Abstract
The effects of repeated treatment with the phosphodiesterase-4 (PDE4) inhibitors rolipram, piclamilast, and 4-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)pyridine (CDP840), which differ in their interactions with high- and low-affinity binding conformers of the enzyme, were contrasted to those of acute treatment on cAMP signaling, hippocampal cell proliferation, and immobility in the forced-swim test in rats. Repeated treatment with rolipram (1 and 3 mg/kg), piclamilast (0.3 and 1 mg/kg), or CDP840 (10 and 30 mg/kg) for 16 days increased cAMP and phosphorylation of cAMP response element binding protein (pCREB) in hippocampus and prefrontal cortex. In addition, repeated treatment with the PDE4 inhibitors increased proliferation and survival of newborn cells in the hippocampus and produced antidepressant-like effects on behavior, as evidenced by decreased immobility in the forced-swim test. Acute treatment with rolipram (3 mg/kg), piclamilast (1 mg/kg), or CDP840 (30 mg/kg) induced transient increases in cAMP and pCREB in hippocampus and prefrontal cortex, but the dose and time dependence of these effects did not parallel the behavioral effects. Compared with rolipram and piclamilast, repeated treatment with CDP840 exerted lesser effects on neural and behavioral measures, probably because of its weak interaction with the high-affinity binding conformer of PDE4. This suggests the relative importance of the high-affinity binding conformer in the mediation of the long-term effects of PDE4 inhibition on cAMP/pCREB signaling, hippocampal cell proliferation, and antidepressant-like effects on behavior.
Introduction
Phosphodiesterase-4 (PDE4) regulates cAMP signaling and is involved in many aspects of cell function (Conti et al., 2003; Houslay and Adams, 2003; O'Donnell and Zhang, 2004). Altered AMP signaling has been suggested to contribute to the pathophysiology of neuropsychiatric illnesses such as major depression, schizophrenia, and dementia (Tanis and Duman, 2007). Consistent with this idea, it has been found that PDE4 inhibitors such as 4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone (rolipram), which increase cAMP signaling, exert antidepressant- and antipsychotic-like effects, as well as memory-enhancing effects, on behavior (O'Donnell and Zhang, 2004; Blokland et al., 2006; Siuciak, 2008).
Antidepressant-like effects of PDE4 inhibitors have been demonstrated using a number of preclinical models, including reversal of the effects of reserpine and potentiation of yohimbine toxicity (Wachtel and Löschmann, 1986; Wachtel and Schneider, 1986), increases in reinforcement rate of rats under a differential reinforcement of low rate schedule (O'Donnell, 1993), and reduction of immobility in the forced-swim and tail-suspension tests (O'Donnell and Zhang, 2004). Clinical studies have demonstrated antidepressant effects of rolipram; however, side effects and toxicity have limited its clinical utility (Zeller et al., 1984; Fleischhacker et al., 1992).
Through the examination of the behavioral phenotypes of mice deficient in one of the four PDE4 subtypes, some understanding of their relative roles has begun to emerge. Mice deficient in PDE4D exhibit an antidepressant-like behavioral phenotype and altered hippocampal long-term potentiation and memory (Zhang et al., 2002; Rutten et al., 2008; Li et al., 2011). PDE4B-deficient mice exhibit an anxiogenic-like profile in a number of behavioral tests (Zhang et al., 2007). In models sensitive to antipsychotic drugs, PDE4B-deficient mice show reduced prepulse inhibition of an acoustic startle response, but reduced sensitivity to the antipsychotic-like effect of rolipram on conditioned avoidance behavior (Siuciak et al., 2007, 2008; Zhang et al., 2007). Although these data suggest a potential role for PDE4B in mediating the antipsychotic-like effects on behavior, the exact nature is unclear. It has been speculated that interaction with DISC1 (Disrupted in schizophrenia 1), which interacts with PDE4B, might be involved in this phenomenon, because DISC1 mutant mice show reduced prepulse inhibition (Clapcote et al., 2007). There are no published data on the behavioral consequences of PDE4A deficiency, and PDE4C is not expressed in the brain.
Although rolipram does not inhibit the PDE4 subtypes with different potencies, its interaction with the enzyme is complex. [3H]Rolipram binds PDE4's catalytic site with two distinct affinities, termed the high- and low-affinity rolipram binding states (HARBS and LARBS, respectively) (Jacobitz et al., 1996). This has been confirmed using [3H]-3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-methoxybenzamide (piclamilast), which binds with equal high affinity to the HARBS and LARBS (Zhao et al., 2003a). Although the LARBS is found in both the brain and peripheral tissues, the HARBS is found only in the brain; consistent with this, the HARBS and LARBS mediate distinct pharmacological effects (Saccomano et al., 1991; Barnette et al., 1996; Zhao et al., 2003b).
PDE4 is involved in signaling pathways affected by antidepressant drugs. Repeated treatment of mice with antidepressants increases PDE4 expression in cerebral cortex and hippocampus, particularly the PDE4D subtype (Dlaboga et al., 2006). There seems to be species dependence because similar treatment of rats has pronounced effects on PDE4A expression (Takahashi et al., 1999; Ye et al., 2000). It is likely that increased PDE4 expression is a compensatory change in response to increased cAMP signaling that results from antidepressant treatment; prior work demonstrated such regulation of PDE4 in cultured neurons (D'Sa et al., 2002; Hajjhussein et al., 2007). Repeated treatment of rats with antidepressant drugs increases the HARBS, but not the LARBS, in cerebral cortex and hippocampus (Zhao et al., 2003b). Consistent with this finding, comparison of the acute behavioral effects of rolipram, piclamilast, and 4-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)pyridine (CDP840), PDE4 inhibitors that differ in their interactions with the HARBS and LARBS (Zhao et al., 2003a), suggests a predominant role for the HARBS in mediating acute antidepressant-like effects on behavior (Zhang et al., 2006).
The effects of antidepressants depend in part on increased neurogenesis in the hippocampus. Inhibition of neurogenesis blocks some, but not all, of the effects of antidepressants on behavior (Santarelli et al., 2003; Holick et al., 2008). Likewise, repeated treatment of mice with rolipram also increases hippocampal neurogenesis; inhibition of rolipram-induced neurogenesis reduces antidepressant-like effects on behavior (Li et al., 2009). The relationship among the ability of PDE4 inhibitors to interact with the HARBS and LARBS to alter cAMP signaling, increase neurogenesis, and produce antidepressant-like effects on behavior has not been well characterized. Comparison of the effects of rolipram, piclamilast, and CDP840 on these measures provides an important insight, because acutely they affect behavior differently, even though all are potent PDE4 inhibitors (Zhang et al., 2006). However, with acute administration, the sedative effects of PDE4 inhibitors interfere with their antidepressant-like effects on behavior; with repeated treatment, this seems to be less of an issue and behavioral effects are more orderly (Li et al., 2009). For this reason, as well as for better relevance to the clinical setting, the present study contrasted the effects of repeated treatment with these three PDE4 inhibitors with their acute effects on behavior in the forced-swim test, hippocampal cell proliferation and survival (an index of neurogenesis), and cAMP signaling. The results suggest the importance of the HARBS in these measures and a close association among increased cAMP signaling, increased hippocampal cell proliferation, and antidepressant-like effects on behavior for the effects of repeated treatment with the PDE4 inhibitors.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 200 to 250 g, were housed individually in clear plastic cages in a room that was maintained at 22°C with a 12-h on/12-h off light cycle with lights on at 7:00 AM. Food and water were freely available. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996) and were approved by the Institutional Animal Care and Use Committee of West Virginia University.
Drug Treatments.
Rolipram was purchased from Sigma-Aldrich (St. Louis, MO); piclamilast and CDP840 were provided by Memory Pharmaceuticals (Montvale, NJ). Dose ranges tested were based on prior studies (Zhang et al., 2006; Li et al., 2009). To assess the effects of repeated treatment, rats were injected intraperitoneally with 1 or 3 mg/kg rolipram, 0.3 or 1 mg/kg piclamilast, 10 or 30 mg/kg CDP840, or the 10% dimethyl sulfoxide/saline vehicle once daily for 16 consecutive days. Two hours after the last treatment, separate groups of rats were subjected to the 5-min forced-swim test, used for measurement of cAMP and pCREB, or started on the bromodeoxyuridine (BrdU) treatment regimen for assessment of hippocampal cell proliferation and survival.
To assess the effects of acute treatment on forced-swim behavior, 0.3, 1, or 3 mg/kg rolipram, 0.3, 1, or 3 mg/kg piclamilast, 3, 10, or 30 mg/kg CDP840, or vehicle was administered to separate groups of rats, and the time of immobility was assessed either 0.5 or 2 h post-treatment. To assess the effects of acute treatment on the neurochemical measures, 3 mg/kg rolipram, 1 mg/kg piclamilast, 30 mg/kg CDP840, or vehicle was administered, and rats were killed 0.5, 1, 2, 3, or 4 h later.
cAMP and pCREB.
To prevent postmortem changes in cAMP and pCREB, rats were killed by focused microwave fixation (O'Callaghan and Sriram, 2004) (Muromachi Microwave Applicator, TMW-4012C, 10-kW output; Stoelting, Kiel, WI). For cAMP measurement, hippocampus and prefrontal cortex were homogenized in ice-cold 0.1 N hydrochloric acid and centrifuged at 13,000g for 50 min at 4°C. cAMP in the supernatant was measured by enzyme-linked immunosorbent assay (Assay Designs, Ann Arbor, MI) and normalized to protein content (Bio-Rad Laboratories, Hercules, CA).
For CREB and pCREB measurement, hippocampus and prefrontal cortex were homogenized in ice-cold radioimmunoprecipitation assay lysis buffer (Upstate, Temecula, CA) and centrifuged at 10,000g for 30 min at 4°C. Solubilized samples were mixed with equal volumes of Laemmli sample buffer and heated to 100°C for 2 min. Equal amounts of sample protein were loaded onto gels for SDS-polyacrylamide gel electrophoresis. After separation by electrophoresis, proteins in the gels were transferred to nitrocellulose membranes, which were incubated overnight at 4°C with primary antibodies against CREB or pCREB (i.e., phosphorylated at Ser-133; Millipore Corporation, Billerica, MA) and then with Alexa Fluor 680-conjugated secondary antibody for 30 min at room temperature (Invitrogen, Carlsbad, CA). An Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) was used for quantifying fluorescence. Treatment-induced changes in CREB and pCREB were assessed within individual gels/immunoblots by densitometry. The immunoblotting data for CREB and pCREB were normalized to β-actin, which also was measured by SDS-polyacrylamide gel electrophoresis/immunoblotting (Sigma-Aldrich), and are expressed as percentage of control.
Hippocampal Cell Proliferation and Survival by Flow Cytometry.
To evaluate the effects of repeated treatment with PDE4 inhibitors on hippocampal cell proliferation, rats were administered 100 mg/kg BrdU i.p. 2 h after the last injection of PDE4 inhibitors to label dividing cells; rats were killed and BrdU labeling was quantified 24 h later. To evaluate the effects on hippocampal cell survival, rats were administered 100 mg/kg BrdU i.p, four times at 2-h intervals 1 day after the last injection of the PDE4 inhibitors; rats were killed and BrdU labeling was quantified 5 weeks later.
BrdU incorporation into cells in the hippocampus was measured by flow cytometry as described previously (Bilsland et al., 2006; Balu et al., 2009). Rats were killed by decapitation, and hippocampi were dissected, placed in 1.5-ml Eppendorf tubes containing Dulbecco's phosphate-buffered saline (Invitrogen) and finely minced. An enzymatic cocktail comprised of digestive solution (2.5 U/ml papain, 1 U/ml dispase, and 250 U/ml DNase I) was added to each tube, which was then incubated at 37°C for 30 min. The samples were triturated and washed twice with Dulbecco's modified Eagle's medium/10% fetal bovine serum and then once with Dulbecco's phosphate-buffered saline.
The samples were centrifuged at 500g for 5 min and stained using a fluorescein isothiocyanate BrdU flow kit (BD Biosciences, San Jose, CA). Precipitated cells were suspended in BD Biosciences Cytofix/Cytoperm buffer and incubated for 30 min on ice. After washing twice with 1 ml of BD Biosciences perm wash buffer, the cells were further permeabilized by resuspension with Cytoperm Plus buffer on ice for 10 min. After washing, the cells were refixed in Cytofix-Cytoperm buffer for 5 min and then incubated with DNase for 1 h at 37°C. After washing with BD Biosciences buffer, the cells were labeled with 50 μl of fluorescein isothiocyanate-conjugated anti-BrdU 1:50 for 20 min. After another wash, cells were labeled with 20 μl of 7-aminoactinomycin D at room temperature. The BrdU-positive cells were counted by a FACSCalibur and analyzed using Cellquest Prosoftware (BD Biosciences).
Forced-Swim Behavior.
The forced-swim test was performed as described previously (Zhang et al., 2006); it consisted of a 15-min pretest and a 5-min test 24 h later. Rats were place individually in a Plexiglas cylinder 45 cm high × 20 cm in diameter filled with water 22 to 23°C to a depth of 30 cm. During the 5-min test, the time of immobility, defined as floating in an upright position with the only movement being that necessary for an animal to keep its head above water, was recorded.
Data Analysis and Statistics.
Data were analyzed by one-way analysis of variance followed by Dunnett's test. Data are expressed as means ± S.E.M. A p value < 0.05 was considered statistically significant.
Results
Effects of Repeated Treatment with Rolipram, Piclamilast, and CDP840 on cAMP and pCREB.
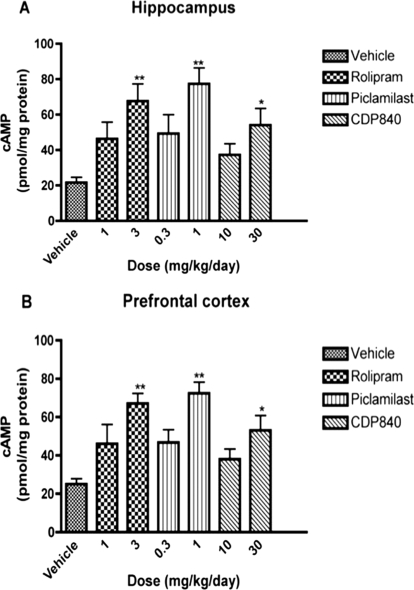
Repeated treatment for 16 days with the PDE4 inhibitors increased cAMP in the hippocampus and prefrontal cortex (Fig. 1). Significant increases were observed only at the higher doses of rolipram (3 mg/kg), piclamilast (1 mg/kg), or CDP840 (30 mg/kg) that were tested. At the higher doses, rolipram, piclamilast, and CDP840 increased cAMP by 204, 250 and 145%, respectively, in hippocampus and by 168, 189, and 112%, respectively, in prefrontal cortex.
Fig. 1.
Effects of repeated treatment with rolipram, piclamilast, or CDP840 on cAMP in rat brain. Treatment for 16 days with rolipram, piclamilast, or CDP840 increased cAMP in hippocampus (A) and prefrontal cortex (B). Bars represent means ± S.E.M. (n = 3–4 per group). **, P < 0.01; *, P < 0.05 versus corresponding control.
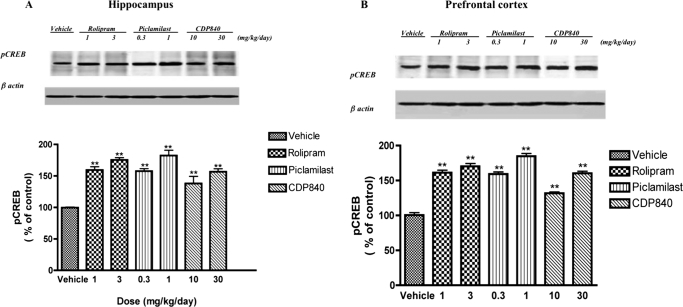
Parallel effects of repeated treatment with the PDE4 inhibitors on pCREB were observed (Fig. 2). However, both the lower and higher doses tested were effective: doses of 1 and 3 mg/kg rolipram increased pCREB in hippocampus by 59 and 75%, respectively; doses of 0.3 and 1 mg/kg piclamilast increased pCREB in hippocampus by 58 and 82%, respectively; and doses of 10 and 30 mg/kg CDP840 increased pCREB in hippocampus by 36 and 55%, respectively. Doses of 1 and 3 mg/kg rolipram increased pCREB in prefrontal cortex by 61 and 70%, respectively; doses of 0.3 and 1 mg/kg piclamilast increased pCREB in prefrontal cortex by 59 and 85% respectively; and doses of 10 and 30 mg/kg CDP840 increased pCREB in prefrontal cortex by 32 and 60%, respectively. None of these treatments affected CREB expression (data not shown).
Fig. 2.
Effects of repeated treatment with rolipram, piclamilast, or CDP840 on pCREB in rat brain. Treatment for 16 days with rolipram, piclamilast, or CDP840 increased pCREB in hippocampus (A) and prefrontal cortex (B); none of the treatments affected CREB. Bars represent means ± S.E.M. (n = 3–4 per group) and are expressed as percentage of vehicle values determined using densitometry and normalized to β-actin. **, P < 0.01 versus corresponding control. Representative immunoblots for pCREB and β-actin are shown.
Effects of Repeated Treatment with Rolipram, Piclamilast, and CDP840 on Hippocampal Cell Proliferation and Survival.
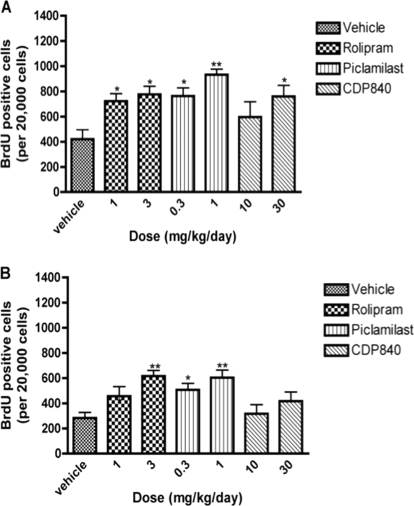
The proliferation and survival of newborn cells in rat hippocampus were measured after repeated treatment with rolipram, piclamilast, or CDP840 (Fig. 3). Sixteen-day treatment with 1 and 3 mg/kg rolipram or 0.3 and 1 mg/kg piclamilast increased both cell proliferation and survival in the hippocampal samples, but the effects on survival only were significant at the higher doses. Repeated treatment with 30 mg/kg CDP840 increased cell proliferation but not survival.
Fig. 3.
Effects of repeated treatment with rolipram, piclamilast, or CDP840 on cell proliferation and survival in rat hippocampus. Treatment for 16 days with rolipram or piclamilast increased cell proliferation (A) and survival (B). CDP840 treatment increased proliferation (A) but not survival (B). Bars represent means ± S.E.M. (n = 3–4 per group). **, P < 0.01; *, P < 0.05 versus corresponding control.
Effects of Repeated Treatment with Rolipram, Piclamilast, and CDP840 on Forced-Swim Behavior.
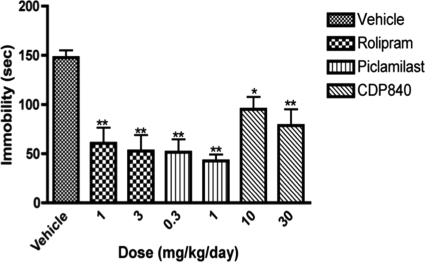
Repeated treatment with PDE4 inhibitors for 16 days produced antidepressant-like effects on the behavior of rats in the forced-swim test in a dose-dependent manner (Fig. 4). Two hours after the last injection, 1 and 3 mg/kg rolipram, 0.3 and 1 mg/kg piclamilast, and 10 and 30 mg/kg CDP840 reduced the time of immobility. At the doses tested, rolipram and piclamilast tended to produce greater reductions in immobility than did CDP840.
Fig. 4.
Effects of repeated treatment with rolipram, piclamilast, or CDP840 on immobility of rats in the forced-swim test. Treatment for 16 days with rolipram, piclamilast, or CDP840 decreased immobility time in a dose-dependent manner. Bars represent means ± S.E.M. (n = 4–5 per group). **, P < 0.01; *, P < 0.05 versus corresponding control.
Effects of Acute Treatment with Rolipram, Piclamilast, and CDP840 on cAMP and pCREB and Immobility in the Forced-Swim Test.
Acute treatment with 3 mg/kg rolipram, 1 mg/kg piclamilast, or 30 mg/kg CDP840 increased cAMP in prefrontal cortex and hippocampus in a time-dependent manner (Table 1). The peak effects of the inhibitors were observed 2 h post-treatment. Similar effects of the PDE4 inhibitors were observed on pCREB in prefrontal cortex and hippocampus (Table 1), with a peak effect also 2 h post-treatment.
TABLE 1.
Effects of acute treatment with PDE4 inhibitors on cAMP and pCREB in hippocampus and prefrontal cortex
Values shown are means ± S.E. expressed as pmol/mg protein cAMP or percentage of control pCREB; n = 6 per group.
| Control | Hours Post-Treatment |
|||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | 4 | ||
| % | pmol/mg protein cAMP | |||||
| Rolipram (3 mg/kg) | ||||||
| Hippocampus | ||||||
| cAMP | 17.5 ± 1.9 | 24.9 ± 3.5 | 47.5 ± 4.5* | 68.9 ± 5.7** | 47.8 ± 3.0* | 23.5 ± 5.0 |
| pCREBa | 100 | 101.8 ± 5.0 | 131.8 ± 8.2 | 182.5 ± 11.0* | 126.3 ± 8.1 | 116 ± 7.8 |
| Prefrontal cortex | ||||||
| cAMP | 18.7 ± 2.7 | 24.8 ± 3.0 | 44.7 ± 5.1* | 61.0 ± 7.3** | 40.3 ± 4.2* | 25.4 ± 6.0 |
| pCREB | 100 | 103 ± 5.9 | 123.5 ± 7.5 | 160.8 ± 10.5* | 127.5 ± 10.9 | 116 ± 7.8 |
| Piclamilast (1 mg/kg) | ||||||
| Hippocampus | ||||||
| cAMP | 21 ± 1.1 | 38.5 ± 4.7 | 58.8 ± 6.1** | 73 ± 7.9** | 49.5 ± 5.5** | 38.5 ± 3.9 |
| pCREB | 100 | 102.3 ± 7.4 | 117.3 ± 11.6 | 157.3 ± 14.8* | 133.0 ± 8.6 | 109.8 ± 7.0 |
| Prefrontal cortex | ||||||
| cAMP | 18.7 ± 2.7 | 38.8 ± 4.9 | 73.3 ± 4.5** | 89.3 ± 6.1** | 52.3 ± 11.5* | 36.0 ± 7.1 |
| pCREB | 100 | 98.5 ± 9.2 | 129.8 ± 9.8 | 156 ± 10.0* | 124.3 ± 5.1 | 101.3 ± 6.8 |
| CDP840 (30 mg/kg) | ||||||
| Hippocampus | ||||||
| cAMP | 22.0 ± 3.9 | 30.8 ± 3.0 | 42.7 ± 6.6* | 51.5 ± 4.1** | 42.6 ± 5.6* | 26.1 ± 2.9 |
| pCREB | 100 | 111.7 ± 2.3 | 135.7 ± 16.6 | 141.3 ± 9.3* | 125.3 ± 7.2 | 102.3 ± 3.6 |
| Prefrontal cortex | ||||||
| cAMP | 23.8 ± 3.7 | 29.2 ± 5.6 | 32.5 ± 2.1 | 52.2 ± 4.1** | 32.3 ± 4.1 | 27.8 ± 4.0 |
| pCREB | 100 | 111.7 ± 2.4 | 117.0 ± 8.2 | 136.3 ± 12.1* | 125.3 ± 7.0 | 102.3 ± 3.5 |
None of the treatments affected CREB.
P < 0.01; *P < 0.05 vs. corresponding control.
Immobility in the forced-swim test was recorded 30 min or 2 h after administration of the PDE4 inhibitors. Doses of 0.3 mg/kg rolipram, 0.3 mg/kg piclamilast, and 3 mg/kg CDP840 decreased immobility in the forced-swim test at 30 min after acute treatment, but not 2 h post-treatment (Table 2). Acute treatment with higher doses of the PDE4 inhibitors did not affect immobility in the forced-swim test at either time point.
TABLE 2.
Effects of acute treatment with PDE4 inhibitors on immobility in the forced-swim test
Values shown are means ± S.E. for times of immobility of separate groups of rats tested in the forced-swim test; n = 6 per group. Rats were treated with PDE4 inhibitors and tested in forced-swim test 0.5 or 2 h post-treatment.
| Immobility |
||
|---|---|---|
| 0.5 h Post-Treatment | 2 h Post-Treatment | |
| s | ||
| Vehicle | 154 ± 10.2 | 138 ± 14.2 |
| Rolipram | 84 ± 12.1* | 144 ± 7.4 |
| 0.3 mg/kg | 174 ± 9.2 | 122 ± 7.0 |
| 1 mg/kg | ||
| 3 mg/kg | 201 ± 8.6 | 127 ± 10.9 |
| Piclamilast | 58 ± 6.24** | 132 ± 5.9 |
| 0.3 mg/kg | ||
| 1 mg/kg | ||
| 3 mg/kg | 129 ± 25.4 | 123 ± 9.3 |
| CDP840 | 91 ± 6.5* | 141 ± 10.6 |
| 3 mg/kg | 122 ± 19.0 | 126 ± 12.5 |
| 10 mg/kg | ||
| 30 mg/kg | 125 ± 15.0 | 123 ± 6.1 |
P < 0.01; *P < 0.05 vs. corresponding control.
Discussion
Repeated treatment with all three PDE4 inhibitors enhanced cAMP signaling in hippocampus and prefrontal cortex, as evidenced by increased cAMP and pCREB. These inhibitors also increased hippocampal cell proliferation and produced antidepressant-like effects on forced-swim behavior. However, rolipram and piclamilast, which bind to the HARBS with high affinity, were effective at increasing cAMP/pCREB signaling and hippocampal cell proliferation at lower doses compared with CDP840, which has lower affinity for the HARBS (Zhao et al., 2003a). Likewise, rolipram and piclamilast decreased the time of immobility in the forced-swim test at lower doses than those required for CDP840 to demonstrate similar effects.
The HARBS and LABRS were characterized in a study that showed that [3H]rolipram binds to recombinant PDE4A with two distinct affinity states with a 500-fold difference in Ki values (Jacobitz et al., 1996). The HARBS is only observed in the central nervous system, whereas the LABRS is present in both the brain and peripheral tissues (Zhao et al., 2003a). Although rolipram exhibits different affinities to the HARBS and LARBS, piclamilast exhibits equal high affinity for both (Zhao et al., 2003a), and CDP840, by contrast, interacts with moderate, but similar, affinity with both states (Zhao et al., 2003a). The present findings suggest that inhibitor interactions with the HARBS are important for mediating neurochemical and behavioral effects of rolipram, piclamilast, and CDP840. Repeated treatment with rolipram or piclamilast increased cAMP signaling and hippocampal cell proliferation and produced antidepressant-like behavioral effects at doses below those at which CDP840 produced similar effects.
Previously, it has been found that repeated treatment with the antidepressant drugs desipramine and fluoxetine increases the HARBS in brain, measured either by [3H]rolipram or [3H]piclamilast binding (Zhao et al., 2003b). By contrast, such treatment does not alter the LARBS in brain. The effects of fluoxetine and desipramine on the HARBS in the brain are prevented when noradrenergic or serotonergic neurons are lesioned with 6-hydroxydopamine or 5,7-dihydroxytryptamine, respectively (Zhao et al., 2003b). This suggests that the neurochemical pathways involved in the mediation of antidepressant activity can influence PDE4 expression and activity. In rats trained to discriminate rolipram from vehicle, only PDE4 inhibitors that have a high affinity for the HARBS generalize to rolipram (Zhang et al., 2006). Likewise, when the effects of acute treatment with PDE4 inhibitors on forced-swim behavior are examined, a potency order of piclamilast > rolipram > CDP840 is observed (Zhang et al., 2006); this is consistent with their relative affinities for the HARBS (Zhao et al., 2003a).
Previous studies have shown that hippocampal neurogenesis plays an important role in some effects of antidepressants that depend on repeated treatment (Santarelli et al., 2003). In addition, the time course of the change in neurogenesis in hippocampus is consistent with the delayed onset of antidepressant efficacy (Sahay and Hen, 2007). The present studies show that repeated treatment with rolipram and piclamilast also increased hippocampal cell proliferation. Previous work has demonstrated that approximately 80% of the increase in BrdU labeling is caused by increased neurogenesis (Li et al., 2009). It is noteworthy that repeated treatment with CDP840 at 10 or 30 mg/kg decreased immobility in the forced-swim test, indicating an antidepressant-like effect, but did not increase hippocampal proliferation. Other studies also have shown that some antidepressant-like behavioral effects do not depend completely on neurogenesis (Airan et al., 2007; David et al., 2007; Wang et al., 2008). This suggests that neurogenesis might not be the only mechanism underlying PDE4 inhibitor-mediated antidepressant-like effects on behavior.
When rats were treated acutely with the PDE4 inhibitors rolipram, piclamilast, or CDP840, there was dissociation between neurochemical and behavioral effects. The time course revealed peak effects at 2 h after acute treatment; by contrast, PDE4 inhibitors decreased immobility in the forced-swim test 30 min post-treatment, but not at 2 h post-treatment. In addition, in contrast to the monotonic dose-response curve for the effects of repeated treatment, acute treatment with higher doses of the PDE4 inhibitors no longer decreased immobility. It is possible that sedative effects of higher doses may confound the results in the forced-swim test after acute treatment, but that some tolerance may develop with repeated treatment.
Previous studies have shown that long-term antidepressant treatment increases cAMP as well as phosphorylation of CREB in specific brain regions. The activated cAMP/pCREB signaling cascade induces increased expression of brain-derived neurotrophic factor in the hippocampus and cerebral cortex (Duman et al., 1997). In addition, repeated administration of either a serotonin- or norepinephrine-selective reuptake inhibitor enhances neurogenesis in adult rodent hippocampus via up-regulation of cAMP/pCREB signaling (Duman et al., 2001). It is noteworthy that cell proliferation is decreased in transgenic mice that express a dominant negative mutant of CREB in the hippocampus (Nakagawa et al., 2002). Furthermore, electroconvulsive shock, an effective antidepressant therapy, also enhances phosphorylation of CREB in rat hippocampus (Jeon et al., 1997). These studies demonstrate that, like proven antidepressants, PDE4 inhibitors produce persistent, enhanced cAMP signaling, altering transcription of neurotrophic factors, increasing hippocampal cell proliferation, and contributing to antidepressant-like effects on behavior.
In summary, it was found that repeated treatment with PDE4 inhibitors increased cAMP/pCREB signaling and hippocampal cell proliferation and also produced antidepressant-like effects on behavior. Comparison of the neurochemical and behavioral effects of rolipram, piclamilast, and CDP840 suggests that interaction with the HARBS, in contrast to the LARBS, mediates these effects. Improved understanding of molecular mechanisms that contribute to the presence of two PDE4 affinity states in the brain may suggest targets for antidepressant drug discovery; potential candidate includes scaffolding proteins (McCahill et al., 2005; Millar et al., 2005) and unique conserved regions of the N terminal of PDE4 (Burgin et al., 2010).
Acknowledgments
We thank Ying Huang and Christopher M. Felton for expert technical assistance. Data for BrdU labeling of hippocampal cells were collected at the West Virginia University Flow Cytometry Core Facility with the consultation and assistance of Drs. Kathleen Brundage and Christopher Cuff.
This research was supported by the National Institutes of Health National Institute of Mental Health [Grants MH051175, MH040694, MH088480]. The West Virginia University Flow Cytometry Core Facility is supported by the National Institutes of Health National Institute for Research Resources [Grants RR016440, RR020866]. J.M.O. has received research funding from Lundbeck Pharmaceuticals and Memory Pharmaceuticals.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179358.
- PDE4
- phosphodiesterase-4
- CREB
- cAMP response element binding protein
- pCREB
- phosphorylated CREB
- BrdU
- bromodeoxyuridine
- HARBS
- high-affinity rolipram binding state
- LARBS
- low-affinity rolipram binding state
- CDP840
- 4-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)pyridine.
Authorship Contributions
Participated in research design: Xiao, O'Callaghan, and O'Donnell.
Conducted experiments: Xiao.
Contributed new reagents or analytic tools: O'Callaghan.
Performed data analysis: Xiao.
Wrote or contributed to the writing of the manuscript: Xiao, O'Callaghan, and O'Donnell.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317:819–823 [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. (2009) Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology 34:1764–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnette MS, Bartus JO, Burman M, Christensen SB, Cieslinski LB, Esser KM, Prabhakar US, Rush JA, Torphy TJ. (1996) Association of the anti-inflammatory activity of phosphodiesterase 4 (PDE4) inhibitors with either inhibition of PDE4 catalytic activity or competition for [3H]rolipram binding. Biochem Pharmacol 51:949–956 [DOI] [PubMed] [Google Scholar]
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. (2006) A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods 157:54–63 [DOI] [PubMed] [Google Scholar]
- Blokland A, Schreiber R, Prickaerts J. (2006) Improving memory: a role for phosphodiesterases. Curr Pharm Des 12:2511–2523 [DOI] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, et al. (2010) Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol 28:63–70 [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. (2007) Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54:387–402 [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. (2003) Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cAMP signaling. J Biol Chem 278:5493–5496 [DOI] [PubMed] [Google Scholar]
- D'Sa C, Tolbert LM, Conti M, Duman RS. (2002) Regulation of cAMP-specific phosphodiesterases type 4B and 4D (PDE4) splice variants by cAMP signaling in primary cortical neurons. J Neurochem 81:745–757 [DOI] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, et al. (2007) Efficacy of the MCHR1 antagonist N- [3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther 321:237–248 [DOI] [PubMed] [Google Scholar]
- Dlaboga D, Hajjhussein H, O'Donnell JM. (2006) Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: comparison with rolipram. Brain Res 1096:104–112 [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. (1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54:597–606 [DOI] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. (2001) Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25:836–844 [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wolf R, Gerlach W, Jaklitsch H, Sastre-y-Hernández M. (1992) A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology 26:59–64 [DOI] [PubMed] [Google Scholar]
- Hajjhussein H, Suvarna NU, Gremillion C, Chandler LJ, O'Donnell JM. (2007) Changes in NMDA receptor-induced cyclic nucleotide synthesis regulate the age-dependent increase in PDE4A expression in primary cortical cultures. Brain Res 1149:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. (2008) Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 33:406–417 [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. (2003) PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobitz S, McLaughlin MM, Livi GP, Burman M, Torphy TJ. (1996) Mapping the functional domains of human recombinant phosphodiesterase 4A: structural requirements for catalytic activity and rolipram binding. Mol Pharmacol 50:891–899 [PubMed] [Google Scholar]
- Jeon SH, Seong YS, Juhnn YS, Kang UG, Ha KS, Kim YS, Park JB. (1997) Electroconvulsive shock increases the phosphorylation of cAMP response element binding protein at Ser-133 in rat hippocampus but not in cerebellum. Neuropharmacology 36:411–414 [DOI] [PubMed] [Google Scholar]
- Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O'Donnell JM, Zhang HT. (2011) Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci 31:172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Huang Y, Amsdell SL, Xiao L, O'Donnell JM, Zhang HT. (2009) Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cAMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34:2404–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill A, McSorley T, Huston E, Hill EV, Lynch MJ, Gall I, Keryer G, Lygren B, Tasken K, van Heeke G, et al. (2005) In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cAMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal 17:1158–1173 [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. (2005) DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310:1187–1191 [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. (2002) Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22:3673–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan JP, Sriram K. (2004) Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods 135:159–168 [DOI] [PubMed] [Google Scholar]
- O'Donnell JM. (1993) Antidepressant-like effects of rolipram and other inhibitors of cyclic adenosine monophosphate phosphodiesterase on behavior maintained by differential reinforcement of low response rate. J Pharmacol Exp Ther 264:1168–1178 [PubMed] [Google Scholar]
- O'Donnell JM, Zhang HT. (2004) Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol Sci 25:158–163 [DOI] [PubMed] [Google Scholar]
- Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL. (2008) Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci 28:625–632 [DOI] [PubMed] [Google Scholar]
- Saccomano NA, Vinick FJ, Koe BK, Nielsen JA, Whalen WM, Meltz M, Phillips D, Thadieo PF, Jung S, Chapin DS. (1991) Calcium-independent phosphodiesterase inhibitors as putative antidepressants: [3-(bicycloalkyloxy)-4-methoxyphenyl]-2-imidazolidinones. J Med Chem 34:291–298 [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. (2007) Adult hippocampal neurogenesis in depression. Nat Neurosci 10:1110–1115 [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809 [DOI] [PubMed] [Google Scholar]
- Siuciak JA. (2008) The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 22:983–993 [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Martin AN. (2007) Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology 192:415–424 [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. (2008) Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology 197:115–126 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terwilliger R, Lane C, Mezes PS, Conti M, Duman RS. (1999) Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J Neurosci 19:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS. (2007) Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med 39:531–544 [DOI] [PubMed] [Google Scholar]
- Wachtel H, Löschmann PA. (1986) Effects of forskolin and cyclic nucleotides in animal models predictive of antidepressant activity: interactions with rolipram. Psychopharmacology 90:430–435 [DOI] [PubMed] [Google Scholar]
- Wachtel H, Schneider HH. (1986) Rolipram, a novel antidepressant drug, reverses the hypothermia and hypokinesia of monoamine-depleted mice by an action beyond postsynaptic monoamine receptors. Neuropharmacology 25:1119–1126 [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Jackson K, O'Donnell JM. (2000) Effects of repeated antidepressant treatment of type 4A phosphodiesterase (PDE4A) in rat brain. J Neurochem 74:1257–1262 [DOI] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernández M. (1984) Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17:188–190 [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O'Donnell JM. (2002) Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology 27:587–595 [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O'Donnell JM. (2007) Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology 33:1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Deng C, Hopper AT, De Vivo M, Rose GM, O'Donnell JM. (2006) Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology 186:209–217 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang HT, O'Donnell JM. (2003a) Inhibitor binding to type 4 phosphodiesterase (PDE4) assessed using [3H]piclamilast and [3H]rolipram. J Pharmacol Exp Ther 305:565–572 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang HT, O'Donnell JM. (2003b) Antidepressant-induced increase in high-affinity rolipram binding sites in rat brain: dependence on noradrenergic and serotonergic function. J Pharmacol Exp Ther 307:246–253 [DOI] [PubMed] [Google Scholar]