Abstract
Catecholamines released from the sympathetic nervous system in response to stress or injury affect expression of inflammatory cytokines generated by immune cells. α1-Adrenergic receptors (ARs) are expressed on innate immune cell populations, but their subtype expression patterns and signaling characteristics are not well characterized. Primary human monocytes, a human monocytic cell line, and monocyte-derived macrophage cells were used to measure expression of the proinflammatory mediator interleukin (IL)-1β responding to lipopolysaccharide (LPS) in the presence or absence of α1-AR activation. Based on our previous findings, we hypothesized that α1-AR stimulation on innate immune cells positively regulates LPS-initiated IL-1β production. IL-1β production in response to LPS was synergistically higher for both monocytes and macrophages in the presence of the selective α1-AR agonist (R)-(−)-phenylephrine hydrochloride (PE). This synergistic IL-1β response could be blocked with a selective α1-AR antagonist as well as inhibitors of protein kinase C (PKC). Radioligand binding studies characterized a homogenous α1B-AR subtype population on monocytes, which changed to a heterogeneous receptor subtype expression pattern when differentiated to macrophages. Furthermore, increased p38 mitogen-activated protein kinase (MAPK) activation was observed only with concurrent PE and LPS stimulation, peaking after 120 and 30 min in monocytes and macrophages, respectively. Blocking the PKC/p38 MAPK signaling pathway in both innate immune cell types inhibited the synergistic IL-1β increase observed with concurrent PE and LPS treatments. This study characterizes α1-AR subtype expression on both human monocyte and macrophage cells and illustrates a mechanism by which increased IL-1β production can be modulated by α1-AR input.
Introduction
The innate immune system is important as the first line of defense against a pathological challenge or damage to tissue. It comprises a group of cells and other mechanisms that act as the body's first means of protection against infection or irritation, working to remove the injurious stimuli and promote healing. The sympathetic nervous system is an important regulatory mechanism for the innate immune response. Specifically, production of the endogenous catecholamines epinephrine and norepinephrine are increased during times of anxiety, pathogenic challenge, or injury and are known to play a role in many physiological responses including alteration of the innate immune response (Calcagni and Elenkov, 2006). For example, norepinephrine has been shown to have a variety of effects on innate immune responses including alteration of cytokine levels after a bacterial endotoxin challenge (Elenkov et al., 2008). Moreover, prolonged stimulation of the sympathetic nervous system leads to increased inflammation with a corresponding decrease in the ability to fight infections (Johnson et al., 2005). In addition, in many chronic inflammatory diseases such as rheumatoid arthritis and multiple sclerosis, increased sympathetic activity is correlated with a heightened disease progression (Brosnan et al., 1985; Capellino and Straub, 2008). Understanding how sympathetic input regulates innate immune cytokine production may prove useful in treating or slowing the progression of these and many other chronic inflammatory disorders.
Effects of epinephrine and norepinephrine are mediated through three AR families that are further characterized into nine receptor subtypes (α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3; see review by Guimarães and Moura, 2001). All three AR families are found to be expressed on many types of immunocompetent cells involved with innate immune responses and are generally thought to play an anti-inflammatory role. For example, activation of α2- or β-AR types have been shown to be responsible for many anti-inflammatory immune cell responses observed after norepinephrine treatment (Farmer and Pugin, 2000; Romero-Sandoval et al., 2005). However, increasing evidence is found in the literature characterizing increased cytokine production by α2-AR stimulation (Spengler et al., 1990; Felsner et al., 1995; Haskó et al., 1995; Flierl et al., 2007) or modified by selective β-AR activation (Grisanti et al., 2010). This demonstrates the importance for determining AR expression profiles from a finite cell population that correspond to specific immune responses. Immunomodulatory α1-AR characteristics are the least studied of all three AR families. α1-AR stimulation seems to possess a proinflammatory effect on immune system responses, which is correlative to many chronic inflammatory disease states (Maestroni, 2000; Heijnen et al., 2002; Perez et al., 2009). For example, in children with polyarticular juvenile rheumatoid arthritis, α1-AR expression on peripheral blood mononuclear cells is responsible for increasing production of the proinflammatory cytokine IL-6 (Heijnen et al., 1996). Other investigations have implicated α1-AR activation to be important for increasing inflammatory responses in an established model of multiple sclerosis (Brosnan et al., 1985). Therefore, understanding α1-AR mechanisms that modulate innate immune cytokine production is perhaps important for the development of therapeutic strategies that would selectively regulate inflammatory responses in diseases for which immunocompetent cells play an essential pathological function.
Based on our previous findings in which cytokine/chemokine changes from human monocytes after simultaneous α1-AR and Toll-like receptor (TLR) 4 stimulation were screened using an antibody array, we investigated the hypothesis that α1-AR modulated innate immune responses have proinflammatory outcomes, which depend on the selective signaling characteristics of α1-AR subtypes expressed on immunocompetent cells (Grisanti et al., 2011). Using primary and immortalized (THP-1) human monocytes as a monocytic model system as well as phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells as a monocyte-derived macrophage cell model (Tsuchiya et al., 1982), we examined effects of concurrent α1-AR stimulation on LPS-induced inflammatory responses. LPS is a component of the Gram-negative bacterial cell wall that activates TLR4 subtypes expressed on immunocompetent cells, which leads to the production of many proinflammatory cytokines including IL-6, IL-8, IL-1β, and tissue necrosis factor α. Our results not only document a unique switching of α1-AR subtype expression during myeloid cell development, but are also the first to demonstrate a mechanism for α1-AR-mediated increases in IL-1β generation from immunocompetent cells that have been pathogenically challenged to initiate an inflammatory response.
Materials and Methods
Materials.
Bisindolylmaleimide II (Bis II), LPS (Escherichia coli serotype O26:B6), (R)-(−)-phenylephrine hydrochloride (PE), staurosporine (STS), PMA, phentolamine, [2-[(2,6-dimethoxyphenoxyethyl)aminomethyl]-1,4-benzodioxane hydrochloride] (WB-4101), 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB 203580), 5-methylurapidil (5-MU), benzamidine, leupeptin, phenylmethylsulfonyl fluoride, and bacitracin were obtained from Sigma-Aldrich (St. Louis, MO). [8-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride] (BMY 7378) and 2-{[β-(hydroxyphenyl)ethyl]aminomethyl}-1-tetralone hydrochloride (BE 2254) were purchased from Tocris Bioscience (Ellisville, MO). (±)-β-([125]Iodo-4-hydroxyphenyl)-ethyl-aminomethyl-tetralone (125I-HEAT) was ordered from PerkinElmer Life and Analytical Sciences (Waltham, MA). All other buffers and chemical reagents were of biological grade and purchased from Thermo Fisher Scientific (Waltham, MA).
Cell Culture.
THP-1 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI medium 1640 (HyClone Laboratories, Logan, UT) with 2 mM l-glutamine adjusted to contain 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, and 10 mM HEPES (complete media), supplemented with 10% heat inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA) under standard cell culture growth conditions (37°C/5% CO2/95% humidified air). To differentiate monocytes into macrophages, THP-1 cells were treated with 200 nM PMA 48 h before all experiments. Cells (1 × 106 per treatment group) were washed with serum-free complete media 30 min before experiments and allowed to become quiescent before preincubation with receptor antagonists (1 h, 500 nM BE 2254) or kinase inhibitors (1 h, 10 nM STS; 1 h, 200 nM Bis II; 16 h, 1 μM SB 203580) followed by treatment with the selective α1-AR agonist PE (10 μM) and/or the TLR4 agonist LPS (25 ng/ml). Preliminary temporal studies were performed to ascertain a time point (3 h) where significant amounts of IL-1β were generated in response to LPS. Final drug concentrations were based on affinity values calculated from competition binding curves (BE 2254), preliminary concentration-response experiments (PE, LPS), or IC50 values of chemical inhibitors (STS, Bis II, SB 203580). Immunocompetent cell viability was assessed by trypan blue exclusion staining. More than 99% cell viability was observed for all treatments after 3 or 24 h and did not significantly differ between experimental groups (data not shown).
Isolation of Primary Human Monocytes.
The lymphocyte layer was obtained from the peripheral blood of healthy adults. In brief, human monocytes were separated by centrifugation at 600g for 20 min at 22°C using a 30 to 45 to 60% Percoll density gradient. The monocyte-enriched fraction was removed from the 30 to 45% gradient interface, washed, then resuspended in complete media containing 10% heat inactivated fetal bovine serum and incubated overnight under standard cell culture growth conditions. A total of 1 × 106 cells/ml were washed in serum-free complete medium and allowed to become quiescent for 30 min before the addition of PE (10 μM) and/or LPS (25 ng/ml).
Membrane Preparation for Receptor Binding.
A crude membrane preparation was performed on untreated THP-1 cells and PMA-differentiated THP-1 cells as described previously (Grisanti et al., 2010). In short, cells were collected in a 50-ml conical tube, followed by two washings at 500g using cold Hank's balanced salt solution. Cells were then resuspended in water containing a protease inhibitor cocktail (10 μg/ml benzamidine, 10 μg/ml leupeptin, 20 μg/ml phenylmethylsulfonyl fluoride, and 10 μg/ml bacitracin). The cells were disrupted by freezing at −80°C for 20 min followed by homogenization of the thawed suspension using 25 strokes from a loose-fitting Dounce homogenizer (B) pestle. The mixture was then centrifuged at 2100g for 15 min to remove nuclear debris. After centrifugation, HEM buffer (20 mM HEPES, 1.4 mM EGTA, 12.5 mM MgCl2, pH 7.4) was added, and the mixture was recentrifuged at 30,000g for 15 min. The final pellet was resuspended in HEM buffer containing 10% glycerol and stored at −80°C until use for radioligand binding. Protein concentrations were determined using the method of Bradford as described previously (Grisanti et al., 2010).
Radioligand Binding.
Radioligand binding was performed using crude THP-1 or PMA-differentiated THP-1 cell membranes as described previously (Grisanti et al., 2010). In brief, saturation binding experiments were performed using the selective α1-AR radioligand antagonist 125I-HEAT. Cell membranes were allowed to equilibrate for 1 h at 37°C with increasing concentrations of 125I-HEAT (0.5–0.01 nM) in a 250-μl total volume of HEM buffer. A saturable concentration (100 μM) of the α-AR antagonist phentolamine was used to determine nonspecific binding. Total binding was stopped by filtering the equilibrated cell membranes through Whatman (Clifton, NJ) GF/B filters that had been soaked in 0.1% bovine serum albumin and 0.3% polyethylenimine to reduce nonspecific binding to the filter. This was followed by washing the membrane-bound filter five times with 5 ml of cold (4°C) HEM buffer to remove any unbound drug. Total and nonspecific binding to cell membrane preparations was determined from the remaining radioactive counts. cpm values were plotted as a function of the 125I-HEAT concentration and from each rectangular hyperbola, specific binding site densities (Bmax) as well as the equilibrium dissociation constant (Kd) of 125I-HEAT for these putative α1-ARs were calculated using nonlinear regression analysis (Prism version 5.04 for Windows; GraphPad Software, Inc., San Diego CA). Competitive radioligand binding was performed using a single concentration (0.1 nM) of 125I-HEAT with increasing concentrations of the selective α1-AR antagonist BE 2254 or the subtype-selective α1-AR antagonists 5-MU, WB-4101, and BMY 7378 in a 250-μl total volume of HEM buffer. Specific radioactive counts were plotted as a function of the competitive receptor antagonist concentration, and nonlinear regression analysis was used to determine the concentration of receptor antagonist that reduced specific 125I-HEAT binding by 50% (IC50). Equilibrium dissociation constants (Ki) of each competitive α1-AR antagonist for specific 125I-HEAT binding sites were calculated using the method of Cheng and Prusoff as described previously (Grisanti et al., 2010).
Plasmids and Transfections.
A dominant-negative (DN) p38 MAPK and empty vector DNA plasmid constructs have been described previously (Whitmarsh et al., 1995) and were kindly provided by Dr. Philip Howe (The Cleveland Clinic Foundation, Cleveland, OH). THP-1 cells were individually transfected with DN p38 MAPK or empty vector constructs (5 × 106 cells/0.5 μg of DNA) by electroporation using the Amaxa Nucleofector system according to the manufacturer's instructions (Amaxa Biosystems, Gaithersburg, MD). Forty eight hours after transfection, cells were treated 3 h with PE (10 μM) and/or LPS (25 ng/ml) and subsequently lysed for immunoblot analysis.
Immunoblot Hybridization.
After drug treatments, an equal number of cells were lysed using a modified radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% SDS, 1.0% Triton X-100, 1.0% sodium deoxycholate, 5 mM EDTA) containing 1.0% protease and phosphatases inhibitor cocktails (Sigma-Aldrich). Equal amounts of total cell lysates were resolved by SDS-polyacrylamide gel electrophoresis (12% for IL-1β and 10% for MAPK analysis) then transferred to polyvinylidene difluoride membranes (Millipore Corporation, Billerica, MA). Polyacrylamide gels were loaded with 60 or 20 μg of protein/well for IL-1β and MAPK immunoblots, respectively. Expression was measured by immunoblotting overnight at 4°C with diluted antibodies against human IL-1β (2 μg/ml; R&D Systems, Minneapolis, MN), extracellular signal-regulated kinase (ERK; 1:1000; Cell Signaling Technology, Danvers, MA), phospho-ERK (1:1000; Cell Signaling Technology), c-Jun N-terminal kinase (JNK; 1:1000; Cell Signaling Technology), phospho-JNK (1:1000; Cell Signaling Technology), phopho-p38 (1:1000; Cell Signaling Technology), p38 (1:1000; Cell Signaling Technology), α1A-AR (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), α1B-AR (1:1000; Santa Cruz Biotechnology, Inc.), α1D-AR (1:1000; Santa Cruz Biotechnology, Inc.), or actin (1:1000; Santa Cruz Biotechnology, Inc.). After washing, polyvinylidene difluoride membranes were incubated at 22°C for 90 min with the appropriate diluted horseradish peroxidase-linked secondary antibody (1:5000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Bound antibody was visualized by chemiluminescent imaging (Thermo Fisher Scientific) and documented by digital photography (UVP, Inc., Upland, CA). Pixel values were normalized to β-actin or nonphosphorylated MAPK and compared with basal expression levels. Protein concentrations were measured using the method of Bradford as described previously (Grisanti et al., 2010).
Enzyme-Linked Immunosorbent Assay.
After drug treatment, an equal number of THP-1 cells were centrifuged at 600g for 5 min to pellet the cells. The supernatant was then collected and stored at −20°C until use for ELISA. Concentrations of IL-1β in the culture media were determined using the human IL-1β/IL-1F2 Quantikine HS ELISA (R&D Systems) according to the manufacturer's instructions. The minimal and maximal IL-1β detection limit of the standard curve that ran with each ELISA was 0.06 and 8 pg/ml, respectively.
Statistical Analyses.
A Wald-Wolfowitz runs test was used to determine whether the data differed significantly from a linear relationship (p < 0.05). For each experiment, the fitted iterative nonlinear regression curve that best represented the data was determined using a partial f test (p < 0.05). Significance among groups was tested using an unpaired t test or one-way analysis of variance followed by a Tukey's multiple comparison test (p < 0.05). All values are reported as the mean ± S.E.M. of n experiments, performed in duplicate. Each n represents an individual experiment from an independent cell preparation or passage.
Results
α1-AR Stimulation Increases IL-1β Production in Human Monocytes Responding to LPS.
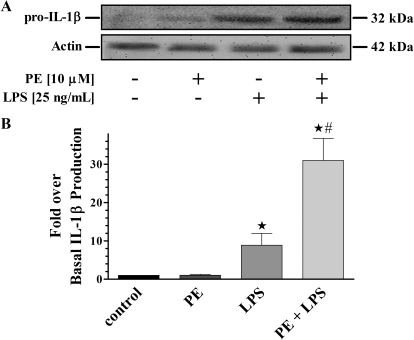
We first sought to determine the ex vivo effects of simultaneous α1-AR activation on inflammatory cytokine responses generated by pathogenically primed primary monocytes isolated from human blood. Based on our previous report (Grisanti et al., 2011), primary monocytes were treated for 3 h with LPS in the presence or absence of PE, then probed for changes in the level of IL-1β production (Fig. 1). Immunoblot analysis showed no change from basal in IL-1β generation for primary monocytes treated with PE alone. However, an anticipated significant (p < 0.05) increase over basal in generated IL-1β was observed for cells treated with LPS only. More importantly, an unexpected synergistic increase in IL-1β production is shown from cells treated concurrently with PE and LPS, which was significantly different (p < 0.05) from both control and LPS-only treatments.
Fig. 1.
α1-AR modulation of IL-1β production from LPS-challenged primary human monocytes. A, representative immunoblot of resolved total cell lysates from isolated primary human monocytes. The 42-kDa actin band is shown as a loading control. B, quantitative analysis of all immunoblots (n = 7) showed increased expression of IL-1β from cells treated only with LPS (8.9 ± 3.1-fold). In addition, there was a synergistic increase in IL-1β generation from cells treated with PE plus LPS (31.0 ± 5.8-fold). There were no differences in IL-1β production from cells treated with PE (1.0 ± 0.6-fold) alone. ★, p < 0.05 versus control; #, p < 0.05 versus LPS.
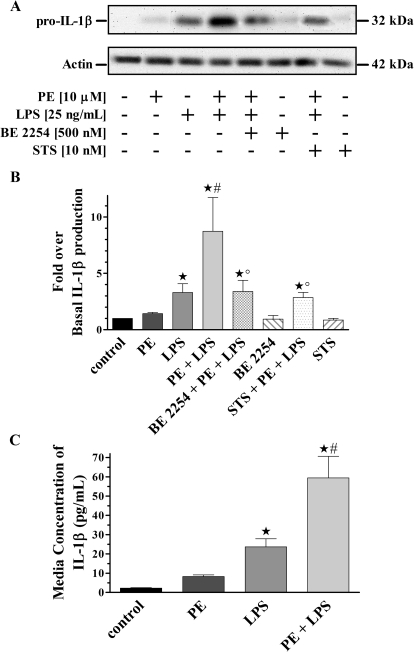
To confirm our ex vivo findings and establish an in vitro monocyte model system for further receptor-signal transduction investigations, THP-1 cells were treated in the same manner as described for primary human monocytes. Resolved THP-1 cell lysates were then probed for changes in IL-1β production (Fig. 2, A and B). Immunoblot analysis again showed no change from basal in generated IL-1β from THP-1 cells treated with PE alone. Likewise, a significant (p < 0.05) increase over basal in IL-1β production was observed for cells treated with LPS only. Moreover, a synergistic increase in IL-1β production was observed from cells treated with both PE and LPS, which was significantly different (p < 0.05) from both control and LPS-only treatments. In addition, this PE-immunomodulatory IL-1β response was blocked in the presence of the selective α1-AR antagonist BE 2254, to levels that were significantly (p < 0.05) different from control as well as concurrent PE and LPS treatments. However, IL-1β amounts were similar to levels generated using LPS only, indicating a specific α1-AR-mediated event. Treatment of cells with BE 2254 alone showed no difference in IL-1β amounts from control.
Fig. 2.
α1-AR mediated synergistic IL-1β production from a human monocyte cell line. A, representative immunoblot of resolved THP-1 total cell lysates. The 42-kDa actin band is shown as a loading control. B, quantitative analysis of all immunoblots (n = 5) showed increased IL-1β expression from monocytes treated with LPS alone (3.3 ± 0.8-fold). There was a synergistic increase in IL-1β generated from cells treated with PE plus LPS (8.7 ± 3.0-fold). There were decreased IL-1β levels from monocytes pretreated with BE 2254 (3.4 ± 1.0-fold) or STS (2.8 ± 0.4-fold). There were no IL-1β differences from cells treated with PE (1.0 ± 0.6-fold), BE 2254 (0.9 ± 0.3-fold), or STS (0.9 ± 0.2-fold) alone. C, quantitative ELISA analysis for secreted amounts of IL-1β from THP-1 cells (n = 5). There was increased IL-1β secretion from monocytes treated only with LPS (23.6 ± 4.2 pg/ml). There were also synergistic IL-1β increases from monocytes treated with PE plus LPS (59.5 ± 11.1 pg/ml). There were no differences in IL-1β secreted from cells treated with PE alone (8.2 ± 0.9 pg/ml) compared with basal (2.3 ± 0.2 pg/ml). ★, p < 0.05 versus control; #, p < 0.05 versus LPS; °, p < 0.05 versus PE + LPS.
To test the hypothesis that α1-AR modulation of IL-1β production in LPS-challenged monocytes is mediated through classic Gαq-initiated signal transduction pathways, we pretreated with the nonspecific PKC inhibitor STS (Fig. 2, A and B). Synergistic increases in IL-1β production were not observed in lysates from cells pretreated with STS followed by concurrent PE and LPS incubation. However, the measured amount of IL-1β generated was significantly different (p < 0.05) from control and similar to levels observed with LPS alone. Furthermore, pretreatment with STS alone did not change IL-1β amounts compared with control.
Pro-IL-1β processing into the bioactive 17-kDa secreted form is a result of constitutive caspase-1 activation through a one-time stimulation of TLR4 receptors (Netea et al., 2009). As a result, we expect that heightened pro-IL-1β generation observed from treated monocytic cell lysates will correlate to the same increase of secreted cytokine. To test this hypothesis the amount of secreted IL-1β was quantified using ELISA analysis from conditioned media of treated THP-1 cells (Fig. 2C). Similar to quantifying levels of pro-IL-1β (32 kDa) obtained using immunoblot analysis, there were no changes in the amounts of secreted IL-1β from cells treated with PE alone. However, there were significant (p < 0.05) increases in amounts of IL-1β secreted from LPS-treated cells compared with control. More importantly, we observed a synergistic IL-1β increase from the media of cells treated with PE plus LPS, which was significantly (p < 0.05) different from amounts secreted basally or from LPS-only treatment.
Concurrent PE and LPS Stimulation Leads to PKC-Dependent p38 MAPK Phosphorylation in Human Monocytes and Macrophages.
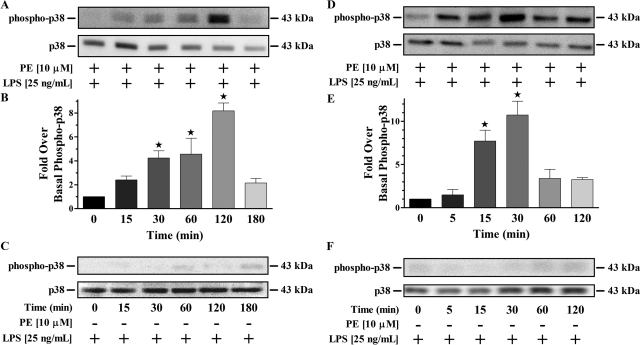
α1-AR stimulation has been shown to increase MAPK activity, leading to greater proinflammatory cytokine production from fibroblasts (Perez et al., 2009). Therefore, we wanted to determine whether monocyte MAPK pathways were being activated by α1-AR agonists in the presence of LPS. Temporal responses of THP-1 cells treated with LPS alone or simultaneously with PE and LPS over 3 h were performed then analyzed for MAPK activation (Fig. 3, A–C). No temporal differences above basal for phosphorylated ERK or JNK were observed from monocytes stimulated with LPS only or concomitantly with PE and LPS (data not shown). Conversely, p38 MAPK was temporally phosphorylated at significant (p < 0.05) levels 30 min after simultaneous PE and LPS treatment, with peak activation occurring at 2 h (Fig. 3, A and B). This response was specific for concurrent α1-AR and TLR4 stimulation because activation of p38 MAPK was not observed at any time point for cells treated with LPS alone (Fig. 3C). Further assessments of p38 MAPK activation in monocytes were subsequently evaluated after a 2-h treatment with receptor agonists.
Fig. 3.
Temporal p38 MAPK responses in monocytes/macrophages initiated by concurrent α1-AR and TLR4 stimulation. A, representative immunoblot of resolved THP-1 total cell lysates incubated concurrently with PE and LPS. B, quantitative analysis of all immunoblots (n = 3) showed increased phosphorylated p38 MAPK from monocytes treated for 30 min (4.2 ± 0.6-fold), 60 min (4.6 ± 1.3-fold), and 120 min (8.2 ± 0.6-fold). There were no differences in activated p38 MAPK generated from monocytes treated for 15 min (2.4 ± 0.3-fold) and 180 min (2.1 ± 0.4-fold). C, representative immunoblot (n = 3) of resolved THP-1 total cell lysates incubated with LPS alone. D, representative immunoblot of resolved PMA-differentiated THP-1 total cell lysates incubated concurrently with PE and LPS. E, quantitative analysis of all immunoblots (n = 3) showed increased phosphorylated p38 MAPK from macrophages treated for 15 min (7.7 ± 1.2-fold) and 30 min (10.7 ± 1.6-fold). There were no differences in activated p38 MAPK generated from macrophage cells treated for 5 min (1.5 ± 0.6-fold), 60 min (3.4 ± 1.0-fold), and 120 min (3.2 ± 0.2-fold). F, representative immunoblot (n = 3) of resolved PMA-differentiated THP-1 total cell lysates incubated with LPS alone. In all immunoblots, the 43-kDa nonphosphorylated p38 MAPK band is shown as a loading control. ★, p < 0.05 versus control.
We also wanted to determine whether α1-AR activation initiates p38 MAPK in human monocyte-derived macrophages. Therefore, temporal responses of PMA-differentiated THP-1 cells to treatments of PE and/or LPS were performed over 2 h (Fig. 3, D–F). No temporal differences in phosphorylated ERK or JNK were observed from macrophages individually or concomitantly treated with PE and LPS compared with control (data not shown). Conversely, p38 MAPK was temporally phosphorylated at significant (p < 0.05) levels above basal starting 15 min after concurrent treatment with PE and LPS, with peak activation occurring at 30 min (Fig. 3, D and E). Moreover, activation of p38 MAPK in macrophages was not observed at any time point using LPS alone (Fig. 3F). Subsequent assessment of p38 MAPK activation in macrophages was evaluated after a 30-min receptor agonist treatment.
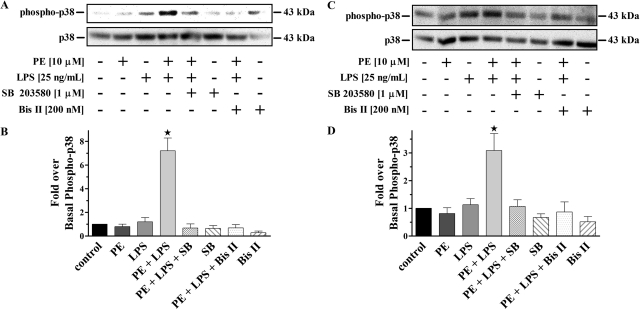
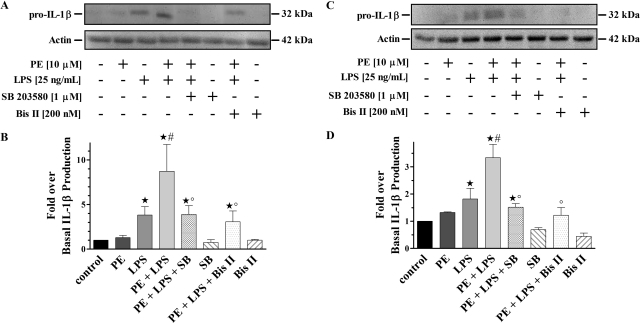
To directly associate α1-AR stimulation to p38 MAPK activation in LPS-challenged monocytes and macrophages, we preincubated with the selective inhibitor SB 203580 (Fig. 4). Immunoblot analysis showed no changes from basal in the generation of phospho-p38 from cells individually treated with PE or LPS alone. However, there was significant (p < 0.05) increases in phospho-p38 production observed from cells simultaneously treated with PE and LPS compared with control. Moreover, this increased phospho-p38 MAPK could be blocked to similar levels as control by preincubation with SB 203580. In parallel experiments, pretreatment with the selective PKC inhibitor Bis II completely inhibited phospho-p38 MAPK generation in the presence of concurrent PE and LPS treatment, to levels that were no different from control. Treatment of cells with SB 203580 or Bis II alone did not alter phospho-p38 MAPK basal levels.
Fig. 4.
Selective inhibition of α1-AR mediated PKC/p38 MAPK activation in LPS-challenged monocytes/macrophages. A, representative immunoblot of resolved THP-1 total cell lysates. B, quantitative analysis of all immunoblots (n = 3) showed phosphorylated p38 MAPK increases from monocytes treated with PE plus LPS (7.2 ± 1.1-fold). There were no differences from control for any other treatments. C, representative immunoblot of resolved PMA-differentiated THP-1 total cell lysates. D, quantitative analysis of all immunoblots (n = 3) showed increases in phosphorylated p38 MAPK from macrophages treated with PE plus LPS (3.1 ± 0.6-fold). There were no changes from control for any other treatments. The 43-kDa nonphosphorylated p38 MAPK band is shown as a loading control for all immunoblots. ★, p < 0.05 versus control.
Synergistic IL-1β Production in Human Monocytes and Macrophages Is Associated with a PKC/p38 MAPK-Dependent Mechanism.
To test whether the synergistic IL-1β response caused by α1-AR stimulation in LPS-challenged monocytes and macrophages is coupled through PKC/p38 MAPK activation we again preincubated with the selective inhibitors SB 203580 and Bis II (Fig. 5). Immunoblot analysis showed no basal differences in the amount of IL-1β generated from cells treated with PE alone as well as the expected significant (p < 0.05) increase from cells treated only with LPS. In addition, synergistic production of IL-1β was observed from cells concurrently treated with PE and LPS, which was significantly (p < 0.05) greater than amounts generated basally and with LPS alone. Cells preincubated with SB 203580 showed no IL-1β differences from levels produced with LPS alone. However, SB 203580-preincubated cells displayed significant (p < 0.05) changes in generated IL-1β compared with control or concurrent PE and LPS treatments. In parallel experiments, cells preincubated with Bis II showed significant (p < 0.05) modifications of generated IL-1β. Amounts of IL-1β from cells treated with SB 203580 or Bis II alone were no different from basal.
Fig. 5.
PKC/p38 MAPK activation is associated with α1-AR-mediated synergistic IL-1β production from LPS-challenged monocytes/macrophages. A, representative immunoblot of resolved THP-1 total cell lysates. B, quantitative analysis of all immunoblots (n = 3) showed increases in generated IL-1β from monocytes treated with LPS alone (3.8 ± 0.9-fold). There was synergistic IL-1β production from monocytes treated with PE plus LPS (8.7 ± 3.0-fold). Monocytes pretreated with SB 203580 or Bis II showed differences in generated IL-1β (3.9 ± 1.0 and 3.1 ± 1.2-fold, respectively). There were no IL-1β changes after treatment with PE (1.3 ± 0.2-fold), SB 203580 (0.7 ± 0.3-fold), or Bis II (1.0 ± 0.1-fold) only. C, representative immunoblot of resolved PMA-differentiated THP-1 total cell lysates. D, quantitative analysis of all immunoblots (n = 3) showed increases in IL-1β generated from macrophages treated with LPS alone (1.8 ± 0.4-fold). There was synergistic IL-1β production from macrophages treated with PE plus LPS (3.3 ± 0.5-fold). Macrophages pretreated with SB 203580 or Bis II showed changes in IL-1β levels (1.5 ± 0.1 and 1.2 ± 0.3-fold, respectively). There were no IL-1β differences produced from macrophages treated with PE (1.3 ± 0.1-fold), SB 203580 (0.7 ± 0.1-fold), or Bis II (0.4 ± 0.1-fold) only. The 42-kDa actin band is shown as a loading control in all immunoblots. ★, p < 0.05 versus control; #, p < 0.05 versus LPS; °, p < 0.05 versus PE + LPS.
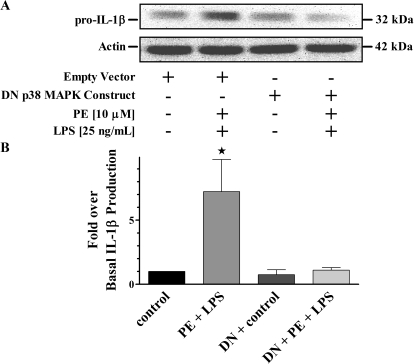
We also applied a molecular biological approach using a previously described DN form of p38 MAPK to corroborate our results using p38 MAPK pharmacological inhibitors (Whitmarsh et al., 1995). Figure 6 shows results from immunoblot analysis of resolved THP-1 cell lysates that had been transfected with either empty vector or the DN p38 MAPK plasmid construct followed by concurrent treatment with PE and LPS. For both sets of transfected cells there was the expected ≈3-fold increase of generated IL-1β over basal in the presence of LPS alone with no differences from control after treatment with only PE (data not shown). Moreover, there were significant (p < 0.05) increases in amounts of IL-1β produced from cells transfected with the empty vector then treated simultaneously with PE and LPS compared with control (Fig. 6). Conversely, cells transfected with the DN p38 MAPK plasmid construct then concurrently treated with PE and LPS showed no differences in the amounts of IL-1β generated compared with basal expression from similarly transfected cells.
Fig. 6.
p38 MAPK activation is necessary for the α1-AR synergistic modulation of IL-1β production. A, representative immunoblot of resolved THP-1 total cell lysates overexpressing the empty vector or DN p38 MAPK plasmid constructs. The 42-kDa actin band is shown as a loading control. B, quantitative analysis of all immunoblots (n = 3) normalized to basal levels of cells transfected with empty vector showed increases in IL-1β generated from similarly transfected cells treated with PE plus LPS (7.2 ± 2.5-fold). There were no IL-1β differences in DN p38 MAPK-transfected cells observed from control (0.8 ± 0.4-fold) or PE plus LPS treatments (1.1 ± 0.2-fold). ★, p < 0.05 versus empty vector control.
Characterization of α1-AR Subtype Expression on Human Monocytes and Macrophages.
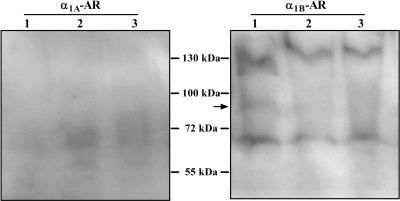
Genomic α1-AR subtype expression patterns have previously been reported for human monocytes; however, we were interested in subtype characteristics of the mature membrane protein (Rouppe van der Voort et al., 1999; Heijnen et al., 2002). We initially probed lysates from nonstimulated THP-1 cells with commercial antibodies whose epitopes were selectively generated for each α1-AR subtype. Shown in Fig. 7 are the results from three independent lysate preparations probed with subtype-selective α1A- and α1B-AR antibodies. Resolved lysates probed with the α1B-AR antibody were the only immunoblots that showed somewhat consistent weak specific banding at the expected size of ≈88 kDa, although numerous nonspecific bands were also identified. Conversely, membranes immunoblotted with the selective α1A-AR antibody showed no specific banding at the expected size of the mature membrane protein. Similar nonspecific results were also found when probing THP-1 cell lysates with the subtype-selective α1D-AR antibody (data not shown).
Fig. 7.
Immunoblot analysis of α1-AR protein expression from THP-1 cell lysates. Independent, untreated THP-1 cell lysate preparations (n = 3) were resolved and probed with antibodies that recognize the α1A- or α1B-AR subtypes. In all preparations the putative specific band for the α1B-AR was observed around the predicted ≈ 88-kDa size (arrow). No specific α1A-AR protein band was detected in any independent monocyte preparation.
To reliably characterize α1-AR subtype expression on human monocytes we used the iodinated α1-AR antagonist 125I-HEAT to identify specific binding sites on untreated THP-1 cell membrane preparations. 125I-HEAT labeled a saturable, homogenous, and specific high-affinity binding site on THP-1 membranes with a total site number (Bmax) of 309 ± 72 fmol/mg protein (n = 4; data not shown). The equilibrium dissociation constant (Kd) of 125I-HEAT calculated for these specific binding sites was 632 ± 234 pM (n = 4), which is similar to 125I-HEAT affinity values used to identify α1-AR specific binding sites in other systems (Goetz et al., 1995). Competition binding assays were also performed using selective α1-AR antagonists. Table 1 summarizes affinity values calculated from individually generated inhibition curves using these α1-AR antagonists to compete for specific monocyte 125I-HEAT binding sites. When the selective α1-AR antagonist BE 2254 was used to compete for specific radiolabeled binding sites, a one-site competition curve was best-fit to the data with a calculated equilibrium dissociation constant (Ki) of 5.3 ± 1.0 nM (n = 3). This value corresponds to the Kd of the analogous iodinated compound (125I-HEAT) and is similar to the calculated affinity of BE 2254 when used to identify α1-AR binding sites in other membrane preparations (Goetz et al., 1995). Subtype-selective α1-AR antagonists were used to establish the characteristics of these monocyte α1-AR binding sites. A one-site model fit best when the α1A-AR subtype-selective antagonists 5-MU or WB-4101 as well as the α1D-AR subtype-selective antagonist BMY 7378 were used to competitively displace specific monocyte 125I-HEAT binding sites. The low-affinity estimates of 5-MU, WB-4101, and BMY 7378 for specific monocyte 125I-HEAT binding sites do not correspond with the high-affinity values of these subtype-selective α1-AR antagonists calculated for the α1A- or α1D-AR in other systems (Gross et al., 1988; Goetz et al., 1995).
TABLE 1.
Equilibrium dissociation constants (Ki) of α1-AR antagonists for 125I-HEAT specific binding sites on human monocytes and macrophages
Mean values ± S.E.M. (in nM) calculated from individually generated inhibition curves of n competitive binding assays. For each individual experiment, the fitted iterative nonlinear regression curve that best represented the data (i.e., one- vs. two-site fit) was determined using a partial f test (p < 0.05).
| BE 2254 (n = 3) | 5-MU (n = 4) | WB-4101 (n = 4) | BMY 7378 (n = 4) | |
|---|---|---|---|---|
| nM | ||||
| Monocyte (THP-1) | ||||
| Ki | 5.3 ± 1.0 | 10,864 ± 1920 | 435 ± 85 | 1079 ± 212 |
| Macrophage (PMA-differentiated THP-1) | ||||
| Ki | 11.0 ± 0.0* | |||
| KiH | 1.3 ± 4.6 | 1.0 ± 0.9 | ||
| KiL | 85,507 ± 4332 | 1371 ± 605 | ||
n = 1.
To determine whether this characterized α1-AR subtype expression pattern is retained in monocyte-derived macrophages, radioligand binding studies were performed on membranes prepared from PMA-differentiated THP-1 cells. Subsequent radioligand saturation binding analysis on these macrophage membrane preparations documented a saturable, homogenous, and specific high-affinity 125I-HEAT binding site (data not shown). The number of specific binding sites was calculated to be 97 ± 32 fmol/mg protein, and the estimated Kd of 125I-HEAT for these macrophage binding sites was 468 ± 67 pM (n = 4). This calculated 125I-HEAT affinity for specific macrophage binding sites is similar to estimated Kd values that identified α1-AR populations on monocytes and in other systems (Goetz et al., 1995). Competition binding assays were also performed using the subtype-selective α1-AR antagonists 5-MU and BMY 7378 (Table 1). Both receptor antagonists competitively displaced specific 125I-HEAT binding sites on macrophage membranes that best fit a two-site model, suggesting a heterogeneous α1-AR subtype profile.
Discussion
Based on previous studies, we hypothesized that α1-AR modulation of TLR4 responses would have proinflammatory outcomes dependent on the selective signaling characteristics of expressed α1-AR subtypes (Grisanti et al., 2011). In agreement with our findings, α1-AR activation seems to play a proinflammatory role in many situations. For example, α1-AR expression is increased in several animal models of chronic human diseases with known inflammatory etiologies such as juvenile rheumatoid arthritis and multiple sclerosis (Brosnan et al., 1985; Capellino and Straub, 2008). Other studies from patients with juvenile rheumatoid arthritis have linked heightened α1-AR expression on peripheral blood leukocytes to an increased production of the proinflammatory cytokine IL-6 when stimulated with PE (Heijnen et al., 1996). Likewise, in this article we characterized α1-AR-mediated synergistic increases in IL-1β levels from LPS-challenged human monocytes (Fig. 1). We used immunoblot analysis to quantitate pro-IL-1β production as a measure of this synergistic α1-AR response. Differences in IL-1β levels, as measured by ELISA, from conditioned media of LPS alone and LPS plus PE-treated monocytes, were comparable (≈3-fold) to differences observed for pro-IL-1β generation in similarly treated cells (Fig. 2). These results correlate quantitation of bioactive IL-1β secreted into the media to the same levels of procytokine generation using immunoblot analysis, validating use of this latter technique in our studies. Furthermore, α1-AR-mediated synergistic effects between primary and immortalized monocytes were similar (≈3-fold) compared with LPS treatment alone (Figs. 1 and 2). This observation is analogous to previously described potentiated IL-1β responses caused by simultaneous stimulation of TLR4 and β1-AR subtypes in both primary monocytes and THP-1 cells (Grisanti et al., 2010) and justifies use of these immortalized cells for subsequent signal transduction investigations.
Previous studies using radioligand binding analysis documented no specific α1-AR binding from a human mononuclear cell preparation, attributing earlier reports describing α1-AR activity to platelet contamination (Casale and Kaliner, 1984). Subsequent investigations have identified genomic expression of α1B- and α1D-AR subtypes from THP-1 total RNA preparations; however, mature translational expression was not examined (Rouppe van der Voort et al., 1999). α1-AR expression in the immune system has been shown to be increased by neuroendocrine mediators such as glucocorticoids and cytokines and β-AR stimulation (Rouppe van der Voort et al., 1999, 2000; Heijnen et al., 2002). Activation of TLR4 in our model system may induce α1-AR expression, which could explain why many researchers did not previously detect α1-AR specific binding. Nonetheless, our studies document mature α1-AR expression from nonstimulated human monocyte and macrophage cell preparations (Table 1). Commercial subtype-selective α1-AR antibodies have been reported to be nonspecific when tested in genetically modified animals (Jensen et al., 2009). Therefore, radioligand binding assays remain the only consistent method of quantitating α1-AR expression. We described a one-site, low-affinity profile of the selective α1A-AR antagonists 5-MU and WB-4101 for specific 125I-HEAT binding sites on untreated monocyte membranes, suggesting expression of the α1B- or α1D-AR subtype. One-site, low-affinity values of the selective α1D-AR antagonist for specific 125I-HEAT binding sites on these same preparations indicates the absence of mature α1D-AR subtypes, which reinforces our conclusions for homogenous α1B-AR protein expression, yet contrasts previous reports characterizing α1D-AR transcripts from monocytes (Rouppe van der Voort et al., 1999).
In macrophages, a two-site, high- and low-affinity profile of 5-MU was calculated for specific 125I-HEAT binding sites, confirming mature expression of the α1A-AR and suggesting the presence of α1B- or α1D-AR subtypes. A similar two-site, high- and low-affinity profile of BMY 7378 was calculated for specific 125I-HEAT binding sites on these same cells, which confirms mature expression of the α1D-AR and validates our α1A-AR subtype observation using 5-MU. Although α1B-AR expression could be represented as part of the low-affinity binding population for both 5-MU and BMY 7378, previous reports have shown consistent 3- to 4-fold lower binding affinities of BMY 7378 for both recombinant and endogenously expressed α1A-AR subtypes compared with the α1B-AR (Yoshio et al., 2001). The extremely low-affinity population (KiL) identified by BMY 7378 in our human macrophage system correlates well with the calculated BMY 7378 value for α1A-AR subtypes in the previous report (Yoshio et al., 2001). At this time, an effective subtype-selective α1B-AR antagonist is not available to validate (monocyte) or rule out (macrophage) α1B-AR expression.
Parallel investigations characterized temporal increases in p38 MAPK activation from monocytes and macrophages concurrently treated with LPS and PE (Fig. 3). Significant increases over basal were shown for monocyte p38 MAPK activation starting at 15 min and peaking at 120 min. Significant phospho-p38 MAPK temporal increases were also observed using our macrophage cell model at 15 min followed by a shorter 30-min peak. Slower and persistent MAPK activation by Gαq-coupled receptors have been shown to be β-arrestin-dependent (Ahn et al., 2004). Moreover, direct interaction of the β-arrestin 1 isoform and MAPK kinase 3, which is specific for p38 MAPK activation has been described previously (McLaughlin et al., 2006). Although there have been reports characterizing the importance of β-arrestin interactions for internalization and recycling of α1A-AR subtypes, β-arrestin-dependent α1-AR signaling pathways have not been described in the literature (Pediani et al., 2005).
Our study goes further using pharmacological and molecular biological approaches to characterize the signal-transduction properties of this cooperative relationship between α1-AR and TLR4 activation to generate a greater cytokine response. The synergistic IL-1β effect shown in our study depends on α1-AR stimulation as evidenced by a reversal of this response in the presence of a selective α1-AR antagonist (Fig. 2). Moreover, inhibition of PKC using STS or Bis II blocked the synergistic increases in IL-1β production observed with PE plus LPS treatment to levels that were no different from cells treated with LPS alone (Figs. 2 and 5). Likewise, p38 MAPK inhibition decreased synergistic IL-1β production after PE plus LPS treatment to levels comparable with LPS only (Fig. 5). These results indicate that although PKC/p38 MAPK activation is not important for TLR4-initiated IL-1β generation the synergistic production of this proinflammatory cytokine with concurrent α1-AR stimulation in monocytes and macrophages is PKC/p38 MAPK-dependent. These data are also the first to document functional α1-AR expression on human monocytes and macrophages and to link their activation to an immunomodulatory increase in proinflammatory cytokine production. It is noteworthy that the homogeneous α1B-AR subtype expression profile in monocytes changed to a heterogeneous population of α1A- and α1D-AR subtypes when differentiated into macrophages. Remarkably, the α1-AR-mediated signaling pathway that regulates TLR4 cytokine production remained the same in both cell types. This observation points to the previously reported signaling redundancy as well as highly inducible nature of α1-AR subtypes in immune cell populations (Rouppe van der Voort et al., 1999, 2000; Heijnen et al., 2002).
TLR4-mediated increases in IL-1β from human lymphocytes have been documented to occur through both pretranscriptional and post-transcriptional mechanisms (Netea et al., 2009). In cardiac myocytes, α1-AR activation promotes the generation of IL-6 through a combination of transcription factor activation and increased mRNA stability (Perez et al., 2009). Similar mechanisms likely occurred in our studies because quantities of both proactive and bioactive IL-1β increased with concurrent α1-AR and TLR4 activation, indicating the generation of new protein. TLR4 effector pathways are typically linked to the myeloid differentiation primary-response protein 88 signaling complex, which activates the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) to regulate IL-1β transcription (Akira and Takeda, 2004). Although it is possible that positive α1-AR regulation of TLR4 signaling occurs upstream of PKC and p38 MAPK, the majority of literature points to alterations in the NF-κB or MAPK signaling pathways. However, increased Ca2+ concentrations in macrophages have been shown to initiate myeloid differentiation primary-response protein 88 signaling through direct interaction and phosphorylation of transforming growth factor β-activated kinase 1, an important activator of both NF-κB and p38 MAPK pathways (Liu et al., 2008b). As a result, Gαq-mediated enhanced Ca2+ levels initiated by α1-AR stimulation could activate p38 MAPK, which was shown to be necessary for the potentiated IL-1β response in our systems. Conversely, TLR4 inflammatory signaling can also occur independent of NF-κB through p38 MAPK-dependent mobilization of activator protein-1 or ETS domain-containing protein Elk-1 transcription factors (Hodgkinson et al., 2008; Smolinska et al., 2008). Activated p38 MAPK shown in our results may be positively regulating TLR4-initiated IL-1β production through these surrogate transcription factors. Alternatively, α1-AR-mediated phospho-p38 MAPK characterized in our studies could have direct positive effects on NF-κB activity by enhancing inhibitor of κB degradation or mediating acetylation of the RelA transcription factor subunit (Liu et al., 2008a; Pan et al., 2010).
Increased IL-1β from innate immunocompetent cells have been implied in the pathogenesis of rheumatoid arthritis, type 1 diabetes, multiple sclerosis, and Alzheimer's disease (Licastro et al., 2000; Audoy-Rémus et al., 2008; Capellino and Straub, 2008; Bradshaw et al., 2009). Therefore, α1-AR modulation of TLR4 signaling characterized in our investigations may prove to be a useful therapeutic strategy for the management of human diseases with known chronic inflammatory etiologies.
Acknowledgments
We thank Brett McGregor for technical support and Julie Horn and Deb Kroese for administrative assistance.
This study was supported by the National Science Foundation [Grant 0235146]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM066726]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK62865]; the National Institutes of Health Centers of Biomedical Research Excellence program [Grant RR016471]; and the North Dakota Experimental Program to Stimulate Competitive Research program through the National Science Foundation [Grant EPS-0447679] (to J.E.P). The Integrative and Organ Systems Pharmacology internship of L.A.G. at the University of Nebraska Medical Center was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R25-GM074089].
A preliminary report of these findings was presented previously: Grisanti LA and Porter JE (2009) α1-Adrenergic receptor modulation of LPS induced inflammation in normal and PMA differentiated THP-1 cells, at the Annual Meeting of the American Society for Pharmacology and Experimental Therapeutics; 2009 April 18–22; New Orleans, LA. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.178012.
- AR
- adrenergic receptor
- BE 2254
- 2-{[β-(hydroxyphenyl)ethyl]aminomethyl}-1-tetralone hydrochloride
- Bis II
- bisindolylmaleimide II
- BMY 7378
- 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride
- DN
- dominant-negative
- ELISA
- enzyme-linked immunosorbent assay
- ERK
- extracellular signal-regulated kinase
- 125I-HEAT
- (±)-β-([125]Iodo-4-hydroxyphenyl)-ethyl-aminomethyl-tetralone
- HEM buffer
- 20 mM HEPES, 1.4 mM EGTA, 12.5 mM MgCl2, pH 7.4
- IL
- interleukin
- JNK
- c-Jun N-terminal kinase
- Kd
- radioligand equilibrium dissociation constant
- Ki
- competitive receptor antagonist equilibrium dissociation constant
- LPS
- lipopolysaccharide
- MAPK
- mitogen-activated protein kinase
- 5-MU
- 5-methylurapidil
- NF-κB
- nuclear factor κ-light-chain-enhancer of activated B cells
- PE
- (R)-(−)-phenylephrine hydrochloride
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- SB 203580
- 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole
- STS
- staurosporine
- THP-1
- human monocyte cell line
- TLR
- Toll-like receptor
- WB-4101
- 2-[(2,6-dimethoxyphenoxyethyl)aminomethyl]-1,4-benzodioxane hydrochloride.
Authorship Contributions
Participated in research design: Grisanti, Combs, and Porter.
Conducted experiments: Grisanti, Woster, and Dahlman.
Contributed new reagents or analytic tools: Dahlman, Sauter, and Combs.
Performed data analysis: Grisanti, Woster, Combs, and Porter.
Wrote or contributed to the writing of the manuscript: Grisanti, Sauter, Combs, and Porter.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. (2004) Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279:35518–35525 [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511 [DOI] [PubMed] [Google Scholar]
- Audoy-Rémus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallières L. (2008) Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci 28:10187–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. (2009) Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Goldmuntz EA, Cammer W, Factor SM, Bloom BR, Norton WT. (1985) Prazosin, an α1-adrenergic receptor antagonist, suppresses experimental autoimmune encephalomyelitis in the Lewis rat. Proc Natl Acad Sci USA 82:5915–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I. (2006) Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann NY Acad Sci 1069: 62–76 [DOI] [PubMed] [Google Scholar]
- Capellino S, Straub RH. (2008) Neuroendocrine immune pathways in chronic arthritis. Best Pract Res Clin Rheumatol 22:285–297 [DOI] [PubMed] [Google Scholar]
- Casale TB, Kaliner M. (1984) Demonstration that circulating human blood cells have no detectable α1-adrenergic receptors by radioligand binding analysis. J Allergy Clin Immunol 74:812–818 [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Kvetnansky R, Hashiramoto A, Bakalov VK, Link AA, Zachman K, Crane M, Jezova D, Rovensky J, Dimitrov MA, et al. (2008) Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? J Immunol 181:1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer P, Pugin J. (2000) β-Adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IκB/NF-κB pathway. Am J Physiol Lung Cell Mol Physiol 279:L675–L682 [DOI] [PubMed] [Google Scholar]
- Felsner P, Hofer D, Rinner I, Porta S, Korsatko W, Schauenstein K. (1995) Adrenergic suppression of peripheral blood T cell reactivity in the rat is due to activation of peripheral α2-receptors. J Neuroimmunol 57:27–34 [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, et al. (2007) Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 449:721–725 [DOI] [PubMed] [Google Scholar]
- Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL., Jr (1995) BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur J Pharmacol 272:R5–R6 [DOI] [PubMed] [Google Scholar]
- Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. (2010) Pro-inflammatory responses in human monocytes are β1-adrenergic receptor subtype dependent. Mol Immunol 47:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti LA, Perez DM, Porter JE. (2011) Modulation of immune cell function by α1-adrenergic receptor activation, in Advances in Adrenergic Receptor Biology (Wang Q. ed), Academic Press, Maryland Heights, MO: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Hanft G, Rugevics C. (1988) 5-Methyl-urapidil discriminates between subtypes of the α1-adrenoceptor. Eur J Pharmacol 151:333–335 [DOI] [PubMed] [Google Scholar]
- Guimarães S, Moura D. (2001) Vascular adrenoceptors: an update. Pharmacol Rev 53:319–356 [PubMed] [Google Scholar]
- Haskó G, Elenkov IJ, Kvetan V, Vizi ES. (1995) Differential effect of selective block of α2-adrenoreceptors on plasma levels of tumour necrosis factor-α, interleukin-6 and corticosterone induced by bacterial lipopolysaccharide in mice. J Endocrinol 144:457–462 [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe van der Voort C, van de Pol M, Kavelaars A. (2002) Cytokines regulate α1-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J Neuroimmunol 125:66–72 [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe van der Voort C, Wulffraat N, van der Net J, Kuis W, Kavelaars A. (1996) Functional α1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol 71:223–226 [DOI] [PubMed] [Google Scholar]
- Hodgkinson CP, Patel K, Ye S. (2008) Functional Toll-like receptor 4 mutations modulate the response to fibrinogen. Thromb Haemost 100:301–307 [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. (2009) Ten commercial antibodies for α-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol 379:409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135:1295–1307 [DOI] [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. (2000) Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol 103:97–102 [DOI] [PubMed] [Google Scholar]
- Liu S, Feng G, Wang GL, Liu GJ. (2008a) p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-κB pathway. Eur J Pharmacol 584:159–165 [DOI] [PubMed] [Google Scholar]
- Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. (2008b) CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood 112:4961–4970 [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. (2000) Dendritic cell migration controlled by α1b-adrenergic receptors. J Immunol 165:6743–6747 [DOI] [PubMed] [Google Scholar]
- McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. (2006) Platelet-activating factor-induced clathrin-mediated endocytosis requires β-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol 176:7039–7050 [DOI] [PubMed] [Google Scholar]
- Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113:2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WW, Li JD, Huang S, Papadimos TJ, Pan ZK, Chen LY. (2010) Synergistic activation of NF-κB by bacterial chemoattractant and TNFα is mediated by p38 MAPK-dependent RelA acetylation. J Biol Chem 285:34348–34354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediani JD, Colston JF, Caldwell D, Milligan G, Daly CJ, McGrath JC. (2005) β-Arrestin-dependent spontaneous α1a-adrenoceptor endocytosis causes intracellular transportation of α-blockers via recycling compartments. Mol Pharmacol 67:992–1004 [DOI] [PubMed] [Google Scholar]
- Perez DM, Papay RS, Shi T. (2009) α1-Adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: involvement of p38 mitogen-activated protein kinase and nuclear factor-κB. Mol Pharmacol 76:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, McCall C, Eisenach JC. (2005) α2-Adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci 25:8988–8994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. (1999) Neuroendocrine mediators up-regulate α1b- and α1d-adrenergic receptor subtypes in human monocytes. J Neuroimmunol 95:165–173 [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. (2000) Noradrenaline induces phosphorylation of ERK-2 in human peripheral blood mononuclear cells after induction of α1-adrenergic receptors. J Neuroimmunol 108:82–91 [DOI] [PubMed] [Google Scholar]
- Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. (2008) Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Mol Immunol 45:990–1000 [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. (1990) Stimulation of α-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol 145:1430–1434 [PubMed] [Google Scholar]
- Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. (1982) Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 42:1530–1536 [PubMed] [Google Scholar]
- Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. (1995) Integration of MAP kinase signal transduction pathways at the serum response element. Science 269:403–407 [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. (2001) Affinity of serotonin receptor antagonists and agonists to recombinant and native α1-adrenoceptor subtypes. Jpn J Pharmacol 86:189–195 [DOI] [PubMed] [Google Scholar]