Exocytosis of synaptic vesicles is a central step in neuronal signal transmission (reviewed in 1). On arrival of an action potential, voltage gated Ca2+ channels in the synaptic plasma membrane open. The influx of Ca2+ ions triggers fusion of synaptic vesicles with a delay of less than 1 ms and increases the fusion rate by more than four orders of magnitude (2). During the past 2 decades, major progress has been made in deciphering the molecular events underlying exocytosis of synaptic vesicles. Although details are still controversial, it is widely accepted that membrane fusion itself is catalyzed by the synaptic SNARE proteins, including the R-SNARE synaptobrevin/vesicle-associated membrane protein that resides on synaptic vesicles and the Q-SNAREs syntaxin-1 and synaptosomal-associated protein-25 (SNAP-25) that reside in the presynaptic plasma membrane. On contact, these SNAREs form a helical complex that is initiated at the membrane-distal N-terminal ends and progresses toward the C-terminal transmembrane anchors, thus pulling the membranes together. The tight coupling of fusion to Ca2+ influx is mediated by the protein synaptotagmin, a resident of synaptic vesicles. Synaptotagmin-1 bears two Ca2+-binding C2 domains that interact with both SNAREs and acidic membrane lipids in a Ca2+-dependent manner. However, despite intense research by many groups, it is still unclear exactly how the enormous acceleration by Ca2+ is achieved at the molecular level. Kyoung et al. (3) now provide us with a unique assay in which some of the key steps have been reconstructed in vitro, thus opening the door toward unraveling synaptic membrane fusion.
Reconstitution of a complex cellular event in vitro, such as protein-mediated membrane fusion, is an important step toward a full mechanistic understanding, and despite the increasing sophistication of cell-based assays, there is still no alternative to this approach. Indeed, there is a long tradition in reconstituting fusion events using both biological and artificial membranes; such assays have been instrumental in unraveling the mechanisms underlying membrane merger, particularly in the field of viral fusion proteins (4). Similarly, the presence of appropriate combinations of SNAREs suffices to mediate fusion of artificial membranes: Liposomes containing synaptobrevin-2 are capable of lipid mixing with a second population of liposomes that contain SNAP-25 and syntaxin-1A (5). Although this observation has been reproduced numerous times, major problems persist. First, the fusion rate is extremely slow (at least 5 orders of magnitude slower than neuronal exocytosis). Second, including the Ca2+ sensor synaptotagmin-1 in the experiments only results in a very moderate acceleration of fusion (reviewed in 6 and 7) that is by no means even close to the increase of four orders of magnitude observed in neurons (2).
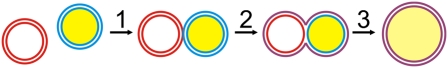
A major limitation of these experiments is the “bulk” nature of the measurements. In typical fusion assays, liposomes are labeled with two spectrally separated fluorescent lipid analogs. These lipid analogs mix on membrane fusion, resulting in a change of fluorescence attributable to Förster resonance energy transfer (FRET) (5). This process is usually measured in a cuvette, where total fluorescence is measured over time after mixing of the two populations. Although this assay is simple and very reliable, it is not possible to distinguish docking from fusion with this approach. This is a serious limitation because the first membrane tethering step (Fig. 1; step 1) is rate-limiting for native SNAREs (8, 9). Thus, any effect on fusion downstream of tethering, as is proposed for synaptotagmin-1 (6), cannot be reliably measured. Furthermore, ensemble averaging does not allow for distinguishing between vesicle populations exhibiting different kinetics, and multiple rounds of fusion cannot be differentiated. Last but not least, testing for nonleakiness (an important criterion for biological fusion) requires different dyes, is difficult to carry out in the same experiment, and is thus frequently omitted.
Fig. 1.
Cascade of membrane fusion events that can be distinguished by the assay of Kyoung et al. (3). Step 1 is liposome tethering. Step 2 is hemifusion with the outer membrane leaflet mixed and the inner leaflet still intact. Step 3 is full-membrane fusion accompanied by content mixing.
In single-liposome fusion assays, membrane tethering can be easily discerned from actual lipid mixing and multiple rounds of membrane fusion can also be readily observed. The first researchers to realize single vesicle Ca2+-triggered membrane fusion experimentally were Lee et al. (10). In this study, Q-SNARE liposomes were immobilized via biotin-avidin linkage (11) on a glass surface. This allowed for measuring lipid mixing with R-SNARE liposomes containing the Ca2+ sensor synaptotagmin-1 using fluorescence microscopy. Surprisingly, membrane fusion was accelerated already at 10 μM Ca2+ (10), which is far lower than observed previously in vitro. Even more surprisingly, higher concentrations resulted in a decrease of the fusion efficiency. This paradox was attributed to “back binding” by synaptotagmin to its “own” membrane at higher Ca2+ concentrations, resulting in functional inactivation (12). Notwithstanding these important findings and methodological advances, content leakage and hemi/full-membrane fusion were not addressed in this study.
Kyoung et al. (3) have now overcome these limitations in a very elegant manner. As in the study by Lee et al. (10), Q-SNARE liposomes were immobilized on a glass slide and synaptotagmin/R-SNARE liposomes were subsequently introduced. Membrane fusion was then monitored by a cunning triple-fluorescence microscopy approach. First, Ca2+ was introduced together with the fluorescent dye cascade blue, which allowed for monitoring the Ca2+ diffusion through the microscopy chamber. Second, one of the liposome populations contained a self-quenching concentration of the red fluorescent lipid analog 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine 4-chlorobenzenesulfonate. Thus, lipid mixing could be monitored because dilution of the lipids resulted in decreased self-quenching and an increase in fluorescence. Third, a self-quenching concentration of the green fluorophore sulforhodamine-B was encapsulated in the lumen of one of the liposome populations. Leakage and content mixing could hence be directly followed by monitoring dispersion and/or increase of the fluorescence. Because these three fluorophores are spectrally separated, Ca2+ diffusion, as well as membrane tethering and lipid and content mixing, could be followed simultaneously.
The results contain a few surprises. First, SNAREs alone mediated some tethering but hardly sufficed to catalyze membrane fusion: Only 20% of the tethered liposomes fused within ∼30 min. Tethering was enhanced in the presence of complexin [as reported previously (9, 13)] or with synaptotagmin (7, 14). In the presence of synaptotagmin-1, Ca2+ dramatically increased fusion. Fusion consisted of a fast phase with a time constant well below the 200-ms time resolution and a slower phase with a subsecond time scale. Very interestingly, disrupting Ca2+ binding to the C2B-domain of synaptotagmin-1 only affected the fast phase of membrane fusion, whereas the total fusion efficiency remained unaltered. Because bulk assays do not allow discrimination between these two phases, this explains why the C2B mutant was indistinguishable from the WT in some studies (7, 12). A striking difference that remains to be clarified is the nonphysiologically low Ca2+ sensitivity: Kyoung et al. (3) require more than 3 mM for acceleration, whereas Lee et al. (10) require only 10 μM.
The triple-fluorescence single-liposome assay of Kyoung et al. (3) provides a powerful tool for future work. Most importantly, it will facilitate studying the role of other regulatory proteins, such as Munc18, Munc13, and Rab3-interacting molecule (1, 6). Also, with direct evidence that trans-SNARE complex formation can, in fact, mediate tethering, it will be very interesting to determine how far the SNARE motifs are coiled up and how further zippering is arrested. Particularly attractive is the perspective of combining the assay of Kyoung et al. (3) with an earlier single-molecule FRET approach from the same group (15).
The study by Kyoung et al. (3) introduces a unique tool for studying membrane fusion. Most importantly, or perhaps even worryingly, they show profoundly different kinetics of lipid and content mixing, asking for a reevaluation of the many studies on membrane fusion that only looked at lipid mixing. The triple-fluorescence single-liposome fusion assay may allow resolution of some of the questions that have been occupying the SNARE field for many years.
Acknowledgments
Work on membrane fusion in our laboratory is supported by US National Institutes of Health Grant P01 GM072694 and Deutsche Forschungsgemeinschaft Grant SFB803. G.v.d.B. is financially supported by the Human Frontier Science Program.
Footnotes
The authors declare no conflict of interest.
See companion article on pages E304 and 11737.
References
- 1.Jahn R, Scheller RH. SNAREs—Engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Rhee JS, et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc Natl Acad Sci USA. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyoung M, et al. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc Natl Acad Sci USA. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 6.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 7.van den Bogaart G, et al. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat Struct Mol Biol. 2011 doi: 10.1038/nsmb.2061. 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 9.Smith EA, Weisshaar JC. Docking, not fusion, as the rate-limiting step in a SNARE-driven vesicle fusion assay. Biophys J. 2011;100:2141–2150. doi: 10.1016/j.bpj.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HK, et al. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 2010;328:760–763. doi: 10.1126/science.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boukobza E, Sonnenfeld A, Haran G. Immobilization in surface-tethered lipid vesicles as a new tool for single biomolecule spectroscopy. J Phys Chem B. 2001;105:12165–12170. [Google Scholar]
- 12.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat Struct Mol Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 13.Seiler F, Malsam J, Krause JM, Söllner TH. A role of complexin-lipid interactions in membrane fusion. FEBS Lett. 2009;583:2343–2348. doi: 10.1016/j.febslet.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araç D, et al. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 15.Brunger AT, Weninger K, Bowen M, Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem. 2009;78:903–928. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]