Abstract
The NADPH oxidase (Nox) subunits 1, 2 (gp91 phox), and 4 are the major sources for reactive oxygen species (ROS) in vascular tissues. In conditions such as ischemia-reperfusion and hypoxia, both ROS and adenosine are released, suggesting a possible interaction. Our aim in this study was to examine the A3 adenosine receptor (A3AR)-induced vascular effects and its relation to ROS and Nox1, 2, and 4 using aortic tissues from wild-type (WT) and A3AR knockout (A3KO) mice. The selective A3AR agonist 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (Cl-IBMECA) (10−10–10−5 M) induced contraction of the aorta from WT but not from A3KO mice, and this contraction was inhibited by the Nox inhibitor apocynin (10−5 M) and the ROS scavengers superoxide dismutase-polyethylene glycol and catalase-polyethylene glycol (100 U/ml each). Cl-IBMECA-induced contraction was not affected by the mast cell degranulator compound 48/80 (100 μg/ml) or the stabilizer cromolyn sodium (10−4 M). In addition, Cl-IBMECA (10−7 M) increased intracellular ROS generation by 35 ± 14% in WT but not in A3KO aorta, and this increase was inhibited by apocynin (10−5 M), diphenyleneiodonium chloride (10−5 M), and the A3AR antagonist 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate (MRS1523) (10−5 M). Furthermore, Cl-IBMECA selectively increased the protein expression of the Nox2 subunit by 150 ± 15% in WT but not in A3KO mice without affecting either Nox1 or 4, and this increase was inhibited by apocynin. The mRNA of Nox2 was unchanged by Cl-IBMECA in either WT or A3KO aortas. In conclusion, A3AR enhances ROS generation, possibly through activation of Nox2, with subsequent contraction of the mouse aorta.
Introduction
Adenosine is an autacoid that plays an important role in the regulation of cardiovascular functions. The cardiovascular effects of adenosine are mediated by activation of four well known cell surface receptors (A1, A2A, A2B, and A3) (Tabrizchi and Bedi, 2001; Mustafa et al., 2009). The role of adenosine receptors (ARs) in vascular contraction and relaxation has been studied in several species, A2AAR and A2BAR showing vasorelaxant effects and A1AR showing vasoconstricting effects (Tabrizchi and Bedi, 2001; Ansari et al., 2007a, 2009). However, the physiological role of A3AR in vascular responses is not fully characterized, although its role in myocardial ischemia and reperfusion injury has been demonstrated (Maddock et al., 2003; Zatta et al., 2006). We previously demonstrated that activation of A3AR leads to endothelium-dependent aortic contraction through cyclooxygenase-1 (COX-1) using A3AR knockout (A3KO) mice (Ansari et al., 2007b). A3AR has also been shown to inhibit or negatively modulate coronary flow in isolated mouse heart (Talukder et al., 2002), to cause vasoconstriction in hamster arterioles (Shepherd et al., 1996), and to reverse vascular hyporeactivity after hemorrhagic shock in rats (Zhou et al., 2010).
NADPH oxidases (Nox) are the major source of reactive oxygen species (ROS) in the vasculature that play both physiological and pathophysiological roles in the control of vascular tone (Carlstrom et al., 2009). The family of NADPH oxidases consists of seven members, Nox1–Nox5 and Doux1 and Doux2 (Schröder, 2010). Among these, Nox1, Nox2, and Nox4 are of relevance in the cardiovascular system. Nox5 is not expressed in rodents because of gene deletion (Kawahara et al., 2007). Endothelial cells express Nox2 and Nox4, whereas vascular smooth muscle cells express Nox1 and Nox4 (Görlach et al., 2000; Cheng et al., 2001; Sorescu et al., 2004). In conditions such as ischemia-reperfusion and hypoxia, both ROS and adenosine are released, suggesting a possible interaction between them (Zatta et al., 2006; Gebremedhin et al., 2010). It is becoming increasingly clear that adenosine may exhibit some of its actions through modulation of Nox activity (Ribé et al., 2008; Carlstrom et al., 2009; Jajoo et al., 2009; Nadeem et al., 2009).
The question arises whether A3AR-induced contraction in mouse aorta is mediated through ROS from Nox. Our data indicate that A3AR activation using Cl-IBMECA leads to aortic contraction in wild-type (WT) but not in A3AR knockout (A3KO) mice. In addition, Cl-IBMECA induced intracellular ROS generation through selective activation of the Nox2 subunit in WT but not in A3KO mice without affecting Nox1 or 4. The Nox inhibitor apocynin inhibited A3AR-induced aortic contraction, ROS generation, and Nox2 activation in the aorta of WT mice but not in A3KO mice.
Materials and Methods
All of the experimental protocols were performed according to the guidelines and approval of the Animal Care and Use Committee at West Virginia University. A3KO (male/female) mice were generated, as described previously (Salvatore et al., 2000) and backcrossed 12 generations to the C57BL/6 background. The corresponding WT (C57BL/6) mice were purchased from Harlan (Indianapolis, IN). A3KO and their corresponding WT mice (10–12 weeks old) were used in this study.
Preparation of Isolated Mouse Aorta.
The mice were sacrificed by deep anesthesia with pentobarbital sodium (65 mg/kg i.p.) followed by thoracotomy. The aorta was gently removed and cleaned of fat and connective tissue. The aorta was cut transversely into four rings that measured 3 to 4 mm in length with extreme care to avoid damaging the endothelium. The rings were mounted vertically between two wire hooks and then suspended in 10-ml organ baths containing Krebs-Henseleit buffer (KH buffer, pH 7.4) of the following composition: 118 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose, and 2.5 mM CaCl2. The KH buffer was maintained at 37°C with continuous bubbling of 95% O2 and 5% CO2. The aortic rings were equilibrated for 90 min with a resting force of 1 g, with changes of the bathing solution at 15-min intervals. The changes in isometric tension were monitored continuously with a fixed-range precision force transducer (TSD, 125 C; BIOPAC Systems, Inc., Goleta, CA) connected to a differential amplifier (DA 100B; BIOPAC Systems, Inc.). The data were recorded on a digital acquisition system and analyzed using Acknowledge 3.5.7 software (BIOPAC Systems, Inc.).
At the end of the equilibration period, aortic rings were contracted with 50 mM KCl to check their viability. The tissues were then contracted with phenylephrine (PE, 10−7 M) to produce consistent submaximal (∼90%) response in our experiments. The aortic rings were then washed several times with KH buffer and allowed to equilibrate for 30 min before the experimental protocol began.
Experimental Protocol.
All experiments were performed in endothelium-intact aortic rings, because we previously showed that activation of A3AR leads to endothelium-dependent aortic contraction because of the higher expression of A3AR in endothelium compared with that in aortic smooth muscle in mouse (Ansari et al., 2007b). The cumulative concentration-response curves for the selective A3AR agonist Cl-IBMECA (10−10–10−5 M) were run in parallel in aortic rings from both WT and A3KO mice. Inhibitors and antagonists [apocynin, 10−5 M; DPI, 10−5 M; 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate (MRS1523), 10−5 M; superoxide dismutase-polyethylene glycol (PEG-SOD), and catalase-polyethylene glycol (PEG-catalase), 100 U/ml each; cromolyn sodium, 10−4 M; and compound 48/80, 100 μg/ml] were added 30 min before aortic contraction with PE and were present throughout the experiments.
ROS Generation in Mouse Aorta.
For reactive oxygen species generation, mouse aortic tissues were cut into 3- to 4-mm-lengths and were first preincubated for 75 min at 37°C in 1 ml of KH buffer. After this incubation, the aortic tissues were incubated with 100 μM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) for 30 min at 37°C. DCFH-DA forms a fluorescent product, 2′,7′-dichlorofluorescein (DCF), intracellularly upon oxidation with ROS (Wang and Joseph, 1999). Fluorescence caused by DCF in each well was measured and recorded for 15 min at 485 nm (excitation) and 530 nm (emission) using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT) with temperature maintained at 37°C. The background fluorescence caused by buffer and DCF was subtracted from the total fluorescence in each well caused by aortic rings in the presence of DCF. Fluorescence intensity units were then normalized by milligrams of wet weight tissue for each aortic ring and expressed as arbitrary fluorescence units per milligram of tissue.
Immunoblotting of Nox1, 2, and 4 in Mouse Aorta.
The aortic tissues from both WT and A3KO mice were processed similar to the organ bath experiments and then incubated in the absence or presence of the Nox inhibitor apocynin (10−5 M) for 30 min before treatment with Cl-IBMECA (10−7 M) for 10 min.
The aortic tissues were homogenized with 6 volumes of ice-cold tissue lysis buffer consisting of 0.05 M Tris-buffered saline (pH 7.4), 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM sodium orthovanadate, and 1 mM sodium fluoride. Homogenized samples were centrifuged for 30 min at 12,000g at 4°C. The protein content of the supernatant was determined using a Bradford protein assay (Bio-Rad Laboratories, Hercules, CA).
Aliquots of the aortic lysates (40 μg of protein/well) were separated on 10% SDS-PAGE. Prestained protein molecular markers (20–112 kDa) were run in parallel. Proteins were transferred to nitrocellulose membranes and incubated with 5% milk for 1 h to block nonspecific binding sites. Membranes were then probed with either anti-gp91 phox (anti-Nox2) mouse monoclonal IgG (BD Biosciences, San Jose, CA) or anti-Nox4 rabbit polyclonal IgG (Abcam, Cambridge, MA) at a dilution of 1:1000 or anti-Nox1 rabbit polyclonal IgG (Abcam) at a dilution of 1:500, followed by incubation with secondary antibodies for 1 h (horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit at 1:10,000 dilution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After extensive washing, membranes were then stripped and reprobed with monoclonal anti-β-actin antibody (Santa Cruz Biotechnology, Inc.) at a dilution of 1:10,000. For detection of bands, the membranes were treated with a ECL Plus (for Nox2, Nox4, and β-actin) or Advance (for Nox1) Kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 1 min and subsequently exposed to ECL Hyperfilm. Relative band intensities were quantified by densitometry (ImageJ 1.43u; National Institutes of Health, Bethesda, MD), and each sample was normalized to the β-actin values. Western blot values are expressed as a percentage of control after densitometric analysis.
Real-Time PCR for Nox2 in Mouse Aorta.
Mouse aortic rings were treated as described previously, and then tissues were snap-frozen in liquid nitrogen and kept at −80°C. Total RNA was isolated from the aortic rings by using TRI reagent (MRC Inc., Cincinnati, OH) followed by purification of the RNA in aqueous phase and removal of genomic DNA by an RNeasy Plus Micro Kit (QIAGEN, Hilden, Germany). This was followed by conversion of 0.5 μg of total RNA into cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions in a total volume of 20 μl. Real-time PCR was performed on an ABI PRISM 7300 Detection System (Applied Biosystems) using TaqMan Universal Master Mix (Applied Biosystems). In brief, the reaction volume (20 μl) included 10 μl of 2× TaqMan Universal Master Mix, 4 μl of cDNA, and 1 μl of 20× FAM-labeled TaqMan gene expression assay. TaqMan inventoried Assays-on-Demand gene expression products were purchased from Applied Biosystems. A Mm01287743_m1 assay was used for the Nox2 gene. An Hs999999_s1 assay was used for ribosomal RNA (18S rRNA) as an endogenous control. The fold difference in expression of target cDNA was determined by using the comparative CT method. The fold difference in gene expression of the target was calculated as described previously (Livak and Schmittgen, 2001).
Drugs Used.
Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma-Aldrich (St. Louis, MO). DCFH-DA, MRS1523, Cl-IBMECA, and DPI were dissolved in dimethyl sulfoxide, whereas apocynin, acetylcholine, PE, PEG-SOD, and PEG-catalase were dissolved in distilled water. Dimethyl sulfoxide final concentration in the organ bath had no effect by itself on the aortic rings.
Data Analysis.
All of the experimental values are presented as means ± S.E.M. (n = number of animals). The comparison among different groups was analyzed by ANOVA followed by the Tukey multiple comparisons test method as a post hoc test. Comparison between two groups was assessed by an unpaired t test. P < 0.05 was taken as significant. The EC50 for aortic contraction was obtained from individual curves by nonlinear regression (curve-fit) graphic analysis. All of the statistical analyses were performed using GraphPad Prism (version 3.0; GraphPad Software Inc., San Diego, CA).
Results
Effect of A3AR Activation on Contractility of WT/A3KO Mouse Aorta.
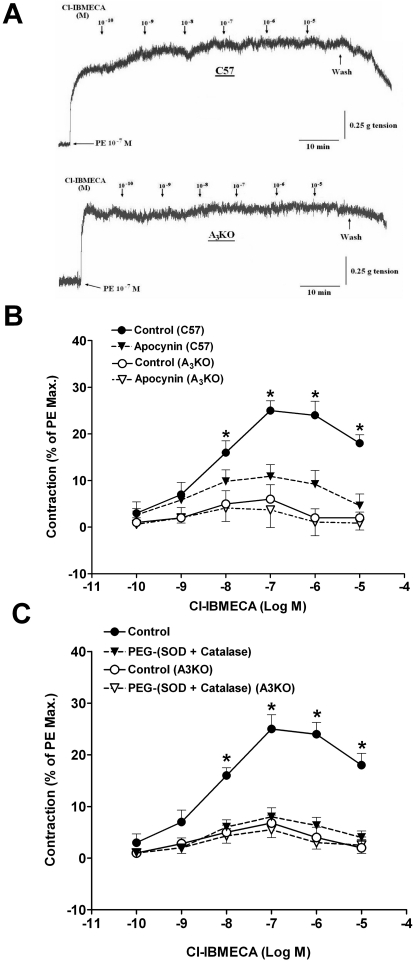
Incubation of WT aortic rings with increasing concentrations of the selective A3AR agonist Cl-IBMECA produced a concentration-dependent contraction with a pEC50 of 8.22 ± 0.27 (Fig. 1, A and B). The contraction induced by Cl-IBMECA was insignificant in A3KO aortic rings. At 10−7 M, Cl-IBMECA increased contraction significantly by 25% in WT, while producing a negligible effect (∼6%) in A3KO mouse aorta (Fig. 1A). The contraction induced by Cl-IBMECA in WT aortic rings was significantly inhibited by preincubation with the Nox inhibitor, apocynin (10−5 M). These results suggest that A3AR-mediated contraction is dependent on Nox activation in mouse aorta.
Fig. 1.
Vascular responses to Cl-IBMECA in WT and A3KO mouse aorta. A, tracing showing the responses to Cl-IBMECA in WT and A3KO mouse aortic rings. B, effect of apocynin on Cl-IBMECA-induced contraction in WT and A3KO mouse aorta. C, effect of PEG-(SOD + catalase) on Cl-IBMECA-induced contraction in WT and A3KO mouse aorta. Data are expressed as the mean ± S.E.M. (n = 6). *, p < 0.05 compared with the A3AR KO control group using one-way ANOVA followed by a Tukey multiple comparison post hoc test.
To further test the involvement of the ROS in the contraction induced by A3AR activation, PEG-SOD, and PEG-catalase as ROS scavengers were tested against Cl-IBMECA-induced contraction (Fig. 1C). Preincubation with PEG-(SOD + catalase) inhibited the contraction induced by Cl-IBMECA in WT aortic rings without affecting that of the A3KO, indicating that A3AR activation causes contraction through the release of ROS.
The role of mast cells in Cl-IBMECA-mediated contraction was also investigated. Preincubation of aortic rings with compound 48/80, a mast cell degranulator, or cromolyn sodium, a mast cell stabilizer, did not affect the baseline or Cl-IBMECA-induced contraction (data not shown). These data suggest that mast cells may not play a significant role in either control of aortic tone or A3AR-mediated contraction in this investigation.
Effect of A3AR Activation on ROS Generation from WT/A3KO Mouse Aorta.
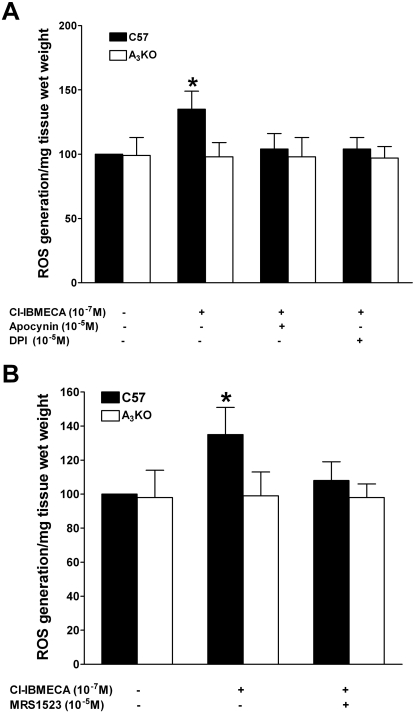
Incubation of WT aortic rings with 10−7 M Cl-IBMECA increased the amount of intracellular ROS production in WT by 35 ± 14% compared with that in corresponding A3KO aortas (Fig. 2A). In contrast, Cl-IBMECA did not increase ROS production in A3KO aorta. Preincubation with Nox inhibitors apocynin (10−5 M) or DPI (10−5 M) prevented this increase in ROS production (Fig. 2A), indicating that the source of ROS activated by Cl-IBMECA is Nox. Likewise, preincubation with the selective A3AR inhibitor MRS1523 (10−7 M) leads to inhibition of ROS production induced by Cl-IBMECA (Fig. 2B), confirming that A3AR activation induces ROS production through Nox.
Fig. 2.
Effects of apocynin and DPI (A) and MRS1523 (B) on Cl-IBMECA-induced ROS generation in WT and A3KO mouse aorta. Data are expressed as the mean ± S.E.M. (n = 4). *, p < 0.05 compared with the corresponding WT control group using an unpaired t test.
Effects of A3AR Activation on Nox1, 2, and 4 Protein Expression in WT/A3KO Mouse Aorta.
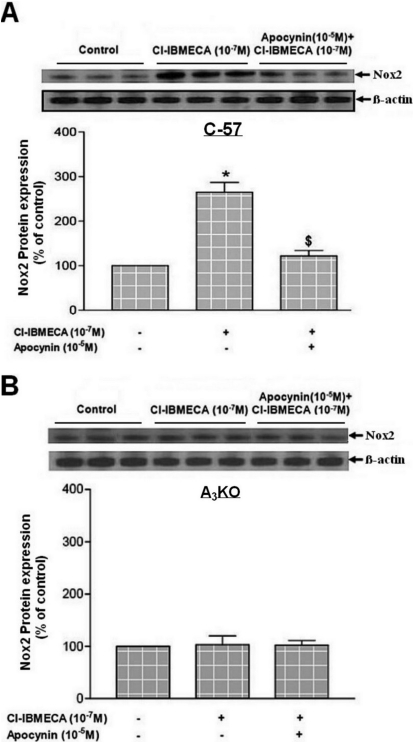
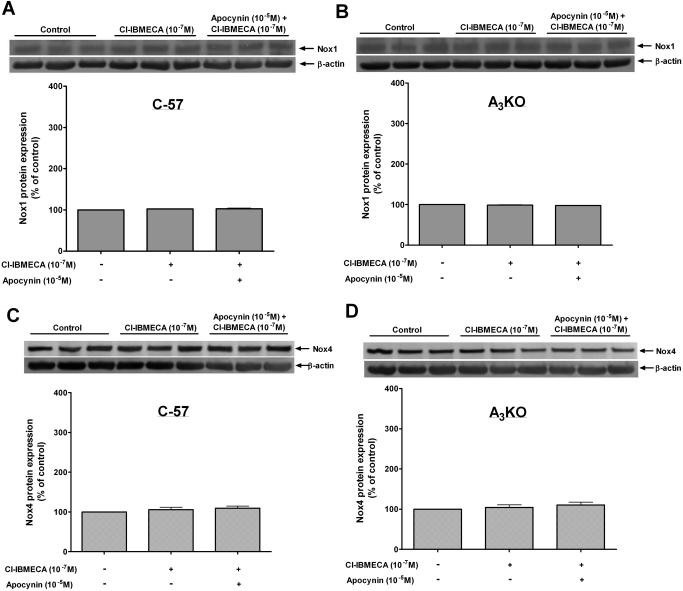
For further confirmation of the role of Nox in the contraction and ROS generation induced by A3AR activation, the protein expression levels of Nox1, 2, and 4 subunits were examined. Activation of A3AR with Cl-IBMECA (10−7 M) selectively increased the expression of Nox2 in WT aorta by 150 ± 15% compared with that in the control (Fig. 3A). This increase in Nox2 expression was inhibited by preincubation with apocynin (10−5 M), whereas neither Cl-IBMECA alone nor with apocynin had an effect on Nox2 expression in A3KO mice aorta (Fig. 3B). On the other hand, Cl-IBMECA (10−7 M) had no effect on the expression of Nox1 (Fig. 4, A and B) or Nox4 (Fig. 4, C and D) either in WT or in A3KO mice aorta. These data confirm the selective activation of the Nox2 subunit by A3AR, leading to ROS generation and contraction in mouse aorta.
Fig. 3.
Effects of Cl-IBMECA on protein expression of Nox2 subunit in WT (A) and A3KO (B) mouse aorta. Data are expressed as the mean ± S.E.M. (n = 3). *, p < 0.05 compared with control; $, p < 0.05 compared with Cl-IBMECA alone using one-way ANOVA followed by a Tukey multiple comparisons post hoc test.
Fig. 4.
Effects of Cl-IBMECA on protein expression of Nox1 in WT (A), Nox1 in A3KO (B), Nox4 in WT (C), and Nox4 in A3KO (D) mouse aorta. Data are expressed as the mean ± S.E.M. (n = 3).
Effects of A3AR Activation on Nox2 mRNA Expression in WT/A3KO Mouse Aorta.
Because Nox2 protein expression was selectively increased by A3AR activation in WT but not in A3KO mouse aorta, we examined the change in Nox2 mRNA expression induced by Cl-IBMECA. Of interest, the mRNA expression of Nox2 was not affected by Cl-IBMECA in either WT or A3KO mouse aorta (data not shown), suggesting that A3AR activation enhances Nox2 protein expression at a post-transcriptional level.
Discussion
This is the first study to show that Nox are involved in A3AR-induced contraction of mouse aorta. However, other studies have shown a relationship between adenosine receptors and Nox in other tissues (Carlstrom et al., 2009; Gebremedhin et al., 2010) but not in aorta. Our findings show that A3AR activation using the selective agonist Cl-IBMECA causes contraction of aorta in WT mice but not in A3KO mice. This A3AR-mediated contraction of aorta was inhibited by a Nox inhibitor (apocynin), in addition to ROS scavengers PEG-SOD and PEG-catalase, and was not affected by the mast cell degranulator compound 48/80 or the stabilizer cromolyn sodium. We also found that A3AR activation leads to increased intracellular ROS generation, which was inhibited by apocynin, DPI, and the selective A3AR antagonist, MRS1523. Furthermore, the protein expression of Nox2 subunit was selectively increased by Cl-IBMECA in WT but not in A3KO mice aorta without affecting either Nox1 or 4.
We used the pharmacological selective A3AR agonist Cl-IBMECA, in addition to A3KO mice to confirm that A3AR activation induces aortic contraction in WT mice. The role of A3AR in the control of vascular tone has also been demonstrated by our laboratory (Ansari et al., 2007b). In another study, infusion of adenosine in A3KO mice has been shown to cause a significant decrease in blood pressure compared with that in WT mice (Zhao et al., 2000). In addition, the effects of A3AR activation on vascular tone have also been demonstrated through inhibition or negative modulation of coronary flow in isolated mouse heart (Talukder et al., 2002), vasoconstriction in hamster arterioles (Shepherd et al., 1996), and reversal of vascular hyporeactivity after hemorrhagic shock in rats (Zhou et al., 2010).
It should be noted that mast cells can be stimulated by A3AR to release histamine and thromboxane, leading to either vasoconstriction (Shepherd et al., 1996) or a short-lasting hypotension in conscious rats (Van Schaick et al., 1996). Our data show that neither the mast cell degranulator (compound 48/80) nor stabilizer (cromolyn sodium) affected A3AR-induced contraction, suggesting that mast cells may not play a significant role in our model.
In the present work, apocynin significantly reduced the contraction induced by Cl-IBMECA, suggesting that A3AR-induced contraction in mouse aorta involves Nox. We have shown previously that COX-1 plays a role in this A3AR response (Ansari et al., 2007b); therefore, it is likely that there may be a relationship between Nox and COX-1. Nox activity has been shown to be activated through the arachidonic acid pathway in cardiac fibroblasts (Colston et al., 2005). In addition, ROS derived from Nox can induce COX-2 protein in human neutrophils (Vega et al., 2006), suggesting an interaction between Nox and COX pathways. Taken together, these data along with our data indicate that A3AR may play an important role in the regulation of vascular tone, mainly through endothelium-dependent pathways.
A3ARs have been shown to be involved in the modulation of diseases involving ROS generation such as ischemia-reperfusion injury (Maddock et al., 2003; Zatta et al., 2006), suggesting that A3AR effects may be related to ROS generation. It has been shown that adenosine constricts renal arterioles of WT but not Nox KO mice (Carlstrom et al., 2009), whereas its cerebral vasodilation in rat involves Nox (Gebremedhin et al., 2010). Furthermore, A3ARs have been shown to modulate Nox activity in monocytes (Broussas et al., 1999) and in prostate cancer cells (Jajoo et al., 2009). Our results show that A3AR induces ROS production that was inhibited by apocynin and DPI. In addition, Cl-IBMECA induced selective protein expression of Nox2 subunit only in WT but not in A3KO animals without affecting Nox1 or 4, suggesting that A3AR induces ROS generation, possibly through the activation of Nox2.
We previously demonstrated that activation of A3AR leads to endothelium-dependent aortic contraction (Ansari et al., 2007b). However, because Nox isoforms are expressed in both endothelium and vascular smooth muscle, further studies are needed to differentiate the role of each cell type in A3AR-induced ROS generation.
Of interest, the protein expression of Nox2 was increased by Cl-IBMECA, but the mRNA was not affected, suggesting that activation of A3AR enhances Nox2 protein expression through post-transcriptional response, such as changes in protein translation and/or turnover. This finding may also partly explain the rapid change in Nox2 protein expression by short-term exposure to Cl-IBMECA. However, a direct correlation between this rapid change in Nox2 protein and vascular contraction requires further confirmation. Rapid changes in gene and protein expression patterns have been shown within minutes of tissue ischemia after surgical tumor excision (Spruessel et al., 2004). In addition, up-regulation of different cellular proteins due to changes in the translational level or protein turnover without changes in mRNA expression has been shown (Burd et al., 2008; Yoshimura et al., 2008).
Vascular Nox are activated within minutes of stimulation (Seshiah et al., 2002), with different species of ROS produced, mainly superoxide anions (O2−). This O2− can enhance vasoconstriction by rapidly converting nitric oxide to the much less vasodilator peroxynitrite (Koppenol et al., 1992). The ROS indicator DCFH-DA used in this study is nonspecific because it can detect several ROS such as O2−, hydrogen peroxide (H2O2), and hydroxyl radicals (Biziukin et al., 1995). However, because most Nox isoforms produce O2− as a main ROS; therefore, our data indicate that A3AR possibly enhances O2− production through Nox2, which may play an important role in the vasoconstriction induced by A3AR in the mouse aorta. Further studies are needed to identify the various ROS produced by the activation of A3AR.
In the literature, some concerns were raised about the selectivity of Nox inhibitors such as apocynin (Schlüter et al., 2008) and DPI (Stuehr et al., 1991), which may affect the results obtained with these inhibitors in our study. Therefore, use of these inhibitors solely may present some arguments about their specific targets. To address this issue, our studies used not only these inhibitors but also ROS generation and Nox subunit protein expression as tools to confirm the relationship between A3AR and Nox.
Because ROS are involved in several diseases and A3AR modulators have been tested in some diseases such as renal cancer (Jajoo et al., 2009), a better understanding of the relationship between A3AR and ROS generation, possibly through Nox, may result in potential therapeutic targets in cardiovascular pathophysiological situations involving higher oxidative stress. In addition, this study shows that Nox inhibitors can be used to attenuate the vasoconstrictor responses to A3AR; therefore, enhancement of the vasodilator responses of adenosine through A2A/BAR (relaxing receptors) could be beneficial in conditions such as hypertension and coronary artery diseases.
In conclusion, A3AR activation induces contraction of the mouse aorta that is dependent on ROS generation, possibly mediated through Nox2.
This study was supported by National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL027339, HL094447, HL071802].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180828.
- AR
- adenosine receptor
- COX-1
- cyclooxygenase-1
- KO
- knockout
- Nox
- NADPH oxidase(s)
- ROS
- reactive oxygen species
- Cl-IBMECA
- 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- WT
- wild type
- A3KO
- A3AR knockout
- KH buffer
- Krebs-Henseleit buffer
- PE
- phenylephrine
- DPI
- diphenyleneiodonium chloride
- MRS1523
- 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate
- PEG-SOD
- superoxide dismutase-polyethylene glycol
- PEG-catalase
- catalase-polyethylene glycol
- DCFH-DA
- 2′,7′-dichlorofluorescin diacetate
- DCF
- 2′,7′-dichlorofluorescin
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: El-Awady, Ansari, and Mustafa.
Conducted experiments: El-Awady, Ansari, and Fil.
Contributed new reagents or analytic tools: Tilley.
Performed data analysis: El-Awady and Ansari.
Wrote or contributed to the writing of the manuscript: El-Awady, Ansari, and Mustafa.
References
- Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. (2007a) Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292:H719–H725 [DOI] [PubMed] [Google Scholar]
- Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. (2007b) Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol 293:H3448–H3455 [DOI] [PubMed] [Google Scholar]
- Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. (2009) A1 adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 297:H1032–H1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biziukin AV, Korkina LG, Velichkovskii BT. (1995) Comparative use of 2,7-dichlorofluorescein diacetate, dihydrorhodamine 123, and hydroethidine for studying oxidative metabolism of phagocytosing cells. Bull Exp Biol Med 119:347–351 [PubMed] [Google Scholar]
- Broussas M, Cornillet-Lefèbvre P, Potron G, Nguyen P. (1999) Inhibition of fMLP-triggered respiratory burst of human monocytes by adenosine: involvement of A3 adenosine receptor. J Leukoc Biol 66:495–501 [PubMed] [Google Scholar]
- Burd CJ, Kinyamu HK, Miller FW, Archer TK. (2008) UV radiation regulates Mi-2 through protein translation and stability. J Biol Chem 283:34976–34982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE. (2009) Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296:R72–R79 [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. (2001) Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131–140 [DOI] [PubMed] [Google Scholar]
- Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. (2005) H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett 579:2533–2540 [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Weinberger B, Lourim D, Harder DR. (2010) Adenosine can mediate its actions through generation of reactive oxygen species. J Cereb Blood Flow Metab 30:1777–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. (2000) A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87:26–32 [DOI] [PubMed] [Google Scholar]
- Jajoo S, Mukherjea D, Watabe K, Ramkumar V. (2009) Adenosine A3 receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia 11:1132–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T, Quinn MT, Lambeth JD. (2007) Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5:834–842 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Maddock HL, Gardner NM, Khandoudi N, Bril A, Broadley KJ. (2003) Protection from myocardial stunning by ischaemia and hypoxia with the adenosine A3 receptor agonist, IB-MECA. Eur J Pharmacol 477:235–245 [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Morrison RR, Teng B, Pelleg A. (2009) Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 193:161–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Ponnoth DS, Ansari HR, Batchelor TP, Dey RD, Ledent C, Mustafa SJ. (2009) A2A adenosine receptor deficiency leads to impaired tracheal relaxation via NADPH oxidase pathway in allergic mice. J Pharmacol Exp Ther 330:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribé D, Sawbridge D, Thakur S, Hussey M, Ledent C, Kitchen I, Hourani S, Li JM. (2008) Adenosine A2A receptor signaling regulation of cardiac NADPH oxidase activity. Free Radic Biol Med 44:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. (2000) Disruption of the A3 adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275:4429–4434 [DOI] [PubMed] [Google Scholar]
- Schlüter T, Steinbach AC, Steffen A, Rettig R, Grisk O. (2008) Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80:271–279 [DOI] [PubMed] [Google Scholar]
- Schröder K. (2010) Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol 10:122–126 [DOI] [PubMed] [Google Scholar]
- Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. (2002) Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91:406–413 [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Linden J, Duling BR. (1996) Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res 78:627–634 [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassègue B, Griendling KK, et al. (2004) Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a Nox1-based NADPH oxidase. Circ Res 95:773–779 [DOI] [PubMed] [Google Scholar]
- Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, Spangenberg J, Zornig C, Juhl HH, David KA. (2004) Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques 36:1030–1037 [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. (1991) Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J 5:98–103 [DOI] [PubMed] [Google Scholar]
- Tabrizchi R, Bedi S. (2001) Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther 91:133–147 [DOI] [PubMed] [Google Scholar]
- Talukder MA, Morrison RR, Jacobson MA, Jacobson KA, Ledent C, Mustafa SJ. (2002) Targeted deletion of adenosine A3 receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol 282:H2183–H2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaick EA, Jacobson KA, Kim HO, IJzerman AP, Danhof M. (1996) Hemodynamic effects and histamine release elicited by the selective adenosine A3 receptor agonist 2-Cl-IB-MECA in conscious rats. Eur J Pharmacol 308:311–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A, Chacón P, Alba G, El Bekay R, Martín-Nieto J, Sobrino F. (2006) Modulation of IgE-dependent COX-2 gene expression by reactive oxygen species in human neutrophils. J Leukoc Biol 80:152–163 [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616 [DOI] [PubMed] [Google Scholar]
- Yoshimura FK, Luo X, Zhao X, Gerard HC, Hudson AP. (2008) Up-regulation of a cellular protein at the translational level by a retrovirus. Proc Natl Acad Sci USA 105:5543–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatta AJ, Matherne GP, Headrick JP. (2006) Adenosine receptor-mediated coronary vascular protection in post-ischemic mouse heart. Life Sci 78:2426–2437 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Makaritsis K, Francis CE, Gavras H, Ravid K. (2000) A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta 1500:280–290 [DOI] [PubMed] [Google Scholar]
- Zhou R, Chen F, Li Q, Hu DY, Liu LM. (2010) Stimulation of the adenosine A3 receptor reverses vascular hyporeactivity after hemorrhagic shock in rats. Acta Pharmacol Sin 31:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]