Abstract
The role of μ-opioid receptor (MOR) down-regulation in opioid tolerance remains controversial. In this study, we used a novel knock-in mouse to examine how changing the extent of MOR down-regulation alters the development of morphine tolerance. These mice express a mutant MOR, degrading MOR (DMOR), that differs from the wild-type (WT) MOR in two ways: 1) unlike the recycling WT MOR, the mutant DMOR is targeted for degradation after its internalization, thus facilitating down-regulation; and 2) unlike the WT MOR, DMOR is efficiently internalized in response to morphine activation. We found that both WT MOR and DMOR mice develop tolerance to morphine, but DMOR mice exhibit a more rapid onset of tolerance and show receptor down-regulation. WT MOR mice develop morphine tolerance more slowly but even once profoundly tolerant show no receptor down-regulation. Furthermore, WT mice show significantly more morphine dependence than DMOR mice after long-term treatment as indicated by withdrawal. Taken together these data indicate that tolerance mediated by receptor down-regulation manifests differently both at the behavioral and biochemical level than does the actual morphine tolerance that occurs in WT mice and that loss of receptor function is not a major contributor to morphine tolerance in WT MOR mice.
Introduction
Although extensively studied, the mechanisms underlying the development of analgesic tolerance to morphine remain unclear. Much of the remaining controversy stems from the debate over whether tolerance to MOR agonists is a consequence of direct changes to the receptor protein, such as receptor desensitization/down-regulation, or occurs through alterations and adaptations in signaling cascades or neural circuitry independent from the receptor protein.
There are data to support both hypotheses. Repeated or continuous exposure to morphine causes a decrease in the antinociceptive effect of drug in many different paradigms, a phenomenon termed “tolerance.” In parallel, responses of individual neurons to MOR agonists in several areas of the central nervous system (CNS) that are of instrumental importance for nociception (Connor et al., 1999; Hack et al., 2003; Zeng et al., 2006; Fyfe et al., 2010) have been shown to be decreased after long-term morphine treatment, a phenomenon described as “desensitization.” Thus, there has been a tendency to equate desensitization to the effects of the drug (tolerance) to desensitization/down-regulation of the receptor per se. However, cells and circuits can adapt in ways that make them less responsive to the same dose of drug, thus appearing “desensitized,” even in the absence of loss of receptor function (Madhavan et al., 2010). Hence, these studies alone do not differentiate between desensitization of receptors and desensitization to drug as the mechanism that produces tolerance. Furthermore, despite numerous studies, there is still no consensus on the effect of long-term morphine treatment on either MOR protein levels (Davis et al., 1975; Nishino et al., 1990; Goodman et al., 1996; Petruzzi et al., 1997; Ray et al., 2004; Sim-Selley et al., 2007) or on MOR coupling to G protein (Sim et al., 1996; Bohn et al., 2000; Kirschke et al., 2002). Indeed, even when loss of MOR function/number has been detected, it is not clear whether this loss contributed to morphine tolerance at the behavioral level.
In this study, we directly address how the loss of receptor function in response to morphine affects the development of tolerance. We accomplished this by generating a novel knock-in mouse that expresses a mutant MOR, degrading MOR (DMOR), which differs from the wild-type (WT) MOR in two distinct ways. First, unlike the recycling WT MOR, after internalization in response to ligands that drive endocytosis of WT MOR, the DMOR is degraded rather than recycled. Second, unlike WT MOR, DMOR is efficiently desensitized and internalized in response to morphine. We find that whereas tolerance to morphine occurs in both WT MOR and DMOR mice, the rate of onset of tolerance and the molecular mechanisms underlying tolerance differ in these two genotypes. In particular, we find that receptor down-regulation contributes to tolerance in DMOR but not WT MOR mice and that WT MOR mice develop a significantly greater degree of dependence on morphine than DMOR mice.
Materials and Methods
Materials.
[35S]guanosine 5′-(γ-thio)triphosphate ([35S]GTPγS) (250 mCi; 9.25 MBq) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), morphine sulfate, naloxone HCl, GDP, HEPES, dl-dithiothreitol, Tricine, magnesium chloride, EDTA, saponin, and M1 anti-FLAG mouse monoclonal antibody were purchased from Sigma-Aldrich (St. Louis, MO). [3H]DAMGO was purchased from PerkinElmer Life and Analytical Sciences. Calcium chloride, Tris base, sodium hydroxide, sodium chloride, and hydrochloric acid were purchased from Thermo Fisher Scientific (Waltham, MA). FuGENE 6 Transfection Reagent was purchased from Roche Diagnostics (Indianapolis, IN). Papain was purchased from Worthington Biochemicals (Freehold, NJ). Serum extender, Neurobasal medium, and GlutaMAX were purchased from Invitrogen (Carlsbad, CA). Vectashield with 4,6-diamidino-2-phenylindole was purchased from Vector Laboratories (Burlingame, CA).
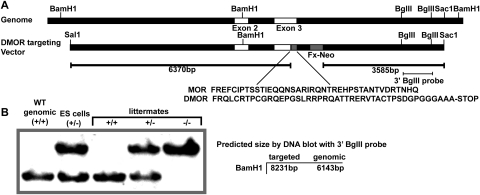
Generation of Transgenic DMOR Mice.
The DMOR knock-in mice were generated using homologous recombination in embryonic stem cells to modify the Oprm1 (MOR) gene. The DMOR mutation was exactly as reported previously (Finn and Whistler, 2001). Mouse genomic DNA clones were derived from a pBK2 library (129.SvEv strain). An ∼12.5-kb SalI-SacI fragment of genomic DNA was used to generate the targeting vector. In this targeting vector, the wild-type MOR C-terminal sequence (FREFCIPTSSTIEQQNSARIRQNTREHPSTANTVDRTNHQ) was replaced with the DMOR sequence (FRQLCRTPCGRQEPGSLRRPRQATTRERVTACTPSDGPGGGAAA). A 2.1-kb cassette containing G418 resistance flanked by lox P sites was inserted into a SpeI site in the intron downstream of exon 3. The targeting vector was linearized with SacI and transfected into embryonic stem cells (TC-1) by electroporation. Clones were selected by G418 resistance. Of the 135 clones screened by polymerase chain reaction, 12 were homologous recombinants (9%). These clones were confirmed by Southern blot analysis. A positive clone was injected into C57BL/6 blastocysts to create chimeric animals. F1 agouti progeny were genotyped for transmission of the mutant allele. These mice were used to generate a transgenic line of DMOR knock-in mice. Mice were genotyped using polymerase chain reaction for the genomic MOR C-terminal DNA sequence. For all experiments, male and female F1 progeny knock-in DMOR and WT littermate mice, 7 to 11 weeks old, were used. All animal experiments were performed in accordance with the Ernest Gallo Clinic and Research Center Institutional Animal Care and Use Committee guidelines.
Primary Striatal Neuronal Cultures and Confocal Microscopy Imaging.
WT mice on the second postnatal day were sacrificed by rapid decapitation, and striatal tissue was dissected out and pooled in dissection fluid (161 mM NaCl, 5 mM KCl, 0.5 mM MgSO4, 3 mM CaCl2, 4.5 mM HEPES, 5.5 mM glucose, and 5.7 μM phenol red, pH 7.4). Pooled striatal tissue was digested in papain solution (dissection fluid supplemented with 0.2 mg/ml l-cysteine, 1 mM CaCl2, 0.5 mM EDTA, 20 units/ml papain, and 3 mM NaOH, dissolved at 37°C, and sterile-filtered) for 30 min, followed by inhibition of digestion with inhibition solution (0.1 ml/ml fetal bovine serum, 22 mM glucose, 2 μl/ml serum extender, 2.5 mg/ml bovine serum albumin, and 2.5 mg/ml trypsin inhibitor, in minimal essential medium, sterile-filtered) and trituration by using pulled glass pipettes in Steve's medium (0.1 ml/ml fetal bovine serum, 22 mM glucose, and 2 μl/ml serum extender, in minimal essential medium, sterile-filtered). Neurons were plated on polylysine-treated coverslips in six-well Falcon culture dishes in Neurobasal A medium containing B27 (1:25), GlutaMAX supplements (1:100), penicillin, and streptomycin. Cultures were maintained for 9 days in vitro (37°C, 7% CO2 pressure), with 50% of the medium changed after day 1 and 7. On day 10, neurons were transfected with either 1 μg of FLAG-tagged DMOR or WT MOR DNA (pcDNA3.1 construct, cytomegalovirus promoter for high protein expression) using FuGENE transfection reagent. FLAG-tagged WT MOR or DMOR was detected 48 h after transfection by addition of primary anti-FLAG M1 antibodies to the growth medium and left for 30 min at 37°C to label a pool of receptors that had reached the surface. The neurons were then treated with DAMGO (1 μM) or morphine (5 μM) for 30 min or left untreated. A subset of neurons was fixed in 4% paraformaldehyde in PBS to assess internalization. Another set were washed three times for 1 min with ice-cold PBS without calcium to remove antibody from surface receptors (anti-FLAG M1 antibodies depend on calcium for binding to the FLAG epitope) and then incubated for an additional 120 min in the presence of naloxone (1 μM) to allow receptor recycling and prevent any additional internalization before fixation as above. After fixation, neurons were permeabilized in 50 mM Tris-HCl, pH 7.5, with 0.1% Triton X-100, 100 mM CaCl2, and 3% nonfat milk powder for 20 min. Neurons were then incubated for 20 min with Alexa 488 goat anti-mouse IgG2b (2 ng/ml; Invitrogen) to detect antibody-labeled receptors. Coverslips were washed in PBS and mounted on glass slides in Vectashield with 4,6-diamidino-2-phenylindole, and images were acquired using similar gain settings on a Zeiss confocal microscope (LSM 510 Meta, 46× objective; Carl Zeiss Inc., Thornwood, NY).
Analgesic Response: Tail-Flick Reflex to Heat Irradiation.
Mice were tested for antinociception using the radiant heat tail-flick procedure. Mice with robust tail-flick reflexes and baseline latencies of 2.0 through 4.5 s were included in the study; a maximum latency of 12 s was set as the cutoff time to minimize damage to the tail. The maximum effect of the drug on the tail-flick reflex was achieved between 20 and 30 min after subcutaneous injection of the drug (data not shown). Subsequent one-point analgesic assessments were performed at the 30-min time point. Dose response was measured by cumulative drug addition, assessed 20 min after each subcutaneously administered dose, three doses per animal. Data are presented as maximal possible effect (MPE) = 100 × (latency after drug − baseline latency)/(cutoff − baseline latency).
Tolerance Induction Protocol: Long-Term Moderate-Dose Morphine Tolerance Induction.
WT MOR and DMOR mice were treated twice daily with morphine (10 mg/kg s.c.) for 7 days, and antinociception was assessed 30 min after the morning dose every other day. The mean MPE ± S.E.M. is presented; n ≥ 22 animals/group. Statistical significance was investigated by repeated measures ANOVA with Dunnett's test.
Neuronal Membrane Preparations.
Animals were euthanized by cervical dislocation, and the CNS was divided by gross anatomy into spinal cord, brainstem (minus cerebellum), and brain, frozen on dry ice, and stored at −80°C until further use. Tissue was homogenized in 10 volumes to weight of 50 mM Tris-HCl, pH 7.4, followed by a 15-min 1500g centrifugation. This procedure was repeated once, and the pooled supernatants were centrifuged at 30,000g for 30 min. The pellets were resuspended in 10 volumes to weight of 50 mM Tris-HCl, pH 7.4, and the pellet was implanted once more. Finally, the pellet was resuspended in 7 volumes to weight of potassium phosphate buffer (50 mM KPO4-K2PO4, pH 7.4) and snap-frozen in liquid nitrogen. Samples were kept at −80°C until use. Protein concentrations were determined with a protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Radioligand Binding.
Saturation binding assays were performed on membranes (85 μg of protein/well) incubated in 50 mM potassium phosphate buffer with 0.1% bovine serum albumin and with increasing concentrations of [3H]DAMGO (0.01–10 nM, 1 mCi/ml) in a total of 200 μl/reaction, for 90 min at 25°C in a 96-well plate. The reactions were terminated by rapid filtration over 96-well GF/B glass fiber filters (presoaked 30 min in Tris-HCl buffer; PerkinElmer Life and Analytical Sciences) using a MultiScreenHTS Vacuum Manifold (Millipore Corporation, Billerica, MA). Filters were washed five times with 200 μl of ice-cold 50 mM Tris-HCl (pH 7.4), dried, subjected to overnight extraction in 50 μl of MicroScint scintillation fluid (PerkinElmer Life and Analytical Sciences), and then counted using a Packard TopCount counter. Nonspecific binding was measured in the presence of 1 μM naloxone and amounted to average <15% of total binding. Binding parameters were determined by nonlinear regression analysis of specific binding using Prism (GraphPad Software Inc., San Diego, CA). Data are presented as means ± S.E.M. of at least three independent experiments (pooled tissue from at least eight animals included in each experiment) performed in triplicate. Significance was established for DMOR by one-way ANOVA with Dunnett's test and for WT versus DMOR with a nonparametric Mann-Whitney test.
GTPγS Binding to Neuronal Membranes.
Concentration-effect curves of [35S]GTPγS binding included nine drug doses between 0.1 and 100,000 nM morphine, 45 μM guanosine 50-diphosphate, 0.05 nM [35S]GTPγS, 10 μg of protein, and assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 10 μg/ml saponin) in a final volume of 1 ml using 3-ml polypropylene tubes. Assays were incubated at 25°C for 90 min. Reactions were terminated by transfer to GF/B glass fiber filters (presoaked for 30 min in ice-cold Tris-HCl buffer) with rapid filtration under vacuum in 12-well format, followed by three washes with 3 ml of ice-cold 50 mM Tris-HCl buffer (pH 7.4). Bound radioactivity was determined by using a Packard scintillation counter for 35S after overnight extraction of the filters in 5 ml of ScintiVerse II scintillation fluid. Data are reported as means ± S.E.M. of at least two independent experiments each (pooled tissue from at least eight animals were included in each experiment) performed in triplicate. Percentage stimulation is defined as [(stimulated binding − basal binding)/basal binding] × 100. Nonlinear regression analysis of concentration-effect curves that determined EC50 and Emax values was performed with GraphPad Prism. Significance was established for DMOR by one-way ANOVA with Dunnett's test and for WT versus DMOR with the nonparametric Mann-Whitney test.
Naloxone-Precipitated Morphine Withdrawal.
Mice of both genotypes were treated with morphine (10 mg/kg s.c.) twice per day for 7 days. For the last injection on day 7 all mice (including naive mice) received morphine (10 mg/kg s.c.), and 30 min after the last injection, all mice were administered 0.5 mg/kg naloxone and placed in a clear plastic cylinder for observation. The number of jumps was counted over 15 min. Significance was established using the nonparametric Mann-Whitney test. The 0.5 mg/kg dose of naloxone was chosen because it was the highest dose of naloxone that did not elicit jumping in the mice treated with saline.
Results
Generation and Characterization of Mice Expressing DMOR.
To examine how loss of MOR function through down-regulation could affect morphine responses, we generated knock-in mice expressing a mutant MOR, DMOR, which in cell-based models is internalized and degraded in lysosomes in response to morphine (Finn and Whistler, 2001). In these mice, the cytoplasmic tail of the WT MOR has been replaced by that of the δ-opioid receptor (Fig. 1A). Mice expressing DMOR were identified by DNA blot (Fig. 1B). The mutant mice were viable, had no gross phenotypic abnormalities, and showed normal baseline pain responses (tail-flick latency 2.7 ± 0.1 s for WT MOR and 2.8 ± 0.1 s for DMOR). These mice are distinct from our previously reported recycling MOR mice, in which the receptor was engineered to recycle (not degrade) after internalization in response to morphine.
Fig. 1.
Generation of DMOR knock-in mice. A, schematic diagram of the targeting strategy. A Sal1-Sac1 genomic fragment containing the MOR sequence was modified to contain the DMOR sequence (inset). A cassette containing resistance to G418 and flanked by lox P sites (Fx-Neo) was inserted in the intron downstream of exon 3 for selection of embryonic stem (ES) clones. B, detection of homologous recombinants. Genomic DNA was digested with BamHI and subjected to DNA hybridization with a ∼1.1-kb BglII fragment (see A). Targeted loci were confirmed by the presence of a band at ∼8 kb. The intact locus gave a band of ∼6 kb.
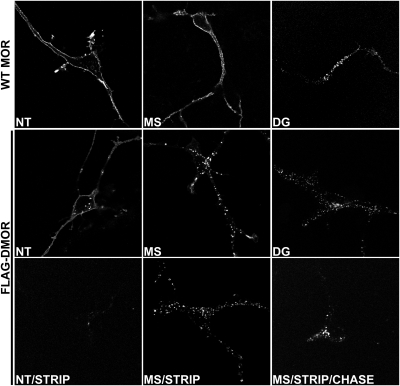
The agonist-promoted internalization and postendocytic fate of DMOR has been thoroughly studied in HEK293 models (Finn and Whistler, 2001). To assess whether DMOR trafficking in neurons was comparable to that in HEK293 cells, we examined trafficking of WT MOR and DMOR in striatal neurons transiently transfected with FLAG-tagged versions of these receptors (pcDNA3.1, cytomegalovirus promoter). As reported previously, the WT MOR is internalized in response to activation by DAMGO but not morphine (Fig. 2, top). Akin to WT MOR, DMORs were localized primarily on the plasma membrane in untreated cells (Fig. 2, DMOR NT) where they could be efficiently stripped of antibody (Fig. 2, DMOR NT/STRIP). DMORs were efficiently internalized in response to the peptide agonist DAMGO (1 μM) (Fig. 2, DMOR DG). However, in contrast to the WT MOR, morphine (5 μM) also efficiently promoted DMOR internalization (Fig. 2, DMOR MS). Internalization of DMOR after morphine treatment was confirmed by the inability to strip off antibody after morphine treatment (Fig. 2, DMOR MS/STRIP). Because morphine promoted efficient internalization of DMOR (but not MOR) (Fig. 2, WT MOR MS), we next examined the postinternalization trafficking properties of DMOR in response to morphine. Neurons were treated with morphine (5 μM) to promote robust internalization of DMOR, and remaining surface receptors were stripped of antibody (Fig. 2, DMOR MS/STRIP). The fate of the pool of internalized receptors protected from the strip was examined after a 120-min chase. The MOR antagonist naloxone was included in the growth medium during this chase period to prevent reinternalization of any DMORs that were recycled to the surface. As expected, DMOR was retained in intracellular vesicles (Fig. 2, DMOR MS/STRIP/CHASE), suggesting that this receptor is impaired for recycling in neurons just as it is in HEK293 cells (Finn and Whistler, 2001). These data show that, unlike the WT MOR, the DMOR is endocytosed in response to morphine. Furthermore, once internalized in response to morphine, the DMOR is recycling-deficient.
Fig. 2.
DMOR trafficking in primary striatal cultures. Striatal cultures were transfected with a FLAG-tagged version of WT MOR or DMOR. Cells were incubated with M1 anti-FLAG antibodies to label surface receptors and treated with DAMGO (DG, 1 μM, 30 min) or morphine (MS, 5 μM, 30 min). Morphine promoted endocytosis of DMOR but not WT MOR. After drug treatment, residual surface receptors were stripped of antibody (NT/STRIP, MS/STRIP) confirming that morphine promoted internalization of the DMOR. Antagonist (naloxone, 1 μM, 120 min) was then added after the morphine treatment and the strip to allow receptor recycling for 120 min and prevent any additional internalization (MS/STRIP/CHASE). Internalized DMOR failed to return to the surface (MS/STRIP/CHASE).
Acute Morphine Antinociception in WT MOR versus Knock-In DMOR Mice.
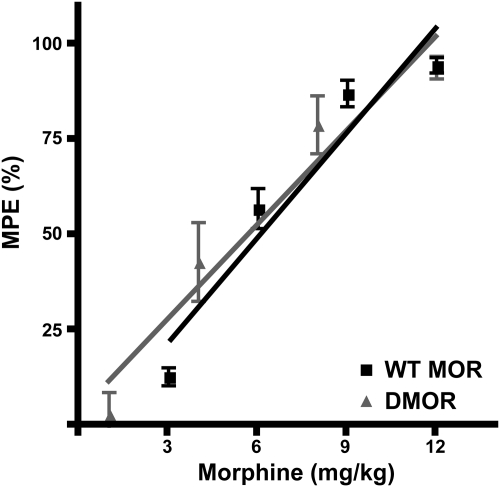
We next compared the acute antinociceptive potency of morphine in the tail-flick paradigm in WT MOR and DMOR mice (n = 8 mice/group). There were no significant differences in the antinociceptive effect of morphine between the two genotypes (Fig. 3). Drug doses could, thus, be kept equal for both genotypes throughout the study.
Fig. 3.
Antinociceptive dose response to morphine in WT MOR and DMOR mice. Antinociceptive responses were determined with the tail-flick test, and data are reported as mean MPE ± S.E.M. Mice (≥19/genotype) were given subcutaneous injections of four accumulative doses of morphine and assessed for antinociception 20 min after each injection. MPE was calculated with the formula: 100 × (drug latency − baseline latency)/(cutoff − baseline latency). ■, WT MOR; ▴, DMOR.
Assessment of Receptor Number and Ligand Affinity in Naive WT MOR and Knock-In DMOR Mice.
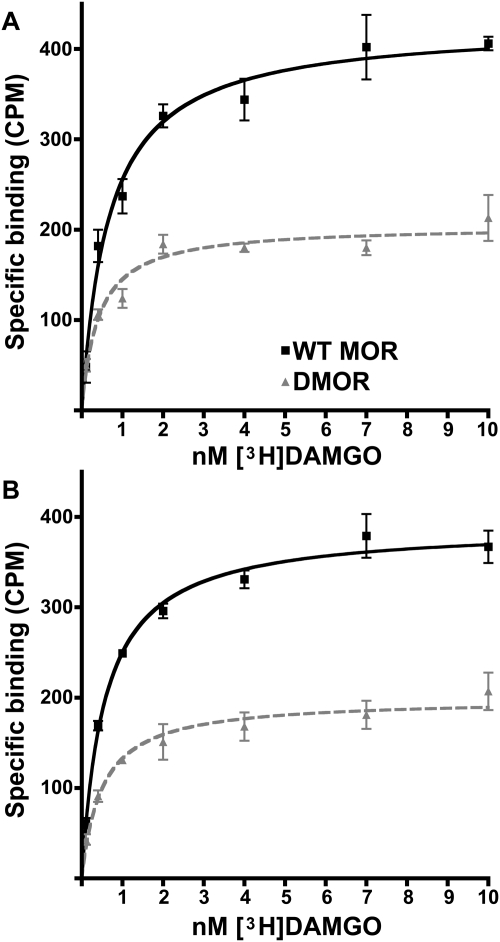
DMOR is not recycled after internalization (Fig. 2) and is degraded in lysosomes after internalization (Finn and Whistler, 2001). Hence, one might anticipate that receptor number could be different in WT MOR versus DMOR mice, even in opioid drug-naive mice, especially in regions of the CNS with high endogenous opioid tone. To examine this possibility, we examined MOR number and affinity in naive WT MOR and DMOR mice in brainstem and spinal cord, CNS regions important for antinociception. Indeed, naive DMOR mice had significantly lower levels of receptors in both brainstem (Fig. 4A; Table 1) and in spinal cord (Fig. 4B; Table 1) than did WT MOR mice (p < 0.0001). The number of receptors in DMOR mice as a fraction of that in WT MOR mice was brainstem 48% and spinal cord 52%. No significant differences in affinity were detected in different CNS regions or in WT versus knock-in DMOR animals (Table 1). This result is consistent with our observation that the DMOR mutation, which is confined entirely to the cytoplasmic tail of the receptor (Fig. 1A), did not affect ligand affinity in cell-based models (Finn and Whistler, 2001). Together, these data suggest that the reduced receptor levels in DMOR animals are not sufficient to alter either baseline pain latencies or acute morphine effects on antinociception as assessed by the tail-flick paradigm (Fig. 3).
Fig. 4.
Saturation radioligand binding of [3H]DAMGO to brainstem and spinal cord membrane homogenates from naive WT MOR and DMOR mice. Brain membranes were prepared as described under Materials and Methods, and binding was performed at 25°C for 90 min. Cold naloxone (10 μM) was used to determine nonspecific binding. Results are displayed as the average of specific binding ± S.E.M. from three separate experiments (pooled tissue from at least eight animals per experiment) done in triplicate for brainstem (A) and spinal cord (B). Nonlinear regression was performed with GraphPad Prism. ■, WT MOR; ▴, DMOR.
TABLE 1.
[3H]DAMGO Bmax and KD in naive and long-term tolerant animals
Data are means ± S.E.M.
| Naive |
Long-Term Tolerant |
|||
|---|---|---|---|---|
| WT MOR | DMOR | WT MOR | DMOR | |
| Bmax (fmol receptor/mg protein) | ||||
| Brainstem | 85 ± 3.4 | 41 ± 1.8* | 85 ± 4.6 | 24 ± 1.8‡ |
| Spinal cord | 77 ± 2.0 | 40 ± 2.0* | 73 ± 3.2 | 14 ± 1.0# |
| KD (nM) | ||||
| Brainstem | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.2 ± 0.1 |
| Spinal cord | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.3 ± 0.1 |
Significantly lower compared with WT naive, p < 0.0001.
Significantly lower compared with DMOR naive, p < 0.01.
Significantly lower compared with DMOR naive, p < 0.05.
Assessment of Receptor Coupling in Naive WT MOR and Knock-In DMOR Mice.
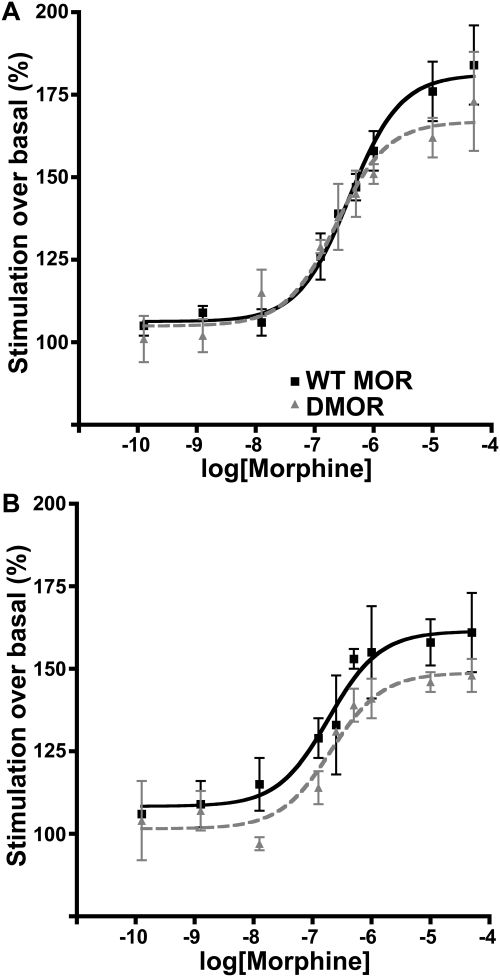
We next examined whether DMOR showed alterations in G protein coupling efficiency compared with WT MOR in brainstem (Fig. 5A) and spinal cord (Fig. 5B). The Emax values for DMOR were not significantly lower than those for WT MOR (Table 2). Together, these data suggest that there is substantial receptor reserve and that loss of more than 50% of the receptors in naive DMOR mice compared with WT MOR mice (Table 1) was insufficient to change the maximal response to morphine either in vitro (Fig. 5) or in vivo (Fig. 3).
Fig. 5.
Receptor coupling by GTPγS in brain membrane homogenates from naive WT MOR and DMOR mice. Brain membranes were prepared as described under Materials and Methods, and morphine stimulation was performed at 25°C for 90 min. Results are displayed as the average of percentage morphine stimulation over basal ± S.E.M. from at least two separate experiments (pooled tissue from at least eight animals per experiment) in triplicate in membranes from brainstem (A) and spinal cord (B). Nonlinear regression was performed with GraphPad Prism Percentage basal = 100 × (stimulated − background)/(basal − background). ■, WT MOR; ▴, DMOR.
TABLE 2.
Morphine stimulated GTPγS Emax and EC50 in naive and long-term tolerant animals
Data are means ± S.E.M.
| Naive |
Long-Term Tolerant |
|||
|---|---|---|---|---|
| WT MOR | DMOR | WT MOR | DMOR | |
| Emax (% basal) | ||||
| Brainstem | 181 ± 4 | 167 ± 5 | 171 ± 2 | 126 ± 4‡ |
| Spinal cord | 161 ± 5 | 149 ± 4 | 153 ± 3 | 121 ± 5# |
| EC50 (nM) | ||||
| Brainstem | 433 ± 69 | 311 ± 72 | 460 ± 58 | 308 ± 95 |
| Spinal cord | 299 ± 82 | 282 ± 68 | 236 ± 52 | 530 ± 160 |
Significantly lower compared with DMOR naive, p < 0.05.
Significantly lower compared with DMOR naive, p < 0.01.
Effect of Long-Term Moderate-Dose Morphine on Antinociception, Receptor Number, and Receptor Coupling in WT MOR and Knock-In DMOR Mice.
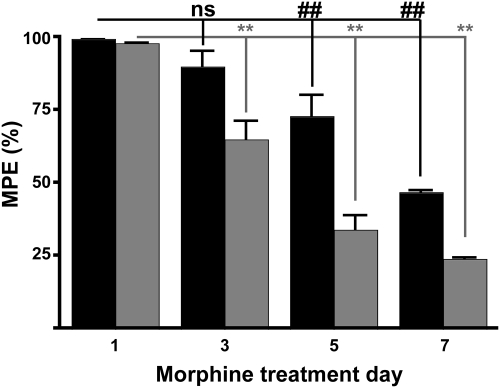
Several groups have shown that repeated, long-term, administration of moderate doses of morphine can produce a profound antinociceptive tolerance in WT mice (Bohn et al., 2000). However, it is not clear whether receptor down-regulation or uncoupling accompanies the tolerance produced by these more moderate dosing paradigms as it does for some high-dose paradigms (Sim-Selley et al., 2007) (Supplemental Fig. 1). To evaluate the relationship between receptor down-regulation and/or receptor uncoupling and behavioral tolerance to these moderate morphine doses, WT and DMOR mice were treated with morphine (10 mg/kg s.c.) twice daily for 7 days, and tail-flick latency was tested after the morning dose every other day. This morphine dose produced >90% MPE in both WT MOR and DMOR mice on day 1 (Fig. 6, day 1; see dose response in Fig. 3). DMOR mice (Fig. 6, ▩) developed tolerance more quickly and to a greater extent over the 7-day period studied than WT MOR mice (Fig. 6, ■) and were tolerant by day 3 (p < 0.01, p < 0.01, and p < 0.01, DMOR day 1 versus day 3, 5, and 7, respectively, repeated-measures ANOVA with Dunnett's multiple comparison test, n = 22 animals/group). WT MOR mice showed tolerance by day 5 (p > 0.05, p < 0.05, and p < 0.01, WT day 1 versus day 3, 5, and 7, respectively, repeated-measures ANOVA with Dunnett's multiple comparison test, n = 22 animals/group).
Fig. 6.
Tolerance to the antinociceptive effects of morphine after long-term repeated subcutaneous morphine in WT MOR and DMOR mice. WT MOR (■), and DMOR (▩) mice were treated twice daily with morphine (10 mg/kg s.c.) for 7 days, and antinociception was assessed 30 min after the morning dose every other day. Mean MPE ± S.E.M. is presented; n ≥22 animals/group. Group statistics by repeated-measures ANOVA with Dunnett's test: ##, and **, p < 0.01; ns, no significant difference; comparison with day 1.
These data demonstrate that opioid tolerance occurs in both genotypes, although faster and to a greater extent in DMOR than in WT mice. We hypothesized that tolerance in the DMOR mice would be accompanied by receptor desensitization and down-regulation based on the trafficking pattern of this receptor in neurons when stimulated by morphine (Fig. 2). However, it was not clear whether it would accompany tolerance in WT mice, although we suspected that it would not. To test this hypothesis, tissue was collected and processed from the morphine-treated animals (Fig. 6) on day 7, for which both genotypes showed substantial tolerance, and receptor number and coupling were compared with those of drug-naive mice. Only DMOR mice showed changes in Bmax after morphine treatment. In particular, in brainstem and spinal cord, DMOR mice showed a 41% reduction (from average 41 ± 1.8 to 24 ± 1.8 fmol/mg protein) and a 65% reduction (from average 40 ± 2.0 to 14 ± 1.0 fmol/mg protein) in Bmax, respectively (Table 1). WT MOR animals showed no significant changes in receptor number in these same areas (100 and 95%, respectively, on average, compared with control animals) (Table 1). These data suggest that receptor down-regulation may contribute to morphine tolerance in DMOR but not WT mice. This loss of receptor number was also reflected in a loss of receptor coupling in DMOR mice (Table 2). Of importance, however, this moderate-dose long-term morphine treatment did not cause significant uncoupling of receptors in WT animals although animals were profoundly tolerant (Fig. 6). DMOR mice showed a 61% reduction in Emax in brainstem and a 57% reduction in Emax in spinal cord (from an average of 167 ± 5 to 126 ± 4% stimulated/basal and from 149 ± 4 to 121 ± 5% stimulated/basal, respectively) (Table 2). In sharp contrast, WT MOR mice showed no significant alterations in coupling (88 and 87%, respectively, on average in brainstem and spinal cord) (Table 2).
Some morphine dosing paradigms have previously been shown to promote receptor desensitization (Sim-Selley et al., 2007). Indeed, we also found that these very high doses of morphine (produced through repeated subcutaneous pellet implantation) did cause antinociceptive tolerance (Supplemental Fig. 1A) that was accompanied by a reduction in MOR G protein coupling in the periaqueductal gray not only in DMOR mice but also in WT mice (Supplemental Fig. 1, B and C). Nevertheless, whereas some desensitization can be induced in WT mice through these high doses of morphine, it is not a prerequisite for the development of tolerance in WT mice (Fig. 6). Thus, it is not clear whether the desensitization that occurs with the high-dose paradigm is contributing to tolerance or whether it is an artifact of or an additional compensatory response to these extreme doses of morphine.
Effect of Receptor Down-Regulation on Precipitated Withdrawal.
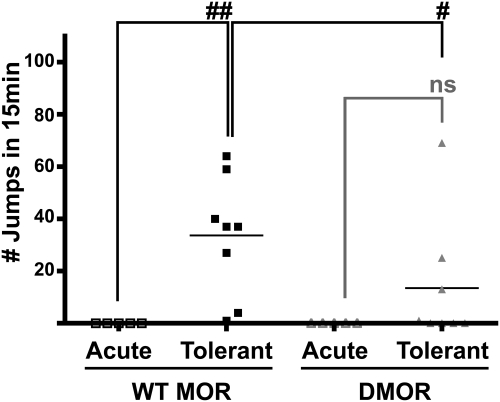
Although both WT and DMOR mice display antinociceptive tolerance after long-term moderate-dose morphine treatment (Fig. 6), assessment of receptor function in the two genotypes indicates that the mechanisms underlying tolerance are different. In particular, tolerance in DMOR mice is accompanied by down-regulation of receptors, whereas tolerance in WT mice is not. Several groups have proposed that tolerance to morphine in WT animals is caused primarily by compensatory homeostatic adaptations that mask the presence of morphine in the presence of drug and manifest as withdrawal upon removal of the drug (for review, see Nestler, 2004; Berger and Whistler, 2010). In contrast, tolerance mediated by loss of receptor function should produce little to no withdrawal upon removal of morphine, because removal of an agonist from a nonfunctional receptor should have no behavioral effect. Thus, we expected that the two genotypes would show differences in their levels of dependence. In particular, we predicted that signs of withdrawal would not accompany tolerance mediated by loss of receptor function in the DMOR animals. To examine this hypothesis, mice of both genotypes were made tolerant to morphine (as in Fig. 6) and dependence after these treatments was assessed by precipitated withdrawal (Fig. 7). Control mice received no morphine before test day. On the test day, all mice were administered 10 mg/kg morphine followed 30 min later by naloxone (0.5 mg/kg s.c.), and withdrawal-induced jumping was assessed. Naloxone at this dose did not elicit jumping in mice of either genotype that had only the single dose of morphine on the test day (Fig. 7, WT MOR Acute and DMOR Acute). In contrast, naloxone elicited jumping in every morphine-tolerant WT mouse (Fig. 7, WT MOR Tolerant). Of importance, naloxone induced significantly less jumping in DMOR mice as a group (Fig. 7, WT MOR 33 ± 8 jumps on average, DMOR 14 ± 9 jumps on average, p < 0.05, Mann-Whitney nonparametric test of WT MOR versus DMOR), and only in some but not all DMOR mice (in four of eight animals). Furthermore, the jumping produced in DMOR mice after receiving morphine through the high-dose pellet implantation protocol was no different from that produced by the long-term moderate-dose protocol (Supplemental Fig. 2). In contrast, WT MOR mice show significantly increased withdrawal-induced jumping after the high dose of morphine compared with the moderate-dose tolerance paradigm (33 ± 8 versus 65 ± 8, Mann-Whitney nonparametric test of WT MOR moderate-dose versus high-dose morphine) (compare Supplemental Fig. 2 with Fig. 7) despite the fact that some WT MOR uncoupling was found after the former tolerance induction paradigm (Supplemental Fig. 1).
Fig. 7.
Morphine dependence after long-term moderate-dose morphine in WT MOR and DMOR mice. WT MOR and DMOR mice received twice daily injections of morphine (10 mg/kg) for 6 days before the test day. On the test day, morphine-naive (□, WT MOR Acute; ▵, DMOR Acute) or morphine-treated (■, WT MOR Tolerant; ▴, DMOR Tolerant) animals were given injections of 10 mg/kg morphine followed by 0.5 mg/kg naloxone 30 min later. Withdrawal was scored as number of jumps over 15 min. Numbers of jumps ± S.E.M. are presented. The Mann-Whitney nonparametric test was used for WT MOR versus DMOR statistics, and the Wilcoxon signed-rank test was used for intragenotype statistics. #, p < 0.05; ##, p < 0.01; ns, no significant difference. □, WT MOR Acute; ▵, DMOR Acute; ■, WT MOR Tolerant; ▴, DMOR Tolerant.
Discussion
In this study, we used a novel knock-in mouse, DMOR, which expresses a mutant MOR that internalizes and down-regulates in response to morphine, to compare morphine tolerance induced by receptor uncoupling and down-regulation with tolerance induced in WT mice. Unlike the WT MOR, DMOR is efficiently internalized when activated by morphine and is unable to recycle back to the plasma membrane (Fig. 2). We show that these DMOR mice develop antinociceptive tolerance to morphine faster than WT mice do (Fig. 6), and it is accompanied by both receptor uncoupling and receptor down-regulation (Tables 1 and 2). In contrast, WT MOR mice acquire significant antinociceptive tolerance to morphine (Fig. 6) without any significant down-regulation or uncoupling of MOR (Tables 1 and 2). Furthermore, WT mice showed a significantly higher degree of precipitated withdrawal-induced jumping compared with DMOR animals, again indicating that the mechanism mediating tolerance in the two genotypes is different.
Several pieces of evidence allude to more than one mechanism underlying morphine tolerance, even in WT mice. For example WT MOR mice quickly recover from high-dose “acute tolerance” (Huidobro-Toro and Way, 1978; Kim et al., 2008). In contrast, it takes mice several weeks to fully recover from tolerance induced by moderate doses of long-term morphine (Cox et al., 1975), which are comparable with the long-term moderate-dose treatment we used here and in many of our other studies. In another example, competitive N-methyl-d-aspartate receptor blockers completely block tolerance to moderate doses of long-term morphine (10 mg/kg) but only partially block tolerance to higher doses (20 or 40 mg/kg) (Allen and Dykstra, 2000).
Down-regulation and/or uncoupling is not necessary for antinociceptive tolerance to morphine to occur (Fig. 6) (Kirschke et al., 2002). Nevertheless, receptor down-regulation and/or receptor uncoupling can accompany tolerance induced by some dosing paradigms (Supplemental Fig. 1) (Sim-Selley et al., 2007). Taken together, these data suggest that receptor uncoupling and possibly even down-regulation could contribute to tolerance to high doses but not to moderate doses of drug. However, it is not possible to determine conclusively whether the MOR uncoupling/desensitization/down-regulation observed in some higher-dosing paradigms is contributing to more profound tolerance to these higher drug doses, whether it is a mechanism compensating for these higher doses or whether the desensitization is merely an artifact or epiphenomenon associated with the higher doses but has no effect on behavioral tolerance.
Together these data indicate that tolerance to morphine in WT mice, at least that which occurs to moderate doses of long-term morphine, is primarily mediated by mechanisms other than receptor desensitization/down-regulation. As we and others have proposed previously, tolerance in WT mice is probably due to homeostatic compensatory changes in signal transduction downstream of the receptors that mask receptor activity in the presence of drug and manifest as withdrawal upon removal of the drug. One key homeostatic adaptation that contributes to morphine tolerance in WT mice is superactivation of adenylyl cyclase signaling (for review, see Nestler, 2004; Berger and Whistler, 2010). The importance of this adaptation not only to second messenger signaling but also to synaptic adaptations and behavioral withdrawal was recently demonstrated in vivo (Madhavan et al., 2010). Superactivation, in turn, may promote additional and diverse changes in gene and protein expression, all of which play a role in the behavioral manifestation of morphine tolerance and dependence.
These compensatory mechanisms are probably occurring, at least to some degree, in the DMOR mice, because we observe some signs of precipitated withdrawal. Thus, we cannot rule out the possibility that the greater tolerance in the DMOR mice is a consequence of an additive effect of the homeostatic adaptations that occur in WT MOR mice and the desensitization/down-regulation unique to the DMOR mice. However, withdrawal-induced jumping is substantially reduced in DMOR mice compared with that in WT mice (Fig. 7), suggesting that these homeostatic adaptations are a minor contributor to tolerance in DMOR mice compared with the effect of receptor down-regulation.
Taken together, our results suggest that tolerance to moderate doses of morphine in WT mice is mediated by compensatory homeostatic adaptations in signal transduction independent of any significant receptor desensitization/down-regulation. We found that very high doses of morphine do cause some receptor uncoupling/desensitization even in WT mice. Although this may be an epiphenomenon, it is possible that receptor uncoupling promoted by high doses of morphine can exacerbate tolerance. If so, this suggests a possible therapeutic window, before morphine dose escalation, open for corrective measures that would prevent desensitization. Furthermore, this reasoning of multiple, but not mutually exclusive, mechanisms underlying tolerance might help explain why rotational therapies have shown some promise. In short, the bias of each rotated drug may contribute to varying and perhaps nonoverlapping mechanistic aspects of tolerance/dependence.
We have previously shown the facilitating internalization and recycling (not degradation) of the MOR in response to morphine can prevent or delay many of the compensatory homeostatic adaptations that contribute to tolerance and dependence to moderate long-term doses of morphine (Finn and Whistler, 2001; He et al., 2002; He and Whistler, 2005; Kim et al., 2008; Madhavan et al., 2010). Thus, the data reported here, together with these previous results, show that receptor internalization has dramatically different effects on the development of analgesic tolerance and dependence, depending on the trafficking of the targeted receptor. Receptor internalization protects against the development of tolerance when the receptor is recycled (Finn and Whistler, 2001; He and Whistler, 2005; Zöllner et al., 2008), presumably by titrating signal transduction through the receptor and preventing cellular adaptations. In contrast, in DMOR mice, internalization actually facilitates tolerance, because, unlike the WT MOR (or recycling MOR), the DMOR is down-regulated after internalization. Thus, both internalization and the fate of the receptor after internalization can be key influences on tolerance, depending on receptor fate.
Supplementary Material
Acknowledgments
We thank M. von Zastrow for support in the development of this project and B. Kieffer for supplying the pBK2 plasmid containing the genomic fragment used to generate the targeting vector. We thank Stacy Taylor for mouse colony maintenance and Li He, Richard van Rijn, Amy Chang, Lene Martini, Laura Milan-Lobo, and Ida Enquist for critically reading this manuscript.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA015232, DA019958]; State of California funds for medical research on alcohol and substance abuse through the University of California San Francisco (to J.J.W.); and the European Molecular Biology Organization [Fellowship ALTF1229-2006] (to J.E.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179754.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- MOR
- μ-opioid receptor
- CNS
- central nervous system
- DMOR
- degrading μ-opioid receptor
- WT
- wild type
- GTPγS
- guanosine 5′-(γ-thio)triphosphate
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- kb
- kilobase
- PBS
- phosphate-buffered saline
- MPE
- maximal possible effect
- ANOVA
- analysis of variance
- HEK
- human embryonic kidney
- MS
- morphine sulfate.
Authorship Contributions
Participated in research design: Enquist and Whistler.
Conducted experiments: Enquist and Ferwerda.
Contributed new reagents or analytic tools: Enquist, Kim, Bartlett, Ferwerda, and Whistler.
Performed data analysis: Enquist.
Wrote or contributed to the writing of the manuscript: Enquist and Whistler.
References
- Allen RM, Dykstra LA. (2000) Role of morphine maintenance dose in the development of tolerance and its attenuation by an NMDA receptor antagonist. Psychopharmacology (Berl) 148:59–65 [DOI] [PubMed] [Google Scholar]
- Berger AC, Whistler JL. (2010) How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol 67:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Connor M, Borgland SL, Christie MJ. (1999) Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. Br J Pharmacol 128:1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Ginsburg M, Willis J. (1975) The offset of morphine tolerance in rats and mice. Br J Pharmacol 53:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Akera T, Brody TM. (1975) Saturable binding of morphine to rat brain-stem slices and the effect of chronic morphine treatment. Res Commun Chem Pathol Pharmacol 12:409–418 [PubMed] [Google Scholar]
- Finn AK, Whistler JL. (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32:829–839 [DOI] [PubMed] [Google Scholar]
- Fyfe LW, Cleary DR, Macey TA, Morgan MM, Ingram SL. (2010) Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther 335:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CB, Emilien B, Becketts K, Cadet JL, Rothman RB. (1996) Downregulation of mu-opioid binding sites following chronic administration of neuropeptide FF (NPFF) and morphine. Peptides 17:389–397 [DOI] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. (2003) Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology 45:575–584 [DOI] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. (2002) Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 108:271–282 [DOI] [PubMed] [Google Scholar]
- He L, Whistler JL. (2005) An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol 15:1028–1033 [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Way EL. (1978) Single-dose tolerance to antinociception, and physical dependence on β-endorphin in mice. Eur J Pharmacol 52:179–189 [DOI] [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, et al. (2008) Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol 18:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke C, Schadrack J, Zieglgänsberger W, Spanagel R. (2002) Effects of morphine withdrawal on micro-opioid receptor-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate autoradiography in rat brain. Eur J Pharmacol 446:43–51 [DOI] [PubMed] [Google Scholar]
- Madhavan A, Bonci A, Whistler JL. (2010) Opioid-induced GABA potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci 30:14029–14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47 (Suppl 1):24–32 [DOI] [PubMed] [Google Scholar]
- Nishino K, Su YF, Wong CS, Watkins WD, Chang KJ. (1990) Dissociation of mu opioid tolerance from receptor down-regulation in rat spinal cord. J Pharmacol Exp Ther 253:67–72 [PubMed] [Google Scholar]
- Petruzzi R, Ferraro TN, Kürschner VC, Golden GT, Berrettini WH. (1997) The effects of repeated morphine exposure on mu opioid receptor number and affinity in C57BL/6J and DBA/2J mice. Life Sci 61:2057–2064 [DOI] [PubMed] [Google Scholar]
- Ray SB, Gupta H, Gupta YK. (2004) Up-regulation of mu-opioid receptors in the spinal cord of morphine-tolerant rats. J Biosci 29:51–56 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. (1996) Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci 16:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Scoggins KL, Cassidy MP, Smith LA, Dewey WL, Smith FL, Selley DE. (2007) Region-dependent attenuation of mu opioid receptor-mediated G-protein activation in mouse CNS as a function of morphine tolerance. Br J Pharmacol 151:1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA, Terman GW. (2006) Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci 26:12033–12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, et al. (2008) Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest 118:1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.