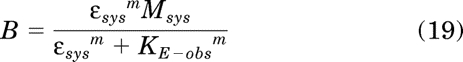 |
In this equation, B is defined as the basal response elicited by a constitutively active receptor in the absence of orthosteric ligand, and KE-obs is defined as:
 |
Rearrangement of eq. 19 yields:
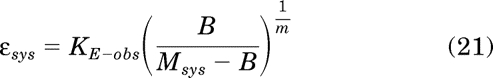 |
This equation can be substituted back into eq. 18 to yield an equation for constitutive receptor activity in the presence of orthosteric ligand:
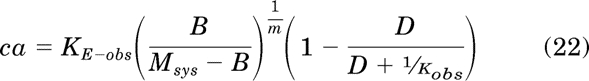 |
Simplification yields:
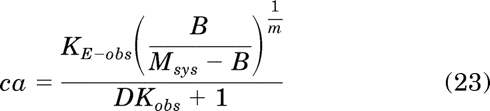 |
The stimulus elicited by the orthosteric ligand-receptor complex (DR*) is simply equivalent to the product of intrinsic efficacy (ε) and receptor occupancy:
 |
Substituting eqs. 23 and 24 for ca and s in eq. 16 yields an equation for the total stimulus (T):
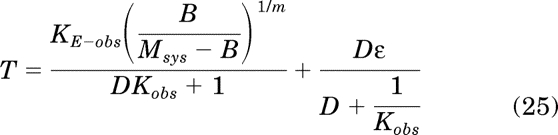 |
Simplification yields:
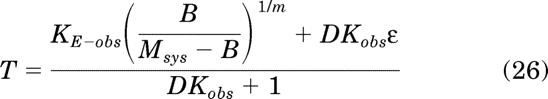 |
To derive an equation for the response, the stimulus (s) in the transducer function of the operational model (eq. 15) is replaced by the total stimulus:
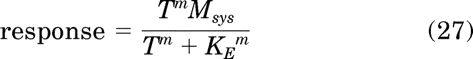 |
Substituting eq. 26 for T and simplifying yields:
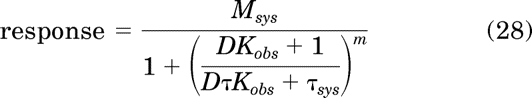 |
in which τ and τsys are defined by:
 |
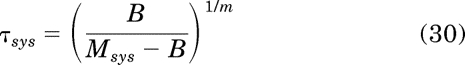 |
It follows from eq. 21 that:
 |
Equation 28 represents the operational model for a constitutively active receptor. There are additional parameters related to this model, which are described next. The parameter τi is defined as:
 |
We define τi-sys as:
 |
Using eqs. 4, 5, 29, 31, 32, and 33, it is possible to define the affinity constants of orthosteric ligands for active and inactive states of the receptor and the constant, Kc, for the isomerization of the free receptor:
 |
 |
 |
The operational model for constitutive activity (eq. 28) can be rewritten in a form that includes Kb by making the appropriate substitution with eq. 34:
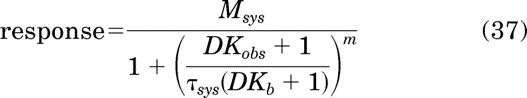 |
Estimation of the Kobs of Orthosteric Ligands When There Is Constitutive Activity.
There is insufficient information in the concentration-response curve of a single orthosteric ligand to estimate its observed affinity constant Kobs, but it is possible to do so if responses are measured before and after inactivation of a fraction of the receptor population (Furchgott, 1966) or in different cells transiently expressing different levels of receptor. It is also possible to estimate the Kobs of a partial agonist if its concentration-response curve is analyzed with that of a full agonist (Stephenson, 1956).
First, we develop an equation based on the operational model (eq. 28) assuming that an irreversible neutral antagonist is used to inactive part of the receptor population. Such an agent has no effect on constitutive receptor activity (τsys). The equation for the constitutively active (ca) component of the total stimulus is derived from eq. 18 by multiplying the receptor occupancy component by the scalar q, which represents the fraction of the receptor population remaining after treatment with an irreversible neutral antagonist:
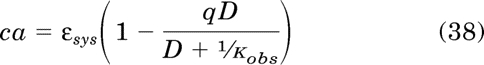 |
Substituting eq. 21 for εsys yields:
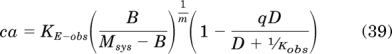 |
The equation for the orthosteric ligand component of the stimulus (s) is derived from eq. 24 by multiplying the receptor occupancy expression by q:
 |
Adding eqs. 39 and 40 yields the total stimulus as defined by eq. 16:
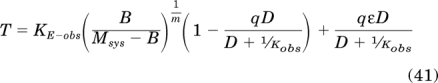 |
Simplification yields:
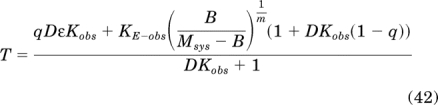 |
Substituting eq. 42 for T in the logistic transducer function of the operational model (eq. 27) yields:
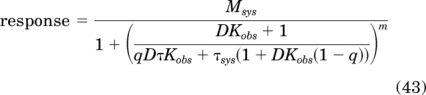 |
This equation is used to estimate the Kobs value of an orthosteric ligand by the method of partial receptor inactivation with an irreversible neutral antagonist as described below.
It is also possible to rearrange eq. 43 into the following form for estimation of the Kb value of the orthosteric ligand:
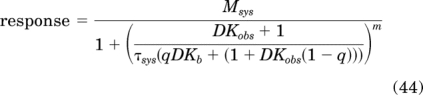 |
The corresponding equation for an irreversible full inverse agonist is developed using the following rationale. After receptor inactivation, constitutive receptor activity is reduced to a fraction of the original value (q), and this effect is adequately reflected by a reduction in the basal response of the system (B) or in the τsys value. Alkylation of the receptor population also reduces the orthosteric ligand component of the stimulus to the same fraction (q) of the original stimulus. The total stimulus, therefore, is given by the following equation:
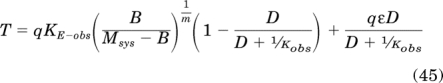 |
Simplification yields:
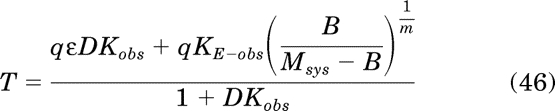 |
Substituting eq. 46 for T in the transducer-response function of the operational model (eq. 27) yields an equation describing the response after partial receptor inactivation with an irreversible inverse agonist:
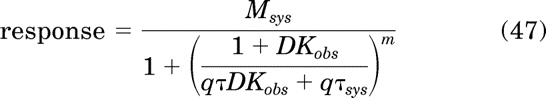 |
To estimate Kobs and τ, global nonlinear regression analysis is used to fit eq. 47 to the concentration-response curves measured before and after receptor inactivation sharing the estimates of Kobs, τ, τsys, Msys, and m between the curves. The parameter q is constrained to 1.0 for the control curve and a unique estimate is obtained for the curve measured after receptor inactivation.
Equation 47 can also be expressed in the following form for the estimation of Kb:
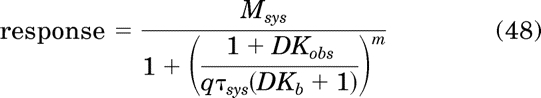 |
The analysis is done sharing the estimates Kobs, Kb, Msys, τsys, and m between the control and “inactivated” curves and obtaining a unique estimate of q for the inactivated curve (q constrained to 1.0 for control).
Equations 47 and 48 can also be used to estimate Kobs and Kb of orthosteric ligands from their concentration-response curves measured in cells transiently expressing high and low levels of a receptor. The data from high and low receptor-expressing cells are analyzed as described above for the control and inactivated conditions, respectively.
Estimation of Relative Efficacy.
Using the method of partial receptor inactivation, it is possible to estimate the observed affinity constant of an orthosteric ligand using eqs. 43 or 47, depending on the nature of the irreversible antagonist. This analysis also provides an estimate of the τ value of the orthosteric ligand. The relative efficacy of one orthosteric ligand (ε) expressed relative to that of another (ε′) is simply equal to the corresponding ratio of τ values:
Substituting in eq. 29 for τ and simplifying proves this equation.
Analysis of Simulated Orthosteric Ligand Concentration-Response Curves
In this section, we generate theoretical concentration-response curves and analyze the simulated data with the operational model for constitutive receptor activity to determine whether it is possible to estimate the Kb and Ka values that were used to simulate the data.
The concentration-response curves were generated as described under Materials and Methods. A theoretical total stimulus was generated assuming that the constitutive stimulus is proportional to the active state of the free receptor associated with the G protein bound with guanine nucleotide (Rs*GX) and that the orthosteric ligand-induced stimulus is proportional to the corresponding active state of the quaternary complex (DRs*GX). The summation of the constitutive and ligand-induced stimuli was defined as the total stimulus (T) and used as input to the operational model (eq. 1). A random error with a range of ± 5% was added to the theoretical data to ensure that our estimation procedure was feasible. In the remainder of this section, we illustrate how to estimate Kobs, relative efficacy, Kb, and Ka.
Estimation of the Kobs and Kb Values of an Orthosteric Ligand Using the Method of Partial Receptor Inactivation.
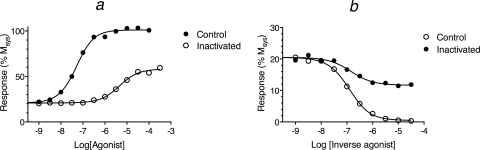
Figure 3a illustrates the theoretical concentration-response curve of a full agonist in a system with constitutive receptor activity. The curves have been simulated under control conditions and after a fraction of the receptor population has been bound with an irreversible neutral antagonist. Receptor inactivation was simulated by multiplying the ligand-receptor component of the stimulus by the scalar, 0.005 (q). A random error (±5%) was also added to the data. The parameters for the simulation are given in the legend to Fig. 3. The “control” and “inactivated” curves were analyzed simultaneously using global nonlinear regression analysis with eq. 43. In the analysis, the estimates of Msys, m, Kobs, τsys, and τ were shared between the two concentration-response curves, and a unique estimate of q was obtained for the inactivated curve. The estimate of q was constrained to a constant value of 1.0 for the control curve. Regression analysis yielded estimates of 5.12 ± 0.08, 101.4 ± 1.0, 1.16 ± 0.13, and 0.0060 ± 0.0005 for log Kobs, Msys, m, and q, respectively, and these were similar to those used to generate the theoretical data (5.08, 100, 1.0, and 0.005, respectively). The estimates of log τ and log τsys were 2.22 ± 0.10 and −0.51 ± 0.11.
Fig. 3.
Simulation of the effects of partial inactivation of the receptor population with an irreversible neutral antagonist on the response to an agonist (a) and an inverse agonist (b). The theoretical curves were generated using the operational model for the total stimulus (eq. 27) with the latter defined as the summation of the active states of the quaternary (DRs*GX) and ternary (Rs*GX) complexes. These were simulated using the quaternary complex model described in Ehlert et al. (2011). The values of log Ka and log Kb for the orthosteric ligands were: agonist, 5.0 and 8.0; inverse agonist, 7.0 and 5.0, respectively. The microscopic state constants for the quaternary complex model were: log Ke, −2.15; log Kg, 2.85; log Kk, 8.00; log Km, 4.60, and log Kc, −4.30. The ratio of G protein to receptor was 10, and the receptor concentration was 40 units. These parameters yield a quaternary complex (DRs*GX) function with a log Kobs of 5.08. The parameters of the operational model were Msys, 100; m, 1.0; and log KE, −1.7.
From the values of Kobs, τ, and τsys it is possible to estimate the Kb value of the orthosteric agonist using eq. 34. This calculation yielded an estimate of 7.84 for log Kb, which is similar to the theoretical value used for the simulation (8.0). Kb can also be estimated by global nonlinear regression analysis of the data in Fig. 3a using eq. 44. When this was done sharing the estimate of Msys, m, Kobs, Kb, and τsys between the two curves and obtaining a unique estimate of q for the control (constrained to one) and “inactivated” curves, a value of 7.84 ± 0.10 was obtained for log Kb.
We also simulated data for an inverse agonist under control conditions and after partial inactivation of 50% of the receptor population (q = 0.5, fraction of residual receptors) (Fig. 3b). The simulated data were analyzed by global nonlinear regression analysis as described above using eq. 43. In this situation, it is impossible to get a reliable estimate of Msys unless the data are analyzed simultaneously with those of an efficacious agonist. For the regression in Fig. 3b we constrained the estimate of Msys to the theoretical value (100%), which is nearly the same as that estimated in Fig. 3a for the efficacious agonist. The estimates of log Kobs, m, and q (6.86 ± 0.09, 1.34 ± 0.28, and 0.42 ± 0.04, respectively) were similar to those used to simulate the data (7.00, 1.0, and 0.5, respectively). The microscopic constants used to simulate the data are given in the legend to Fig. 3.
The value of Kb can be estimated using eq. 34, but in the case of a full inverse agonist, the calculation has substantial error as indicated by the log estimate of 5.56 for the simulated data in Fig. 3b. This estimate differs somewhat from the theoretical value used for the simulation (5.0). The Kb value was also estimated by regression analysis using eq. 44 as described above, which yielded the same unreliable estimate of 5.56 ± 0.34. As described below, more reliable estimates can be made for partial inverse agonists.
Can the agonist data be analyzed using the standard operational model after subtracting the basal response from the total response? Likewise, can the response of the inverse agonist be expressed as percentage of inhibition of basal activity and analyzed with the standard operational model? In the case of the inverse agonist (Fig. 3b), fitting the standard operational model:
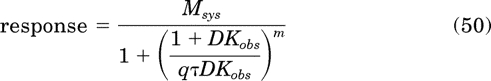 |
to the data with the estimate of Msys constrained so that it cannot exceed complete inhibition of basal activity (i.e., Msys ≤ 20% inhibition) yielded the following estimates of log Kobs (6.45 ± 0.20), m (1.68 ± 0.36), and q (0.19 ± 0.2). These estimates have substantial error compared with the theoretical values (7.0, 1.0, and 0.5), and the increase in the residual sum of squares (RSS) was colossal (10-fold). The reason for the poor fit is that partial receptor inactivation increases the potency of an inverse agonist and reduces the potency of agonists when the transducer slope factor is equal to or greater than one. Thus, the standard operational model does not adequately describe the behavior of inverse agonists. In the case of the full agonist (Fig. 3a), there was little error in the estimate of Kobs and q when eq. 50 was used to analyze the data, but with partial agonists, the error in Kobs can be substantial (3-fold).
Estimation of the Kb Values of a Series of Orthosteric Ligands.
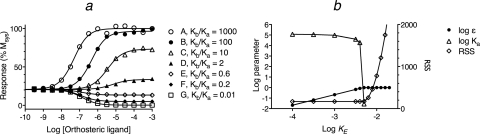
We simulated concentration-response curves for a series of agonists and inverse agonists in a system having constitutive activity to determine whether it were possible to estimate their Kb and Ka values (Fig. 4). The Ka value of each theoretical agonist was 105 M−1 and that of the inverse agonists was 107 M−1. The ratio of Kb/Ka varied from 103 for the most efficacious agonist (A) to 10−2 for the full inverse agonist (G). The complete list of parameters for the simulations is given in the legend to Fig. 4. The theoretical total stimuli were generated using the approach described under Materials and Methods. These were used as input to the logistic-transducer function of the operational model (eq. 1) to generate the simulated concentration-response curves shown in Fig. 4a.
Fig. 4.
Simulation and analysis of the response of orthosteric ligands at a constitutively active receptor. a, the theoretical curves were generated using the operational model for the total stimulus (eq. 27) with the latter defined as the summation of the active states of the quaternary (DRs*GX) and ternary (Rs*GX) complexes. These were simulated using the quaternary complex model described in Ehlert et al. (2011). The log affinity constant of the agonists A to D for the inactive state was 5.0, and that for the inverse agonists (E–G) was 7.0. The Kb value expressed relative to Ka is given in the symbol key. The parameters of the model were the same as those given in the legend to Fig. 3. b, nonlinear regression analysis of the concentration-response curve of agonist A according to eq. 52. During regression analysis, KE was constrained to a range of values shown on the abscissa. The corresponding estimates of log ε and the residual sum of squares are plotted against the constrained value of log KE.
To estimate Kb values, all of the concentration-response curves in Fig. 4a were analyzed simultaneously using global nonlinear regression analysis with a form of eq. 37 in which some of the parameters are expressed as logarithms:
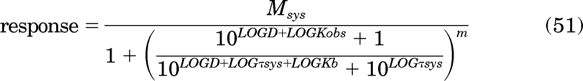 |
In this equation, LOGD, LOGτsys LOGKb, and LOGKobs denote the logarithms of D, τsys Kb and Kobs, respectively. The curves were analyzed simultaneously sharing the estimates of Msys, m, and log τsys among the curves and obtaining unique estimates of log Kobs and log Kb for each orthosteric ligand. Regression analysis will not yield an accurate estimate of the Kobs of a full agonist, and the nonlinear regression algorithm used might not converge on a solution. This is not a problem because the error in the estimate of the Kobs of a full agonist is inversely correlated with τ such that the product of the two (τKobs) is constant over the domain that yields a least-squares fit. Thus, Kobs can be constrained to an arbitrarily low value (lower than the true value) during regression analysis, and the resulting estimate of Kb or the product, τKobs, is accurate (see eq. 34; Kb = τKobs/τsys). In our case, we simply constrained the Kobs value of agonist A to its theoretical value (i.e., 5.08; log reciprocal of the EC50 value for half-maximal formation of DRs*GX). Regression analysis yielded the following estimates of the log Kobs values of the orthosteric ligands B to G: 5.17 ± 0.03, 5.07 ± 0.01, 5.12 ± 0.03, 6.90 ± 0.04, 6.97 ± 0.01 and 6.89 ± 0.01, respectively. These values are nearly identical to those used to simulate the data (5.01, 5.001, 5.001, 7.00, 7.00 and 7.00, respectively). The corresponding estimates of the log Kb values for agonists A to G (7.88 ± 0.02, 6.91 ± 0.01, 5.96 ± 0.01, 5.38 ± 0.03, 6.71 ± 0.04, 6.37 ± 0.01, and 5.16 ± 0.09, respectively) were also similar to their theoretical values (8.0, 7.0, 6.0, 5.301, 6.78, 6.301, and 5.0, respectively). The Kobs and Kb values are listed in Table 1. Regression analysis also yielded estimates of Msys (100.3 ± 0.50), m (1.15 ± 0.10), and log τsys (−0.51 ± 0.005).
TABLE 1.
Theoretical and estimated parameters for the orthosteric ligand concentration-response curves shown in Fig. 4
| Ligand | Theoretical Parameters |
Estimated Parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| Log Kb | Log Ka | Log Kobsa | Log Relative Efficacyb | Log Kb | Log Ka | Log Kobs | Log Relative τc | |
| A | 8.00 | 5.00 | 5.08 | 0.00 | 7.88 ± 0.02 | 4.29 | N.D. | 0.00 |
| B | 7.00 | 5.00 | 5.01 | −0.93 | 6.91 ± 0.01 | 5.14 | 5.17 ± 0.03 | −1.08 |
| C | 6.00 | 5.00 | 5.00 | −1.93 | 5.96 ± 0.01 | 5.06 | 5.07 ± 0.01 | −1.92 |
| D | 5.30 | 5.00 | 5.00 | −2.62 | 5.38 ± 0.03 | 5.10 | 5.12 ± 0.03 | −2.56 |
| E | 6.78 | 7.00 | 7.00 | −3.15 | 6.71 ± 0.004 | 6.94 | 6.90 ± 0.04 | −3.01 |
| F | 6.30 | 7.00 | 7.00 | −3.62 | 6.37 ± 0.01 | 6.99 | 6.97 ± 0.01 | −3.41 |
| G | 5.00 | 7.00 | 7.00 | −4.92 | 5.16 ± 0.09 | 6.91 | 6.89 ± 0.01 | −4.53 |
N.D., not determined.
The log reciprocal of the orthosteric ligand concentration required for half-maximal formation of the DRs*GX complex.
The maximal amount of DRs*GX complex formed by the orthosteric ligand, expressed relative to that of agonist A.
Expressed relative to that of agonist A.
Estimation of the Ka Values of a Series of Orthosteric Ligands.
The estimation of Ka is more complicated than that of Kb. It is impossible, for example, to estimate the Ka of a single orthosteric ligand if the only information available is its concentration-response curve measured before and after partial receptor inactivation. In this section, therefore, a method for estimating the Ka values of the series of orthosteric ligands illustrated in Fig. 4 is described. The method is accurate for those ligands with relative efficacies less than one-third that of the most efficacious ligand in the series.
The process of estimating Ka consists of the following steps:
Determining the maximal estimate of KE-obs that yields a least-squares fit of eq. 52 to the concentration-response curve of the most efficacious agonist.
Estimating a provisional value of ε for each orthosteric ligand by regression analysis with eq. 52 with KE-obs constrained to the maximal estimate for the most efficacious agonist.
Estimating Ka from the provisional ε values of the agonists through a series of calculations using eqs. 2, 3, 32, and 35.
For those agonists having an efficacy value less than one-third that of the most efficacious agonist, the estimate of Ka is valid.
For the most efficacious agonist and agonists with an efficacy not less than one-third that of the most efficacious agonist, the value of Ka represents a minimal, limiting estimate.
The first step involves nonlinear regression analysis of the concentration-response curve of the most efficacious agonist (agonist A; Fig. 4) using the following equation:
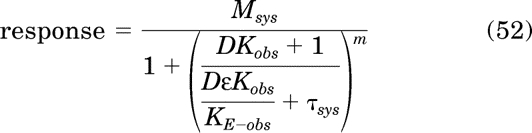 |
This equation represents the operational model for constitutive activity (eq. 28) with τ replaced with ε/KE-obs. The goal is to identify the maximal estimate of KE-obs that yields a least-squares fit. Regression analysis is done iteratively with Msys, m, and Kobs constrained as constants equivalent to those estimated in the Kb analysis described above. An approximate estimate of KE-obs is obtained in the first regression by constraining ε to 1.0 and estimating KE-obs. In the second and subsequent regressions, KE-obs is constrained to a constant 0.1 log unit less than that of the prior regression. For each of these subsequent regressions, log ε is constrained to the range log ε < 0 (1 > ε > 0), and regression analysis yields an estimate of log ε. This stepwise process is continued until the RSS no longer decreases. This occurs when the RSS of a regression is equivalent to that of the prior regression. That the RSS is at a minimum can be verified by doing the regression with log KE-obs constrained to arbitrarily low value (e.g., −4.0) and verifying that the RSS is the same. The maximum value of log KE-obs that yields a minimum value of the RSS is recorded as well as the provisional estimate of log ε for this value of log KE-obs. For agonist A in Fig. 4, these values were −0.03 and −2.4, for log ε and log KE-obs, respectively.
An example of how the RSS changes with a change in the value to which KE-obs is constrained during nonlinear regression is shown in Fig. 4b for agonist A. The plot shows the results of regression analysis using eq. 52 with the parameters Msys, m, and Kobs constrained to constants as described above and KE-obs constrained to various values indicated on the abscissa. A vertical line through each set of points shows the results of a single nonlinear regression step. As the value to which log KE-obs is constrained decreases from a high value (−1.8), the RSS decreases and reaches a stable minimum value when log KE-obs ≤ −2.4. The estimate of log ε is a little less than 0 (ε slightly less than 1.0) when log KE-obs is equivalent to its maximal value for generating a least-squares fit.
Having identified the maximal, limiting estimate for the domain of log KE-obs for agonist A and its associated provisional log ε value, the concentration-response curves of the less efficacious orthosteric ligands are analyzed by nonlinear regression analysis with eq. 52 with Msys, m, and Kobs constrained as described above and log KE-obs constrained to its maximal limiting estimate (−2.4 for the data in Fig. 4). Regression analysis yields the provisional log ε values of the orthosteric ligands. From these estimates of ε, εi values are estimated using eq. 2. Then, estimates of τi are calculated using eq. 32, and finally, Ka is estimated using eq. 35 and the estimates of Kobs, τi, and τsys. These values are listed in Table 1. There was good agreement between these log Ka estimates for ligands B to G (5.14, 5.06, 5.10, 6.94, 6.99, and 6.91, respectively) and those used to simulate the data (5.0, 5.0, 5.0, 7.0, 7.0, and 7.0, respectively). The exception is in the estimation of Ka of the most efficacious agonist (A), which exhibited a log difference of approximately 0.71 in its estimation (log estimate, 4.29; log theoretical estimate, 5.0).
It can be shown that the estimate of Ka approaches Kobs when KE-obs is constrained to an arbitrarily low value during regression analysis with eq. 52. Constraining log KE-obs to an arbitrarily low value also yields a least-squares fit as shown in Fig. 4b. For agonist A in Fig. 4, the true value of Ka lies somewhere between the limiting value of Ka estimated above and the Kobs value. The same can be said of any agonist analyzed that has an efficacy not less than one-third that of the most efficacious agonist in a series.
It is possible to estimate the limiting minimal value of log Ka by the method of partial receptor inactivation using an approach analogous to that illustrated in Fig. 4b. The data in Fig. 3a were analyzed in this manner using a modified form of eq. 43 in which τ was replaced with ε/KE-obs. The ligand and system parameters for the agonist in Fig. 3a are identical to those of agonist A in Fig. 4. This analysis yielded a minimum estimate of log Ka of 4.35, which is similar to that estimated above for agonist A in Fig. 4 (4.29).
If the most efficacious orthosteric ligand in a series is an inverse agonist, the nonlinear regression analysis is done as described above but with the regression equation modified:
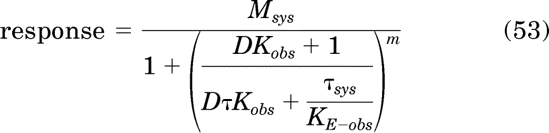 |
This equation is analogous to the operational model for constitutive receptor activity (eq. 28, but with τsys replaced with εsys/KE-obs). The regression analysis requires an estimate of Msys, which can only be determined by the method of partial receptor inactivation with an efficacious agonist or by assuming that the Emax of a full agonist is equivalent to Msys. Thus, it seems unlikely that an experimenter would know Msys without also having the concentration-response curve of an efficacious agonist. Regardless, if Msys is known and the most efficacious ligand is an inverse agonist, a limiting estimate of log KE-obs can be estimated using eq. 53 as in a manner analogous to that described above for the most efficacious agonist. This estimate can be used to calculate the provisional ε values of the inverse agonists from their corresponding τ values. Ultimately, Ka values can be estimated using eqs. 3, 32, and 35 as described above.
Estimation of the Relative Efficacy Values of a Series of Orthosteric Ligands.
It is possible to estimate the relative efficacy of the orthosteric ligands in various ways based on the parameter values estimated so far. For example, the intrinsic relative activity (RAi) of the orthosteric ligand is defined as the product of its observed affinity and efficacy expressed relative to that of a reference orthosteric ligand (Ehlert et al., 1999; Griffin et al., 2007). From eq. 4, it follows that:
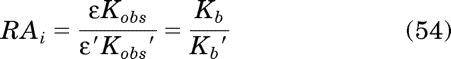 |
in which ′ denotes the parameters of the reference orthosteric ligand. Rearranging eq. 54 yields:
 |
Thus, the parameter estimates (Kb and Kobs) from the initial regression with eq. 51 could be used to estimate relative efficacy using eq. 51 provided that the Kobs value of any full agonist was previously determined. The relative efficacy values of the ligands were determined in this way and there was good agreement between the estimates and the theoretical values (see Table 1). Alternatively, the concentration-response curves could be analyzed with eq. 28 using global nonlinear regression analysis, sharing the estimates of Msys, m, and τsys among the curves and obtaining unique estimates of Kobs and τ for each orthosteric ligand. Again, the Kobs of any full agonist needs to be determined previously and constrained as a constant during regression analysis. The relative efficacy values of the orthosteric ligands could then be estimated from the τ values using eq. 49.
Estimation of the Kb Values of Orthosteric Ligands and the Kobs and Relative Efficacy Values of Partial Agonists Is Easy When the Hill Slope Is Equal to One.
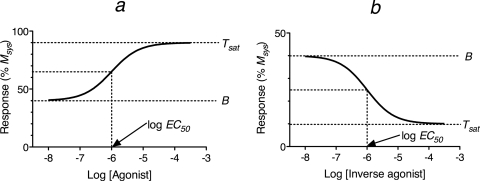
When the Hill slope of the ligand concentration-response curve is equivalent to one, the transducer-slope factor in the operational model is also equal to one, and there are simple relationships between the parameters of the concentration-response curve and Kb, Kobs, and relative efficacy. For these relationships, we define the EC50 value as the concentration of orthosteric ligand that elicits a half-maximal change in response from the basal level caused by constitutive receptor activity (B) to that observed at maximally effective concentrations of the ligand. We define the effect at maximally effective concentrations of the ligand as Tsat (total response at a saturating ligand concentration). For an agonist, this value is equal to the maximum total response above background level without constitutive activity. For an inverse agonist, Tsat represents the difference between the response measured at saturating concentration of inverse agonist and the level of response measured in the absence of constitutive activity. Figure 5 illustrates these terms for an agonist (a) and inverse agonist (b).
Fig. 5.
The definitions of EC50, the basal response of a constitutively active receptor (B), and the total response of the receptor at a maximally effective concentration of the orthosteric ligand (Tsat) are shown for an agonist (a) and an inverse agonist (b).
When the Hill slope of the concentration-response curve is equivalent to one, the Kb value of an orthosteric ligand relative to that of a reference orthosteric ligand can be expressed in terms of the empirical parameters of the concentration-response curve:
In this equation the parameters of the standard orthosteric ligand are denoted by ′. We have previously defined the RAi value as a relative measure of the affinity constant of an agonist for the active state of the receptor (Tran et al., 2009). It can also be shown that the Kb and Kobs values of an orthosteric ligand are described by:
 |
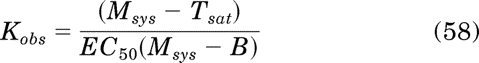 |
Finally, the efficacy of one orthosteric ligand expressed relative to a reference ligand is equivalent to:
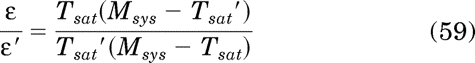 |
where the parameters of the reference orthosteric ligand are denoted by ′. The derivation of eqs. 56 to 59 is given in Appendix. The equations for Kobs and relative efficacy can only be applied in the analysis of partial agonists and inverse agonists, because there is little difference between Msys and the Tsat of a full agonist. In fact, the Tsat of a full agonist could be used as an estimate of Msys in the estimation of Kobs and relative efficacy of partial agonist.
The Tsat and EC50 values of all of orthosteric ligands A to G can be estimated from the parameters of the operational model for constitutive activity (eq. 28) as described in Appendix (see eqs. 61 and 70) or by nonlinear regression analysis using a four-parameter logistic equation:
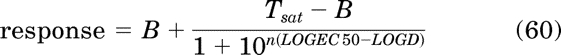 |
In this equation, n denotes the Hill slope of the concentration-response curve and LOGEC50 and LOGD denote the log values of EC50 and D, respectively.
These estimates of Tsat and log EC50 for the data in Fig. 4 are listed in Table 2. The estimates of Kb, Kobs, and relative efficacy were also estimated from the Tsat and EC50 values using eqs. 57 to 59, and these estimates are also listed in Table 2 for ligands B to G. The log efficacy values have been normalized relative to that of agonist B. There is agreement between these estimates of Kb, Kobs, and relative efficacy and the theoretical values listed in Table 1 after normalizing the theoretical efficacy values to that of agonist B. This is expected because the transducer slope factor for this simulation was equal to one (m = 1; see legend to Fig. 4).
TABLE 2.
Estimation of Kb, Kobs, and relative efficacy using equations 57, 58, and 59, respectively
The parameters were estimated from the Tsat and EC50 values of the concentration-response curves shown in Fig. 4. For the calculations, it was assumed that the Tsat of agonist A was a good estimate of Msys. The estimate of the basal response was 20.6%.
| Agonist | Tsat | Log EC50 | Log Kb | Log Kobs | Log Relative Efficacya |
|---|---|---|---|---|---|
| % Msys | |||||
| A | 100 | −7.32 | 8.00 | N.D. | N.D. |
| B | 96.6 | −6.33 | 7.00 | 4.97 | 0.00 |
| C | 73.4 | −5.46 | 6.02 | 4.99 | −1.01 |
| D | 34.0 | −5.08 | 5.37 | 5.07 | −1.74 |
| E | 13.5 | −6.96 | 6.72 | 6.95 | −2.26 |
| F | 5.05 | −6.92 | 6.32 | 7.01 | −2.72 |
| G | 0.28 | −6.90 | 5.03 | 6.99 | −4.00 |
N.D., not determined.
Expressed relative to that of agonist B.
Additional Simulations.
We also simulated concentration-response curves with random error for orthosteric ligands under conditions of constitutive activity with different concentrations of guanine nucleotide and with transducer slope factors (m) that differed from one. In each case, we were able to obtain reasonable estimates of Kb and Ka as well as the receptor population parameters (Kobs and relative efficacy) except for the Ka value of the most efficacious agonist and the Kb value of full inverse agonists. This analysis was done based on the regression eqs. 28, 37, and 52 as described above. With the most efficacious agonist, the limiting minimal estimate of Ka was accurate when the concentration of guanine nucleotide was low, but with real data there is uncertainty in knowing whether the estimate is accurate as described in Ehlert et al. (2011).
We also considered the possibility of two active states of the receptor and a summary of the results of these simulations is given in Appendix.
Analysis of Experimental Data
M2 Muscarinic Receptor-Mediated Phosphoinositide Hydrolysis in HEK 293 Cells Overexpressing Gα15.
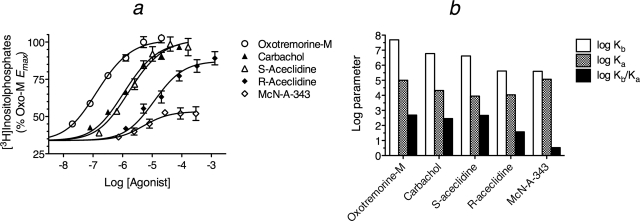
We previously investigated the activity of a group of muscarinic agonists for eliciting [3H]phosphoinositide hydrolysis in HEK 293 cells prelabeled with [3H]inositol and expressing Gα15 and the M2 muscarinic receptor (Griffin et al., 2007). The cells were treated with pertussis toxin and 4-DAMP mustard to inhibit signaling through Gi and to block a small response elicited by an endogenous M3 receptor, respectively. In that study, we analyzed the component of the response above constitutive M2 receptor activity using the standard operational model and a null equation. The background level of [3H]inositolphosphate accumulation in HEK 293 Gα15 cells was approximately 1500 to 2000 cpm. After transient transfection of the M2 receptor, inositolphosphate accumulation in the absence of orthosteric ligand increased to 3500 to 4000 cpm. Thus, approximately 2000 cpm of [3H]inositolphosphates could be attributed to constitutive activity of the M2 receptor. Figure 6a shows the results of our prior study plotted as the total response (constitutive and ligand induced activity) normalized relative to the Tsat of oxotremorine-M (approximately 6000 cpm). The Tsat and log EC50 values (mean ± S.E.M.) of oxotremorine-M, carbachol, S-aceclidine, R-aceclidine, and 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium (McN-A-343) were 100 ± 0.7, 94.9 ± 0.6, 99.7 ± 1.0, 86.1 ± 0.9, and 53.5 ± 1.0 for Tsat and −6.79 ± 0.02, −6.02 ± 0.02, −5.76 ± 0.03, −4.93 ± 0.04, and −5.24 ± 0.11 for log EC50, respectively.
Fig. 6.
M2 muscarinic receptor-mediated stimulation of phosphoinositide hydrolysis in HEK 293 cells expressing Gα15. a, the effects of muscarinic agonists on [3H]inositolphosphate accumulation are shown. The [3H]inositolphosphate level measured in cells not transfected with the M2 receptor was subtracted from the measurements made in cells transfected with the M2 receptor and normalized relative to the Emax of oxotremorine-M. The data are from Griffin et al. (2007) and represent the mean ± S.E.M. of four to six experiments, each done in triplicate. b, analysis of the data in a yielded estimates of the affinity constants of the agonists for the active (Kb) and inactive (Ka) states of the receptor. The Ka value was assumed to be approximately equal to Kobs.
The observed affinity constants of carbachol and oxotremorine-M were determined in competitive binding assays with [3H]N-methylscopolamine on intact HEK cells overexpressing Gα15. The data in Fig. 6a were analyzed as described above to estimate the Kb, the minimum value of Ka, Kobs, and relative efficacy, and these results are given in Table 3. During regression analysis, the log Kobs values of oxotremorine-M and carbachol were constrained to the values determined in the competitive binding experiments with [3H]N-methylscopolamine (5.0 and 4.3, respectively). Regression analysis yielded estimates of Msys (106 ± 2), log τsys (−0.52 ± 0.03), and m (0.63 ± 0.08). Relative efficacy was calculated as the agonist τ value expressed relative to that of oxotremorine-M. The estimates of Kb, Ka, and their ratio are illustrated in the histogram shown in Fig. 6b. Because there is potential ambiguity in the estimate of the Ka value of the most efficacious agonists (oxotremorine-M, carbachol, and S-aceclidine), we assumed that their Ka values were similar to Kobs for the illustration in Fig. 6b.
TABLE 3.
M2 receptor-mediated stimulation of [3H]inositolphosphate accumulation in HEK 293 cells expressing Gα15
The parameters were estimated from the agonist concentration-response curves shown in Fig. 6.
| Agonist | Log Kb | Log Kaa | Log Kobsb | Log Kb/Kac | Log Relative Efficacyd |
|---|---|---|---|---|---|
| Oxotremorine-M | 7.69 ± 0.03 | 3.76 | 5.01 ± 0.08 | 2.7–3.9 | 0.00 |
| Carbachol | 6.78 ± 0.02 | 3.46 | 4.32 ± 0.10 | 2.5–3.3 | −0.24 |
| S-Aceclidine | 6.62 ± 0.04 | 3.00 | 3.95 ± 0.29 | 2.7–3.6 | −0.03 |
| R-Aceclidine | 5.62 ± 0.04 | 4.01 | 4.04 ± 0.04 | 1.6 | −1.11 |
| McN-A-343 | 5.60 ± 0.11 | 5.06 | 5.07 ± 0.11 | 0.54 | −2.16 |
A minimum estimate of log Ka.
The Kobs values of oxotremorine-M and carbachol were estimated in competitive binding experiments on intact HEK 293 cells expressing Gα15 and the M2 muscarinic receptor, and the Kobs values of the remaining agonists were estimated by nonlinear regression analysis using eq. 37.
The range of values represents log Kb/Kobs − log Kb/Ka.
The log τ value of the agonist expressed relative to that of oxotremorine-M.
M3 Muscarinic Receptor-Mediated Phosphoinositide Hydrolysis in HEK Gα15 Cells.
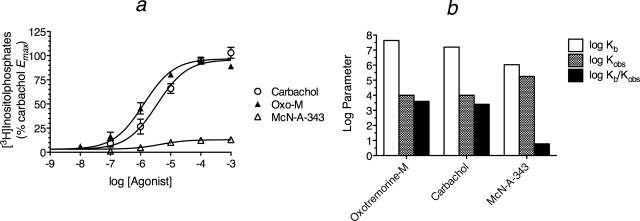
We also transiently expressed the M3 receptor in HEK 293 Gα15 cells and measured phosphoinositide hydrolysis elicited by oxotremorine-M, carbachol, and McN-A-343. The data are plotted as total response (constitutive and ligand induced activity) normalized relative to the Tsat of oxotremorine-M (Fig. 7a). In these experiments, the background level of [3H]inositolphosphate accumulation was similar to that observed in our prior studies on the M2 receptor, but the constitutive activity elicited by transient transfection of the M3 receptor was only approximately 600 to 1000 cpm, and Tsat values of oxotremorine-M and carbachol were much greater (40,000–60,000 cpm of [3H]inositolphosphates). The Tsat and log EC50 values (mean ± S.E.M.) of oxotremorine-M, carbachol, and McN-A-343 were 100 ± 1.3, 110 ± 2.0, and 14.3 ± 0.8 for Tsat and −5.97 ± 0.04, −5.33 ± 0.06, and −5.21 ± 0.23 for log EC50, respectively.
Fig. 7.
M3 muscarinic receptor-mediated stimulation of phosphoinositide hydrolysis in HEK 293 cells expressing Gα15. a, the effects of muscarinic agonists on [3H]inositolphosphate accumulation are shown. The [3H]inositolphosphate level measured in cells not transfected with the M3 receptor was subtracted from the measurements made in cells transfected with the M3 receptor and normalized relative to the Emax of carbachol. The data represent the mean ± S.E.M. of three experiments, each done in triplicate. b, analysis of the data in a yielded estimates of the affinity constants of the agonists for the active (Kb) and inactive (Ka) states of the receptor. The Ka value was assumed to be approximately equal to Kobs.
The Kobs values of carbachol and oxotremorine-M were estimated by the method of partial receptor inactivation using a 5-min incubation with the aziridinium ion of 4-DAMP mustard (1.0 μM) as a means of inactivating a fraction of the receptor population. Treatment with 4-DAMP mustard had little effect on the basal [3H]inositolphosphate response in HEK 293 Gα15 cells transfected with the M3 receptor. The data were analyzed as described above in connection with Fig. 3 to yield a log Kobs value for carbachol and oxotremorine-M of 3.74 ± 0.14 and 4.00 ± 0.13, respectively.
The data in Fig. 7a were analyzed for estimation of Kb, Kobs, relative efficacy, and the minimum estimate of Ka as described above, and the corresponding parameter estimates are listed in Table 4. Regression analysis yielded estimates of Msys (99.1 ± 2.03), log τsys (−1.74 ± 0.25), and m (0.85 ± 0.08). During the analysis, the log Kobs values of carbachol and oxtremorine-M were constrained to those estimated by the method of partial receptor inactivation. The Kb, Ka, and Kb/Ka values of the agonists are illustrated in Fig. 7b and listed in Table 4. For the histogram, it was assumed that the Ka values of oxotremorine-M and carbachol were approximately equal to Kobs. Although the Emax value of carbachol was greater than that of oxotremorine-M, a reasonable fit of eq. 28 was obtained sharing the estimate of Msys. The parameters yielded a lower estimate for the efficacy of carbachol (0.66) relative to oxotremorine-M (see Table 4).
TABLE 4.
M3 receptor-mediated stimulation of [3H]inositolphosphate accumulation in HEK 293 cells expressing Gα15
The parameters were estimated from the agonist concentration-response curves shown in Fig. 7.
| Agonist | Log Kb | Log Kaa | Log Kobsb | Log Kb/Kac | Log Relative Efficacyd |
|---|---|---|---|---|---|
| Oxotremorine-M | 7.64 ± 0.27 | 2.68 | 4.00 ± 0.13 | 3.6–4.9 | 0.00 |
| Carbachol | 7.20 ± 0.27 | 3.31 | 3.74 ± 0.14 | 3.4–3.9 | −0.18 |
| McN-A-343 | 6.03 ± 0.21 | 5.25 | 5.25 ± 0.20 | 0.78 | −2.85 |
A minimum estimate of log Ka.
The Kobs values of carbachol and oxotremorine-M were estimated by the method of partial receptor inactivation as described in the text, and that of McN-A-343 by regression analysis of the data in Fig. 7 using eq. 37.
The range of values represents log Kb/Kobs − log Kb/Ka.
The log τ value of each agonist normalized relative to that of oxotremorine-M.
Discussion
Most receptors evolved to be active when occupied by an endogenous ligand and inactive when unoccupied. Because high activity is associated with an optimum structure, multiple active states would reduce activity from the optimum, and multiple inactive states with variable affinity would engender ligand induction of an inactive state over an active one. Thus, discrete active and inactive states with high and low affinity for the endogenous ligand, respectively, are likely properties of an efficient, ligand-dependent molecular switch. That evolution has selected efficient receptors can be seen in the uniform quantal currents of single ligand-gated ion channels (Colquhoun and Sakmann, 1985). The ensemble average of their individual random behaviors yields a continuum of predictable, ligand-dependent activity at the level of the receptor population. The methods of this article describe how to estimate orthosteric ligand affinity constants for active and inactive states of a constitutively active G protein-coupled receptor by analyzing a response downstream from receptor activation.
We have previously shown that the product of affinity and efficacy is proportional to the affinity constant of the agonist for the active state of the receptor (Tran et al., 2009). When an agonist concentration-response curve is analyzed with the operational model, it is always possible to estimate the product of the parameters, Kobs and τ (Griffin et al., 2007; Ehlert, 2008). This product is proportional to both the product of affinity and efficacy (Ehlert et al., 1999) and the affinity constant of the active state (Tran et al., 2009). The proportionality constant for the latter (τsys) is a function of the inherent activity of the free receptor (Kc) and the sensitivity of the downstream signaling pathway (KE-obs), and it can be estimated from the basal response of the system using the operational model (see eqs. 28 and 30). Thus, by dividing the product, τKobs, by τsys, it is possible to estimate the affinity constant of an agonist for the active state of the receptor. Analogous reasoning shows that it is possible to estimate the affinity constant of the inactive state by dividing the product, τiKobs, by τi-sys. It is impossible, however, to obtain a reliable estimate of τiKobs for the most efficacious agonist in a series unless additional information is obtained. Likewise, it is difficult to estimate τKobs for a full inverse agonist.
An advantage of estimating the microscopic affinity constants of orthosteric ligands for receptor states is that the latter are purely receptor-dependent properties. Although the nature of the G protein, other effectors that come in contact with the receptor, and the concentration of GTP can change the activation state and observed affinity of the receptor population, these have no effect on the structure of receptor states, per se, nor on the estimation of Kb and Ka. Thus, a unique set of receptor states can give rise to a continuum of observed affinities and efficacies (Ehlert, 2000).
Although the existence of agonist bias in receptor signaling (Kenakin, 2011) may seem to conflict with the simple picture given above, it implies a set of discrete receptor states that differentially signal through different effectors (e.g., G proteins, G protein-coupled receptor kinases). It follows that the different effectors report the activity of different receptor states. Thus, the G protein is not so much a determinant of drug-receptor activity so much as a window onto the different receptor states that generate unique activities. Using our approach it is possible to estimate the affinity constants of these states by analyzing responses elicited through different G proteins. Even if the estimate of the affinity constant of the active state represents a weighted average of multiple active states, it would still represent a receptor-dependent property characteristic of the particular response.
Constitutive receptor systems offer an advantage for drug discovery in that these systems enable the detection of inverse agonist activity and are more sensitive to agonist activity (Chen et al., 1999). Because most potent antagonists are likely to exhibit selectivity for the inactive state of the receptor, constitutively active receptor systems are useful for the direct detection of antagonist activity (Chen et al., 2000). We show that constitutively active systems are also useful in the estimation of microscopic affinity constants for active and inactive states of the receptor. Constitutive activity can be achieved by overexpression of the receptor or by increasing the sensitivity of the transduction mechanism. The latter can be accomplished by overexpressing the G protein, which has been described for several receptors including the D2 dopamine (Senogles et al., 1990) and M3 muscarinic (Burstein et al., 1997) receptors. Although overexpression of receptors and G proteins may lead to nonphysiological signaling and a change in the parameters of the receptor population (i.e., Kobs and ε), the estimation of Kb for the receptor state that interacts with the overexpressed G protein should be unaffected.
The observed affinity constants (Kobs) of carbachol and oxotremorine-M were approximately 10-fold higher at the M2 receptor compared with the M3. There was little difference between M2 and M3 receptors with regard to the Kb and Ka values of the agonists, however, although the range of the Ka estimate was large (0.7 and 1.3 log units for carbachol and oxotremorine-M, respectively). The increase in observed affinity at the M2 receptor can be attributed to the greater constitutive activity of the M2 receptor relative to the M3 in HEK 293 cells expressing Gα15. When expressed relative to the τ value of oxotremorine-M, the τsys value of the M2 receptor (0.21%) was 10-fold greater than that of the M3 receptor (0.023%). The consequences of eq. 8 suggest a comparable difference in the Kobs value of a highly efficacious agonist.
Analysis of single-channel recordings of the neuromuscular nicotinic acetylcholine receptor of the adult mouse yielded estimates of 5.0 × 107 and 7.1 × 103 M−1 for the affinity constants of acetylcholine for the open and closed states (Auerbach, 2010). The difference in affinity (7000-fold) corresponds to approximately 5.2 kcal · mol−1 of available energy for inducing the open state of the channel. We estimated affinity constants of 1.6 × 107 and 2.0 to 5.5 × 103 M−1 for carbachol at the active and inactive states of the M3 receptor, which corresponds to a 2900- to 7800-fold difference in affinity (4.7–5.3 kcal · mol−1). The agreement between the microscopic affinity constants of acetylcholine and its carbamate analog at nicotinic and muscarinic receptors suggest that natural selection has sculpted optimal binding pockets for acetylcholine on these proteins (see also Jackson, 1989).
The estimate of the Kb/Ka ratios of McN-A-343 at M2 and M3 receptors was very low (approximately 3.5- to 6.0-fold, 0.15–0.37 kcal · mol−1), indicating that this M1 and M4 selective muscarinic agonist has little selectivity for the active states of M2 and M3 receptors and that it would be unlikely for this agonist to elicit a response at these receptors unless there were significant constitutive activity or a highly sensitive system to amplify low constitutive activity. These results have implications for native systems, such as the guinea pig ileum, where the compound exhibits an Emax of approximately 30% that of carbachol through activation of the M3 receptor. Perhaps there is substantial constitutive activity in ileal smooth muscle that is prevented from eliciting contraction by inhibitory K+ currents (e.g., Ca2+-activated K+ channels). Inhibition of KCa channels with charybdotoxin elicits spontaneous contraction of guinea pig ileum (Hong et al., 1997). A small 3- to 5-fold selectivity for the active state could generate a substantial contraction in a smooth muscle with a moderate threshold for the response caused by K+ channel activity that is almost satisfied by constitutive receptor activity.
Our method for the estimation of Ka is limited in the sense that it is never possible to estimate the Ka of the most efficacious agonist in a series, only agonists with Kb/Ka ratios less than one-third that of the most efficacious agonist. Ehlert et al. (2011) describe this limitation in detail with regard to the estimation of RIi. Our estimates of the Kb values of agonists for the M3 receptor were more variable than those of the M2 receptor. This can be attributed to 1) the greater relative error in the estimation of the very low constitutive activity of the M3 receptor, 2) the limited number of agonists run in the M3 receptor assay, and 3) the wider spacing of the agonist concentrations in the M3 assay. Accurate estimation of Kb depends on an accurate estimate of constitutive activity (τsys) and the transducer slope factor (m) in the operational model. The error in the estimate of the latter parameter is greatly reduced with more agonists having a range of efficacies and a tighter geometric spacing of agonist concentrations.
Our method of estimating the receptor-dependent Kb and Ka values of orthosteric ligands through the analysis of downstream responses at G protein-coupled receptors has widespread application in drug discovery, particularly when defined receptor-effector systems are used (Stewart et al., 2010).
Appendix
Summary of the Analysis of Simulations Involving Two Active States of the Receptor
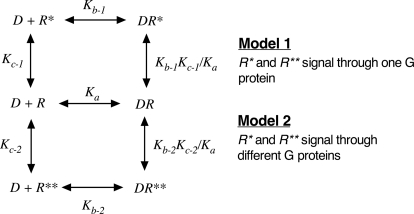
Using techniques described previously (Ehlert, 2008; Tran et al., 2009), we also investigated a model having two active states that signal through the same G protein (model 1) and another having two active states that signal through two different G protein to elicit two different responses (model 2) (see Fig. 8).
Fig. 8.
Model for a receptor with two active states (R* and R**). In model 1, both active states signal through the same G protein to elicit the same response. In model 2, each active state signals through a different G protein to elicit a different response.
The results of our analysis showed that the behavior of the first model depends on the activity of the two states, their relative abundances in the absence of orthosteric ligand, and the selectivity of the orthosteric ligand for the two states. If the relative abundances (Kc-1 = Kc-2) and the activities of the two active states are the same, then the estimate of the Kb value of an orthosteric agonist is approximately equal to that of the state for which it has higher affinity, particularly when Kb-1/Kb-2 or Kb-2/Kb-1 is more than 10. At lower ratios, there is more discrepancy, but the difference is never large because of the lack of a large difference between Kb-1 and Kb-2 under this condition. If there is a difference in the abundance (Kc-1 ≠ Kc-2) or the activity of the two states, however, then the estimate of Kb could range from Kb-1 to Kb-2, even when Kb-1 > Kb-2. For example, if an active state with much lower affinity is much more abundant or has much greater activity, then the estimate of Kb could approximate Kb-2 even if Kb-1 > Kb-2.
When there are different states that differentially couple to different G proteins to elicit different measurable responses, then the estimate of Kb-1 or Kb-2 is accurate, provided that it is possible to measure the constitutive activity associated with each active state. If not, it is possible to obtain a reliable estimate of RAi as described previously (Ehlert, 2008; Tran et al., 2009).
Derivation of Equations 56 to 59
Our strategy involves first deriving equations for Tsat, B, and EC50 in terms of the parameters of the operational model for constitutive activity when the transducer slope factor is equivalent to one (m = 1). These expressions can then be substituted into eqs. 56 to 59 under Results to determine whether the latter are equalities.
Tsat can be determined by evaluating the limit of the operational model for constitutive activity (eq. 28) with m = 1 as D approaches infinity:
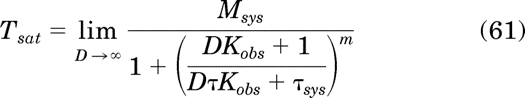 |
Taking the limit yields:
 |
When m = 1, eq. 62 reduces to:
 |
The equation for the basal response elicited by constitutive activity (B) in the absence of an orthosteric ligand can be derived by evaluating the operational model for constitutive activity (eq. 28) when D = 0:
 |
When m = 1, eq. 64 reduces to:
 |
The expression for EC50 can be derived by first considering the range of responses elicited by an orthosteric ligand in the presence of constitutive receptor activity. For an orthosteric agonist, this range spans from B to Tsat and is equivalent to Tsat − B. An EC50 response for an agonist occurs when the total response is equivalent to half of this dynamic range plus the basal response:
In this equation, response50 denotes the total response observed at the EC50 concentration of orthosteric agonist. For an orthosteric inverse agonist, the dynamic range is B − Tsat, and an EC50 response occurs when half of this dynamic range is subtracted from the basal response:
Both eqs. 66 and 67 reduce to the following form:
If the term, response50, in eq. 68 is substituted with the operational model for constitutive activity (eq. 28) with EC50 substituted for D, the following equation is generated:
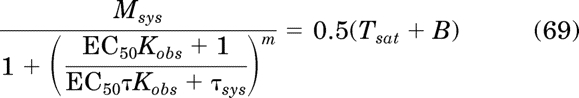 |
Substituting eqs. 62 and 64 for Tsat and B and solving for EC50 yields:
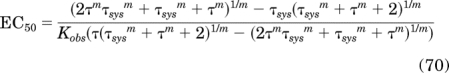 |
When m = 1, eq. 70 reduces to:
 |
Now it is possible to substitute eqs. 63, 65, and 71 for Tsat, B, and EC50, respectively, into eqs. 56 to 59 to determine whether the latter are equalities. Beginning with eq. 56, making the appropriate substitutions yields:
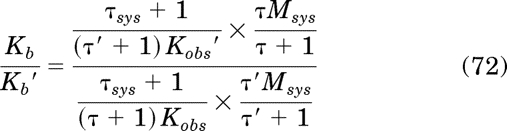 |
Simplification yields:
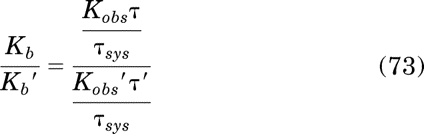 |
Substitution of eq. 34 for τKobs/τsys yields the equality Kb/Kb′ = Kb/Kb′, which verifies eq. 56. Substituting eqs. 65 and 71 for Tsat and EC50 in eq. 57 yields:
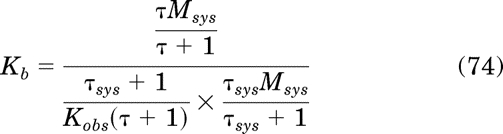 |
Simplification yields the equality Kb = Kb, which verifies eq. 57. Substituting in eqs. 63, 65, and 71 for Tsat, B, and EC50, respectively, in eq. 58 yields the following expression:
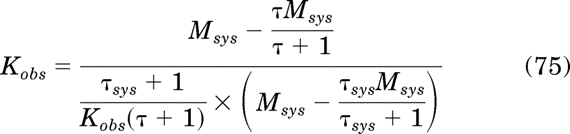 |
Simplification yields the equality Kobs = Kobs, which proves eq. 58. Finally, eq. 56 can be rewritten in the following form because it has been shown the RAi is equivalent to the product of the observed affinity and efficacy of one orthosteric ligand, expressed relative to that of another:
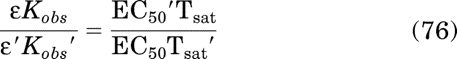 |
Substituting in eq. 58 for Kobs yields:
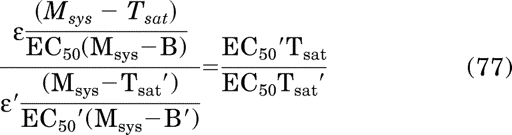 |
This equation reduces to eq. 59, which proves the latter.
Estimation of Initial Parameter Values for Nonlinear Regression Analysis
Below, we describe equations for estimating the initial parameter values for nonlinear regression analysis of concentration-response curves using eq. 51 and the log forms of eqs. 28, 43, and 44. The parameter estimates are denoted by ″ and expressed in terms of the empirical parameters of the concentration-response curve. The definitions of the variables are given under Results.
Initial Parameter Estimates for Equation 43.
Equation 43 is first modified so that the independent variable D and the parameters τ, τsys, and Kobs are expressed as logarithms:
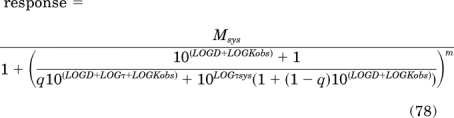 |
In this equation, LOGD, LOGτ, LOGτsys, and LOGKobs denote the log values of D, τ, τsys, and Kobs.
If the orthosteric ligand is an agonist, the initial parameter estimates of Msys, q, and LOGτ are calculated as:
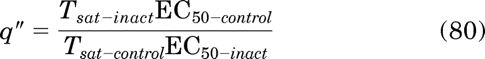 |
 |
in which, Tsat-full denotes the Tsat of a full agonist, and the subscripts “control” and “inact” denote parameters of the concentration-response curves measured before and after partial receptor inactivation, respectively. If the orthosteric ligand is an inverse agonist, Msys must be constrained to a constant value previously estimated from the analysis of agonist data. The initial estimates of q and LOGτ for an inverse agonist are calculated as:
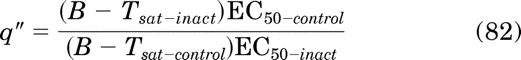 |
 |
The remaining initial parameter values are estimated in the same manner for both agonists and inverse agonists:
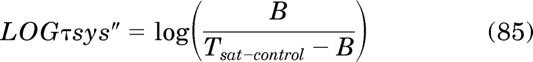 |
Initial Parameter Estimates for Equations 28, 44, and 51.
Equations 28 and 44 are first modified by expressing the independent variable D and the parameters τ, τsys, and Kb as logarithms as in eqs. 87 and 88, respectively:
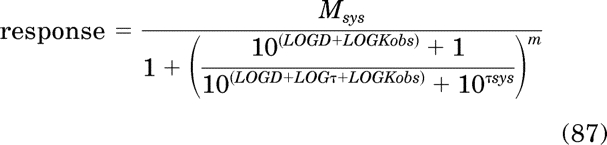 |
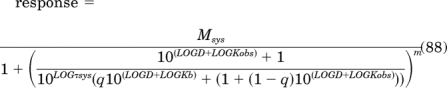 |
in which LOGKb denotes the log affinity constant of the active state of the receptor. The initial value of LOGKb (LOGKb″) is estimated as:
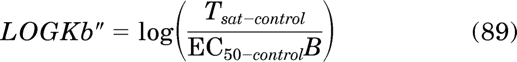 |
The initial values of the other parameters in eq. 87 and all of the other parameters in eqs. 88 and 51 are estimated as described above.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM 69829].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179309.
- McN-A-343
- 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium
- HEK
- human embryonic kidney
- G418
- (2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol
- RSS
- residual sum of squares
- 4-DAMP mustard
- N-(2-chloroethyl)-4-piperidinyl diphenylacetate.
Authorship Contributions
Participated in research design: Ehlert, Suga, and Griffin.
Conducted experiments: Ehlert, Suga, and Griffin.
Performed data analysis: Ehlert, Suga, and Griffin.
Wrote or contributed to the writing of the manuscript: Ehlert.
References
- Auerbach A. (2010) The gating isomerization of neuromuscular acetylcholine receptors. J Physiol 588:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162 [DOI] [PubMed] [Google Scholar]
- Burstein ES, Spalding TA, Brann MR. (1997) Pharmacology of muscarinic receptor subtypes constitutively activated by G proteins. Mol Pharmacol 51:312–319 [DOI] [PubMed] [Google Scholar]
- Chen G, Jayawickreme C, Way J, Armour S, Queen K, Watson C, Ignar D, Chen WJ, Kenakin T. (1999) Constitutive receptor systems for drug discovery. J Pharmacol Toxicol Methods 42:199–206 [DOI] [PubMed] [Google Scholar]
- Chen G, Way J, Armour S, Watson C, Queen K, Jayawickreme CK, Chen WJ, Kenakin T. (2000) Use of constitutive G protein-coupled receptor activity for drug discovery. Mol Pharmacol 57:125–134 [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol 369:501–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ. (2000) Ternary complex model, in Biomedical Applications of Computer Modeling (Christopoulos A. ed) pp 21–85, CRC Press, Boca Raton, FL [Google Scholar]
- Ehlert FJ. (2008) On the analysis of ligand directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch Pharmacol 377:549–577 [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Sawyer GW, Bailon R. (1999) A simple method for estimation of agonist activity at receptor subtypes: comparison of native and cloned M3 muscarinic receptors in guinea pig ileum and transfected cells. J Pharmacol Exp Ther 289:981–992 [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Suga H. (2011) Analysis of functional responses at G protein-coupled receptors: Estimation of relative affinity constants for the inactive receptor state. J Pharmacol Exp Ther 338:658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF. (1966) The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv Drug Res 3:21–55 [Google Scholar]
- Griffin MT, Figueroa KW, Liller S, Ehlert FJ. (2007) Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o, Gs, and G15. J Pharmacol Exp Ther 321:1193–1207 [DOI] [PubMed] [Google Scholar]
- Hille B. (2001) Ion Channels of Excitable Membranes, 3rd Ed, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- Hong SJ, Roan YF, Chang CC. (1997) Spontaneous activity of guinea pig ileum longitudinal muscle regulated by Ca(2+)-activated K+ channel. Am J Physiol Gastrointest Liver Physiol 272:G962–G971 [DOI] [PubMed] [Google Scholar]
- Jackson MB. (1989) Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci USA 86:2199–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302 [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416 [DOI] [PubMed] [Google Scholar]
- Senogles SE, Spiegel AM, Padrell E, Iyengar R, Caron MG. (1990) Specificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted system. J Biol Chem 265:4507–4514 [PubMed] [Google Scholar]
- Stephenson RP. (1956) A modification of receptor theory. Br J Pharmacol 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GD, Sexton PM, Christopoulos A. (2010) Detection of novel functional selectivity at M3 muscarinic acetylcholine receptors using a Saccharomyces cerevisiae platform. ACS Chem Biol 5:365–375 [DOI] [PubMed] [Google Scholar]
- Surya A, Foster KW, Knox BE. (1995) Transducin activation by the bovine opsin apoprotein. J Biol Chem 270:5024–5031 [DOI] [PubMed] [Google Scholar]
- Tran JA, Chang A, Matsui M, Ehlert FJ. (2009) Estimation of relative microscopic affinity constants of agonists for the active state of the receptor in functional studies on M2 and M3 muscarinic receptors. Mol Pharmacol 75:381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]