Abstract
Death of pancreatic β cells is a pathological hallmark of type 1 diabetes (T1D). However, the molecular mechanisms of β cell death and its regulation are poorly understood. Here we describe a unique regulatory pathway of β cell death that comprises microRNA-21, its target tumor suppressor PDCD4, and its upstream transcriptional activator nuclear factor-κB (NF-κB). In pancreatic β cells, c-Rel and p65 of the NF-κB family activated the mir21 gene promoter and increased miR-21 RNA levels; miR-21 in turn decreased the level of PDCD4, which is able to induce cell death through the Bax family of apoptotic proteins. Consequently, PDCD4 deficiency in pancreatic β cells renders them resistant to death, and PDCD4 deficiency in NOD or C57BL/6 mice conferred resistance to spontaneous diabetes and diabetes induced by autoimmune T cells or the β cell toxin streptozotocin (STZ). Thus, the NF-κB−microRNA-21−PDCD4 axis plays a crucial role in T1D and represents a unique therapeutic target for treating the disease.
Keywords: autoimmunity
Apoptosis of pancreatic β cells is a critical component of the pathological process of autoimmune type 1 diabetes (T1D); however, the relevant death mediators and regulators that function in β cells have not been well established (1, 2). The transcription factor nuclear factor-κB (NF-κB) is activated by survival factors and cytokines such as TNF-α and IL-1β during T1D, but its role in β cell death is complex and controversial. Whereas NF-κB prevents apoptosis in most cell types, it can both promote and inhibit islet β cell death (3, 4). This appears to be related to the nature of the death-inducing conditions involved. For example, TNF-α–induced β cell death is inhibited by NF-κB (4, 5), whereas that induced by interleukin (IL)-1β is promoted by NF-κB (6, 7). In either case, the molecular mechanisms of the NF-κB effect have not been well characterized.
PDCD4 (programmed cell death protein 4) is a translational and transcriptional regulator of gene expression and tumor suppressor (8–10). It inhibits cap-dependent translation through its interaction with the eukaryotic initiation factors (eIF) 4A and eIF4G (10, 11). On the other hand, PDCD4 also controls gene transcription in the nucleus (12–16). However, although PDCD4 is predominantly localized to the nucleus and is able to inhibit AP-1, its mechanisms of action in transcription are poorly understood. The recent discovery that PDCD4 interacts with the DNA binding domain of the transcription factor Twist-1, inhibiting its DNA binding ability, provides a potential mechanism for the PDCD4 effect (17). We initially cloned the rat Pdcd4 (i.e., DUG) from insulinoma cells undergoing apoptosis (10) and generated Pdcd4 knockout C57BL/6 mice by germ line gene targeting (18). As we reported, Pdcd4 knockout mice develop normally, but are defective in certain immune responses and eventually succumb to spontaneous lymphomas (12, 18).
MicroRNAs (miRNAs, or miRs) are small noncoding RNAs that control the translation of mRNAs in a manner similar to short interfering RNAs (siRNAs), i.e., by promoting the degradation of target mRNAs or preventing their translation (19, 20). MicroRNA-21 (miR-21) targets PDCD4 mRNA posttranscriptionally, blocking production of the PDCD4 protein (21–23). This miRNA is up-regulated in many cancers, including lymphoma and leukemia and therefore has been called an “oncomiR.” MiR-21 up-regulation is believed to be responsible for the loss of PDCD4 protein in a variety of human cancers (24–28). Additionally, miR-21 up-regulation is also associated with the development of a variety of inflammatory diseases including colitis and psoriasis (29, 30).
We report here that the three aforementioned factors, NF-κB, PDCD4, and miR-21, form a unique regulatory axis that controls islet β cell death during T1D. This axis is involved in NF-κB–mediated protection of pancreatic β cells and may serve as a unique therapeutic target for treating T1D.
Results and Discussion
PDCD4 Deficiency in Nonhematopoietic Cells Reduces the Susceptibility to Type 1 Diabetes.
To determine the potential roles of PDCD4 in the pathogenesis of type 1 diabetes, we generated Pdcd4-deficient nonobese diabetic (NOD) mice by backcrossing Pdcd4-deficient B6 mice to the NOD background as described in Materials and Methods. Compared with age-matched wild-type (WT) littermates, we found that young (<2 mo old) Pdcd4−/− NOD mice had normal body, thymus, and spleen weights and did not exhibit any gross abnormalities. By flow cytometry, we found that the percentages of cells expressing CD4, CD8, B220, and CD11c in the spleen and thymus of Pdcd4−/− and WT mice were similar (Fig. S1A). We also found that there was no significant difference in the expression of the T-cell activation markers CD44, CD62L, and CD25 between Pdcd4−/− and WT cells (Fig. S1B). PDCD4 expression has been shown to be up-regulated upon induction of apoptosis in several cell types (31, 32). We found that Pdcd4 deficiency did not affect anti-CD3– or LPS-induced apoptosis of splenocytes (Fig. S2A). Splenocyte proliferation was also similar between WT and Pdcd4−/− NOD cells (Fig. S2B).
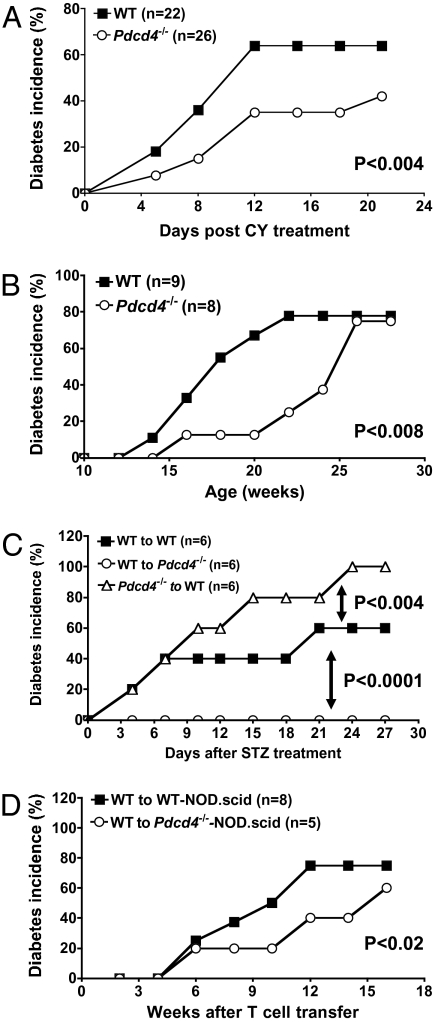
NOD mice spontaneously develop autoimmune diabetes, which shares many immunological and pathological features with human T1D (33). The T1D in these mice can be significantly accelerated and synchronized by cyclophosphamide (CY). To determine the effect of PDCD4 deficiency on T1D, we monitored the development of spontaneous and CY-induced diabetes in Pdcd4−/− NOD mice. Age- and sex-matched wild-type littermates were used as controls. Remarkably, we found that Pdcd4−/− NOD mice were significantly resistant to both spontaneous and CY-induced diabetes (Fig. 1 A and B). Specifically, 2 wk after the CY treatment, ∼70% of WT NOD mice developed diabetes. By contrast, the incidence of the disease decreased to ∼40% in the Pdcd4−/− group (Fig. 1A). Similarly, spontaneous diabetes was significantly delayed in Pdcd4−/− NOD mice compared with their WT controls (Fig. 1B). Consistent with these clinical findings, histochemical analysis of pancreatic sections of WT and Pdcd4−/− mice revealed significant differences. Insulitis, characterized by peri- and intraislet infiltration by inflammatory cells, was less severe in Pdcd4−/− than in WT mice (Fig. S3 A and B). Taken together, these results indicate that PDCD4 plays a crucial role in accelerating T1D.
Fig. 1.
PDCD4 deficiency in nonhematopoietic cells exacerbates type 1 diabetes. (A) Six- to 8-wk-old WT (filled square, n = 22) and Pdcd4−/− (open circle, n = 26) NOD mice were injected with 200 mg/kg cyclophosphamide to induce diabetes. In all experiments in this study, mice were considered diabetic if the blood glucose levels equaled or exceeded 250 mg/dL on two consecutive tests. Data presented are accumulated diabetes incidence pooled from three independent experiments. (B) WT (filled square, n = 9) and Pdcd4−/− (open circle, n = 8) NOD mice were monitored for the development of spontaneous diabetes for 28 wk. Data presented are accumulated diabetes incidence pooled from two independent experiments. (C) Bone marrow chimeric B6 mice (n = 6) were generated by injecting WT or Pdcd4−/− bone marrow cells into WT or Pdcd4−/− mice as described in Materials and Methods. Eight weeks later, mice were injected with low-dose STZ to induce diabetes. Mice were monitored for the development of diabetes for 27 d. Results are accumulated diabetes incidence and are representative of two independent experiments. (D) A total of 1.5 × 107 splenic T cells isolated from WT NOD mice were injected into WT NOD.scid mice (n = 8) and Pdcd4−/− NOD.scid mice (n = 5) through the tail vein. Mice were monitored for the development of spontaneous diabetes for 16 wk. Results are accumulated diabetes incidence and are representative of two independent experiments. The differences between the two groups are statistically significant for all panels as determined by the Kaplan–Meier test.
PDCD4 is constitutively expressed by both hematopoietic and nonhematopoietic cells. It is important to determine which cells express PDCD4, which plays a role in T1D. This may be accomplished by generating bone marrow or lymphocyte chimeric mice. Thus, chimeric mice were generated by injecting Pdcd4−/− or WT bone marrow cells into irradiated Pdcd4−/− or WT recipients. Mice were then injected with low-dose streptozotocin (STZ) to induce T1D (34). We found that PDCD4 deficiency in hematopoietic cells significantly exacerbated the diabetes, whereas PDCD4 deficiency in nonhematopoietic cells alleviated it (Fig. 1C). The accumulated incidence of diabetes was decreased from 60% in WT mice to 0% in mice with PDCD4 deficiency in nonhematopoietic cells (P < 0.0001). On the other hand, the accumulated incidence of diabetes was increased from 60% in mice that received WT bone marrow cells to 100% in mice that received Pdcd4−/− bone marrow cells (P < 0.004). Similarly, following adoptive transfer of WT T cells into WT NOD.scid or PDCD4-deficient NOD.scid mice, the spontaneous T1D was significant decreased in PDCD4-deficient NOD.scid recipient mice (P < 0.02, Fig. 1D). Taken together, these results indicate that PDCD4 expressed by hematopoietic cells plays an opposite role of that of nonhematopoietic cells in T1D.
Increased Th1, Th2, and Th17 Cytokine Gene Expression in Pdcd4−/− T Cells.
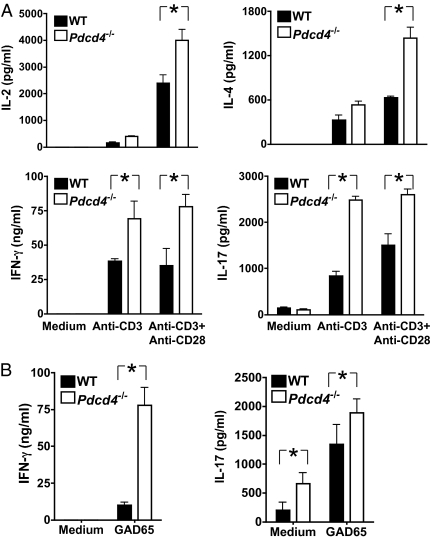
T cells are a subset of hematopoietic cells that play important roles in T1D. To determine the potential effect of PDCD4 deficiency in T cells, we examined their cytokine expression in vitro upon stimulation with anti-CD3 or islet antigen GAD65. We found that, upon stimulation with anti-CD3, Pdcd4−/− NOD splenocytes, pancreatic lymph node cells, and islet infiltrating T cells produced significantly more Th17 (IL-17A), Th1 (IL-2 and IFN-γ), and Th2-type cytokines (IL-4) (Fig. 2A and Figs. S4 and S5). Consistent with this finding, IL-17A and IFN-γ were also significantly increased in Pdcd4−/− T-cell cultures stimulated with murine GAD65 (Fig. 2B and Figs. S4 and S5). In both pancreatic lymph nodes and islets, the frequency of CD4+CD44+ T cells was increased, whereas that of CD4+CD25+ T cells unchanged in PDCD4-deficienct mice compared with WT mice (Fig. S6). These results indicate that PDCD4 deficiency in hematopoietic cells may promote autoreactive T-cell activation and therefore exacerbate diabetes, which is consistent with results from the chimeric experiment shown in Fig. 1C. On the other hand, we found that the production of proinflammatory cytokines (IL-6, IL-12, and TNF-α) by myeloid cells following stimulation with LPS was not significantly different between WT and Pdcd4−/− groups (Fig. S7).
Fig. 2.
PDCD4 deficiency increases cytokine production. Mice were treated as in Fig. 1A and killed 22 d after the CY injeciton. Splenocytes were isolated from WT and Pdcd4−/− mice (n = 3) and cultured at 1.5 million per well in 96-well plates with or without (control) plate-bound anti-CD3 mAb (1 μg/mL) and anti-CD28 mAb (5 μg/mL) (A) or GAD65 (20 μg/mL) (B). Two days later, culture supernatants were collected and cytokine concentrations were determined by quantitative ELISA. The differences between WT and Pdcd4−/− cultures are determined by ANOVA (P < 0.01). Data are representative of three separate experiments.
These results indicate that PDCD4 may selectively regulate Th cell differentiation. To test this possibility, WT and PDCD4-deficient splenic CD4+ T cells were purified and cultured under Th1-, Th2-, Th17-, or Treg-inducing conditions. We found that PDCD4 deficiency did not significantly affect the differentiation of Th1, Th2, Th17, or Treg cells (Fig. S8). Consistent with these results, the frequency of Tregs was not altered in PDCD4-deficient mice (Fig. S9A). These data indicates that the increased Th1, Th2, and Th17 responses in PDCD4-deficient cells is likely related to the PDCD4 effect on gene expression, not cell differentiation.
PDCD4 Deficiency Prevents Islet β Cell Death, Whereas PDCD4 Overexpression Induces It.
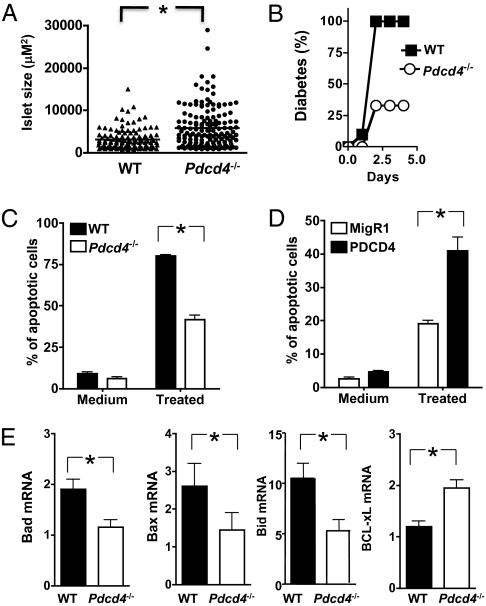
Because PDCD4 expressed by nonhematopoietic cells accelerates autoimmune diabetes (Fig. 1) and PDCD4 is up-regulated during apoptosis in INS-1 insulinoma cells (10), we examined the impact of PDCD4 deficiency on the death and survival of pancreatic islet β cells. Compared with control animals, the mean pancreatic islet size in 12-wk-old Pdcd4−/− NOD mice were significantly increased (Fig. 3A). To determine the effect of PDCD4 deficiency on streptozococin-induced killing of β cells in vivo, we subjected WT and Pdcd4−/− B6 mice to one-time high-dose STZ treatment. Two days after the STZ treatment, 100% of WT mice developed diabetes. By contrast, the incidence of the disease was reduced to ∼30% in the Pdcd4−/− group (Fig. 3B).
Fig. 3.
PDCD4 deficiency in islet β cells renders them resistant to death, whereas PDCD4 overexpression sensitizes them to death. (A) Pancreatic sections from 12-wk-old WT and Pdcd4−/− NOD mice (n = 4) were fixed in 10% formalin, embedded in paraffin, sectioned (three sections from each animal, 120 μM apart), stained with antiinsulin antibody, and examined by microscopy using ImageJ software. Each data point represents an islet. Mean size of islets was shown as the horizontal bar. (B) Resistance of Pdcd4−/− mice to high-dose streptozotocin-induced islet toxicity. WT (n = 10) and Pdcd4−/− (n = 9) B6 mice were injected once with 160 mg/kg STZ, and diabetes was monitored as in Fig. 1. The difference between the two groups is statistically significant (P < 0.03). (C) Islet β cells from Pdcd4−/− mice are resistant to cytokine-induced death. Islet β cells were isolated from WT and Pdcd4−/− mice (n = 6) and were cultured in medium or with IFN-γ (100 U/mL) and TNF-α (10 ng/mL) (treated) for 3 d. Cells were stained with annexin V and 7AAD, and the degree of apoptosis was analyzed by flow cytometry. (D) Islet β–TC-6 cells were infected with retroviruses encoding GFP (MigR1) or PDCD4 and GFP (PDCD4). They were then either left untreated or treated with cytokines as in D for 3 d, and apoptosis was analyzed by flow cytometry as in D. (E) PDCD4 deficiency alters the expression of apoptosis-related genes. Islet β cells were isolated from WT and Pdcd4−/− B6 mice (n = 6) and treated with IFN-γ (100 U/mL) and TNF-α (10 ng/mL). After 24 h, total RNAs were extracted and mRNA expression were analyzed by real-time RT-PCR. Data are representative of three separate experiments. The difference between the two groups is statistically significant, *P < 0.01.
Next, we examined the effect of PDCD4 deficiency and overexpression in a model of cytokine-induced death of islet β cells in vitro. Inflammatory cytokines such as TNF-α plus IFN-γ directly induce apoptosis of islet β cells, simulating insulitis-induced injury in T1D (35). Compared with WT islet β cells, PDCD4-deficient cells were significantly less sensitive to apoptosis (Fig. 3C). Conversely, PDCD4 overexpression in β–TC-6 cells increased their susceptibility to cytokine-induced apoptosis (Fig. 3D). Consistent with these results, the mRNA levels of proapopotic genes (Bad, Bax, and Bid) were significantly decreased in PDCD4-deficient islet β cells, whereas that of antiapoptotic gene Bclxl was increased (Fig. 3E).
The NF-κB−miR-21−PDCD4 Axis That Controls Islet β Cell Death.
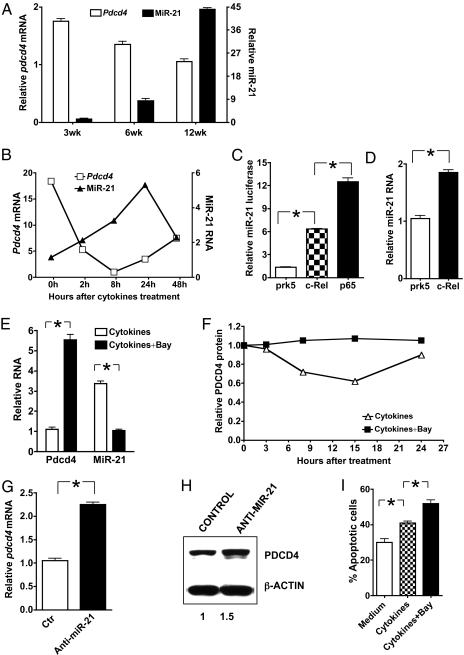
NF-κB inhibition in islet β cells accelerates the development of T1D, and NF-κB prevents β cell death induced by TNF-α plus IFN-γ (4, 5). However, the molecular mechanisms underlying the NF-κB effect are not clear. PDCD4 is negatively regulated by miR-21 (23), and at least in myeloma cells, miR-21 expression is regulated by NF-κB (36, 37). We therefore hypothesized that NF-κB might regulate β cell death through miR-21 and PDCD4. To test this hypothesis, we first examined the relationship between miR-21 and PDCD4 levels in pancreatic islets of NOD mice. In the NOD mice, periinsulitis begins to affect a small number of islets 6–8 wk after birth and spreads to the majority of islets around the age of 13–14 wk. At the latter time point, the β cell mass decreases significantly, resulting in elevation of blood glucose and development of diabetes. By comparing miR-21 and PDCD4 levels in the islets of female NOD mice during the first 12 wk of their lives when the blood glucose levels were normal, we discovered a striking inverse relationship between the two. In islets of 6- and 12-wk-old animals, the levels of miR-21 were significantly higher than that of 3-wk-old mice (Fig. 4A). In contrast, the level of PDCD4 was significantly decreased in the islets of older animals. This inverse relationship was recapitulated in β–TC-6 cells treated with inflammatory cytokines TNF-α and IFN-γ in vitro. The expression of miR-21 increased, whereas that of PDCD4 decreased (Fig. 4B). Because the PDCD4 we detected in the inflamed islets might also be from infiltrating T cells, we analyzed the T-cell infiltration levels in 3-wk- and 12-wk-old NOD mice. We found that although infiltrating T cells were present in NOD islets, they were far outnumbered by islet cells. Specifically, the infiltrating T cells in the islets of 3-wk-old and 12-wk-old NOD mice accounted for only ∼1.1 and ∼9% of all cells, respectively (Fig. S9B). We then separated islet cells from infiltrating cells by gradient Ficoll centrifugation and analyzed PDCD4 expression by real-time RT-PCR. We found that the expression of PDCD4 was significantly decreased in purified islet cells of 12-wk-od NOD mice compared with those of 3-wk-old NOD mice (Fig. S9C).
Fig. 4.
The NF-κB−miR-21−PDCD4 axis protects islet β cells from death. (A) PDCD4 down-regulation correlates with miR-21 up-regulation in the islets of NOD mice. Pancreatic islet β cells were isolated from 3-, 6-, and 12-wk-old NOD mice (three mice per time point). Total RNA (for PDCD4) and short-fragment–enriched RNA (for miR-21) were extracted as described in Materials and Methods. The levels of PDCD4 mRNA and miR-21 were determined by real-time RT-PCR. (B) PDCD4 down-regulation correlates with miR-21 up-regulation in the islet β–TC-6 cells. β–TC-6 cells were treated with IFN-γ (100 U/mL) and TNF-α (10 ng/mL) for the indicated times. The levels of PDCD4 mRNA and miR-21 were determined by real-time RT-PCR. (C) NF-κB activates the Mir21 promoter. β–TC-6 cells were transiently transfected with the Mir21 promoter luciferase construct together with an expression vector for full-length murine c-Rel or p65, or the empty vector pRK5 as indicated. After 24 h, cells were treated with IFN-γ and TNF-α for 8 h and the luciferase activities measured. The promoter activity is presented as fold increase over cells transfected with empty vector. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was included as an internal control. (D) NF-κB–c-Rel induces miR-21 expression in β–TC-6 cells. β–TC-6 cells were transfected with a c-Rel expression plasmid or empty vector using Lipofectamine LTX reagents. Cells were then stimulated with IFN-γ and TNF-α for 8 h. MiR-21 expression was determined as in Fig. 4A. (E and F) NF-κB inhibition down-regulates miR-21, whereas it up-regulates PDCD4 expression in β–TC-6 cells. β–TC-6 cells were treated with IFN-γ and TNF-α with or without NF-κB inhibitor Bay. Cells were collected 8 h after the treatment and the expression of PDCD4 mRNA and miR-21 (E) were determined by real-time RT-PCR. To determine the level of PDCD4 protein, cells were collected at the indicated times, and Western blot was performed using anti-PDCD4. The relative level of PDCD4 protein was quantified by densitometry (F). (G and H) Knocking down miR-21 up-regulates PDCD4 expression in β–TC-6 cells. β–TC-6 cells were transfected with siRNA for miR-21 or a control siRNA (Ambion). Cells were then treated with IFN-γ and TNF-α for 8 h, and PDCD4 mRNA (G) and protein (H) were determined by real-time RT-PCR and Western blot, respectively. (I) NF-κB inhibition promotes β–TC-6 cells from cytokine-induced apoptosis. β–TC-6 cells were treated with IFN-γ and TNF-α with or without the NF-κB inhibitor Bay for 3 d. Cells were stained with annexin V and 7AAD and analyzed by flow cytometry. For all panels, data are representative of three separate experiments. The difference between the two groups is statistically significant, *P < 0.01.
We next examined the functional relationships among NF-κB, miR-21, and PDCD4 in β cells. We found that overexpression of c-Rel and p65 increased both Mir21 promoter activity and the level of miR-21 RNA in β–TC-6 cells (Fig. 4 C and D). Conversely, inhibition of NF-κB with Bay reversed the cytokine-induced miR-21 expression, whereas significantly increased PDCD4 mRNA and protein levels (Fig. 4 E and F). On the other hand, inhibition of miR-21 with miR-21 siRNA also reversed the cytokine-induced repression of PDCD4 mRNA and protein levels (Fig. 4 G and H). Finally, inhibition of NF-κB with Bay protected β–TC-6 cells from cytokine-induced apoptosis (Fig. 4I). Taken together, these results indicate that NF-κB regulates β cell death through the miR-21–PDCD4 axis (Fig. 5).
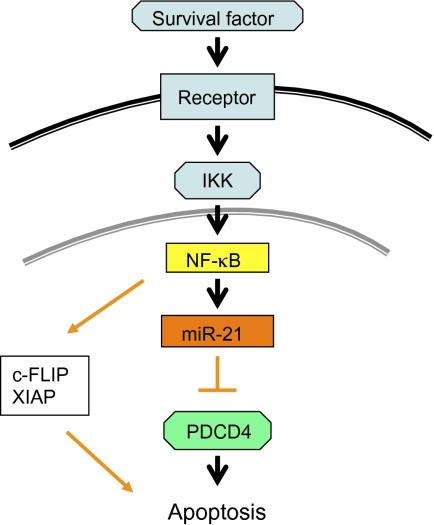
Fig. 5.
NF-κB protects islet β cells through the miR-21–PDCD4 axis. Survival factors activate NF-κB through IKK (by degrading the inhibitor protein IκB). After activation, NF-κB translocates into the nucleus and turns on its target genes, including Mir21. MiR-21 in turn degrades PDCD4 and protects the islet β cells from PDCD4-induced death.
Islet-infiltrating leukocytes produce proinflammatory cytokines, such as IL-1β, TNF-α, and IFN-γ and play a central role in the development of T1D. Prolonged exposure of β cells to inflammatory cytokines leads to apoptosis or necrosis, which in turn causes diabetes. However, the molecular mechanisms that control islet cell death during T1D are not clear. Eukaryotic cells contain hundreds of noncoding RNAs called microRNAs that bind to the 3′ untranslated region of mRNAs, affecting their stability and/or translation. miRNAs have been shown to play a central role in many physiological and pathological processes in humans. miRNAs are also important regulators of β cell apoptosis and functions (38). Our results indicate that an important function of the miR-21–PDCD4 pathway is to mediate the protective effect of NF-κB in islet β cells. It has been reported that inflammatory cytokines such as TNF-α and IFN-γ are important death inducers in T1D. On the basis of our data, we propose that the antiapoptotic function of NF-κB in islet β cells may be mediated by the down-regulation of PDCD4, whose transcription and/or translation is inhibited by miR-21 (Fig. 5). Thus, targeting the miR-21−PDCD4 pathway may represent a unique strategy for treating autoimmune T1D.
Materials and Methods
Mice.
C57BL/6 (B6) mice that carry a Pdcd4 gene mutation were generated as we described previously (18). To generate NOD mice with the Pdcd4 gene null mutation, Pdcd4−/−, B6 mice were backcrossed to NOD mice for nine generations. All mice were housed in the University of Pennsylvania animal care facilities under pathogen-free conditions and all procedures were preapproved by the institutional animal care and use committee (IACUC).
Induction and Evaluation of Diabetes.
For CY-induced diabetes, NOD mice were injected intraperitoneally with 200 mg/kg of CY once a week on two occasions. For high-dose STZ-induced diabetes, B6 mice were injected intraperitoneally once with 160 mg/kg of STZ. For low-dose STZ-induced diabetes, B6 mice were injected intraperitoneally with 40 mg/kg of STZ for 5 consecutive days. Both STZ and CY were purchased from Sigma. Mice were tested in a blinded manner every other day for urinary glucose levels using the Keto-Diastix kit (Bayer) and every other week for blood glucose levels. They were considered diabetic if the urinary glucose levels were >500 mg/dL or the blood glucose levels were >250 mg/dL on at least two consecutive tests.
Flow Cytometry and Antibodies.
Flow cytometric analyses were used to determine the rate of apoptosis of β cells. After treatment, cells were stained with annexin V and 7AAD (BD Biosciences). Stained cells were analyzed on a FACS-Calibur flow cytometer (BD Biosciences). Data were analyzed with FlowJo software.
Cell Culture and Cytokine Assays.
For cytokine assays, splenocytes were cultured at 1.5 × 106 cells/well in 0.2 mL of DMEM with 10% FBS in the presence or absence of 20 μg/mL GAD65 peptide or anti-CD3 and anti-CD28. Culture supernatants were collected 48 h later, and cytokine concentration was determined by quantitative ELISA. All purified antibodies and recombinant cytokines were purchased from BD Biosciences.
Real-Time RT-PCR.
Total RNA was extracted using TRIzol (Invitrogen) and small RNA species were extracted using mirVana miRNA isolation kit (Ambion) according to the manufacturer's instructions. Reverse transcription was performed using oligo dT primers or specific primers for miR-21 and control U6 (Ambion). Real-time PCR was carried out in an Applied Biosystems 7500 system using Power SYBR Green PCR master mix (Applied Biosystems). Relative levels of gene expression were determined using GAPDH (for PDCD4, Bad, Bid, BCL-xL, and Bax) or U6 (for miR-21) as the control. The primers for PDCD4 are as follows: forward, 5′-atggatatagaaaatgagcagac-3′ and reverse, 5′-ccagatctggaccgcctatc-3′. The primers for Bad, Bid, BCL-xL, and Bax were synthesized as previously described (39, 40).
Immunoblotting.
Cells were lysed using RIPA lysis buffer containing protease inhibitors. Samples were loaded to 12% SDS/PAGE gels and subjected to electrophoresis. Proteins were transferred to nitrocellulose membranes and subsequently probed for PDCD4 using the polyclonal anti-PDCD4 (Tebu-Bio; Rockland; 600–401-965) or for β-actin using the monoclonal antibody AC-15 (Sigma).
Bone Marrow and T-Cell Transfers.
Bone marrow chimeric mice were generated by irradiating wild-type and Pdcd4−/− recipient mice twice with 500 rads spaced 3 h apart, followed by i.v. injection of 107 bone marrow cells from wild-type or Pdcd4−/− C57BL/6 or NOD mice. Repopulation of the immune system was monitored by flow cytometric analysis of the blood. As we reported, in the chimeric mice so generated, ∼90% of the T cells and >95% of the B cells and myeloid cells were derived from donor bone marrow 8–9 wk after the cell transfer (41). For T-cell transfer, 1.5 × 107 T cells isolated from spleens were injected into NOD.scid mice through the tail vein.
Transient Transfection.
For siRNA transfection, β–TC-6 cells were transfected using siPORT NeoFX Transfection Agent (Ambion). Cells were left to recover for 24 h before treatment with IFN-γ plus TNFα for the indicated times. For luciferase assays, β–TC-6 cells were transiently transfected with miR-21 promoter–luciferase construct (37) together with the c-Rel or p65 expression vector or empty vector using Lipofectamine LTX transfection reagent (Invitrogen). After 24 h, cells were treated with or without IFN-γ plus TNFα for 8 h and the luciferase activities of total cell lysates measured using the Dual-Luciferase Reporter Assay system (Promega). Cotransfection of the Renilla–luciferase expression vector pRL-TK (Promega) was used as an internal control for all reporter assays.
Mouse Islet Isolation.
Islets were isolated from murine pancreata as follows. A total of 4 mL collagenase P solution (1 mg/mL) was slowly injected into the common bile duct after occlusion of the distal end proximal to the duodenum. The distended pancreas was excised and the digestion was performed in a water bath at 37 °C for 30 min. The collagenase digest was then subjected to a Ficoll gradient separation to facilitate the harvesting of islets. Islets were hand picked and counted under an inverted microscope.
Recombinant Retroviruses.
The recombinant PDCD4 retrovirus was generated by inserting the murine PDCD4 cDNA into the XhoI and EcoRI site of the MigR1 vector and by transfecting 293T cells with the recombinant MigR1 plasmid. In the recombinant viral genome, the PDCD4 cDNA lies directly 5′ of an internal ribosome entry site (IRES), which is followed by a cDNA encoding enhanced green fluorescent protein (EGFP).
Statistical Analysis.
The significance of the differences in disease severity and immune parameters were determined by the Kaplan–Meier test, paired Student's t test, or ANOVA.
Supplementary Material
Acknowledgments
The authors thank Dr. Peng Wang and Dr. Warren Pear for reagents and/or valuable discussions and Jennifer Devirgiliis, the Islet Cell Biology Core of the Penn Diabetes Center, and the Morphology Core of the Center for Molecular Studies in Digestive and Liver Diseases at the University of Pennsylvania for technical support. This work was supported by National Institutes of Health Grants DK070691, AI50059, AI069289, and DK19525.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101450108/-/DCSupplemental.
References
- 1.Kim YH, et al. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: No role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol. 1999;29:455–465. doi: 10.1002/(SICI)1521-4141(199902)29:02<455::AID-IMMU455>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimberg H, et al. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 2001;50:2219–2224. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, et al. NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA. 2007;104:1913–1918. doi: 10.1073/pnas.0610690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang I, et al. Nuclear factor kappaB protects pancreatic beta-cells from tumor necrosis factor-alpha-mediated apoptosis. Diabetes. 2003;52:1169–1175. doi: 10.2337/diabetes.52.5.1169. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Hohmeier HE, Gasa R, Tran VV, Newgard CB. Selection of insulinoma cell lines with resistance to interleukin-1beta- and gamma-interferon-induced cytotoxicity. Diabetes. 2000;49:562–570. doi: 10.2337/diabetes.49.4.562. [DOI] [PubMed] [Google Scholar]
- 7.Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem. 2000;275:36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- 8.Shibahara K, et al. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166:297–301. doi: 10.1016/0378-1119(95)00607-9. [DOI] [PubMed] [Google Scholar]
- 9.Yoshinaga H, Matsuhashi S, Fujiyama C, Masaki Z. Novel human PDCD4 (H731) gene expressed in proliferative cells is expressed in the small duct epithelial cells of the breast as revealed by an anti-H731 antibody. Pathol Int. 1999;49:1067–1077. doi: 10.1046/j.1440-1827.1999.00995.x. [DOI] [PubMed] [Google Scholar]
- 10.Goke A, et al. DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem Biophys Res Commun. 2002;297:78–82. doi: 10.1016/s0006-291x(02)02129-0. [DOI] [PubMed] [Google Scholar]
- 11.Yang HS, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheedy FJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 13.Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: Recent advances in the elucidation of function and regulation. Biol Cell. 2009;101:309–317. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 14.Bitomsky N, Wethkamp N, Marikkannu R, Klempnauer KH. siRNA-mediated knockdown of Pdcd4 expression causes upregulation of p21(Waf1/Cip1) expression. Oncogene. 2008;27:4820–4829. doi: 10.1038/onc.2008.115. [DOI] [PubMed] [Google Scholar]
- 15.Leupold JH, et al. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–4562. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- 16.Yang HS, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiota M, et al. Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res. 2009;69:3148–3156. doi: 10.1158/0008-5472.CAN-08-2334. [DOI] [PubMed] [Google Scholar]
- 18.Hilliard A, et al. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol. 2006;177:8095–8102. doi: 10.4049/jimmunol.177.11.8095. [DOI] [PubMed] [Google Scholar]
- 19.Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The microRNA world: Small is mighty. Trends Biochem Sci. 2003;28:534–540. doi: 10.1016/j.tibs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 23.Frankel LB, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 24.Belair C, Darfeuille F, Staedel C. Helicobacter pylori and gastric cancer: Possible role of microRNAs in this intimate relationship. Clin Microbiol Infect. 2009;15:806–812. doi: 10.1111/j.1469-0691.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 25.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Lee CG. MicroRNA and cancer—focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramantieri L, et al. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12(6A):2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho WC. OncomiRs: The discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. doi: 10.1053/j.gastro.2008.07.068. e24. [DOI] [PubMed] [Google Scholar]
- 30.Bostjancic E, Glavac D. Importance of microRNAs in skin morphogenesis and diseases. Acta Dermatovenerol Alp Panonica Adriat. 2008;17:95–102. [PubMed] [Google Scholar]
- 31.Hwang SK, et al. Aerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter mice. Gene Ther. 2007;14:1353–1361. doi: 10.1038/sj.gt.3302983. [DOI] [PubMed] [Google Scholar]
- 32.Lankat-Buttgereit B, Lenschen B, Schmidt H, Göke R. The action of Pdcd4 may be cell type specific: Evidence that reduction of dUTPase levels might contribute to its tumor suppressor activity in Bon-1 cells. Apoptosis. 2008;13:157–164. doi: 10.1007/s10495-007-0153-x. [DOI] [PubMed] [Google Scholar]
- 33.Toyoda H, Formby B. Contribution of T cells to the development of autoimmune diabetes in the NOD mouse model. Bioessays. 1998;20:750–757. doi: 10.1002/(SICI)1521-1878(199809)20:9<750::AID-BIES8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Flodström M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999;48:706–713. doi: 10.2337/diabetes.48.4.706. [DOI] [PubMed] [Google Scholar]
- 35.Suk K, et al. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: A key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 36.Vinciguerra M, et al. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology. 2009;49:1176–1184. doi: 10.1002/hep.22737. [DOI] [PubMed] [Google Scholar]
- 37.Zhou R, Hu G, Gong AY, Chen XM. Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. 2010;38:3222–3232. doi: 10.1093/nar/gkq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roggli E, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59:978–986. doi: 10.2337/db09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobascio AM, Klinger FG, De Felici M. Isolation of apoptotic mouse fetal oocytes by AnnexinV assay. Int J Dev Biol. 2007;51:157–160. doi: 10.1387/ijdb.062203al. [DOI] [PubMed] [Google Scholar]
- 40.He CH, et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest. 2005;115:1039–1048. doi: 10.1172/JCI23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilliard BA, et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.