Abstract
Despite its long history of use and abuse in human culture, the molecular basis for alcohol action in the brain is poorly understood. The recent determination of the atomic-scale structure of GLIC, a prokaryotic member of the pentameric ligand-gated ion channel (pLGIC) family, provides a unique opportunity to characterize the structural basis for modulation of these channels, many of which are alcohol targets in brain. We observed that GLIC recapitulates bimodal modulation by n-alcohols, similar to some eukaryotic pLGICs: methanol and ethanol weakly potentiated proton-activated currents in GLIC, whereas n-alcohols larger than ethanol inhibited them. Mapping of residues important to alcohol modulation of ionotropic receptors for glycine, γ-aminobutyric acid, and acetylcholine onto GLIC revealed their proximity to transmembrane cavities that may accommodate one or more alcohol molecules. Site-directed mutations in the pore-lining M2 helix allowed the identification of four residues that influence alcohol potentiation, with the direction of their effects reflecting α-helical structure. At one of the potentiation-enhancing residues, decreased side chain volume converted GLIC into a highly ethanol-sensitive channel, comparable to its eukaryotic relatives. Covalent labeling of M2 positions with an alcohol analog, a methanethiosulfonate reagent, further implicated residues at the extracellular end of the helix in alcohol binding. Molecular dynamics simulations elucidated the structural consequences of a potentiation-enhancing mutation and suggested a structural mechanism for alcohol potentiation via interaction with a transmembrane cavity previously termed the “linking tunnel.” These results provide a unique structural model for independent potentiating and inhibitory interactions of n-alcohols with a pLGIC family member.
Keywords: cys-loop receptor, Gloeobacter violaceus
Humans have produced and consumed ethanol for industrial, recreational, and medical purposes for millennia, with a broad spectrum of effects on human health. In fact, ethanol is one of several alcohols that cause intoxication and anesthesia. Despite the widespread historical role of alcohol in society, the molecular basis of alcohol pharmacology remains unclear.
Current models of alcohol action on the central nervous system support a role for proteins including ion channels. Indeed, intoxicating concentrations of ethanol modulate several ion channels in vitro (1). The phenomenon of alcohol cutoff, by which n-alcohols increase in potency with carbon chain length up to a certain size (2), supports the presence of discrete protein sites capable of binding alcohols with increasing affinity up to a certain molecular volume (3). Alcohol cutoffs vary among ion channels and can be altered by point mutations (4), supporting the presence of specific alcohol binding sites in proteins. Further evidence for direct binding comes from alcohol analogs, such as diazirine derivatives (5) and methanethiosulfonate (MTS) reagents (6), which can covalently modify accessible protein residues. Labeling with MTS reagents has been further shown to mimic alcohol effects (6).
Many of the receptors associated with pharmacologically relevant effects of alcohol belong to the pentameric ligand-gated ion channel (pLGIC) family. Alcohols enhance function of glycine receptors (GlyRs) (7) and most γ-aminobutyric acid type A receptors (GABAARs) (8), whereas nicotinic acetylcholine receptors (nAChRs) exhibit potentiation by short-chain but inhibition by long-chain n-alcohols (9). Modulation of pLGICs occurs at ethanol concentrations approaching the legal impairment limit (1) and at even lower concentrations of long-chain n-alcohols (7–9). Genetic and pharmacological targeting of pLGICs in mice further support a role for these channels in mediating pharmacologically relevant effects of alcohol (10). Elucidating the precise binding interactions of alcohol with ion channels in vivo, however, has proven complex: many channels are heteromers of subunits with varying alcohol sensitivities, and their expression varies by tissue and with alcohol exposure (11). Furthermore, alcohol modulation of G proteins (12) and protein kinases (13) may modify ion channel function indirectly.
Structural models of pLGICs based on cryoelectron microscopy (14) and crystallography of partial (15) or truncated (16) homologs reveal a common topology consisting of five subunits arranged semisymmetrically around a conductive pore. Each subunit contains a transmembrane domain of four membrane-spanning helices (M1–M4) linked by loops of variable length. The M2 helices line the channel pore; to simplify comparisons, M2 residues are often numbered in prime notation, beginning at the N-terminal intracellular end (≈1′) and progressing to the C-terminal extracellular end (≈20′) (Fig. S1). Models of several pLGICs support gating transitions in which the transmembrane domain undergoes a wringing motion, reorganizing the helical interfaces within and between each subunit (17, 18).
Functional studies implicate residues in the transmembrane domains of several pLGICs as direct targets for alcohol. Transmembrane domain mutations, particularly at the 15′ position in M2, alter alcohol sensitivity (19) and cutoff (4) in GABAARs and GlyRs, and MTS labeling causes irreversible alcohol-like modulation (6, 20). Similar studies of nAChRs identified distinct residues (at the 16′ and 17′ positions in M2) mediating inhibitory and excitatory effects of n-alcohols (21). A photoactivatible octanol derivative also incorporates into the nAChR at the C-terminal end of M2, supporting a role for this region in direct binding of long-chain n-alcohols (5). Molecular models suggest these residues surround a water-filled pocket (22) in which alcohol binding could preferentially stabilize the open state (23, 24).
Recent crystal structures of the prokaryotic pLGIC GLIC in a presumed open (25, 26) or desensitized (27, 28) state pose unique opportunities to characterize ion channel modulation via a simplified model system. The similarity of GLIC to eukaryotic pLGICs was recently confirmed by the crystal structure of the Caenorhabditis elegans glutamate-gated chloride channel α (GluCl) (29). GLIC-mediated currents can be recorded in heterologous expression systems (30), and its homomeric assembly and limited intracellular domain restrict possible mechanisms of modulation. Furthermore, GLIC crystal structures contain ordered water and detergent molecules in close proximity to the M2 helices (25, 26), possibly mimicking interactions with alcohol. Recent functional (31) and structural (32) characterizations of GLIC inhibition by general anesthetics, as well as the GlyR-like allosteric modulation of a GLIC/GlyR chimera described in the accompanying article (33), further support the relevance of this prokaryotic channel to pLGIC modulation. We proposed that GLIC comprises a simplified, structurally accessible model system for structure and function of pLGICs, including modulation by n-alcohols.
Results and Discussion
GLIC Exhibits Differential Modulation by n-Alcohols.
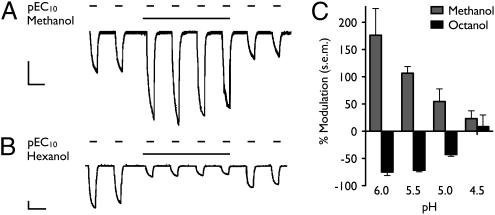
Despite structural and functional similarities between GLIC and eukaryotic pLGICs, modulation of GLIC by alcohols has not been previously demonstrated. An anesthetic concentration (34) of methanol (590 mM) did not directly activate GLIC during preapplication at neutral pH; however, in the presence of methanol, proton-gated GLIC currents elicited at pH 5.5 (≈pEC10) were potentiated approximately twofold (Fig. 1A). An anesthetic concentration of hexanol (570 μM) similarly had no direct effect on oocyte currents but instead inhibited proton-gated GLIC currents at pH 5.5 by more than 50% (Fig. 1B).
Fig. 1.
Modulation by n-alcohols of GLIC currents in Xenopus laevis oocytes. Current traces show successive GLIC activations by pH 5.5 (≈pEC10) in the presence and absence of (A) 590 mM methanol or (B) 570 μM hexanol. (Scale bars, 2 μA, 5 min). (C) Modulation by either short-chain n-alcohols (590 mM methanol, gray) or long-chain n-alcohols (36 μM octanol, black) was more pronounced at higher pH (lower level of activation). Errors are SEM, n = 2–30.
Both potentiation and inhibition by n-alcohols were more pronounced at lower levels of channel activation (Fig. 1C). At pH 4.5, where GLIC conducts more than 50% of its maximal current, modulation by either alcohol was negligible. Neither potentiation nor inhibition by n-alcohols was use-dependent, because the quantity of modulation did not change with successive activations in the prolonged presence of alcohol (Fig. 1 A and B). Both these features are consistent with alcohol actions on mammalian pLGICs (7, 35).
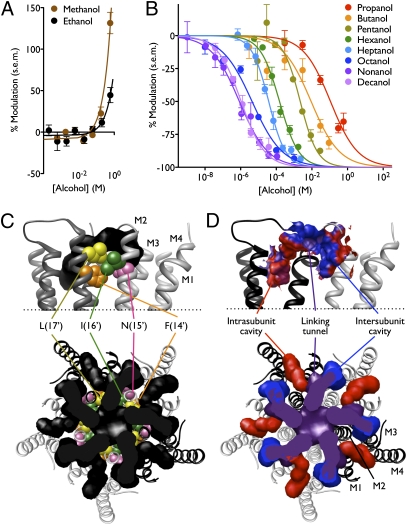
The profile of n-alcohol modulation of GLIC is reminiscent of eukaryotic pLGICs. In particular, nAChRs exhibit potentiation by short-chain and inhibition by long-chain n-alcohols (9). To characterize the pattern of chain length-dependent modulation in GLIC, we measured concentration-dependent modulation of proton-gated GLIC currents at pH 5.5 by a range of n-alcohols. High concentrations of both methanol and ethanol potentiated GLIC, with negligible modulation below 590 mM (Fig. 2A). Conversely, n-alcohols larger than ethanol inhibited GLIC, their potency increasing with chain length from propanol to nonanol (Fig. 2B). Decanol had similar potency to nonanol (Fig. 2B), suggesting that it could still be accommodated by the inhibitory site but that its increased volume did not enhance binding. By one common definition, the largest alcohol exhibiting increased potency compared with shorter-chain alcohols constitutes the alcohol cutoff (4), in this case nine carbons—a profile similar to nAChRs (36), GlyRs (7), and GABAARs (8).
Fig. 2.
Profile of GLIC modulation by n-alcohols. (A) GLIC currents were potentiated by high concentrations of methanol or ethanol. Because it was not possible to collect data for complete concentration response relationships, curves represent linear regression fits. (B) GLIC currents were inhibited by n-alcohols larger than ethanol in a dose-dependent manner. Potency of inhibition by long-chain n-alcohols (propanol and larger) increased with chain length up to nonanol. Curves represent nonlinear regression fits as described in SI Methods. In A and B, errors are SEM, n = 2–18. (C) Region of GLIC (Protein Data Bank ID 3EAM) (25) transmembrane domain surrounding M2 residues previously implicated in alcohol modulation of eukaryotic pLGICs, shown as spheres: L(17′) (L241, yellow) and I(16′) (I240, green), homologous to the L263 “excitatory site” and L262 “inhibitory site” in M2 of the α2 nAChR (21); and N(15′) (N239, pink), homologous to S267 in the α1 GlyR (19). Position F(14′) (F238, orange), demonstrated in this study to strongly influence alcohol modulation of GLIC, is also shown. Cavities (black) neighboring the implicated residues were calculated using the Hollow script as described in SI Methods. Upper: Two GLIC subunits (gray, white) viewed from the plane of the membrane in the channel pore; cavity regions corresponding to the pore lumen are removed for clarity. Transmembrane helices M1–M4 are labeled in one subunit. Lower: Full pentameric channel transmembrane domain and cavities, viewed from the extracellular side. (D) Views as in C. Upper: Solvent-excluded surfaces surrounding cavities defined in C, with polar (blue), nonpolar (red), and intermediate (purple) regions colored by residue hydrophobicity. For clarity, M2 helices are not shown. Lower: Internal cavities as in C with intrasubunit (red) and intersubunit (blue) regions colored independently of linking tunnels and pore lumen (purple). Transmembrane helices are labeled in one subunit.
Residues Implicated in Alcohol Modulation Border Multiple Internal Cavities.
We used the known crystal structure of GLIC to model residues implicated in alcohol action. Alcohol has been proposed to occupy water-filled amphiphilic cavities in proteins (37). Furthermore, ion channel residues associated with alcohol modulation are proposed to neighbor regions critical to gating, thus converting the limited binding energy of one or more alcohol molecules into changes in the gating energy landscape as efficiently as possible (38). Therefore, we asked whether amino acid residues homologous to those that affect alcohol modulation in pLGICs might border solvent-accessible cavities and/or influence ion channel gating in GLIC.
We focused on the GLIC transmembrane domain, which aligns in a straightforward fashion with those of several pLGICs (39) (Fig. S1). The resulting alignments place amino acid residues involved in alcohol modulation around cavities at the extracellular end of the transmembrane domain (Fig. 2C), a region considered critical for channel gating (17). Each GLIC subunit is associated with one intrasubunit and one intersubunit cavity, connected to one another via narrow channels through a pore-facing linking tunnel (Fig. 2D) (32). The intrasubunit cavity is primarily hydrophobic (Fig. 2D); it is predicted to open onto the hydrophobic core of the lipid bilayer and is partially occupied in the crystal structure by lipids (25). It is also occupied by anesthetic inhibitors in two recent cocrystal structures (32). The intersubunit cavity and linking tunnel are more hydrophilic (Fig. 2D) and are occupied by multiple crystallographically resolved water molecules (25, 26). The intersubunit cavity is accessible to the lipid bilayer, whereas the linking tunnel opens onto the pore lumen (Fig. 2 C and D). When modeled onto the GLIC channel, most M2 residues involved in alcohol modulation cluster around the water-filled intersubunit cavity and linking tunnel (Fig. 2C). Indeed, a cavity homologous to the intersubunit-linking tunnel region was occupied by ethanol in a recent 2-μs molecular dynamics simulation in the α1 GlyR (40).
Specific Mutations in the Pore-Lining Helix Modify Alcohol Potentiation.
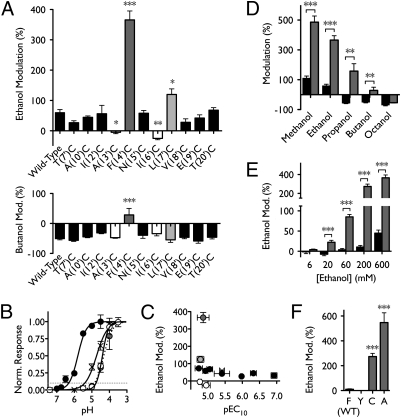
To identify specific residues involved in alcohol modulation of GLIC, and potentially of eukaryotic pLGICs, we performed cysteine-scanning mutagenesis on the M2 helix of GLIC and measured the effect of individual cysteine substitutions on gating and alcohol modulation. We focused on the C-terminal end of the M2 helix, near the extracellular domain, because of past evidence for alcohol modulation sites in this region (Fig. 2C). All mutants tested produced functional channels in oocytes except L(8′)C and H(11′)C. Mutant I(9′)C showed evidence of rapid disulfide bond formation (Fig. S2A, Top) and could not be characterized fully. Modulation by various n-alcohols was tested for all other mutants at their respective EC10 activation levels, as in previous studies of alcohol modulation in GlyRs and GABAARs (19).
Potentiation by high concentrations of ethanol was unchanged in all but four of the mutants tested. Most dramatically, cysteine substitution at F(14′) and L(17′) markedly enhanced potentiation (Fig. 3A, Upper). Indeed, the F(14′)C mutation enhanced potentiation by both methanol and ethanol relative to wild type and produced potentiation rather than inhibition by propanol and butanol (Fig. 3D). This mutant was potentiated by ethanol at concentrations as low as 20 mM (Fig. 3E), approximately the blood alcohol concentration considered legally intoxicating in the United States (1). Thus, a single substitution at the 14′ position converts GLIC into a channel that is modulated by pharmacologically relevant concentrations of ethanol in a manner similar to eukaryotic pLGICs (1). Sequence alignments reveal that several ethanol-sensitive pLGICs contain smaller residues at 14′ (Fig. S1), suggesting that side chain volume at this position may influence alcohol sensitivity throughout this protein family.
Fig. 3.
Effects of cysteine substitutions in M2 on n-alcohol modulation. (A) Modulation by 600 mM ethanol (Upper) or 11 mM butanol (Lower) of wild-type GLIC and 11 mutants. Channels exhibiting ethanol potentiation not significantly different from wild type are shown in black; mutants that ablated (13′, 16′; white) or enhanced (14′, 17′; gray) ethanol potentiation are colored independently (significance vs. wild-type, Dunnet's multiple comparison test, analysis of variance). (B) Sample pH response curves showing enhanced proton sensitivity of mutant A(10′)C (black circles) and reduced proton sensitivity of A(13′)C (white circles) and F(14′)C (gray circles, dotted curve) relative to wild type (crosses). Curves represent nonlinear regression fits as described in SI Methods. Dotted line represents 10% activation, at which alcohol modulation was measured. (C) Lack of correlation between changes in gating and ethanol potentiation for wild-type (crosses) and cysteine-substituted channels (circles) colored as in A. (D) Modulation of wild-type (black) and F(14′)C (gray) GLIC by a range of n-alcohols: 590 mM methanol, 600 mM ethanol, 86 mM propanol, 11 mM butanol, and 36 μM octanol. (E) Enhancement of potentiation in mutant F(14′)C (gray) relative to wild type (black) at a range of ethanol concentrations. In D and E, significance is vs. wild type, unpaired t test. (F) Potentiation by 200 mM ethanol of a variety of mutants substituted at position 14′ (significance vs. wild-type, Dunnet's multiple comparison test, analysis of variance). Errors are SEM, n = 2–17. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine the physicochemical requirements for the ethanol effect observed in F(14′)C, we tested alternative substitutions at the 14′ position. Decreasing hydrophobicity by substituting tyrosine at this position did not change ethanol potentiation; on the other hand, decreasing side chain volume by substituting alanine enhanced ethanol potentiation to an even greater degree than cysteine (Fig. 3F). This alternative substitution also removes the possibility for disulfide bond formation, indicating that covalent cross-linking is not required for the enhancement of ethanol effects observed in F(14′)C. Also consistent with cysteine substitution, alanine substitution dramatically shifted the chain-length cutoff for potentiation: mutant F(14′)A was potentiated by alcohols as large as hexanol, whereas longer-chain alcohols had similar effects as on wild-type GLIC (Fig. S3A and SI Discussion). Similar to wild-type GLIC, the F(14′)A mutant exhibited greater ethanol potentiation at lower levels of channel activation (Fig. S3B), although an anesthetic concentration (200 mM) of ethanol did potentiate proton-evoked responses in the mutant at all levels of activation tested. The evident enhancement of maximal proton responses by ethanol (Fig. S3C) was further reminiscent of nAChRs (21).
In contrast to the positions at which cysteine enhanced ethanol potentiation (14′ and 17′), which are oriented toward the intersubunit interface in the GLIC crystal structure, substitutions at A(13′) and I(16′), which face the channel pore (Fig. S2B), removed or reversed potentiation by ethanol (Fig. 3A and Fig. S2D, Upper). The pattern of ethanol-enhancing and -suppressing residues was thus consistent with the α-helical structure of the M2 region. Notably, the 17′ and 16′ positions correspond to residues that mediate excitatory and inhibitory effects of n-alcohols, respectively, in the nAChR (21). Inhibition by 11 mM butanol (approximately IC50 for wild-type GLIC) was unchanged in all mutants except F(14′)C (Fig. 3A and Fig. S2D, Lower). Evaluation of longer-chain alcohol effects on this mutant revealed inhibition by 36 μM (≈IC50) octanol that was indistinguishable from wild-type GLIC (Fig. 3D). The consistency of long-chain alcohol effects in all M2 mutants suggests that mechanisms of potentiation and inhibition are independent, as has been proposed for both nAChRs (41) and GABAARs (42).
Mutations in the M2 helix, as expected, also altered pH responses of GLIC; sample curves in Fig. 3B show examples of both enhanced and inhibited proton gating. Given that alcohol modulation varies with the level of channel activation (Fig. 1C), we corrected for variations in gating by calculating the pEC10 for each mutant and using this pH to activate the channel in all modulation experiments. There was no correlation between pEC10 and ethanol potentiation (Fig. 3C), indicating that variations in these two channel properties were independent.
Labeling of Specific M2 Residues Mimics Alcohol Potentiation.
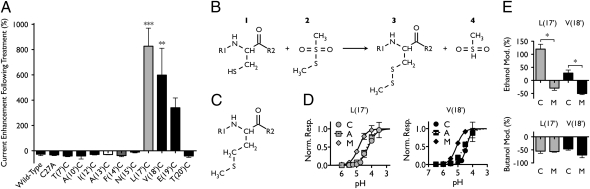
Labeling of introduced cysteines with MTS reagents has been used previously to mimic alcohol binding to specific residues in pLGICs (6). If an alcohol binding site contains an exposed cysteine side chain, covalent labeling with an MTS reagent should result in persistent modification that mimics modulation by an n-alcohol, while reducing or blocking further alcohol modulation (6) (SI Discussion). Given the low potency of ethanol for potentiation of wild-type GLIC, we chose the smallest MTS reagent available, methyl MTS, to mimic the potentiating effect of methanol on wild-type GLIC. Although methyl MTS itself is substantially larger than methanol, potentially limiting its access to reactive residues, the labeling reaction covalently binds just the methanethiol group, leaving behind a methanol analog that differs only by one atom (Fig. 4B).
Fig. 4.
Labeling of cysteine mutants by MTS alcohol analogs. (A) GLIC current amplitudes for wild-type, C27A, and 10 M2 cysteine mutants after treatment with methyl MTS, relative to currents measured immediately before treatment (significance vs. modulation of wild-type GLIC, Dunnett's multiple comparison test, analysis of variance). (B) Reaction scheme showing labeling of cysteine-containing protein (1) by methyl MTS (2), yielding the methanethiolated protein (3) and a rapidly decomposing sulfinic acid (4). (C) Chemical structure of methionine in a protein. (D) Proton response curves for mutants in which L(17′) (gray, Left) or V(18′) (black, Right) was substituted with cysteine (circles), alanine (squares, dotted curve), or methionine (diamonds); curves fitted as in Fig. 3D. (E) Modulation by 600 mM ethanol (Upper) or 11 mM butanol (Lower) of GLIC mutants with cysteine or methionine substitutions at L(17′) (gray) or V(18′) (black) (significance of C vs. M mutants at each position, unpaired t test). Errors are SEM, n = 2–11. *P < 0.05; **P < 0.01; ***P < 0.001.
Application and washout of methyl MTS resulted in significant current enhancement of mutants containing cysteine substitutions at 17′ and 18′ (Fig. 4A). Furthermore, mimicking methyl MTS modification by methionine substitution (Fig. 4C and SI Discussion) enhanced proton gating (Fig. 4D) and selectively abolished ethanol modulation (Fig. 4E), indicating that modification of either position with a methyl MTS-labeled or structurally equivalent residue substitutes for alcohol potentiation. Mutant E(19′)C also showed a trend toward enhancement after methyl MTS treatment that was significant by two-tailed unpaired t test (P < 0.01) but was not significant in the context of all mutants tested (Fig. 4A). The remaining mutants tested were no different from wild type after methyl MTS washout (Fig. 4A), indicating that, under these conditions, either the engineered residues were not accessible to the labeling reagent, or labeling had no effect on channel function. The latter condition is important to consider given the minimal change effected by labeling of a cysteine with methyl MTS, which only adds two nonhydrogen atoms to each residue (Fig. 4B); this distinction may underlie differences between our results and studies using larger, charged cysteine labeling reagents (27).
Wild-type channels exhibited a small decrease in current amplitude after MTS treatment (Fig. 4A), a change that was consistent with overall rundown observed in the latter stages of most GLIC recordings. There is only one cysteine in wild-type GLIC, at position 27 in the extracellular domain; relative currents after methyl MTS treatment of mutant C27A were indistinguishable from wild type (Fig. 4A), indicating that MTS effects are not related to modification of this native cysteine. Mutants I(9′)C and I(16′)C, which demonstrated spontaneous cross-linking (Fig. S2A), were excluded from methyl MTS analysis, owing to the likelihood that MTS reagents might disrupt intersubunit disulfide bonds and require complex interpretation.
The location of MTS-reactive residues in the GLIC structure suggests that the structural basis for alcohol modulation may be conserved in eukaryotic pLGICs. For example, labeling by MTS reagents at position 17′ in the α2 nAChR (21) as well as in GLIC mimics alcohol modulation, suggesting that cavity volume in this region is important in both channel types. The critical position for alcohol potentiation of GlyRs and GABAARs, 15′ (19), does not influence alcohol modulation (Fig. 3A) or mediate current enhancement after methyl MTS labeling (Fig. 4A) in GLIC; however, this residue is one register below position 18′, at which methyl MTS labeling both mimics (Fig. 4 A and D) and blocks subsequent (Fig. 4E) alcohol potentiation. Given that several residues at the extracellular end of M2 are smaller in GlyRs and GABAARs than in GLIC (Fig. S1), the cavity that borders the 18′ position is likely to penetrate deeper into the subunit in the eukaryotic channels, potentially accessing the 15′ position directly below. Recent evidence for enhanced alcohol potentiation in a GLIC chimera containing the α1 GlyR transmembrane domain (33) supports the principle that local changes in this domain substantially influence alcohol modulation.
Transmembrane Cavities Implicated in Alcohol Potentiation of GLIC.
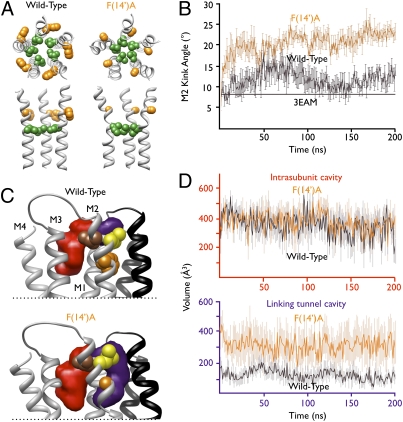
To model the structural basis for alcohol potentiation in GLIC, we performed molecular dynamics simulations of both wild-type GLIC and the potentiation-enhanced mutant F(14′)A. Early in the 200-ns simulation, the 14′ substitution caused a structural change with a dramatic increase in the kink angle of the M2 helix (Fig. 5A), around the level of the hydrophobic gate at the 9′ position (43). Over the second half of the simulation the kink angle was consistently larger in the F(14′)A mutant than in the wild-type channel (Fig. 5B). This increased kink angle bent the C-terminal end of the M2 helix away from the pore lumen, increasing the diameter of the pore and spreading the subunits apart from one another above the level of the kink (Fig. 5A). Thus, for example, at the end of 200-ns simulation, Cα atoms of neighboring I(16′) residues are separated by 10.0 ± 0.4 Å in wild-type GLIC but by 11.3 ± 0.6 Å in F(14′)A. Conversely, the enhanced kink angle in the F(14′)A mutant compressed the pore constriction at the level of I(9′), obstructing the ion conduction pathway at this residue (Fig. 5A); this obstruction may account for the reduced pH sensitivity of F(14′) mutants relative to wild-type GLIC (Fig. 3B). It should be noted that the functional state of the GLIC crystal structure is currently controversial; some recent studies suggest it represents a desensitized state (27, 28), whereas its close structural alignment with the open-state structure of GluCl supports its open conducting conformation (29). It remains to be determined how close simulations such as ours may be to a conductive state.
Fig. 5.
Structural correlates of GLIC alcohol potentiation. (A) Wild-type (Left) and F(14′)A (Right) GLIC after 200-ns molecular dynamics simulations. Upper: M2 helices viewed from the extracellular side with kink position 9′ (green) and ethanol-enhancing position 14′ (orange) as spheres. Lower: Equivalent residues in the M2 helices of three subunits viewed from the channel pore. (B) Measurement of M2 helix kink angle over the course of wild-type (black) and F(14′)A (orange) simulations. Solid black line represents kink angle in GLIC crystal structure. (C) Region of GLIC surrounding key M2 positions after 200-ns simulation. Upper: Transmembrane helices from two subunits with positions 14′ (orange), 17′ (yellow), and 18′ (brown) on the proximal (gray) subunit shown as spheres. Average intrasubunit (red) and linking tunnel (purple) cavities associated with the proximal subunit were calculated using Fpocket as described in SI Methods. For clarity, only helices M2 and M3 of the distal (black) subunit are shown; in the proximal subunit, transmembrane helices M1–M4 are labeled. Lower: Equivalent view of mutant F(14′)A. (D) Average volumes across all five instances of each cavity type over the course of wild-type (black) and F(14′)A (orange) simulations. Upper: Similar pattern of intrasubunit cavities in both simulations. Lower: Larger, more variable linking tunnels in mutant F(14′)A relative to wild-type. In B and D, errors are SEM.
The increased separation between subunits in F(14′)A was associated with changes in the transmembrane cavities (Fig. 5 C and D and Fig. S4). Whereas the intrasubunit cavity was of similar volume and variability in both wild-type (335 ± 52 Å3) and F(14′)A mutant (369 ± 75 Å3) simulations (Fig. 5 C and D; red), the so-called linking tunnel between the intra- and intersubunit cavities (32) was substantially larger and more variable throughout the simulation for the F(14′)A mutant (316 ± 98 Å3) relative to wild-type GLIC (117 ± 33 Å3) (Fig. 5 C and D; purple). The intersubunit cavity also displayed substantial mobility but was too variable to permit characterization as a discrete cavity throughout the simulations. Generally, the central channel cavities (consisting of the pore lumen and contiguous linking tunnel region) formed a “T” shape when viewed from the plane of the membrane in the channel pore (Fig. S4A). Conversely, because of the increased kink angle of the M2 helix and widening and deepening of the linking tunnels, the same central cavity formed an irregular “Y” shape in the F(14′)A mutant (Fig. S4B).
The enlargement of the linking tunnel cavity in the F(14′)A mutant offers an indirect explanation for the pharmacology of enhancement. It is notable that the F(14′)C mutant was not itself enhanced by methyl MTS treatment, suggesting that this residue may not be fully accessible or that its labeling is not sufficient to mimic alcohol potentiation. Whether or not the 14′ position is directly accessible to solvent, our simulation data demonstrate the dramatic influence of this residue on the conformation of the C-terminal portion of M2 and thus the volume of nearby cavities, particularly the linking tunnel. The deeper, wider linking tunnel in the mutant could accommodate more alcohol molecules and/or bind them with greater affinity; furthermore, it could accommodate larger alcohols than in the wild type, suggesting a structural basis for the potentiating effects of longer-chain alcohols on 14′-substituted mutants (Fig. 3D and Fig. S2C). Small residue substitutions at L(17′), one register above F(14′) (Fig. 5C), could cause similar changes, potentially accounting for the enhanced ethanol potentiation of mutant L(17′)C (Fig. 3A). Furthermore, the 17′ and 18′ positions, at which methyl MTS labeling mimicked alcohol potentiation (Fig. 4), were located at the periphery of the linking tunnel (Fig. 5C and Fig. S4); methyl MTS modification of either residue could orient the methanethiolate label similarly to an alcohol molecule. Recent simulations of the closely related GlyR indicate that occupation of this cavity could stabilize the open state of the channel (40). Conversely, other regions of the protein—including the intrasubunit cavity that was recently implicated in inhibition by volatile anesthetics (32)—were relatively unaltered in the F(14′)A mutant (Fig. 5C and D), consistent with the existence of an independent mechanism for long-chain alcohol inhibition.
Conclusions
We demonstrated GLIC to be an alcohol-sensitive channel with a profile of modulation similar to some eukaryotic pLGICs. We were able to tune potentiation of GLIC by short-chain alcohols with specific mutations in the pore-lining helix, without influencing an evidently independent mechanism of inhibition by long-chain alcohols. We implicated specific residues at the extracellular end of M2 in alcohol potentiation, consistent with past studies of eukaryotic pLGICs. Mutagenesis at one of these positions (14′) was sufficient to convert GLIC into a potently ethanol-sensitive channel, providing a useful model system for future structural and functional characterization. Furthermore, whereas past studies have conflicted over the relative roles of putative intrasubunit and intersubunit cavities in alcohol modulation of pLGICs, our mutagenesis and molecular modeling suggest a model in which short-chain alcohols occupy a pore-facing pocket that includes the linking tunnel. This cavity primarily occupies the intersubunit interface but borders on residues previously implicated in an intrasubunit alcohol binding region (44); hence, past evidence for both intra- and intersubunit sites of action may in fact implicate the same cavity. These results further establish GLIC as a valuable model system for structural and functional studies of pLGICs.
Methods
Oocyte Electrophysiology.
Oocyte electrophysiology, including molecular biology, oocyte preparation, two-electrode voltage clamp recordings, treatment with alcohols and MTS reagents, and statistics were performed as previously described (20, 30). Specifications are provided in SI Methods.
Modeling and Molecular Dynamics.
Chemical structures were prepared using MarvinSketch 5.3.7 (ChemAxon), and protein structures were represented using the University of California, San Francisco Chimera package (45). Cavities were calculated using the Hollow script (46) or Fpocket package (47) as described in SI Methods. Molecular models of wild-type GLIC and the F(14′)A mutant were built from PDB ID 3EAM with protonation states similar to those described by Bocquet et al. (25). The mutant was optimized using the ROSETTA program (48). Each model was inserted in a lipid bilayer, relaxed, and finally simulated for 200 ns as described in detail in SI Methods. The M2 kink angle was computed with a custom VMD (49) script.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institutes on Alcohol Abuse and Alcoholism Grants T32 AA007471, R01 AA06399, and R01 AA013378, Swedish Research Council Grant VR 2010-491, and European Research Council Grant ERC 209825.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104480108/-/DCSupplemental.
References
- 1.Harris RA, Trudell JR, Mihic SJ. Ethanol's molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pringle MJ, Brown KB, Miller KW. Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol Pharmacol. 1981;19:49–55. [PubMed] [Google Scholar]
- 3.Franks NP, Lieb WR. Partitioning of long-chain alcohols into lipid bilayers: Implications for mechanisms of general anesthesia. Proc Natl Acad Sci USA. 1986;83:5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wick MJ, et al. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: Evidence for an alcohol receptor? Proc Natl Acad Sci USA. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem. 2000;275:29441–29451. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- 6.Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakahiro M, Arakawa O, Nishimura T, Narahashi T. Potentiation of GABA-induced Cl- current by a series of n-alcohols disappears at a cutoff point of a longer-chain n-alcohol in rat dorsal root ganglion neurons. Neurosci Lett. 1996;205:127–130. doi: 10.1016/0304-3940(96)12397-1. [DOI] [PubMed] [Google Scholar]
- 9.Bradley RJ, Sterz R, Peper K. The effects of alcohols and diols at the nicotinic acetylcholine receptor of the neuromuscular junction. Brain Res. 1984;295:101–112. doi: 10.1016/0006-8993(84)90820-5. [DOI] [PubMed] [Google Scholar]
- 10.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: Contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem Res. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Ye JH. The role of G proteins in the activity and ethanol modulation of glycine-induced currents in rat neurons freshly isolated from the ventral tegmental area. Brain Res. 2005;1033:102–108. doi: 10.1016/j.brainres.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Khisti RT, Morrow AL. Regulation of native GABAA receptors by PKC and protein phosphatase activity. Psychopharmacology (Berl) 2005;183:241–247. doi: 10.1007/s00213-005-0161-x. [DOI] [PubMed] [Google Scholar]
- 14.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Brejc K, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 16.Dellisanti CD, Yao Y, Stroud JC, Wang Z-Z, Chen L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- 17.Bertaccini EJ, Trudell JR, Lindahl E. Normal-mode analysis of the glycine α1 receptor by three separate methods. J Chem Inf Model. 2007;47:1572–1579. doi: 10.1021/ci600566j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taly A, et al. Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J. 2005;88:3954–3965. doi: 10.1529/biophysj.104.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihic SJ, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Akabas MH, Harris RA. Functional and structural analysis of the GABAA receptor α 1 subunit during channel gating and alcohol modulation. J Biol Chem. 2005;280:308–316. doi: 10.1074/jbc.M409871200. [DOI] [PubMed] [Google Scholar]
- 21.Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. Sites of excitatory and inhibitory actions of alcohols on neuronal α2β4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- 22.Bertaccini EJ, Shapiro J, Brutlag DL, Trudell JR. Homology modeling of a human glycine α 1 receptor reveals a plausible anesthetic binding site. J Chem Inf Model. 2005;45:128–135. doi: 10.1021/ci0497399. [DOI] [PubMed] [Google Scholar]
- 23.Cheng MH, Coalson RD, Cascio M. Molecular dynamics simulations of ethanol binding to the transmembrane domain of the glycine receptor: Implications for the channel potentiation mechanism. Proteins. 2008;71:972–981. doi: 10.1002/prot.21784. [DOI] [PubMed] [Google Scholar]
- 24.Forman SA, Miller KW. Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth. 2011;58:191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocquet N, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 26.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 27.Parikh RB, Bali M, Akabas MH. Structure of the M2 transmembrane segment of GLIC, a prokaryotic Cys loop receptor homologue from Gloeobacter violaceus, probed by substituted cysteine accessibility. J Biol Chem. 2011;286:14098–14109. doi: 10.1074/jbc.M111.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Gutierrez G, Grosman C. Bridging the gap between structural models of nicotinic receptor superfamily ion channels and their corresponding functional states. J Mol Biol. 2010;403:693–705. doi: 10.1016/j.jmb.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocquet N, et al. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 31.Weng Y, Yang L, Corringer P-J, Sonner JM. Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg. 2010;110:59–63. doi: 10.1213/ANE.0b013e3181c4bc69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nury H, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 33.Duret G, et al. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci USA. 2011;108:12143–12148. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alifimoff JK, Firestone LL, Miller KW. Anaesthetic potencies of primary alkanols: Implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol. 1989;96:9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardoso RA, et al. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- 36.Zuo Y, et al. Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:700–711. [PubMed] [Google Scholar]
- 37.Trudell JR, Harris RA. Are sobriety and consciousness determined by water in protein cavities? Alcohol Clin Exp Res. 2004;28:1–3. doi: 10.1097/01.ALC.0000108648.32241.BD. [DOI] [PubMed] [Google Scholar]
- 38.Howard RJ, et al. Alcohol binding sites in distinct brain proteins: The quest for atomic level resolution. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01502.x. 10.1111/j.1530-0277.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baenziger JE, Corringer P-J. 3D structure and allosteric modulation of the transmembrane domain of pentameric ligand-gated ion channels. Neuropharmacology. 2011;60:116–125. doi: 10.1016/j.neuropharm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Murail S, Wallner B, Trudell JR, Bertaccini E, Lindahl E. Microsecond simulations indicate that ethanol binds between subunits and could stabilize an open-state model of a glycine receptor. Biophys J. 2011;100:1642–1650. doi: 10.1016/j.bpj.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood SC, Forman SA, Miller KW. Short chain and long chain alkanols have different sites of action on nicotinic acetylcholine receptor channels from Torpedo. Mol Pharmacol. 1991;39:332–338. [PubMed] [Google Scholar]
- 42.Ueno S, et al. Tryptophan scanning mutagenesis in TM2 of the GABA(A) receptor alpha subunit: Effects on channel gating and regulation by ethanol. Br J Pharmacol. 2000;131:296–302. doi: 10.1038/sj.bjp.0703504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nury H, et al. One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc Natl Acad Sci USA. 2010;107:6275–6280. doi: 10.1073/pnas.1001832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: Important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–1300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- 45.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 46.Ho BK, Gruswitz F. HOLLOW: Generating accurate representations of channel and interior surfaces in molecular structures. BMC Struct Biol. 2008;8:49. doi: 10.1186/1472-6807-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Guilloux V, Schmidtke P, Tuffery P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinformatics. 2009;10:168. doi: 10.1186/1471-2105-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohl CA, Strauss CEM, Misura KMS, Baker D. Protein structure prediction using Rosetta. Methods Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- 49.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. 27–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.