Abstract
G protein-coupled receptors (GPCRs) play central roles in most physiological functions, and mutations in them cause heritable diseases. Whereas crystal structures provide details about the structure of GPCRs, there is little information that identifies structural features that permit receptors to pass the cellular quality control system or are involved in transition from the ground state to the ligand-activated state. The gonadotropin-releasing hormone receptor (GnRHR), because of its small size among GPCRs, is amenable to molecular biological approaches and to computer modeling. These techniques and interspecies comparisons are used to identify structural features that are important for both intracellular trafficking and GnRHR activation yet distinguish between these processes. Our model features two salt (Arg38-Asp98 and Glu90-Lys121) and two disulfide (Cys14-Cys200 and Cys114-Cys196) bridges, all of which are required for the human GnRHR to traffic to the plasma membrane. This study reveals that both constitutive and ligand-induced activation are associated with a “coincidence detector” that occurs when an agonist binds. The observed constitutive activation of receptors lacking Glu90-Lys121, but not Arg38-Asp98 ionic bridge, suggests that the role of the former connection is holding the receptor in the inactive conformation. Both the aromatic ring and hydroxyl group of Tyr284 and the hydrogen bonding of Ser217 are important for efficient receptor activation. Our modeling results, supported by the observed influence of Lys191 from extracellular loop 2 (EL2) and a four-residue motif surrounding this loop on ligand binding and receptor activation, suggest that the positioning of EL2 within the seven-α-helical bundle regulates receptor stability, proper trafficking, and function.
Introduction
The hypothalamic decapeptide, gonadotropin-releasing hormone (GnRH), binds a cognate G protein-coupled receptor (GPCR) in the pituitary gonadotrope. This event stimulates the synthesis and release of the gonadotropins (luteinizing hormone and follicle-stimulating hormone). Mammalian GnRHRs appear unique among GPCRs because of the absence of an intracellular carboxyl-terminal domain involved in receptor trafficking, desensitization, and arrestin-mediated internalization of other GPCRs (Lefkowitz, 1998). Primate GnRHRs, unlike their rat and mouse orthologs, are poorly trafficked from the endoplasmic reticulum (ER) to the plasma membrane (PM). Approximately half of the newly synthesized receptor molecules are misfolded and retained in the ER (Leaños-Miranda et al., 2002; Conn et al., 2006a; Conn and Janovick, 2009b). This distribution of human GnRHR (hGnRHR) between the ER and PM is highly sensitive to point mutations (Knollman et al., 2005) whose effects on receptor trafficking are the underlying cause of certain forms of disease (Leaños-Miranda et al., 2002, 2003a; Janovick et al., 2006). We found that misfolded and misrouted hGnRHR mutants can be rescued by target-specific pharmacoperones, small cell-permeating molecules that serve as folding templates that enable them to pass the cellular quality control system (QCS) (Janovick et al., 2002; Conn and Janovick, 2009a,b). The small size of the hGnRHR has made it possible to construct hundreds of mutants that have been useful in understanding structures that are important for trafficking to the PM.
Prior studies suggested that the disulfide bridge between Cys114 and Cys196, orthologs of which are common among GPCRs, is an absolute requirement for trafficking of the hGnRHR to the PM (Janovick et al., 2006). Mutants in which either end of the disulfide bridge is converted to Ala are retained in the ER by the QCS and cannot be rescued with pharmacoperones (Conn et al., 2007). Misfolding and misrouting of WT receptor are also caused by failure of formation of the second disulfide bridge between Cys14 and Cys200 (Janovick et al., 2006), but pharmacoperones rescue them by allowing passage through the QCS. When the pharmacoperone is removed, cells expressing hGnRHR[C14A or C200A] regain the ability to bind and respond to agonists, suggesting that, although this structure is important for trafficking, it is not absolutely required for receptor activation by agonist.
Formation of the Cys14-Cys200 bridge is destabilized by residue Lys191 in primates (or Glu191 in many other mammals) (Janovick et al., 2006); in the rat and mouse sequence, there is no orthologous residue, making these receptors one residue shorter than the human sequence (Knollman et al., 2005; Janovick et al., 2006). Rodent GnRHRs traffic to the plasma membrane with much higher efficiency than does the human counterpart. Deletion of the Lys191 residue also rescues the hGnRHR[C14A or C200A] mutants. The action of Lys191 is supported by a four-residue noncontiguous motif (Leu112, Gln208, Leu300, and Asp302) that is replaced in the rat GnRHR sequence by Phe112, Glu207, Val299, and Glu301. Accordingly, inserting Lys191 alone in the rat sequence is without substantial effect on trafficking to the plasma membrane (Knollman et al., 2005; Janovick et al., 2006).
A salt bridge connecting Glu90-Lys121 also seems to be required for trafficking. Disruption of this ion pair in the E90K mutant is the underlying cause in some cases of human hypogonadotropic hypogonadism (Janovick et al., 2009). When rescued by pharmacoperones [which form a surrogate bridge (Janovick et al., 2009)] or by deletion of residue Lys191, this mutant reveals constitutive activity (CA) (Janovick and Conn, 2010), presenting the first reported mutant with CA and the first structural link relating the common requirements for trafficking and receptor activation. The salt bridge that is required to be intact for trafficking results in receptor activation when it is broken; thus, ER retention of this mutant protects the cell from unregulated CA.
We have used this information to construct a model for the receptor, aided by recent advances in the structure of both the inactive rhodopsin and photoactivated opsin in a complex with a G protein fragment (Topiol and Sabio, 2009). In the present study, we provide evidence from computer modeling and site-directed mutagenesis for the existence of a second salt bridge, Arg38-Asp98, and suggest structural features of the inactive and active receptor conformations that explain the observation that Asp2-GnRH, which is inactive with the WT receptor (neither agonist nor antagonist), becomes an agonist with rescued E90K.
Materials and Methods
Modeling of Human GnRH Receptors.
The homology models of human GnRH receptor (UniProt number P30968-1, residues 1-328) in the inactive and active states were developed using crystal structures of bovine rhodopsin in the dark-adapted state (Protein Data Bank code 1U19) and, after photoactivation, cocrystallized with the G protein-derived peptide (Protein Data Bank code 3DQB), respectively. The models were generated as described previously for melanocortin receptors (Chai et al., 2005; Pogozheva et al., 2005). The procedure included the following steps: 1) generation of initial models; 2) loop modeling; 3) ligand docking using experimental restraints; 4) iterative distance geometry refinement of ligand-receptor complexes; 5) energy minimization; and 6) model validation.
Initial precomputed models that were obtained from the Protein Modeling Portal (http://www.proteinmodelportal.org/) showed some misalignments, defects in helices and loops, and lack of correct disulfides. We corrected these problems in the second step of the modeling procedure by combining structural elements from different GPCR templates and using alternative sequence alignments. We modeled the intracellular loop IL3 as extensions of TM5 and TM6 with a short interhelical connection, in accordance with the squid rhodopsin template (Protein Data Bank code 2Z73). Conformations of the N terminus, intracellular loop IL1, and the extracellular loop EL3 were modeled using the rhodopsin structure with the corrected sequence alignments. The long EL2 (residues 181-204) was modeled based on several GPCR templates (Protein Data Bank codes 3EML, 1U19, and 2Z73) to provide the formation of both disulfides while leaving space for docking the decapeptide ligands.
In the third step, an NMR-derived II'β-turn conformation of GnRH, pGlu1-His2-Trp3-Ser4-Tyr5-Gly6-Leu7-Arg8-Pro9-Gly10-NH2 (Protein Data Bank code 1YY1), was selected for the docking experiment, as this conformation is consistent with 1 to 5 and 4 to 10 cyclization in bicyclic ligands with sub-nanomolar affinity (Rivier et al., 2000). This conformation of GnRH was manually docked in the active conformation of the receptor to comply with experimentally identified contacts between GnRH analogs and receptor residues: pGlu1 with Asn212, His2 with Asp98 and Lys121, Tyr5/His5 with Tyr290, d-Trp6 with Cys14, Arg8 with Asp302, Pro9 with Trp101, and Gly10 with Arg38 and Asn102 (Sealfon et al., 1997; Flanagan et al., 2000; Hoffmann et al., 2000; Hövelmann et al., 2002; Coetsee et al., 2008; Millar et al., 2008; Stewart et al., 2008). A similar mode of GnRH docking has been proposed and experimentally validated (Millar et al., 2008). The same ligand-receptor contacts were used for docking of Asp2-GnRH in the inactive receptor conformation.
The ligand-receptor complexes were refined by distance geometry calculations (Güntert and Wüthrich, 1991) with template-derived and experimental structural restraints. The distance-geometry refinement was employed to remove steric overlaps and to maximize the number of H-bonds in the receptor and between the receptor and ligand. In this step, H-bonds that can be formed between polar residues of ligand and receptor were added as distance constraints. In particular, H-bonding interactions with pyroglutamic acid of GnRH involved residues from TM4 (Gln174) and TM6 (Tyr283). Different positions of EL2 were generated with and without docked peptide. Several residues with correlated behavior in multiple sequence alignments were brought into contact during several iterations of the refinement procedure. Models that better satisfied structural restraints and maximized the number of intra- and intermolecular H-bonds were used for the subsequent energy minimization. Energy minimization of ligand-receptor complexes was then performed using the CHARMm potentials (QUANTA; Accelrys, San Diego, CA), with a dielectric constant of ε = 10 and the adopted basis Newton-Raphson minimization method (100 iterations).
To further test the models for their compliance with known experimental data, we docked agonist, buserelin [pGlu1-His2-Trp2-Ser4-Tyr5-d-Ser(tBu)6-Leu7-Arg8-Pro9-NHEt], and GnRH-II (His5Trp7Tyr8GnRH) in the active receptor form, as well as a peptide antagonist cetrorelix [Ac-d-Nal1-d-(pCl)Phe2-d-Pal3-Ser4-Tyr5-d-Cit6-Leu7-Arg8-Pro9-d-Ala10-NH2] in the inactive receptor conformation. Peptides were docked in II'β-turn conformations using poses similar to those of GnRH or Asp2-GnRH. Although GnRH-II is an agonist in cells expressing the GnRHR, it is not normally a ligand in vivo. The peptide docking was followed by the energy minimization of the receptor-ligand complex. Coordinates of the described models can be downloaded from our website (http://mosberglab.phar.umich.edu/resources/).
Materials.
pcDNA3.1 (Invitrogen, Carlsbad, CA), GnRH analog, d-tert-butyl-Ser6-des-Gly10-Pro9-ethylamide-GnRH (buserelin; Hoechst-Roussel Pharmaceuticals, Somerville, NJ), myo-[2-3H(N)]inositol (NET-114A; PerkinElmer Life and Analytical Sciences, Waltham, MA), DMEM, Opti-MEM, Lipofectamine, phosphate-buffered saline (Invitrogen), competent cells (Promega, Madison, WI), and Endofree maxi-prep kits (QIAGEN, Valencia, CA) were obtained as indicated.
Mutant Receptors.
WT and mutant GnRHR cDNAs for transfection were prepared as reported previously (Janovick et al., 2002); the purity and identity of plasmid DNAs were verified by dye terminator cycle sequencing (Applied Biosystems, Foster City, CA). Pharmacoperones In3, Q89, and Q103 (Merck and Company, Boston, MA) were obtained as indicated (Conn and Janovick, 2009a; Janovick et al., 2009). Full chemical structures and the mechanism of action on the hGnRHR have been reported previously (Conn and Janovick, 2009a,b; Janovick et al., 2009).
Transient Transfection.
COS-7 cells were cultured in growth medium (DMEM, 10% fetal calf serum, and 20 μg/ml gentamicin) at 37°C in a 5% CO2 humidified atmosphere. For transfection of WT or mutant receptors into cells, 5 × 104 cells were plated in 0.25 ml of growth medium in 48-well Costar cell culture plates (Corning Life Sciences, Lowell, MA). Twenty-four hours after plating, the cells were washed with 0.5 ml of Opti-MEM and then transfected with WT or mutant receptor DNA with pcDNA3.1 (empty vector) to keep the total DNA constant (100 ng/well). Lipofectamine was used according to the manufacturer's instructions. Five hours after transfection, 0.125 ml of DMEM with 20% fetal calf serum and 20 μg/ml gentamicin was added. Twenty-three hours after transfection, the medium was replaced with 0.25 ml of fresh growth medium. Where indicated, pharmacoperones (indicated concentration) in 1% dimethyl sulfoxide (“vehicle”) were added for 4 h in respective media to the cells and then removed 18 h before agonist treatment (Leaños-Miranda et al., 2003b). In the present study, we used trypan blue exclusion to confirm cell viability after drug exposure.
Inositol Phosphate Assays.
Twenty-seven hours after transfection, cells were washed twice with 0.5 ml of DMEM/0.1% BSA/20 μg/ml gentamicin, “preloaded” for 18 h with 0.25 ml of 4 μCi/ml myo-[2-3H(N)]inositol in inositol-free DMEM, and then washed twice with 0.3 ml of DMEM (inositol-free) containing 5 mM LiCl and treated for 2 h with 0.25 ml of a saturating concentration of buserelin (10−7 M) in the same medium. When constitutive activity was assessed, buserelin was omitted from the assessment period. Total inositol phosphate (IP) was then determined (Janovick et al., 2006). This assay has been validated as a sensitive measure of PM expression for functional receptors when expressed at low amounts of DNA (<100 ng/125 μl) and stimulated by excess agonist (Cook and Eidne, 1997; Leaños-Miranda et al., 2002, 2003b; Janovick et al., 2003a,b; Ulloa-Aguirre et al., 2004; Castro-Fernandez et al., 2005; Knollman et al., 2005; Conn et al., 2006a,b, 2007; Conn and Janovick, 2009a,b).
Binding Assays.
Cells were cultured and plated in growth medium as described above, with the exception of 105 cells in 0.5 ml of growth medium, and added to 24-well Costar cell culture plates (cell transfection and medium volumes were doubled accordingly). Twenty-three hours after transfection, the medium was replaced with 0.5 ml of fresh growth medium with or without pharmacoperone (1 μg/ml In3). Twenty-seven hours after transfection, cells were washed twice with 0.5 ml of DMEM containing 0.1% BSA and 20 μg/ml gentamicin, and then 0.5 ml of DMEM was added. After 18 h, cells were washed twice with 0.5 ml of DMEM/0.1% BSA/10 mM HEPES, and then 2 × 106 cpm/ml 125I-buserelin, prepared in our laboratory (specific activity, 700–800 μCi/μg), was added to the cells in 0.5 ml of the same medium and allowed to incubate at room temperature for 90 min, consonant with maximal binding (Brothers et al., 2002). New receptor synthesis during this period is negligible at room temperature. After 90 min, the media were removed, and radioactivity was measured (Brothers et al., 2003). To determine nonspecific binding, the same concentrations of radioligand were added to similarly transfected cells in the presence of 10 μg/ml unlabeled GnRH.
Statistics.
Data (n ≥ 3) were analyzed with one-way analysis of variance and then the Holm-Sidak test paired with the Student's t test (SigmaStat 3.1; SPSS Inc., Chicago, IL). Means ± S.E.M. are shown.
Results
Two Salt Bridges and Two Cys Bridges Are Predicted in the hGnRHR.
Homology models of hGnRH receptors in the active and inactive states in complexes with GnRH (or GnRH-II or buserelin) and Asp2-GnRH (or cetrorelix), respectively, were developed by distance geometry calculation using structural restraints from rhodopsin templates (Protein Data Bank codes 3DQB and 1U19), NMR structure of GnRH (Protein Data Bank code 1YY1), and experimentally determined ligand-receptor contact points (see Materials and Methods). The transmembrane regions of the models closely resemble those in rhodopsin structures, with a root mean square deviation of 1.31 and 1.84 Å (for 219 Cα atoms) between human GnRH receptor and bovine rhodopsin in inactive and active conformations, respectively. The largest difference is in the position of EL2, which, in the GnRH receptor, is outwardly displaced relative to its position in rhodopsin, exposing the large binding cavity for decapeptide ligands.
In our model, two experimentally identified disulfide bridges that connect EL2 with the N terminus (Cys14-Cys200) and TM3 (Cys114-Cys196) respectively, are present (Fig. 1). We also observed two interhelical ion pairs between TM1 and TM2 (Arg38-Asp98) and between TM2 and TM3 (Glu90-Lys121). The first ion pair is conserved in the majority of GnRH receptors, whereas the second one is specific for mammalian receptors. Based on the model, we hypothesized that correct translocon-assisted folding and association of helices require timely formation of ionic pairs between TM1-TM2 and TM2-TM3 and consequential closure of two disulfide bridges that stabilize the receptor structure enabling its traffic to the plasma membrane.
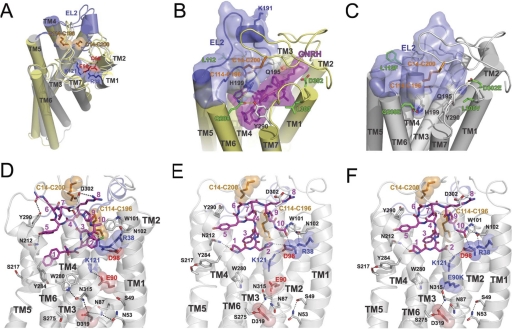
Fig. 1.
A, two Cys bridges (Cys14-Cys200 and Cys114-Cys196) and two ionic pairs (Arg38-Asp98 and Glu90-Lys121) in homology model of human GnRH receptor in the inactive conformation. Inactive (gray) and active (yellow) states were modeled using rhodopsin crystal structures (Protein Data Bank codes 1U19 and 3DQB, respectively). EL2 loops (residues 181–204) are colored blue, Cys residues are colored orange, Arg and Lys residues are colored blue, and Asp and Glu residues are colored red. Superposition of active and inactive conformation shows that the largest conformational changes include outward rigid body movement of TM6, movement of TM5 toward TM6, tilting of TM7, and shift of its middle part toward TM2. B and C, the proposed movement of the EL2 in the wild-type and mutant human GnRH receptor. B, homology models of the wild-type human receptor (yellow) in active state with GnRH decapeptide (purple) show the upper position of the EL2 (blue), which is attached to the receptor by two disulfide bridges (Cys14-Cys200 and Cys114-Cys196). C, homology model of the ΔLys191, L112F, Q208E, L300V, and D302E mutant of the human receptor in the inactive state (gray) with EL2 loop (blue) inserted between helices and filling the ligand binding pocket. Deletion of charged Lys191 permits the movement of EL2 inside the seven-helical bundle. Substitutions in noncontiguous four-residue motif that interacts with EL2 (Gln208 forms H-bond with His199, Val300 allows reorientation of Tyr290 that interacts with His199, and Glu302 forms H-bond with Gln195) may facilitate the inward movement of EL2. Multiple interaction of EL2 with helices may stabilize the receptor structure. D to F, rearrangement of ionic bridges and H-bond networks during activation of wild-type and mutant human GnRH receptor. D, model of the inactive conformation of receptor in complex with peptide antagonist cetrorelix. Two ionic bridges are formed between TM1-TM2 (Arg38-Asp98) and TM2-TM3 (Glu90-Lys121). The H-bond network that is formed in TM1-TM3-TM6-TM7 (Asn53-Asn87-Asp319-Asp315-Trp280) additionally stabilizes the receptor structure. Antagonist interacts with Arg38-Asp98 salt bridge, but not with Lys121. E, model of the active conformation of the receptor complexed with the natural decapeptide agonist GnRH. The ionic bridge Glu90-Lys121 is broken, and Lys121 together with Asp98, interact with His2 of GnRH. Arg38 forms H-bond with Gly10-NH2 of the ligand. Rearranged residues (Asn53-Ser49 and Asn87-Glu90-Asn315-Glu319-Ser275) form H-bond network in TM1-TM2-TM3-TM6-TM7. Tyr284 rotates and forms H-bond with Ser217. F, model of the E90K mutant of human GnRH receptor in complex with Asp2-GnRH decapeptide, which acts as an agonist. Salt bridge Glu90-Lys121 is broken, and Lys121 interacts with Asp2 of the ligand and also forms an ionic pair with Asp98. H-bond network is similar to one in the active conformation of the wild-type receptor. Lys90 is involved not only in H-bonding with Asn315 but also in the ionic attraction with Asp319 and repulsion with Lys121. These ionic and H-bonding interactions of Lys90 probably stabilize the active conformation, which leads to CA. Interaction of decapeptide ligands (colored purple) in all three models are in good agreement with the experimental data.
During the refinement procedure, we tested several conformations of the large EL2 to understand the molecular mechanisms underlying the observed importance of Lys191, Cys14-Cys200 disulfide bridge, and noncontiguous four-residue motif for receptor trafficking and activation. We found that, in the WT receptor, the presence of charged Lys191 in the middle of EL2 does not permit the insertion of the loop between helices, because burial of the highly polar Lys191 side chains is energetically unfavorable and creates steric clashes with the N terminus (Fig. 1B). On the other hand, deletion of the Lys191 residue from the human receptor allows a 7-Å inward shift of the middle part of EL2 (at Gln195) toward the ligand binding pocket (Fig. 1C). Such movement requires only minor change in EL2 conformation, such as reorientation of Cys200 side chain and a small backbone readjustment at the TM4 and TM5 ends. Furthermore, this model shows that four residues from a noncontiguous motif interact with the EL2: Phe112 is located near the Cys114-Cys196 disulfide bridge that links EL2 to TM3; Glu208 and Glu302 may form H-bonds with EL2 residues (His199 and Gln195, respectively); and L300V substitution facilitates the reorientation of Tyr290 interacting with EL2. Therefore, rat-like substitution of these four residues would also favor the inward shift of EL2.
Based on these observations, we hypothesized that the major difference between human and rat receptor may be in the position of EL2. We suggest, that in the WT human receptor, EL2 is loosely packed because of the presence of Lys191 unlocking the ligand binding cavity. In this case, the presence of both Cys14-Cys200 and Cys114-Cys196 disulfide bridges would be critical to maintain the correct receptor structure able to pass QCS and function at the plasma membrane. In contrast, EL2 in rat (and mouse) receptor lacking Lys191 is likely inserted inside the binding pocket, forming multiple interactions with the seven-α-bundle, including those with the four-residue motif. In this case, the Cys114-Cys199 disulfide bridge would be less important, as it would only reinforce the receptor structure already stabilized by the loop insertion. The wide opening of the binding cavity in the human receptor would also facilitate binding of different ligands, including pharmacoperones. The structure of human GnRH receptor may also be stabilized by the H-bonding network formed between residues from TM1 (Asn53), TM2 (Asn87), and TM7 (Asp319), which are conserved in rhodopsin-like GPCRs. The structural and functional importance of these residues for trafficking, activation, and coupling pathways of GnRH receptors has been established (Flanagan et al., 1999). In the human GnRH receptor model, this network may be supplemented by Ser49, Ser275, Trp280, Asn315, Glu90, and Lys121.
Comparison of models of human GnRH receptor and its E90K mutant in inactive and active conformations with different peptide ligands (Fig. 1, D–F) suggests that this hydrogen bond network may rearrange upon ligand binding and receptor structural transitions. In particular, only in the inactive receptor conformation can both ionic pairs, Arg38-Asp98 and Glu90-Lys121, exist and hydrogen bonds between residues Asn53, Asn87, Asn315, Asp319, and Trp280 link all four helices, TM1, TM2, TM6, and TM7 (Fig. 1D). The Glu90-Lys121 pair connecting TM2 and TM3 may also participate in this network via tightly bound water molecules, which have been observed in GPCR crystal structures (Angel et al., 2009). However, during agonist-induced receptor activation, the TM2-TM3 ionic bridge between Glu90-Lys121 becomes broken as a result of rotation of Lys121 toward His2 of the GnRH and formation of an alternative TM2-TM3 ionic bridge between Lys121 and Asp98 (Fig. 1, E and F). Asp98 also may form contact with His2 of the GnRH while weakening its interactions with Arg38 from TM1. The extended H-bond network seems to be broken in the active receptor, but tight connections may be formed between TM7 and TM2 via H-bonding of residues Glu90, Asn87, Asn315, Asp319, and Ser275, which bring TM2 and TM7 closer. Further water-mediated H-bonding network may be formed between conserved residues at the intracellular ends of TM3 (Arg139 from DRY motif), TM5 (Asn231), and TM7 (Tyr323 from the NPxxY motif) (data not shown).
The model of the E90K mutant in complex with Asp2-GnRH (Fig. 1F) represents an active receptor conformation with broken interactions between positively charged Lys121 and Lys90, and an alternative bridge formed between Lys121 and Asp98. In this conformation, Lys121 effectively interacts with Asp2 of the ligand, which behaves as an agonist in the E90K mutant, while Lys90 may form an H-bond with Asn315 and an ionic interaction with negatively charged Asp319. Such interactions would hold TM2 and TM7 together in the conformation appropriate for the activated receptor.
Breaking the Glu90-Lys121 Salt Bridge Leads to Constitutive Activity and Interference with Trafficking.
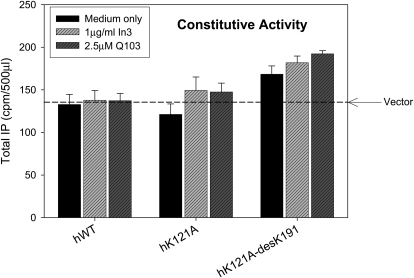
Breaking the Glu90-Lys121 bridge in the E90K mutant results in a misrouted protein retained in the ER. The poor trafficking to the plasma membrane can be improved by pretreatment with pharmacoperones or by deletion of Lys191 (Janovick et al., 2002). When rescued by either of these means, this mutant showed CA, i.e., the increase of agonist-independent IP production (Janovick and Conn, 2010). Accordingly, we examined mutations at the Lys121 end of this salt bridge to determine whether that perturbation also resulted in CA. Mutation of Lys121 to Ala did not produce significant CA either alone or following rescue by pharmacoperones In3 or Q103 (Fig. 2). The additional deletion of Lys191 resulted in modest CA of human K121A-desLys191, particularly when it was combined with pharmacoperone rescue of the double mutant. In this group, agonist-independent IP production increased by 17 to 42% above basal. Mutants K121R or K121R-desLys191 did not show CA with or without rescue with either pharmacoperone (data not shown).
Fig. 2.
Assessment of constitutive activity shown by IP production by mutants of Lys121. COS-7 cells were transfected with 100 ng of WT or mutant cDNA as described under Materials and Methods. Mutants were incubated in media alone or rescued with pharmacoperone (In3, Q103); pharmacoperones In3 and Q103 were washed out, and IP production was measured in response to media alone (no agonist). The dashed horizontal line shows basal level of vector only. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
We previously examined the IP production by activation of Lys121 mutants with the GnRH receptor agonist buserelin (Janovick et al., 2009). Among those single mutants at residue 121 (Ala, Asp, Glu, Gly, Asn, Gln, or Arg), only the conservative substitution, K121R, led to a response to buserelin that was comparable to that of the WT hGnRHR (without pharmacoperone rescue). There was a slight responsiveness of K121A and K121Q and a more modest response of K121G and K121N to buserelin when these mutants were first rescued by pharmacoperone In3. There was virtually no response when Lys121 was converted to the negatively charged Asp or Glu. Further deletion of Lys191 in these mutants resulted in responsiveness from K121R, a more modest response from K121Q > Ala > Asn > Gly and no response from the negatively charged Asp or Glu.
Comparing the results for agonist-stimulated receptor activation and constitutive activation suggests that mutants in position 121, which, when rescued, result in CA, are not necessarily those that show the best response to agonist. Part of this uncoupling may reflect the observation that Lys121 is a potential binding site for agonists of GnRH.
These data are consistent with our modeling of K121A and K121R mutants, which suggests that modest CA observed in the K121A, but not in the K121R mutant, may be related to breaking of the ionic bridge in the K121A mutant. However, the lower CA of K121A in comparison with the E90K mutant can be explained by the lack of additional stabilization of the TM2-TM7 proximity, which in the E90K mutant may be reinforced by Lys90-Asp319 ionic interactions. On the other hand, Arg121, but not Ala121, would interact with the ligand similar to Lys121. This may explain the better response to buserelin of the K121R mutant relative to the K121A mutant.
Breaking the Arg38-Asp98 Salt Bridge Interferes with Trafficking but Does Not Produce Constitutive Activity.
Similar to the Glu90-Lys121 salt bridge, a bridge linking Arg38-Asp98 is predicted by the model. The residues at positions 38 and 98 are highly conserved among mammals and in other orders. In some flies, there is a conservative replacement to the Lys38-Glu98 pair, whereas in some other flies, Arg38 is replaced by Ser38 and the ion pair is broken. In these species, however, a new ion pair appears (Glu98-Lys306) together with Tyr42, which may preserve the structural relations. We have previously shown (Janovick et al., 2009) that Asp98 mutants interfere with both trafficking and responsiveness of hGnRHR to agonist; it is believed to be a site of interaction with the agonist.
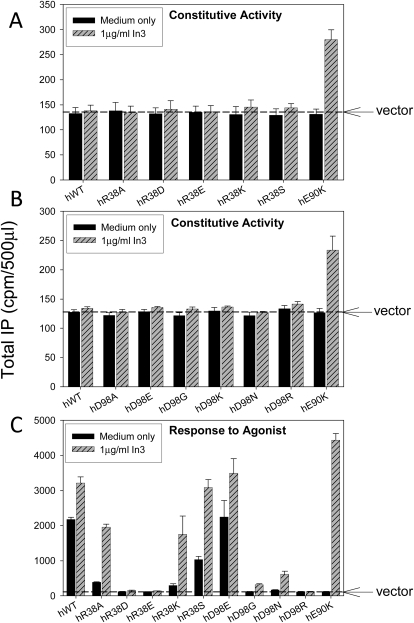
We prepared mutants at both ends (i.e., R38A, Asp, Glu, Lys, and Ser and D98A, Glu, Gly, Lys, Asn, and Arg) of the presumptive Arg38-Asp98 bridge. Unlike mutants of the Glu90-Lys121 salt bridge, the Arg38 and Asp98 mutants do not show measurable CA following pharmacoperone rescue (Fig. 3A and B). R38A and the conservative replacement R38K lose about 75% of their IP response to buserelin stimulation, and R38D and R38E are totally inactive mutants. R38S retains approximately half of its responsiveness (Fig. 3C). The conservative replacement of the negatively charged Asp98 by Glu is unremarkable, although Gly, Asn, and Arg substitutions result in mutants that are inactive and only modestly rescuable by In3, probably because of the interaction of this residue with the agonist.
Fig. 3.
Assessment of constitutive activity shown by IP production of mutants of Arg38 (A) and Asp98 (B). COS-7 cells were transfected with 100 ng of WT or mutant cDNA as described under Materials and Methods. Mutants were incubated in media alone or rescued with pharmacoperone In3 (A and B). In3 was then washed out, and IP production was measured in response to media alone (no agonist added). C, IP production in response to agonist (10−7 M buserelin, a saturating dose) with or without In3 rescue. COS-7 cells were transfected with 20 ng of WT or mutant cDNA plus 80 ng of empty vector. The dashed horizontal line shows the basal level of vector only. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
Restoration of responsiveness of R38A and R38K by In3 suggests that they are misrouted (probably ER-retained mutants) and rescuable, whereas R38D and R38E may be permanently retained or unable to bind agonist or achieve the active configuration needed to interact with G proteins. The ability of pharmacoperones to rescue a substantial amount of the responsiveness of R38A, Lys, and Ser, but not the mutants with charge changes, is reminiscent of the pattern seen with mutants that do not pass the cellular QCS (Conn et al., 2006b).
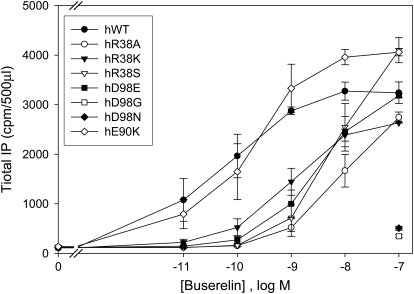
To better understand the functional role of charged residues Arg38 and Asp98, we assessed the dose-response curves in the R38S, Ala, and Lys and D98E, Gly, and Asn mutants (Fig. 4) with buserelin. It seems that relative affinity to buserelin is significantly (>50-fold) decreased for all of these mutants. This contrasts with the unchanged affinity of the E90K mutant to buserelin. These results indicate that, unlike Glu90, both Arg38 and Asp98 are involved in key protein-ligand interactions. Therefore, their substitutions not only destabilize the receptor but also impair agonist binding and/or activation properties of the receptor. This is consistent with our receptor models (Fig. 1, D–E) that support the formation of H-bonding interactions between Asp98 and His2 and between Arg38 and C-terminal amide of buserelin.
Fig. 4.
Dose-response curves of IP production by WT and mutant GnRH receptors in response to buserelin. IP production was assessed in response to the indicated doses of buserelin. COS-7 cells were transiently transfected with 20 ng of WT or mutant cDNA plus 80 ng of empty vector as described under Materials and Methods. WT and mutants were incubated with In3 and then washed out before agonist stimulation with buserelin for the measurement of IP production as described under Materials and Methods. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
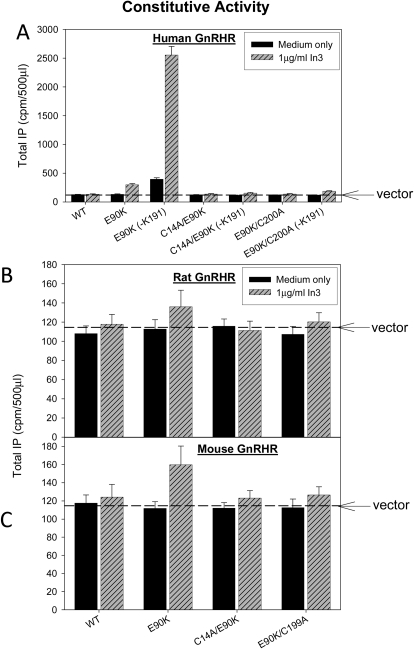
Comparisons among Species: Pharmacoperone In3 Rescues Constitutive Activity in Mutant E90K.
Human, rat, or mouse WT GnRHR did not show CA even after pharmacoperone rescue (Fig. 5). In contrast, the E90K mutants of human and mouse GnRHRs, but not rat GnRHR, show measurable CA after In3 rescue (Fig. 5). We have attributed the appearance of CA to alteration of the relation between TM2 and TM3 in a fashion that may be similar to the events that occur after the binding of the natural ligand (Janovick and Conn, 2010). CA is the most prominent for the human mutant and modest for the rat and mouse mutants. This difference in the ability to rescue is not likely to be attributed to the superior recognition of In3 by the human sequence, because this molecule retains good affinity (1.7 nM) to the rat GnRHR (Chu et al., 2001).
Fig. 5.
Constitutive activity of WT or E90K mutants of human (A), rat (B), and mouse (C) GnRHR. COS-7 cells were transiently transfected with 100 ng of cDNA as described under Materials and Methods. Mutants were incubated in media alone or after rescue with pharmacoperone In3 as described under Materials and Methods; In3 was then washed out, and IP production was measured in response to media alone (no agonist added). The dashed horizontal line shows basal level of vector only. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
These mutagenesis results indicate that differences in CA between species may be related to the different conformational flexibility of receptors. The computational modeling suggests that, in rodent receptors, EL2 may be deeply inserted between helices, whereas in the human receptor, this loop is more polar and probably is shifted from the protein milieu to the water environment. The increased stability of rat and mouse receptor attributed to EL2 interactions with helices may prevent manifestation of receptor activation in the absence of the agonist.
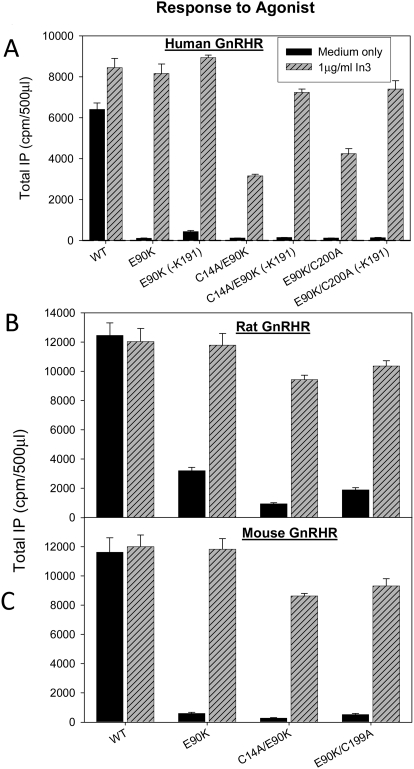
Requirement of the Cys14-Cys200 Disulfide Bridge for Constitutive and Agonist-Induced Receptor Activation.
Different levels of CA among the three species examined led us to consider those physical differences known to exist between them as a possible explanation for this difference. Rat WT GnRHR does not require the formation of Cys14-Cys200 for trafficking as does the human GnRHR (Janovick et al., 2006). The mouse is intermediate in the requirement for this bridge. These data indicate the apparent structural flexibility of a human receptor, which demands an additional stabilization by the second disulfide.
To investigate the connection between the receptor ability to undergo conformational transitions and structural constraints in EL2, we measured responsiveness to agonist of WT and E90K mutants of human (Fig. 6A), rat (Fig. 6B), and mouse (Fig. 6C) sequences after rescue by pharmacoperone In3 and/or deletion of residue Lys191. This was compared with mutants also containing C14A and C200A modifications that preclude formation of the Cys14-Cys200 bridge.
Fig. 6.
Assessment of constitutive activity of WT and mutants (with a broken Cys14-Cys199 (rodent),200 (human) bridge) by IP production. The bridge was broken by inserting Ala in place of Cys14 or Cys200 in human (A), rat (B), or mouse (C) GnRHR mutants. COS-7 cells were transiently transfected with 20 ng of cDNA (with 80 ng of empty vector) as described under Materials and Methods. Mutants were incubated in media alone or rescued with pharmacoperone (In3) as described under Materials and Methods; In3 was then washed out, and IP production was measured in response to media alone (no agonist added). In some cases, residue Lys191 was deleted because this modification is known to increase trafficking of WT and mutant hGnRHRs to the plasma membrane. In all figures, Means ± S.E.M.s are shown for least three independent experiments, each performed in replicates of four.
The human, mouse, and rat E90K sequences show measurable CA after rescue by In3. CA is substantially inhibited by the introduction of mutations C14A or C200A, even though the disulfide bridge is not a requisite for trafficking to the plasma membrane in the rat sequence (Fig. 5) (Janovick et al., 2006). These observations suggest that the Cys bridge is required for CA in all species of E90K mutants examined. Lys191 deletion provides measurable improvement in CA of In3-rescued E90K human receptor mutants. This improvement is highly pronounced for the E90K-ΔLys191 mutant having both disulfide bridges (up to 10-fold increase in IP production; Fig, 5A), and it is noticeable even for receptors with a broken Cys14-Cys200 bridge. The effect of Lys191 deletion is possibly attributed to improved receptor trafficking.
The elimination of the Cys14-Cys200 disulfide bridge similarly affects agonist-induced activation of E90K mutants of human and rodent receptors (Fig. 6). This effect is larger for human and mouse receptors but also significant (up to 4-fold decrease of IP production) for the rat receptor of which trafficking is less dependent of this disulfide (Knollman et al., 2005). All double mutants cannot be fully rescued by the In3 pretreatment. Deletion of Lys191 in human receptor in combination with In3 additionally improves IP production but does not reproduce the function of the WT receptor. A similar experiment cannot be done for the mouse or rat sequence because this residue is not normally present in the natural sequence. These results indicate that the presence of the Cys14-Cys200 disulfide supports ligand-induced activation of receptors from different species.
The receptor model suggests that receptor activation may involve movements of TM2, TM3, TM6, and TM7 together with EL2, which opens the ligand binding pocket for agonist. The Cys14-Cys200 disulfide bridge that restrains the position of EL2 relative to surrounding helices may be critical to provide proper conformational changes associated with constitutive or agonist-induced receptor activation.
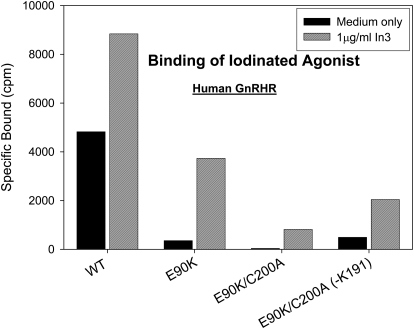
In other experiments, we assessed the impact of breaking the disulfide bridge in the human WT and E90K mutants on the receptor trafficking monitored by the specific binding of radioligand (Fig. 7). These data show that mutant E90K is not present at the plasma membrane, consistent with the previous observation that this mutant is retained in the ER by the cellular quality control system (Brothers et al., 2004). The amount of functional receptor in the plasma membrane increased after pretreatment by In3. E90K mutants that cannot form the Cys14-Cys200 bridge were further impaired in trafficking. This effect was partially reversed by the deletion of Lys191. These data demonstrate that, while considering the role of the Cys14-Cys200 bridge in receptor activation, it is important to consider its effect on receptor trafficking. Thus, the small effect of Lys191 deletion on the constitutive and agonist-induced activity of the E90K mutant may be fully attributed to the improved trafficking of this receptor.
Fig. 7.
Radioligand binding of WT hGnRHR and selected mutants with E90K and broken Cys14-Cys200 bridges. In some cases, Lys191 was also deleted. Cells were transfected with 25 ng of WT or mutant (each with or without Lys191) cDNA (with 75 ng of empty vector) and rescued with or without pharmacoperone In3, as described under Materials and Methods for binding studies. The In3 was then washed out, and specific binding was determined by using 2 × 106 cpm/ml 125I-buserelin for 90 min at room temperature. The tracer was removed, cells were washed twice, and radioactivity was measured. Means ± S.E.M.s for total binding averaged 7%.
Impact of EL2 Interactions on Ligand Binding and Constitutive Activation of E90K Mutant.
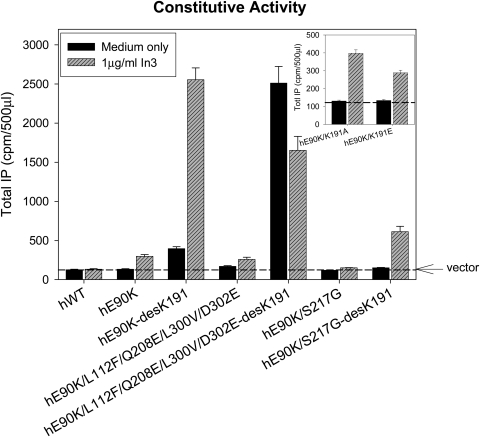
We also examined the impact of replacing the noncontiguous four-residue motif that allows Lys191 to inhibit trafficking (Janovick et al., 2006). When the human motif in hGnRHR[E90K] was replaced with the orthologous rat residues (i.e., L112F, Q208E, L300V, and D302E), this decreased the measured CA but modestly increased the IP production after rescue. The same was true when Lys191 was deleted from this sequence (Fig. 8). It is presumed that this is the result of increased trafficking of these mutants to the plasma membrane. It is noteworthy that the human E90K mutant with rat-like substitutions, including the deletion of Lys191, which demonstrates the biggest CA, has decreased CA after pretreatment by In3. This effect may be attributed to the incomplete removal of the drug, which may inhibit CA of the receptor. Another interpretation may be related to the possibility that folding and stability of the human receptor with rat-like substitutions are more effective in the absence of drug, which may compete with the EL2 for the space in the ligand binding pocket.
Fig. 8.
Assessment of constitutive activity (IP production) by the E90K mutation in the presence of combinations of the rat-motif in the human sequence (L112F, Q208E, L300V, and D302E), S217G, and deletion of residue Lys191. Mutants K191A and K191E are shown in the inset. COS-7 cells were transiently transfected with 100 ng of cDNA as described under Materials and Methods. IP production was measured in response to media alone (no agonist added). The dashed horizontal line shows basal level of vector only. In all figures, Means ± S.E.M.s are shown for least three independent experiments, each performed in replicates of four.
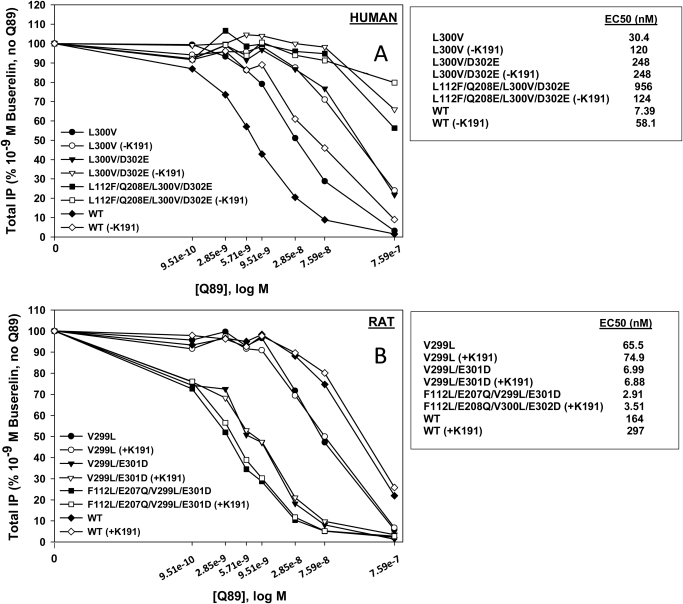
To further explore the structural role of EL2 in human and rat receptors, we assessed the ability of pharmacoperone Q89 to inhibit interactions with agonist of rat and human receptor mutants in which Lys191 was deleted in hGnRHR together with other rat-like replacements in the noncontiguous motif and vice versa. We observed that Q89 binds human receptor with >100-fold higher binding affinity than rat receptor (Fig. 9). We also found that the binding of Q89 to the human receptor (Fig. 9A) was inhibited by Lys191 deletion. Consecutive substitution of the human receptor motif by four rat residues decreased the binding affinity to Q89, resulting in the low rat-like affinity. In contrast, rat GnRHR (Fig. 9B) after substitution with human residues at positions 112, 207 (corresponding to 208 in the human sequence), 299 (300 in the human sequence), and 301 (302 in the human sequence) showed significantly improved Q89 binding, reaching a binding affinity near that for the human sequence. However, the addition of Lys191 in the rat sequence did not demonstrate any additional effect.
Fig. 9.
IP production by WT GnRHR and various mutants expressed in COS-7 cells in response to 10−9 M agonist (buserelin) and in the presence of Q89. A, human: COS-7 cells were transiently transfected with 10 ng of human WT or human mutant cDNA plus 90 ng of empty vector, and IP production was determined as described under Materials and Methods. B, rat: COS-7 cells were transiently transfected with 5 ng of rat WT or rat mutant cDNA plus 95 ng of empty vector, and IP production was determined as described under Materials and Methods. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
These data are consistent with our suggestion regarding the possible obstruction of the ligand binding pocket of rat receptor by EL2 deeply inserted between helices. Blocking the access to the binding pocket would prevent binding of pharmacoperone Q89. On the other hand, steric interference from Lys191 in the human receptor would prevent the insertion of EL2 in the ligand binding pocket. The high accessibility of the binding pocket probably explains the high binding affinity of hGnRHR for Q89. The lack of effect of Lys191 insertion in the rat receptor on the Q89 binding may be attributed to smaller steric hindrances of this residue with the surrounding receptor helices and loops.
To understand the nature of interactions of Lys191 that impair human receptor trafficking, we substituted the positively charged Lys191 to the hydrophobic Met191. This mutation does not change the IP coupling of the receptor (Fig. 10B). Earlier we found that the replacement of Lys191 by uncharged Ala191 or negatively charged Glu191 slightly improved receptor trafficking, but to a much lesser extent than the deletion of Lys191 (Fig. 8, inset) whether In3 was present or not. Thus, our present and previous (Janovick et al., 2006) results suggest that the effect of Lys191 may be steric rather than charge-related. Indeed, the modeling shows that Lys191 may collide with the N terminus upon insertion of the loop inside the receptor α-bundle.
Fig. 10.
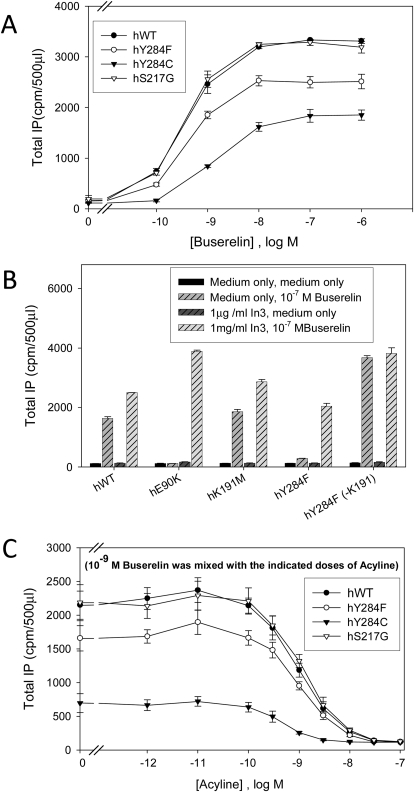
IP production for WT hGnRHR and various mutants in response to agonist after rescue with pharmacoperone In3. COS-7 cells were transiently transfected with 20 ng of WT or mutant cDNA plus 80 ng of empty vector as described under Materials and Methods. A, mutants were rescued with pharmacoperone (In3) as described under Materials and Methods; In3 was then washed out, and IP production was measured in response to the indicated dose of buserelin. B, WT GnRHR or mutants were incubated in media alone or rescued with pharmacoperone In3 as described under Materials and Methods; In3 was then washed out, and IP production was measured in response to medium alone or agonist (10−7 M buserelin). C, mutants were rescued with pharmacoperone (In3) as described under Materials and Methods; In3 was then washed out, and IP production was measured in response to antagonist (acyline) dose-response curve containing agonist (10−9 M buserelin). In all figures, Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
Role of Ser217 and Tyr284 in Ligand Binding and Receptor Activation.
We have previously shown a role for Ser217 in trafficking of the GnRHR (Knollman et al., 2005). In Fig. 8, we show that the S217G mutation reduces CA in the human E90K mutant, suggesting a role for this residue in the active state of the receptor. This effect cannot be fully reversed either by In3 or by the Lys191 deletion (Fig. 8), suggesting that the loss of the ability to produce CA is not likely due to ER retention. In our model of the active conformation (Fig. 1, E and F), Ser217 forms an H-bond with Tyr284, thus stabilizing this active state. The absence of this H-bond may explain the reduced CA of the S217G mutant.
To further explore this possibility and the role of the Ser217-Tyr284 H-bond, we prepared Y284F and Y284C mutants that cannot form this H-bond. The Y284C mutant also lacks the aromatic moiety of the native residue. Agonist buserelin-stimulated activation of both mutants is significantly reduced (Fig. 10A). This effect is more pronounced for the Y284C mutant. Both Y284F (Fig. 10B) and Y284C mutants, as well as the S217G mutant, can be rescued by the pharmacoperone In3 and/or deletion of Lys191 (Fig. 10, A and B) (Knollman et al., 2005). After pretreatment with In3, the S217G mutant demonstrates buserelin-stimulated activation similar to the WT receptor, whereas Y284F and Y284C show 70 and 23% activity, respectively. These results indicate that the effect of the reduced receptor activation by agonist is partially attributed to the impaired trafficking of the mutants.
The larger impairment of the Y284C suggests the importance of both the OH-group and the aromatic ring of Tyr284 for proper receptor trafficking and activation. However, the difference between both Tyr284 mutants may also be related to different binding affinities of these mutants to drugs and peptide ligands. To examine this possibility, we analyzed dose-response curves of these mutants with buserelin, as well as the inhibition of buserelin-induced activity by the high-affinity peptide antagonist acyline (Fig. 10C). These experiments show that the binding affinities of all mutants to peptide agonist buserelin and antagonist acyline were similar to the corresponding affinities of the WT receptor. Considering these results together with the reduced CA of the E90K/S217G double mutant, we conclude that a) the aromatic ring and OH-group of the Tyr284 are essential for receptor activation; and b) the formation of the H-bond between Ser217 and Tyr284 or other polar groups is probably involved in the activation process.
Discussion
Transmembrane proteins expressed in ER, such as GPCRs, are subject to a QCS that assesses receptor structure, retaining some mutants in the ER and promoting the exit from the ER of others (Achour et al., 2008). Correctly folded GPCRs pass the QCS and traffic via the Golgi complex to the PM or other cellular locus where they function appropriately. The QCS is not protein-specific; it recognizes general aspects of misfolding (e.g., exposure of hydrophobic plates in aqueous environments or unformed Cys bridges), often with relatively low affinity. Accordingly, GPCRs or their mutants that are potentially functional but not completely folded may be retained and destined for degradation. As shown in the present report, the requirements for passage through the QCS and for receptor activation may differ. Pharmacoperones are target-specific, low molecular weight structures that enter cells and bind to otherwise misfolded proteins, correct their folding (Janovick et al., 2007), and promote passage through the QCS and arrival at the correct functional location. The functional rescue of several misfolded mutant proteins by small nonpeptide molecules has been demonstrated (Conn et al., 2007).
In the present study, structural models for the hGnRHR in active and inactive conformations were developed and tested. These models feature two salt bridges (Glu90-Lys121 and Arg38-Asp98) and two disulfide bridges (Cys114-Cys196 and Cys14-Cys200). The disulfide bridges are usually critical for stabilization of the native structure of different GPCRs, including hGnRHR, and are assessed by the cellular QCS. The salt bridges are, likewise, the subject of scrutiny by the QCS and are of particular interest because the natural ligand for this receptor articulates with residues in each of the ion pairs, specifically Arg38, Asp98, and Lys121 (Zhou et al., 1995; Flanagan et al., 2000). Accordingly, the binding of GnRH will disrupt both ionic interactions simultaneously, thus presenting the possibility of a coincidence detector. Coincidence mechanisms encode information by detecting the occurrence of simultaneous yet distinct input signals. Detection by coincidence is well known to reduce spurious signals in biological systems and could explain, in the present context, why antagonists do not produce a response. Failure to activate this detector may explain the function of antagonists, which may not interact with Lys121 (Zhou et al., 1995).
This is consistent with the observation that mutants that totally break the Glu90-Lys121 bridge (i.e., E90K) show CA (Janovick and Conn, 2010). In contrast, mutations that break the Arg38-Asp98 bridge do not cause CA but weaken receptor stability (poor trafficking) and impair ligand binding, given that both residues form key contacts with natural ligands. The level of CA for the E90K mutant is modest: the receptor does not produce the same robust response that is observed with agonist occupancy, suggesting that the structure of the receptor is nonidentical with the change in the receptor that occurs when an agonist binds.
Our modeling suggests that CA is probably associated with relative movement of helices accompanied by the rearrangement of the hydrogen bonding network between TMs 1, 2, 3, 6, and 7 and the formation of a tight connection between TM2 and TM7 that may be stabilized by ionic interactions between E90K and Asp319. With the deletion of Lys191 and L112F, Q208E, L300V, and D302E substitutions, the CA of the E90K mutant substantially increases. In this case, the activation process may additionally involve movement of EL2 relative to noncontiguous residues from surrounding helices and loops, as was suggested based on computational modeling of agonist-bound active conformation.
The importance of the region of the GnRHR molecule with these two salt bridges is emphasized by the observation that a ligand (Asp2-GnRH) that was not recognized by WT hGnRHR becomes an agonist for E90K once this molecule is rescued and trafficked to the plasma membrane (Janovick and Conn, 2010). The computational models suggest that, in the active conformation of the E90K mutant, the side chain of Lys90 is rotated toward Asp98, forming a new Lys90-Asp98 ionic bridge. This rotation of the Lys90 favors its electrostatic interactions with Asp2 of the ligand, thus promoting its binding and subsequent induction of the full receptor activation. In the WT receptor, the binding of the Asp2-GnRH may be impeded because of the electrostatic repulsion between Asp98 of the receptor and Asp2 of the ligand.
These studies also suggest that the Cys14-Cys200 disulfide bridge is a requirement for CA and for agonist-induced activation in the three species examined (rat, mouse, and human). E90K mutants of human receptor with a broken bridge cannot be fully rescued by the deletion of Lys191 or by pharmacoperone In3, methods by which trafficking to the membrane of human WT or mutant E90K is enhanced. Furthermore, the CA and agonist-induced activation is also reduced in rat E90K mutants with a broken Cys14-Cys200 bridge, even though this bridge is not a requirement for trafficking of the rat receptor (Janovick et al., 2006). These observations suggest that the activation of GnRHR is more effective when the disulfide bridge is intact. The restraints imposed by this disulfide may prevent loop unfolding when EL2 moves out of the α-helix bundle during receptor activation.
An additional approach relying on the binding of pharmacoperone Q89 to human and rat receptor and their mutants supports the hypothesis that EL2 is inserted more deeply between helices in rat receptor than in human receptor. Such insertion of EL2 into the ligand binding pocket in rat receptor (or human receptor mutants containing rat substitutions) may obscure the binding pocket, which would explain the observed decrease of Q89 binding affinity in both cases. The looser packing of EL2 on the surface of the wild-type human receptor may be the main cause of its structural instability, which is manifested by the increased level of misfolded and misrouted proteins and more pronounced CA, compared with rat receptors.
Finally, we obtained experimental evidence that shows the importance of the aromatic moiety and OH-group of Tyr284 in receptor trafficking and activation, which is not related to the effect on ligand binding. The proposed hydrogen bonding interactions between Tyr284 and Ser217 that may be formed in the active receptor conformation is important for efficient CA or ligand-induced activation (Figs. 8 and 10), although it is not absolutely required. The current data do not rule out the involvement of Ser217 in proper receptor trafficking, possibly by participation in receptor oligomerization, as was suggested previously (Knollman et al., 2005).
Acknowledgments
We thank Jo Ann Binkerd for formatting the manuscript.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK85040] (to P.M.C.); the National Institutes of Health National Center for Research Resources [Grants RR030229, RR000163] (to P.M.C.); the National Institutes of Health National Institute on Drug Abuse [Grant DA003910] (to H.I.M.); and by the National Science Foundation [Grant 0849713] (Division of Biological Infrastructure) (to I.D.P.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180869.
- GnRH
- gonadotropin-releasing hormone
- GnRHR
- gonadotropin-releasing hormone receptor
- hGnRHR
- human GnRHR
- QCS
- quality control system
- ER
- endoplasmic reticulum
- GPCR
- G protein-coupled receptors
- PM
- plasma membrane
- TM
- transmembrane segment
- CA
- constitutive activity
- WT
- wild type
- EL
- extracellular loop
- DMEM
- Dulbecco's modified Eagle's medium
- BSA
- bovine serum albumin
- IP
- inositol phosphate.
Authorship Contributions
Participated in research design: Janovick, Pogozheva, Mosberg, and Conn.
Conducted experiments: Janovick, Pogozheva, Mosberg, and Conn.
Contributed new reagents or analytic tools: Janovick, Pogozheva, Mosberg, and Conn.
Performed data analysis: Janovick, Pogozheva, Mosberg, and Conn.
Wrote or contributed to the writing of the manuscript: Janovick, Pogozheva, Mosberg, and Conn.
Acquired funding for the research: Mosberg and Conn.
References
- Achour L, Labbé-Jullié C, Scott MG, Marullo S. (2008) An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci 29:528–535 [DOI] [PubMed] [Google Scholar]
- Angel TE, Chance MR, Palczewski K. (2009) Conserved waters mediate structural and functional activation of family A (rhodopsin-like) G protein-coupled receptors. Proc Natl Acad Sci USA 106:8555–8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM. (2004) Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18:1787–1797 [DOI] [PubMed] [Google Scholar]
- Brothers SP, Janovick JA, Conn PM. (2003) Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab 88:6107–6112 [DOI] [PubMed] [Google Scholar]
- Brothers SP, Janovick JA, Maya-Nunez G, Cornea A, Han XB, Conn PM. (2002) Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol 190:19–27 [DOI] [PubMed] [Google Scholar]
- Castro-Fernández C, Maya-Núñez G, Conn PM. (2005) Beyond the signal sequence: protein routing in health and disease. Endocr Rev 26:479–503 [DOI] [PubMed] [Google Scholar]
- Chai BX, Pogozheva ID, Lai YM, Li JY, Neubig RR, Mosberg HI, Gantz I. (2005) Receptor-antagonist interactions in the complexes of agouti and agouti-related protein with human melanocortin 1 and 4 receptors. Biochemistry 44:3418–3431 [DOI] [PubMed] [Google Scholar]
- Chu L, Hutchins JE, Weber AE, Lo JL, Yang YT, Cheng K, Smith RG, Fisher MH, Wyvratt MJ, Goulet MT. (2001) Initial structure-activity relationship of a novel class of nonpeptidyl GnRH receptor antagonists: 2-arylindoles. Bioorg Med Chem Lett 11:509–513 [DOI] [PubMed] [Google Scholar]
- Coetsee M, Millar RP, Flanagan CA, Lu ZL. (2008) Identification of Tyr(290(6.58)) of the human gonadotropin-releasing hormone (GnRH) receptor as a contact residue for both GnRH I and GnRH II: importance for high-affinity binding and receptor activation. Biochemistry 47:10305–10313 [DOI] [PubMed] [Google Scholar]
- Conn PM, Janovick JA. (2009a) Drug development and the cellular quality control system. Trends Pharmacol Sci 30:228–233 [DOI] [PubMed] [Google Scholar]
- Conn PM, Janovick JA. (2009b) Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol 299:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PM, Janovick JA, Brothers SP, Knollman PE. (2006a) ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol 190:13–16 [DOI] [PubMed] [Google Scholar]
- Conn PM, Knollman PE, Brothers SP, Janovick JA. (2006b) Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol 20:3035–3041 [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. (2007) G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 59:225–250 [DOI] [PubMed] [Google Scholar]
- Cook JV, Eidne KA. (1997) An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology 138:2800–2806 [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Rodic V, Konvicka K, Yuen T, Chi L, Rivier JE, Millar RP, Weinstein H, Sealfon SC. (2000) Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry 39:8133–8141 [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Zhou W, Chi L, Yuen T, Rodic V, Robertson D, Johnson M, Holland P, Millar RP, Weinstein H, et al. (1999) The functional microdomain in transmembrane helices 2 and 7 regulates expression, activation, and coupling pathways of the gonadotropin-releasing hormone receptor. J Biol Chem 274:28880–28886 [DOI] [PubMed] [Google Scholar]
- Güntert P, Wüthrich K. (1991) Improved efficiency of protein structure calculations from NMR data using the program DIANA with redundant dihedral angle constraints. J Biomol NMR 1:447–456 [DOI] [PubMed] [Google Scholar]
- Hoffmann SH, ter Laak T, Kühne R, Reiländer H, Beckers T. (2000) Residues within transmembrane helices 2 and 5 of the human gonadotropin-releasing hormone receptor contribute to agonist and antagonist binding. Mol Endocrinol 14:1099–1115 [DOI] [PubMed] [Google Scholar]
- Hövelmann S, Hoffmann SH, Kühne R, ter Laak T, Reiländer H, Beckers T. (2002) Impact of aromatic residues within transmembrane helix 6 of the human gonadotropin-releasing hormone receptor upon agonist and antagonist binding. Biochemistry 41:1129–1136 [DOI] [PubMed] [Google Scholar]
- Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, Sauer DR, Haviv F, Greer J, Conn PM. (2007) Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol 272:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Conn PM. (2010) Salt bridge integrates GPCR activation with protein trafficking. Proc Natl Acad Sci USA 107:4454–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. (2003a) Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305:608–614 [DOI] [PubMed] [Google Scholar]
- Janovick JA, Knollman PE, Brothers SP, Ayala-Yáñez R, Aziz AS, Conn PM. (2006) Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem 281:8417–8425 [DOI] [PubMed] [Google Scholar]
- Janovick JA, Maya-Nunez G, Conn PM. (2002) Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87:3255–3262 [DOI] [PubMed] [Google Scholar]
- Janovick JA, Patny A, Mosley R, Goulet MT, Altman MD, Rush TS, 3rd, Cornea A, Conn PM. (2009) Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol 23:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Ulloa-Aguirre A, Conn PM. (2003b) Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine 22:317–327 [DOI] [PubMed] [Google Scholar]
- Knollman PE, Janovick JA, Brothers SP, Conn PM. (2005) Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem 280:24506–24514 [DOI] [PubMed] [Google Scholar]
- Leaños-Miranda A, Janovick JA, Conn PM. (2002) Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:4825–4828 [DOI] [PubMed] [Google Scholar]
- Leaños-Miranda A, Janovick JA, Maya-Nunez G, Ulloa-Aguirre A, Conn PM. (2003a) Misrouted proteins as a novel disease etiology and therapeutic target: rescue of hypogonadotripic hypogonadism-causing and manufactured mutants as a proof of principle, in Molecular Endocrinology: Methods and Protocols (Kumar RA. ed), Humana Press, Totowa, NJ [Google Scholar]
- Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. (2003b) Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab 88:3360–3367 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. (1998) G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273:18677–18680 [DOI] [PubMed] [Google Scholar]
- Millar RP, Pawson AJ, Morgan K, Rissman EF, Lu ZL. (2008) Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front Neuroendocrinol 29:17–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI. (2005) Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry 44:11329–11341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier JE, Jiang G, Struthers RS, Koerber SC, Porter J, Cervini LA, Kirby DA, Craig AG, Rivier CL. (2000) Design of potent dicyclic (1–5/4–10) gonadotropin releasing hormone (GnRH) antagonists. J Med Chem 43:807–818 [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP. (1997) Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18:180–205 [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Sellar R, Wilson DJ, Millar RP, Lu ZL. (2008) Identification of a novel ligand binding residue Arg38(1.35) in the human gonadotropin-releasing hormone receptor. Mol Pharmacol 73:75–81 [DOI] [PubMed] [Google Scholar]
- Topiol S, Sabio M. (2009) X-ray structure breakthroughs in the GPCR transmembrane region. Biochem Pharmacol 78:11–20 [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. (2004) Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5:821–837 [DOI] [PubMed] [Google Scholar]
- Zhou W, Rodic V, Kitanovic S, Flanagan CA, Chi L, Weinstein H, Maayani S, Millar RP, Sealfon SC. (1995) A locus of the gonadotropin-releasing hormone receptor that differentiates agonist and antagonist binding sites. J Biol Chem 270:18853–18857 [DOI] [PubMed] [Google Scholar]