Abstract
The mesolimbic dopaminergic system, especially the nucleus accumbens, has received attention for its involvement in the reinforcing and addictive properties of cocaine and other drugs of abuse. It is generally accepted that the ability of cocaine to inhibit the dopamine transporter (DAT) is directly related to its reinforcing actions. However, mice with a genetic deletion of the DAT (DAT-KO mice) still experience the rewarding effects of cocaine. These behavioral findings suggest that there is an alternate site for cocaine reinforcement. We demonstrate here that modulation of the serotonergic system in the ventral tegmental area, where the mesolimbic dopamine system originates, is a target of cocaine action. The ultimate effect of this serotonin mechanism in animal models with sustained elevations of dopamine may be a feed-forward enhancement of dopamine levels in the nucleus accumbens.
Cocaine is one of the most powerful drugs of abuse known. Despite this fact, to this date there are no effective medications for cocaine abuse, dependence, or withdrawal. Consequently, there is intense interest in better understanding the neural mechanisms of cocaine addiction in the hope that such knowledge will help develop more effective therapeutic strategies designed at reducing cocaine abuse.
The mesolimbic dopamine system, especially the nucleus accumbens (NAc), has received particular attention for its involvement in the reinforcing and addictive properties of cocaine and other drugs of abuse (1-4). Although cocaine also binds to serotonin and norepinephrine transporters, the predominant hypothesis has been that the reinforcing effects of cocaine are related to its ability to inhibit the dopamine transporter (DAT), especially in the NAc (1, 5-7). The essential role of the DAT in cocaine reinforcement has been challenged because mice lacking the DAT (DAT-KO mice) still self-administer cocaine (8) and exhibit cocaine-conditioned place preference (CPP) (9). These behavioral findings suggest that an alternate site for cocaine reinforcement exists.
Several lines of evidence have suggested that the interaction of cocaine with the serotonin transporter (SERT) may be involved in cocaine self-administration in these mice, including cocaine binding (8), c-Fos activation (8), and lack of cocaine reinforcement in DAT/SERT double knockout mice (9). Serotonin has also been implicated in the paradoxical calming effect that psychostimulants have on locomotor hyperactivity in DAT-KO mice (10). However, a role for the norepinephrine transporter (NET) in the effects of cocaine has also been postulated, and a decrease in the clearance rate of dopamine by means of NET inhibition by cocaine in the NAc has been suggested (11). Nevertheless, Budygin et al. (12) have found that cocaine does not affect dopamine clearance when perfused locally into the NAc of DAT-KO mice, and dopamine cell bodies in the ventral tegmental area (VTA) have been implicated as important targets of cocaine reinforcement in these mice (12).
Neuroanatomical studies have shown a high density of serotonin immunoreactive fibers in the VTA (13), and the existence of a functional relationship between serotonin and dopamine neurons in the mesolimbic dopamine system has been confirmed (14-17). Psychostimulant-induced neuroadaptations in the VTA have also been reported to play an important role in the sensitization process (18-21). In addition, 5-HT1B serotonin receptors have been shown to modulate the effects of cocaine (22, 23).
The aim of this study was to elucidate the alternative cocaine mechanism present in DAT-KO mice that was responsible for its reinforcing actions. We demonstrate here that a sensitivity to serotonin that is not present in naive wild-type animals is present in DAT-KO mice and can be induced by chronic treatment with a DAT inhibitor. Investigation of this action of cocaine may be helpful in understanding the neurobiology of cocaine abuse and dependence and eventually in the discovery of effective treatments.
Materials and Methods
Animals. Mice with a deletion of the gene (Slc6a3) encoding the DAT were used in these studies. Homozygote DAT knockout mice and wild-type littermates derived from the crossing of heterozygous DAT 129SvJ/C57BL mice were used (24). Animal care was in accordance with Wake Forest University's Institutional Animal Care and Use Committee and in compliance with National Institutes of Health guidelines for the care and use of experimental animals.
Microdialysis. Microdialysis guide-cannulas (CMA/7 Guide Cannula; CMA/Microdialysis AB, Stockholm, Sweden) were stereotaxically implanted, one in the NAc area (anterior, +1.2 mm; lateral, +0.6 mm; vertical, -4.2 mm) and another one in the ipsilateral VTA area (anterior, -3.0 mm; lateral, +0.4 mm; vertical, -4.0 mm), relative to bregma and dura surface. Concentric microdialysis probes (membrane length 1 mm, CMA/7, CMA/Microdialysis AB, Stockholm, Sweden) were implanted while animals were recovering from anesthesia. Experiments were conducted in freely moving mice ≈24 h after surgery. The probes were perfused with artificial cerebrospinal fluid (148 mM NaCl/2.7 mM KCl/1.2 mM CaCl2/0.85 mM MgCl2, pH 7.4) at a constant flow rate of 1.0 μl/min. Samples were collected every 20 min and analyzed for dopamine by high-performance liquid chromatography coupled to electrochemical detection. Microdialysis data were calculated as the percentage change from baseline concentration, with 100% being defined as the average of the last three samples. The effect of cocaine, fluoxetine, citalopram, and desipramine on extracellular concentration of dopamine in the VTA or NAc areas was assessed by one-way or two-way analysis of variance (ANOVA) for repeated measures, with genotype as the between-subject factor and time as the within-subject factor. Values of P < 0.05 were considered statistically significant.
Fast Scan Cyclic Voltammetry (FSCV). Coronal mouse brain slices (400 μm thick) containing the VTA were prepared. Slices were perfused at 1 ml/min with 34°C Kreb's buffer, and carbon-fiber microelectrodes were used. During FSCV dopamine recording, the electrode potential was linearly scanned from -400 to +1,200 mV and back to -400 mV at 300 V/s, repeated every 100 ms. Dopamine release was evoked every 10 min by 30-pulse, 30-Hz stimulations (350 μA, 4 ms). In each case, dopamine was identified by its characteristic background substracted cyclic voltammogram. The oxidation currents were converted to concentration by electrode calibration with 10 μM dopamine at the end of the experiment. Uptake rates were compared by using a Student t test. P < 0.05 was considered significant.
CPP. The CPP apparatus consisted of white and black chambers (21 × 28 cm) connected by an anteroom (21 × 12 cm) with guillotine doors. During the preconditioning phase, mice were allowed access for 15 min to both chambers. During the conditioning phase, mice received an i.p. dose of either cocaine (20 mg/kg), fluoxetine (15 mg/kg), or saline and were confined to one chamber of the apparatus for 15 min. Mice were returned to their home cage for 8 h and then given an injection of either drug or saline, whichever they had not yet received, and were placed in the opposite chamber. Pairing was randomized across groups. This process was repeated for 4 days. On day 5, mice were placed in the anteroom and the doors opened. CPP was assessed by the amount of time spent in each chamber over a 15-min observation period. Data were analyzed with a Student t test. P < 0.05 was considered significant.
Results
Elevated Dopamine via SERT Blockade in DAT-KO Mice. We used microdialysis to test the hypothesis that activation of the serotonin system is related to the increase in NAc dopamine induced by systemic cocaine administration in DAT-KO mice. In accordance with previous studies (8, 11), baseline extracellular dopamine concentrations in the NAc of DAT-KO (14 ± 2 fmol/μl; n = 35) were higher than those in wild-type (2 ± 0.4 fmol/μl; n = 26) mice. Also, as previously reported (11), cocaine (20 mg/kg, i.p.) elevated extracellular dopamine concentrations in the NAc of wild-type (F7,21 = 6.63, P < 0.01) and surprisingly also of DAT-KO mice (F7,28 = 6.33, P < 0.01) (Fig. 1a). No difference in the effect of cocaine was found between genotypes (F1,7 = 0.50, P = 0.51). The discovery of cocaine-induced dopamine elevations in the NAc made it obvious why cocaine was reinforcing in DAT-KO mice, but the question remained: How is dopamine being elevated by cocaine in the absence of the DAT? A previous study (11) hypothesized that cocaine may increase dopamine in the NAc of DAT-KO mice through inhibition of the NET. However, we observed that the selective NET inhibitor desipramine (10 mg/kg, i.p.) did not change extracellular dopamine in the NAc of either wild-type (F7,21 = 2.82, P = 0.23) or DAT-KO animals (F7,21 = 0.52, P = 0.69) (Fig. 1d). To test the involvement of a serotonergic mechanism in cocaine actions, the effects of two SERT inhibitors, fluoxetine (15 mg/kg, i.p.) and citalopram (10 mg/kg, i.p.), on extracellular dopamine in the NAc were evaluated. Systemic administration of fluoxetine (Fig. 1b) or citalopram (Fig. 1c) increased dopamine in the NAc of DAT-KO mice (F7,35 = 4.77, P < 0.01 and F7,21 = 8.19, P < 0.01, respectively), while having no effect in wild-type animals (F7,21 = 0.38, P = 0.93 and F7,21 = 1.47, P = 0.22, respectively) (Fig. 1 b and c). Thus, serotonin transporter blockade mimicked the effects of cocaine in DAT-KO mice.
Fig. 1.
Serotonin transporter blockade mimics the effects of cocaine in DAT-KO mice. Effect of cocaine (20 mg/kg, i.p.) (a), fluoxetine (15 mg/kg, i.p.) (b), citalopram (10 mg/kg, i.p.) (c), and desipramine (10 mg/kg, i.p.) (d) administration on extracellular concentrations of dopamine in the NAc of wild-type (○) and DAT-KO (•) mice. Arrows represent the time of administration of each drug. Data are mean ± SEM values from four to six separate animals for each group and are expressed as percentages of the corresponding basal values.
Lack of Cocaine Effect in the NAc of DAT-KO Mice. A previous voltammetry study (12) excluded the possibility that, after genetic deletion of the DAT, the NET or SERT actively takes up dopamine in the NAc. Here, we studied the local effect of cocaine administration through the dialysis probe placed in the NAc on extracellular dopamine. Other authors (25) have demonstrated previously that the action of similar cocaine concentrations on dopamine terminals as in the NAc and in the cell bodies of the VTA is independent of local anesthetic activity. Cocaine (0.1-3 mM) significantly increased extracellular dopamine in wild-type animals (F15,45 = 6.48, P < 0.0001), but no changes were observed in DAT-KO mice (F15,45 = 1.51, P = 0.14, genotype × drug F1,6 = 13.65, P < 0.01) (Fig. 2). Therefore, and as it was previously proposed, the mechanism of cocaine interaction with dopamine neurotransmission does not take place at the level of presynaptic terminals in the DAT-KO mice. These results indicate that cocaine does not inhibit dopamine uptake in the NAc of DAT-KO mice, suggesting the possibility that the VTA could be involved in the systemic cocaine-induced dopamine increase observed in the NAc (12).
Fig. 2.
Cocaine does not inhibit dopamine uptake in the NAc of DAT-KO mice. The effects of cocaine (0.1, 1, and 3 mM) locally applied in the NAc on extracellular concentrations of dopamine in wild-type (○) and DAT-KO (•) mice are shown. Arrows represent the time of administration of each different concentration of cocaine through the microdialysis probe located in the NAc area. Data are mean ± SEM values from four to five separate animals for each group and are expressed as percentages of the corresponding basal value.
Fluoxetine Mimics Cocaine in DAT-KO Mice. Our next approach was to investigate whether the VTA could be involved in the cocaine-induced NAc dopamine increase in DAT-KO mice. By using dual-probe microdialysis, we evaluated the effects of cocaine, perfused through the dialysis probe located in the vicinity of the VTA (25), on extracellular concentrations of dopamine simultaneously in the VTA and in the NAc. In wild-type animals, administration of cocaine into the VTA significantly decreased extracellular dopamine in the NAc (F11,33 = 13.67, P < 0.01) (Fig. 3a). It is likely that this reduction is the result of a dopamine autoreceptor-mediated feedback inhibition by elevated dopamine in the VTA (26) (Fig. 3b). In DAT-KO mice, when cocaine was locally applied into the VTA, a significant increase was observed in extracellular dopamine in the NAc (F11,55 = 5.24, P < 0.001) (Fig. 3a). Thus, the response of NAc dopamine to cocaine in the VTA was opposite between wild-type and DAT-KO mice (F1,7 = 31.08, P < 0.01) (Fig. 3a). Fluoxetine, also administered locally into the VTA, mimicked cocaine effects in DAT-KO mice. It (10-100 μM) caused a significant increase in extracellular dopamine in the mutant mice (F11,44 = 8.58, P < 0.001) whereas no changes in wild-type animals were observed (F11,33 = 1.86, P = 0.08) (Fig. 3c).
Fig. 3.
Cocaine and fluoxetine act identically in the VTA to elevate dopamine in the NAc of DAT-KO mice. Effect of local administration of cocaine (0.1, 1, and 3 mM) through the microdialysis probe located in the VTA on extracellular dopamine measured in the NAc (a) or in the VTA (b) of wild-type (○) and DAT-KO mice (•). Effect of local administration of fluoxetine (10, 50, and 100 μM) through the microdialysis probe located in the VTA on extracellular dopamine measured in the NAc (c) or in the VTA (d) of wild-type (○) and DAT-KO mice (•). Arrows represent the time of administration of each different concentrations of the drugs through the microdialysis probe located in the VTA area. Data are mean ± SEM values from four to six separate animals for each group and are expressed as percentages of the corresponding basal value.
Taking advantage of the microdialysis probe implanted in the VTA to deliver drugs, we were also able to monitor VTA dopamine levels. Baseline extracellular dopamine concentrations in VTA of DAT-KO (12 ± 4 fmol/μl; n = 12) were higher than those in wild-type animals (2 ± 0.5 fmol/μl; n = 10). As expected, in wild-type mice there was an increase in VTA dopamine with cocaine due to DAT inhibition (F11,33 = 8.10, P < 0.001) (Fig. 3b) whereas there was no effect of fluoxetine (F11,33 = 0.35, P = 0.96) (Fig. 3d). Local administration of cocaine (Fig. 3b) or fluoxetine (Fig. 3d) in the VTA of DAT-KO animals significantly increased extracellular dopamine in this area. (F11,55 = 5.24, P < 0.001 and F11,33 = 3.97, P < 0.001, respectively). This effect does not occur in naive wild-type mice. The expected feedback inhibition of NAc dopamine in DAT-KO mice is absent because of a pronounced decrease in the activity of impulse-regulating dopamine autoreceptors in the VTA (27) (Fig. 3 a and c). These data demonstrate that cocaine and fluoxetine act identically in the VTA to elevate dopamine in the NAc of DAT-KO mice.
SERT Does Not Clear Dopamine in DAT-KO Mice. To rule out the possibility that the SERT is an alternative site for dopamine clearance in DAT-KO mice, the effect of fluoxetine on dopamine signals was monitored by fast-scan cyclic voltammetry (28). In NAc (12) and VTA slices, fluoxetine (10 μM) had no effect on dopamine clearance (0.06 ± 0.02 s-1 vs. 0.07 ± 0.01 s-1; P = 0.18) (Fig. 4). Therefore, SERT inhibition does not affect dopamine clearance. This result leaves elevated VTA serotonin as the most likely mediator of cocaine effects.
Fig. 4.
Lack of fluoxetine effect on dopamine clearance in VTA slices from DAT-KO mice. Dopamine efflux was measured by fast scan cyclic voltammetry in response to 30-pulse, 30-Hz (350 μA, 4 ms) stimulation in DAT-KO VTA slices. Control (○); fluoxetine (•). Data are plotted every 10th point for visual clarity. (Inset) There is no change in the rate of dopamine clearance, reported as a rate constant k, before and after drug administration.
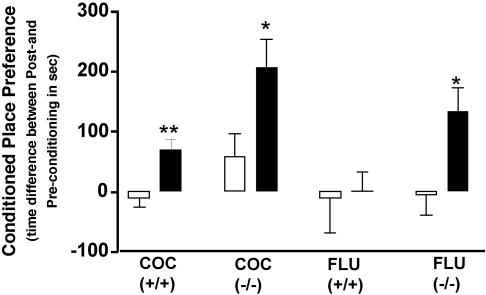
Fluoxetine Is Reinforcing in DAT-KO Mice. The relationship between cocaine reward and serotonin in DAT-KO mice has been suggested by the elimination of cocaine-CPP in DAT/SERT double knockout mice (9, 29). Here, we tested the hypothesis that SERT inhibition is reinforcing in DAT-KO mice. CPP was evaluated after pairings of saline (10 ml/kg), cocaine (20 mg/kg), or fluoxetine (15 mg/kg) with a given chamber for 4 consecutive days. As demonstrated (9, 28), we found that both wild-type and DAT-KO mice showed significant place preference for the chamber paired with cocaine (t = 3.58, P < 0.01 and t = 2.29, P < 0.05, respectively) (Fig. 5). As postulated, DAT-KO (t = 2.58, P < 0.05), but not wild-type animals (t = 0.18, P = 0.85), also exhibited significant place preference for the chamber paired with fluoxetine (29) (Fig. 5). This reinforcing effect of fluoxetine in DAT-KO mice is consistent with the increase in NAc dopamine levels observed after fluoxetine administration.
Fig. 5.
Reinforcing effect of cocaine and fluoxetine in DAT-KO mice. Place preference conditioning of wild-type (+/+) and DAT-KO (-/-) mice (n = 8-10) to saline (open bars) or to cocaine (COC) or fluoxetine (FLU) (filled bars) administration over a 4-day period. Time scores shown represent differences between post- and preconditioning time spent in the saline- or drug-paired environment. *, P < 0.05; **, P < 0.01, between drug and saline.
Pharmacological Blockade of DAT. Neurochemical studies (8, 30) in DAT-KO mice have demonstrated that the absence of the DAT confers to these animals a persistent hyperdopaminergic tone. A long-acting, high affinity cocaine analog, 2β-propanoyl-3β-(4-tolyl)-tropane (PTT) (31, 32), was given to wild-type mice for 10 days (3 mg/kg, i.p.) to approximate the elevated dopamine tone observed in DAT-KO mice. Microdialysis experiments were performed in these animals to test the hypothesis that high affinity blockade of DAT, and subsequent elevations of dopamine, could induce the appearance of this new effect of SERT inhibition. Baseline dopamine concentrations in the NAc of PTT-treated animals 24 h after the last PTT injection were significantly higher than in the control group (3 ± 0.2 vs. 1.3 ± 0.4 fmol/μl, t = 4.88, P < 0.01). Fig. 6a shows that, indeed, subchronic administration of PTT induced a switch in the brain such that fluoxetine (15 mg/kg, i.p.) now significantly elevated dopamine in the NAc (F7,23 = 1.68, P < 0.01), as it did in DAT-KO mice. The difference in the time course of systemic fluoxetine between DAT-KO (Fig. 1b) and PTT-treated animals (Fig. 6a) can be explained by slower dopamine dynamics in the extrasynaptic space in the absence of the DAT (12). Fig. 6b shows the time course for this fluoxetine effect to appear. Three days of PTT treatment (3 mg/kg, i.p., once per day) was not enough to have the new effect of SERT inhibition, and only after 6 days of PTT treatment did fluoxetine significantly increase dopamine levels in the NAc (267 ± 60%, P < 0.01). To test the possibility that the effect of fluoxetine on dopamine levels in the NAc of PTT-treated animals may be due to a lack of uptake and not elevated basal levels of dopamine, that is, that fluoxetine may always have a marginal effect on synaptic dopamine release that is not detected because of rapid reuptake, we evaluated fluoxetine effects in the presence of an acute dose of PTT, at a time when DAT blockade is present. Fluoxetine (15 mg/kg, i.p.) was administered 80 min after acute PTT (3 mg/kg, i.p.) treatment (Fig. 6c). During this time, dopamine transporters were blocked by PTT (30, 31), and dopamine levels were significantly increased (661.61 ± 164.32%, P < 0.0001, n = 4), but fluoxetine did not elevate dopamine further (Fig. 6b).
Fig. 6.
Pharmacological blockade of the DAT induces a “switch” in serotonin-mediated NAc dopamine response. (a) The effect of fluoxetine (15 mg/kg, i.p.) on extracellular dopamine in naive animals treated for 10 days with the long-acting DAT blocker PTT (3 mg/kg, i.p., once per day). Dopamine was measured in the NAc of control (○) and PTT-treated (•) mice (n = 4). The arrow represents the administration time of fluoxetine. Data are mean ± SEM values and are expressed as percentages of the corresponding basal value. (b) Time course of PTT treatment for new fluoxetine effect on dopamine to appear in the NAc. The bars represent the maximal effect of fluoxetine (15 mg/kg, i.p.) on dopamine dialysate levels after PTT (3 mg/kg, i.p., once per day) treatments for either 3 (n = 4), 6 (n = 5), or 10 (n = 4) days. **, P < 0.01. Data are mean ± SEM values and are expressed as percentages of the corresponding basal value. (c) Acute effect of PTT treatment (•) and subsequent administration of fluoxetine. Fluoxetine did not increase dopamine levels (n = 4). PTT (3 mg/kg, i.p.) significantly increased dopamine levels (P < 0.01) but did not reveal the fluoxetine switch. ○, Control animals received a saline injection followed by fluoxetine (n = 4). Data are mean ± SEM values and are expressed as percentages of the corresponding basal value.
Discussion
The observations that DAT-KO mice self-administer cocaine (8) and display CPP for cocaine (9, 29) demonstrated that targets other than the DAT could contribute to the rewarding properties of cocaine. Several laboratories have been attempting to find out the basis of cocaine reward in this mouse (8, 11, 12, 29). In a previous study (12), our laboratory began to narrow the possibilities by showing that, contrary to published hypotheses (11), cocaine did not alter dopamine uptake in the NAc of DAT-KO mice. The main findings from the present study are that a serotonin mechanism is present in the VTA of DAT-KO mice that, when activated, leads to an increase in dopamine levels in the NAc, just as cocaine does by DAT blockade in wild-type animals. This serotonin mechanism is not present in naive wild-type mice and could be mediating the reinforcing properties of cocaine and fluoxetine in the DAT-KO mice. These findings emerged from an experimental design using dual-probe microdialysis in the NAc and VTA of the mouse as a necessary tool to answer this question. We also used voltammetry in the VTA of DAT-KO mice to examine the pharmacology of dopamine clearance in this area.
The interactions between dopamine and serotonin systems have been an extensive subject of study. Neuroanatomical studies have shown a high density of serotonin immunoreactive fibers in the VTA (13), and there is evidence that the mesolimbic dopaminergic system originating in the VTA is under inhibitory control by the serotonin system, although the situation is complex (13-15). There is evidence suggesting that the serotonin system can negatively modulate cocaine-maintained behaviors. For example, the reinforcing efficacy of cocaine can be enhanced by partial depletion of brain serotonin (33), and increasing serotonin activity may attenuate the reinforcing effects of this drug (34-36). Although serotonin-specific drugs do not seem to have significant reinforcing efficacy (37, 38), serotonin could play a different role in animals with chronically elevated dopamine, as occurs in DAT-KO mice or in wild-type animals after pharmacological blockade of the DAT or other pathophysiological conditions.
Although a serotonin-mediated mechanism has been postulated previously to mediate the rewarding effects of cocaine in animals with a genetic deletion of the DAT (8, 9), noradrenergic neurotransmission has also been implicated. Several studies (39-42) have documented interactions between noradrenergic and dopaminergic neurons through alpha-1 adrenergic receptors. Interestingly, mice lacking the alpha-1b subtype of adrenergic receptors do not exhibit the locomotor or rewarding effects mediated by cocaine (41). There is considerable crosstalk between dopaminergic, noradrenergic, and serotonergic systems that occurs on systemic administration of cocaine (43), and more studies are needed to define the importance of each monoamine and the role of the VTA in the reward process (44).
Genetically altered mice have provided a way to address important questions in biology, and in particular the DAT-KO mice have been a valuable model to understand the mechanisms of action of cocaine and other psychostimulants (44-46). However, to extend our findings to a more “physiological” model, we treated wild-type mice with a long-acting dopamine uptake blocker, PTT (31, 32), and induced the appearance of the same target that leads to elevated dopamine in the NAc after blockade of the serotonin transporter. These results indicate that serotonin does play a different role in animals with reduced dopamine transporter function. This demonstration of the induction of a VTA serotonin “switch” in wild-type animals makes the DAT-KO findings relevant to other cases of chronic DAT blockade. This switch/alteration may be mediated by changes in serotonin receptors and/or effectors regulating VTA dopamine neurons. Of the 14 characterized serotonin receptors (47), there is a sizeable amount of literature supporting a functional role for 5-HT2A and 5-HT2C receptors in the regulation of dopamine neurotransmission (48, 49). Other serotonin receptor subtypes, including 5-HT3 and 5-HT1A, have also been shown to modulate the activity of mesolimbic dopaminergic cells in the VTA (49, 50). In addition, serotonin actions at 5-HT1B receptors in the VTA modulate cocaine-induced dopamine release in then NAc and alter the rewarding and stimulant properties of cocaine (23). A recent report (22) indicated that elevated expression of 5-HT1B receptors in the VTA increased cocaine-induced locomotor hyperactivity and also shifted the dose-response curve for cocaine-CPP to the left, suggesting enhanced rewarding effects of cocaine. The additional use of anatomical and physiological approaches will be necessary to clarify the complex roles of these serotonin receptors in cocaine addiction.
Taken together, these findings describe a serotonin “switch” that occurs in the VTA after reduced DAT function, as in the case of a genetic deletion of the DAT or treatment with a long-acting DAT inhibitor, and mediates the effects of cocaine. In pathophysiological conditions that involve an elevated dopamine tone, this switch in serotonin function may cause cocaine to induce a potentiated enhancement of dopamine levels in the NAc.
Acknowledgments
We thank Dr. M. G. Caron and Dr. J. L. Weiner for valuable comments on the manuscript. We thank Dr. W. C. Wetsel, E. Budygina, and Dr. J. F. Cheer for experimental assistance. This work was supported by Wake Forest University Health Sciences Venture Funds and National Institutes of Health Grants AA11997, AAO14091, and DA06634.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DAT, dopamine transporter; DAT-KO, dopamine transporter knockout; NAc, nucleus accumbens; PTT, 2β-propanoyl-3β-(4-tolyl)-tropane; SERT, serotonin transporter; VTA, ventral tegmental area; NET, norepinephrine transporter; CPP, conditioned place preference.
References
- 1.DiChiara, G. (1995) Drug Alcohol Depend. 38, 95-137. [DOI] [PubMed] [Google Scholar]
- 2.Carboni, E., Imperato, A., Perezzani, L. & Di Chiara, G. (1989) Neuroscience 28, 653-661. [DOI] [PubMed] [Google Scholar]
- 3.Cass, W. A., Gerhardt, G. A., Mayfield, R. D., Curella, P. & Zahniser, N. R. (1992) J. Neurochem. 59, 259-266. [DOI] [PubMed] [Google Scholar]
- 4.Wu, Q., Reith, M. E., Kuhar, M. J., Carroll, F. I. & Garris, P. A. (2001) J. Neurosci. 21, 6338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob, G. F. & Bloom, F. E. (1988) Science 242, 715-723. [DOI] [PubMed] [Google Scholar]
- 6.Volkow, N. D., Wang, G. J., Fischman, M. W., Foltin, R. W., Fowler, J. S., Abumrad, N. N., Vitkun, S., Logan, J., Gatley, S. J., Pappas, N., et al. (1997) Nature 386, 827-830. [DOI] [PubMed] [Google Scholar]
- 7.Kuhar, M. J., Ritz, M. C. & Boja, J. W. (1991) Trends Neurosci. 14, 299-302. [DOI] [PubMed] [Google Scholar]
- 8.Rocha, B. A., Fumagalli, F., Gainetdinov, R. R., Jones, S. R., Ator, R., Giros, B., Miller, G. W. & Caron, M. G. (1998) Nat. Neurosci. 1, 132-137. [DOI] [PubMed] [Google Scholar]
- 9.Sora, I., Hall, F. S., Andrews, A. M., Itokawa, M., Li, X. F., Wei, H. B., Wichems, C., Lesch, K. P., Murphy, D. L. & Uhl, G. R. (2001) Proc. Natl. Acad. Sci. USA 98, 5300-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gainetdinov, R. R., Wetsel, W. C., Jones, S. R., Levin, E. D., Jaber, M. & Caron, M. G. (1999) Science 283, 397-401. [DOI] [PubMed] [Google Scholar]
- 11.Carboni, E., Spielewoy, C., Vacca, C., Nosten-Bertrand, M., Giros, B. & Di Chiara, G. (2001) J. Neurosci. 21, art-RC141. [DOI] [PMC free article] [PubMed]
- 12.Budygin, E. A., John, C. E., Mateo, Y. & Jones, S. R. (2002) J. Neurosci. 22, art-RC222. [DOI] [PMC free article] [PubMed]
- 13.Herve, D., Pickel, V. M., Joh, T. H. & Beaudet, A. (1987) Brain Res. 435, 71-83. [DOI] [PubMed] [Google Scholar]
- 14.Prisco, S. & Esposito, E. (1995) Br. J. Pharmacol. 116, 1923-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Matteo, V., Di Giovanni, G., Di Mascio, M. & Esposito, E. (1999) Neuropharmacology 38, 1195-1205. [DOI] [PubMed] [Google Scholar]
- 16.Di Matteo, V., Cacchio, M., Di Giulio, C., Di Giovanni, G. & Esposito, E. (2002) Pharmacol. Biochem. Behav. 71, 607-613. [DOI] [PubMed] [Google Scholar]
- 17.McMahon, L. R., Filip, M. & Cunningham, K. A. (2001) J. Neurosci. 21, 7781-7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler, E. J. (1992) J. Neurosci. 12, 2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce, R. C. & Kalivas, P. W. (1997) Brain Res. Brain Res. Rev. 25, 192-216. [DOI] [PubMed] [Google Scholar]
- 20.White, F. J. & Kalivas, P. W. (1998) Drug Alcohol Depend. 51, 141-153. [DOI] [PubMed] [Google Scholar]
- 21.Cornish, J. L. & Kalivas, P. W. (2001) Behav. Brain Res. 126, 205-209. [DOI] [PubMed] [Google Scholar]
- 22.Neumaier, J. F., Vincow, E. S., Arvanitogiannis, A., Wise, R. A. & Carlezon, W. A., Jr. (2002) J. Neurosci. 22, 10856-10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castanon, N., Scearce-Levie, K., Lucas, J. J., Rocha, B. & Hen, R. (2000) Pharmacol. Biochem. Behav. 67, 559-566. [DOI] [PubMed] [Google Scholar]
- 24.Giros, B., Jaber, M., Jones, S. R., Wightman, R. M. & Caron, M. G. (1996) Nature 379, 606-612. [DOI] [PubMed] [Google Scholar]
- 25.Chen, N. H. & Reith, M. E. A. (1994) J. Neurochem. 63, 1701-1713. [DOI] [PubMed] [Google Scholar]
- 26.Einhorn, L. C., Johansen, P. A. & White, F. J. (1988) J. Neurosci. 8, 100-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, S. R., Gainetdinov, R. R., Hu, X. T., Cooper, D. C., Wightman, R. M., White, F. J. & Caron, M. G. (1999) Nat. Neurosci. 2, 649-655. [DOI] [PubMed] [Google Scholar]
- 28.Jones, S. R., Garris, P. A., Kilts, C. D. & Wightman, R. M. (1995) J. Neurochem. 64, 2581-2589. [DOI] [PubMed] [Google Scholar]
- 29.Hall, F. S., Li, X. F., Sora, I., Xu, F., Caron, M., Lesch, K. P., Murphy, D. L. & Uhl, G. R. (2002) Neuroscience 115, 153-161. [DOI] [PubMed] [Google Scholar]
- 30.Jones, S. R., Gainetdinov, R. R., Jaber, M., Giros, B., Wightman, R. M. & Caron, M. G. (1998) Proc. Natl. Acad. Sci. USA 95, 4029-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porrino, L. J., Ritz, M. C., Goodman, N. L., Sharpe, L. G., Kuhar, M. J. & Goldberg, S. R. (1989) Life Sci. 45, 1529-1535. [DOI] [PubMed] [Google Scholar]
- 32.Freedland, C. S., Smith, H. R., Hart, S. L., Daunais, J. B., Davies, H. M. L. & Porrino, L. J. (2000) Brain Res. 869, 98-104. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, D. C. S., Loh, E. A., Baker, G. B. & Vickers, G. (1994) Pharmacol. Biochem. Behav. 49, 177-182. [DOI] [PubMed] [Google Scholar]
- 34.Howell, L. L. & Byrd, L. D. (1995) J. Pharmacol. Exp. Ther. 275, 1551-1559. [PubMed] [Google Scholar]
- 35.Czoty, P. W., Ginsburg, B. C. & Howell, L. L. (2002) J. Pharmacol. Exp. Ther. 300, 831-837. [DOI] [PubMed] [Google Scholar]
- 36.Fantegrossi, W. E., Ullrich, T., Rice, K. C., Woods, J. H. & Winger, G. (2002) Psychopharmacology 161, 356-364. [DOI] [PubMed] [Google Scholar]
- 37.Locke, K. W., Levesque, T. R., Nicholson, K. L. & Balster, R. L. (1996) Prog. Neuro-Psychopharmacol. Biol. Psychiatry 20, 1019-1035. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, D. C. S., Phelan, R., Hodges, L. M., Hodges, M. M., Bennett, B., Childers, S. & Davies, H. (1999) Psychopharmacology 144, 389-397. [DOI] [PubMed] [Google Scholar]
- 39.Grenhoff, J. & Svensson, T. H. (1993) Eur. J. Pharmacol. 233, 79-84. [DOI] [PubMed] [Google Scholar]
- 40.Paladini, C. A., Fiorillo, C. D., Morikawa, H. & Williams, J. T. (2001) Nat. Neurosci. 4, 275-281. [DOI] [PubMed] [Google Scholar]
- 41.Drouin, C., Darracq, L., Trovero, F., Blanc, G., Glowinski, J., Cotecchia, S. & Tassin, J. P. (2002) J. Neurosci. 22, 2873-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auclair, A., Cotecchia, S., Glowinski, J. & Tassin, J. P. (2002) J. Neurosci. 22, 9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reith, M. E., Li, M. Y. & Yan, Q. S. (1997) Psychopharmacology 134, 309-317. [DOI] [PubMed] [Google Scholar]
- 44.Gainetdinov, R., Sotnikova, T. & Caron, M. (2002) Trends Pharmacol. Sci. 23, 367-373. [DOI] [PubMed] [Google Scholar]
- 45.Laakso, A., Mohn, A. R., Gainetdinov, R. R. & Caron, M. G. (2002) Neuron 36, 213-228. [DOI] [PubMed] [Google Scholar]
- 46.Jones, S. R., Gainetdinov, R. R., Wightman, R. M. & Caron, M. G. (1998) J. Neurosci. 18, 1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes, N. M. & Sharp, T. (1999) Neuropharmacology 38, 1083-1152. [DOI] [PubMed] [Google Scholar]
- 48.Di, M., V, De Blasi, A., Di Giulio, C. & Esposito, E. (2001) Trends Pharmacol. Sci. 22, 229-232. [DOI] [PubMed] [Google Scholar]
- 49.Gillies, D. M., Mylecharane, E. J. & Jackson, D. M. (1996) Eur. J. Pharmacol. 303, 1-12. [DOI] [PubMed] [Google Scholar]
- 50.Lejeune, F. & Millan, M. J. (1998) Synapse 30, 172-180. [DOI] [PubMed] [Google Scholar]