Abstract
Separate reports that hypertonicity activates p38 via a Rac1–OSM–MEKK3–MKK3–p38 pathway and that p38α contributes to activation of TonEBP/OREBP led us to the hypothesis that Rac1 might activate TonEBP/OREBP via p38. The present studies examine that possibility. High NaCl is hypertonic. We find that siRNA knockdown of Rac1 reduces high NaCl-induced increase of TonEBP/OREBP transcriptional activity (by reducing its transactivating activity but not its nuclear localization). Similarly, siRNA knockdown of osmosensing scaffold for MEKK3 (OSM) also reduces high NaCl-dependent TonEBP/OREBP transcriptional and transactivating activities. Simultaneous siRNA knockdown of Rac1 and OSM is not additive in reduction of TonEBP/OREBP transcriptional activity, indicating a common pathway. However, siRNA knockdown of MKK3 does not reduce TonEBP/OREBP transcriptional activity, although siRNA knockdown of MKK6 does. Nevertheless, the effect of Rac1 on TonEBP/OREBP is also independent of MKK6 because it occurs in MKK6-null cells. Furthermore, we find that siRNA knockdown of Rac1 or OSM actually increases activity (phosphorylation) of p38, rather than decreasing it, as previously reported. Thus, the effect of Rac1 on TonEBP/OREBP is independent of p38. We find instead that phospholipase C-γ1 (PLC-γ1) is involved. When transfected into PLC-γ1–null mouse embryonic fibroblast cells, catalytically active Rac1 does not increase TonEBP/OREBP transcriptional activity unless PLC-γ1 is reconstituted. Similarly, dominant-negative Rac1 also does not inhibit TonEBP/OREBP in PLC-γ1–null cells unless PLC-γ1 is reconstituted. We conclude that Rac1/OSM supports TonEBP/OREBP activity and that this activity is mediated via PLC-γ1, not p38.
Keywords: osmotic stress, MAPK
Hypertonicity (e.g., high NaCl) activates the transcription factor TonEBP/OREBP (also known as NFAT5), resulting in increased expression of osmoprotective genes (1). Hypertonicity increases TonEBP/OREBP activity by increasing its abundance (1), transactivating activity (1), nuclear localization (1), and phosphorylation (2, 3). The regulatory increase of phosphorylation depends on both increased kinase activity and reduced phosphatase activity (4, 5). The stress-activated MAP kinase p38 has been extensively studied in this regard, but understanding its role has been complicated because hypertonicity activates two different p38s, namely p38α (MAPK14) and p38δ (MAPK13), which have opposite effects on TonEBP/OREBP activity: p38α increases TonEBP/OREBP activity and p38δ decreases it (6). Furthermore, the phosphospecific antibodies commonly used to measure p38 activity do not directly distinguish between p38α activity and p38δ activity, nor do some forms of inhibition that have been used (6). In this article, we will continue to refer to “p38” unless p38α and p38δ are distinguished. Hypertonicity activates p38α via the upstream kinase MKK3 (MAP2K3) or MKK6 (MAP2K6) (7), and p38α and MEKK3 (MAP3K3) contribute to hypertonicity-induced activation of TonEBP/OREBP (6, 8, 9). Thus, p38α contributes to TonEBP/OREBP activity.
Rac1 is a Rho GTPase in the Ras superfamily (10). It is a molecular switch that transmits diverse signals from cell-surface receptors to intracellular targets, regulating many cellular activities, including gene transcription, cytoskeleton reorganization, cell growth, migration, and oncogenesis (10). A link between Rac1 and p38 was indicated in a report showing that Rac1 forms a complex with osmosensing scaffold for MEKK3 (OSM), MEKK3, and MKK3 that activates p38 in response to hypertonicity (11). Also, Rac1 and p38α are reported to contribute to the hypertonicity-induced increased expression of TonEBP/OREBP mRNA (12). Putting this information together, it has been proposed that Rac1 and OSM might contribute to activation of TonEBP/OREBP via a MEKK3–MKK3–p38 pathway (13, 14). The initial purpose of the present experiments was to test this hypothesis directly. We confirm that Rac1/OSM contributes to activation of TonEBP/OREBP, but the activation is via phospholipase C-γ1 (PLC-γ1), not p38.
Results
Effect of Rac1 and OSM on the Transcriptional Activity of TonEBP/OREBP.
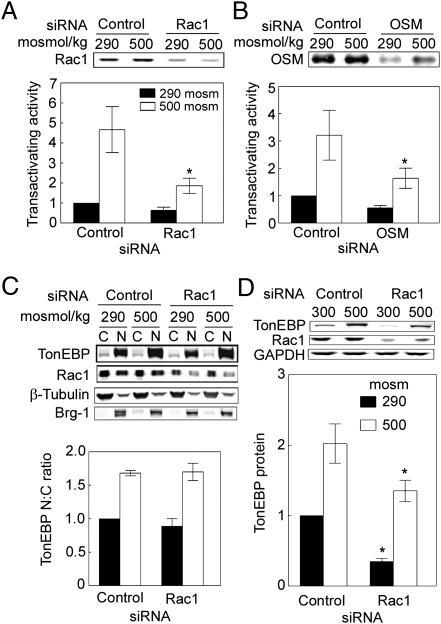
We measured TonEBP/OREBP transcriptional activity in HEK293 cells stably expressing a luciferase reporter containing the osmotic response elements (OREs) that are the target of TonEBP/OREBP in the aldose reductase gene. Raising osmolality from 290 to 500 mosmol/kg by adding NaCl for 16 h increases the transcriptional activity of TonEBP/OREBP by 85-fold (Fig. 1B). siRNA-mediated knockdown of Rac1 or OSM (Fig. 1A) significantly reduces TonEBP/OREBP transcriptional activity at both 290 and 500 mosmol/kg (Fig. 1B). As a control, when the DNA elements in the reporter are mutated to prevent TonEBP/OREBP binding, reporter activity is much lower, is not affected by osmolality, and is not affected by the siRNAs (Fig. 1C). Simultaneous knockdown of both Rac1 and OSM does not inhibit TonEBP/OREBP transcriptional activity any more than knocking down either individually (Fig. 1D), suggesting that Rac1 and OSM act in the same pathway. We conclude that Rac1 and OSM contribute to TonEBP/OREBP transcriptional activity.
Fig. 1.
Rac1 and OSM regulate TonEBP/OREBP transcriptional activity. We used a luciferase reporter containing three OREs in the context of the aldose reductase gene to measure TonEBP/OREBP transcriptional activity. Cells were transfected with Rac1 or OSM siRNAs for 32 h, then osmolality was increased to 500 mosmol/kg (NaCl added) or left at 290 mosmol/kg for 16 h. (A) Specific siRNAs against Rac1 and OSM greatly reduce the abundance of those proteins. (B) Knockdown of either Rac1 or OSM significantly reduces TonEBP/OREBP transcriptional activity, measured in HEK293 cells stably expressing the ORE luciferase reporter. (C) The siRNAs have no significant effect when the reporter contains OREs mutated to prevent binding by TonEBP/OREBP (*P < 0.05 compared with respective control, n = 3). (D) Combined knockdown of both Rac1 and OSM has no greater effect on TonEBP/OREBP transcriptional activity than knocking them down individually (*P < 0.05 compared with control, n = 3).The Rac1 siRNA concentration was reduced (as recommended by Invitrogen) by 60% from 33.3 nM to 13.3 nM to reduce its effect sufficiently so that any additive effect of simultaneously knocking down OSM could be seen.
Effect of Rac1 and OSM on the High NaCl-Induced Increase of TonEBP/OREBP Transactivating Activity.
High NaCl increases the transactivating activity of TonEBP/OREBP in HEK293 cells stably expressing a reporter that contains the TonEBP/OREBP transactivation domain (Fig. 2 A and B). Reporter activity in this assay depends on expression of the TonEBP/OREBP transactivation domain but is independent of expression of native TonEBP/OREBP. siRNA knockdown of Rac1 or OSM significantly reduces this high NaCl-induced increase of TonEBP/OREBP transactivating activity. We conclude that Rac1 and OSM contribute to the high NaCl-induced increase of TonEBP/OREBP transactivating activity.
Fig. 2.
Rac1 and OSM contribute to high NaCl-induced increase of TonEBP/OREBP transactivating activity and TonEBP/OREBP protein abundance but not to TonEBP/OREBP nuclear localization. (A and B) As in Fig. 1, except that we measured TonEBP/OREBP transactivating activity in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system. High NaCl increases TonEBP/OREBP transactivating activity, and the increase is reduced significantly by siRNA against either Rac1 (A) or OSM (B) (*P < 0.05 compared with control at 500 mosmol/kg, n = 3). (C) HEK293 cells were transfected with siRNA against Rac1 for 24 h at 290 mosmol/kg. After incubation for an additional 24 h, the medium was changed for 30 min, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added). Proteins were extracted separately from cytoplasm (C) and nuclei (N) and then analyzed by Western blotting. Note that the subcellular distributions of β-tubulin (cytoplasmic marker) and Brg-1 (nuclear marker) are unaffected by NaCl concentration or siRNA against Rac1, showing consistent separation of cytoplasmic and nuclear proteins. siRNA against Rac1 does not affect nuclear localization of TonEBP/OREBP. N:C, nuclear to cytoplasmic ratio. (D) HEK293 cells were transfected with Rac1 siRNA for 32 h, then osmolality was increased to 500 mosmol/kg (NaCl added) or left at 290 mosmol/kg for 16 h (*P < 0.05 compared with respective control, n = 3). Knockdown of Rac1 significantly reduces TonEBP/OREBP protein abundance.
Effect of Rac1 on High NaCl-Induced Nuclear Localization and Protein Abundance of TonEBP/OREBP.
High NaCl increases the nuclear to cytoplasmic ratio of TonEBP/OREBP (Fig. 2C). However, siRNA knockdown of Rac1 has no significant effect on high NaCl-induced TonEBP/OREBP nuclear localization (Fig. 2C). In contrast, siRNA-mediated knockdown of Rac1 significantly reduces TonEBP/OREBP protein abundance regardless of NaCl concentration (Fig. 2D). We conclude that Rac1 contributes to high NaCl-induced increase of TonEBP/OREBP transcriptional activity by increasing its transactivating activity and protein abundance but not its nuclear localization.
Effects of MKK3 and MKK6 on High NaCl-Induced Activation of TonEBP/OREBP.
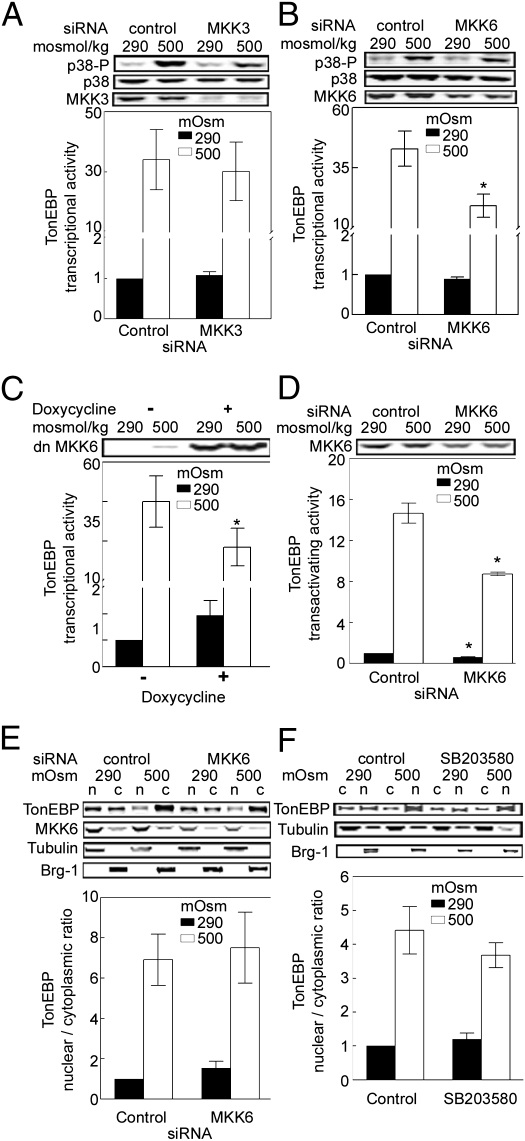
To determine whether Rac1 and OSM activate TonEBP/OREBP through a MEKK3–MKK3–p38 pathway, we tested the effect of siRNA knockdown of MKK3. Knockdown of MKK3 has no significant effect on TonEBP/OREBP transcriptional activity even though it reduces high NaCl-induced phosphorylation of p38 (Fig. 3A), consistent with our previous finding that dominant-negative MKK3 does not reduce TonEBP/OREBP transcriptional activity even though it reduces phosphorylation of p38 (15). On the other hand, MKK6 apparently is involved. siRNA against MKK6 reduces both the high NaCl-induced increase of TonEBP/OREBP transcriptional activity and the high NaCl-induced increase of p38 phosphorylation (Fig. 3B). Dominant-negative MKK6 has the same effect (Fig. 3C). We conclude that MKK3 does not contribute to high NaCl-induced increase of TonEBP/OREBP transcriptional activity but that MKK6 does.
Fig. 3.
MKK6, but not MKK3, contributes to high NaCl-induced increase of TonEBP/OREBP transcriptional activity. (A) HEK293 cells stably expressing an ORE-X luciferase reporter of TonEBP/OREBP activity were transfected with control siRNA or siRNA against MKK3 for 32 h. Then the medium was changed, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added) for 16 h before measuring luciferase activity. (B) As in A, except siRNA was against MKK6 (*P < 0.05 compared with control at 500 mosmol/kg, n = 3). (C) A conditional (Tet-On) vector expressing dominant-negative MKK6-FLAG (dnMKK6) was cotransfected together with an ORE-X reporter of TonEBP/OREBP transcriptional activity into HEK293 cells for 16 h. Then expression of dnMKK6 was induced by adding doxycycline for 24 h before changing the medium, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added) for 16 h (*P < 0.05 compared with control at 500 mosmol/kg, n = 3). (D) MKK6 contributes to high NaCl-induced increase of TonEBP/OREBP transactivating activity. As in B, except that TonEBP/OREBP transactivating activity was measured in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system (*P < 0.05 compared with respective control, n = 3). (E) MKK6 does not contribute to high NaCl-induced nuclear localization of TonEBP/OREBP. As in B, except that NaCl was increased for 30 min before extracting cytoplasmic and nuclear proteins separately for Western blot analysis of TonEBP/OREBP and calculating its nuclear to cytoplasmic ratio. (F) p38 does not contribute to high NaCl-induced nuclear localization of TonEBP/OREBP. HEK293 cells were preincubated with 0.1% DMSO (Control) or 10 μM SB203580 in 0.1% DMSO for 60 min. Then the medium was changed, either maintaining osmolality at 290 mosmol/kg with the inhibitor or increasing it to 500 mosmol/kg with the inhibitor (NaCl added) for 30 min before measuring the TonEBP/OREBP nuclear to cytoplasmic ratio.
Effects of MKK6 and p38 on High NaCl-Induced Increase of TonEBP/OREBP Transactivating Activity and Nuclear Localization.
siRNA against MKK6 significantly reduces TonEBP/OREBP's transactivating activity (Fig. 3D) but does not affect its nuclear localization (Fig. 3E). Thus, MKK6 contributes to high NaCl-induced TonEBP/OREBP transcriptional activity by increasing its transactivating activity, not its nuclear localization. Previous studies have shown that p38 is a major target of MKK6 (7) and that both dominant-negative p38α and the p38 inhibitor SB203580 reduce TonEBP/OREBP transactivating activity (9). Thus, MKK6 apparently increases TonEBP/OREBP transactivating activity via p38α. That being so, the lack of an effect of MKK6 on TonEBP/OREBP nuclear localization suggests that p38 is not involved in high NaCl-induced nuclear localization of TonEBP/OREBP. Consistent with this idea, the p38 inhibitor SB203580 does not significantly affect high NaCl-induced TonEBP/OREBP nuclear localization (Fig. 3F).
Effect of Rac1 and OSM on Phosphorylation of p38.
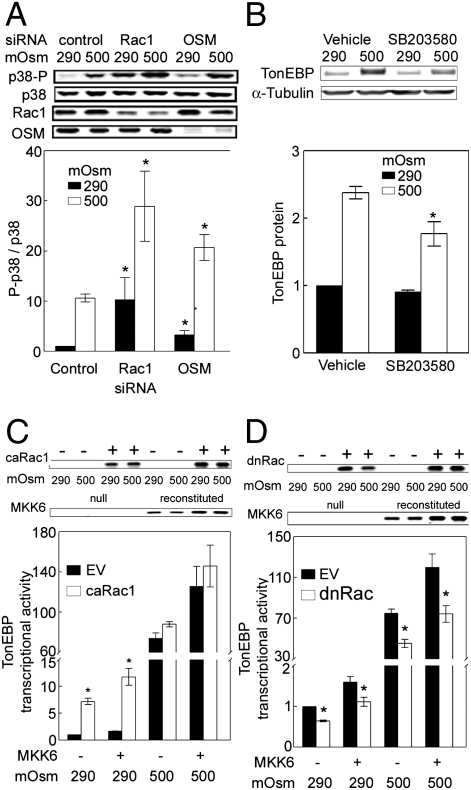
siRNA-mediated knockdown of OSM was reported to inhibit the increase of p38 phosphorylation caused by high sorbitol-induced hypertonicity in HEK293 cells (11). We attempted to extend this observation to high NaCl-induced hypertonicity by testing whether knockdown of OSM also inhibits increased phosphorylation of p38 when hypertonicity is caused by adding NaCl. Contrary to our expectation, we found that siRNA-mediated knockdown of OSM actually increases phosphorylation of p38 both at 290 mosmol/kg and when osmolality is increased to 500 mosmol/kg by adding NaCl (Fig. 4A). siRNA against Rac1 has a similar effect (Fig. 4A). Given this unexpected result, we attempted to reproduce the published effect of sorbitol (11). Using the previously published protocol (11), we found that siRNA knockdown of OSM or Rac1 increases phosphorylation of p38 both at 290 mosmol/kg and when osmolality is increased to 500 mosmol/kg by adding sorbitol (Fig. S1). The effect of knockdown of Rac1 on phosphorylation of p38 is not mediated through Rac2 because we find no evidence that HEK293 cells express Rac2 protein (Fig. S2). We do not know why our result (Fig. S1) is different from the previously published one (11). The p38 inhibitor SB203580 significantly reduces TonEBP/OREBP protein expression at 500 mosmol/kg but not at 300 mosmol/kg (Fig. 4B).We conclude that Rac1 and OSM do not stimulate p38, but may actually inhibit it, and that the stimulation of TonEBP/OREBP by Rac1 and OSM does not depend on p38.
Fig. 4.
siRNA knockdown of Rac1 or OSM increases phosphorylation of p38. (A) HEK293 cells were transfected with either Rac1 or OSM siRNA for 24 h. Then 24 h later, the medium was changed, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added) for 30 min before Western blot analysis of p38 and phospho-p38 (p38-P) (*P < 0.05 compared with respective control, n = 3). (B) SB203580 significantly reduces TonEBP/OREBP protein abundance. HEK293 cells were preincubated with 0.1% DMSO (Vehicle) or 10 μM SB203580 in 0.1% DMSO for 60 min, then osmolality was increased to 500 mosmol/kg with the inhibitor (NaCl added) or left at 290 mosmol/kg with the inhibitor for 8 h (*P < 0.05, compared with control at 500 mosmol/kg, n = 3). (C and D) Expression of Rac1 contributes to transcriptional activity of TonEBP/OREBP independent of MKK6. (Upper) Representative Western blots of expressed transfected MKK6, caRac1, or dominant-negative Rac1 (dnRac1). (C) MKK6−/− MEFs, or the same cells reconstituted with MKK6, were cotransfected with caRac1 or empty vector (EV) and an ORE reporter for 32 h, then the medium was changed, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added) for 16 h before measuring TonEBP/OREBP transcriptional activity (*P < 0.05 vs. EV, n = 3). (D) As in C, except cells were transfected with dominant-negative Rac1 (*P < 0.05 vs. EV, n = 3).
Role of MKK6 in Activation of TonEBP/OREBP by Rac1.
Having found that TonEBP/OREBP activity depends on expression of both Rac1 (Fig. 1B) and MKK6 (Fig. 3B), we tested to see whether the effects are related. Lack of MKK6 [MKK6−/− mouse embryonic fibroblast cells (MEFs)] does not prevent overexpression of catalytically active Rac1 (caRac1) from increasing TonEBP/OREBP transcriptional activity at 290 mosmol/kg (Fig. 4C). caRac1 does not increase TonEBP/OREBP transcriptional activity any further when NaCl is high (Fig. 4C), but that may be because native Rac1 is already activated enough by high NaCl in these cells to obscure the effect of additional activity from caRac1. In addition, dominant-negative Rac1 reduces TonEBP/OREBP transcriptional activity even in the absence of MKK6 expression (Fig. 4D). We conclude that the contribution of Rac1 to TonEBP/OREBP activity is independent of MKK6.
Role of PLC-γ1 in Activation of TonEBP/OREBP by Rac1.
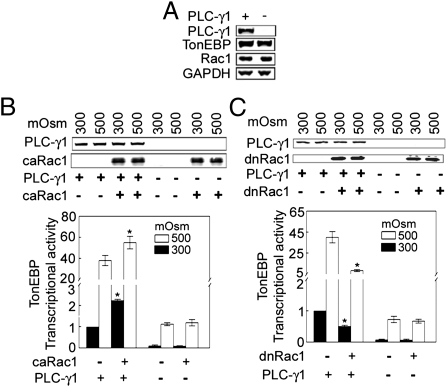
Not finding that MAPK pathways are involved in the activation of TonEBP/OREBP by Rac1, we turned our attention to PLC-γ1 based on reports that PLC-γ1 contributes to activation of TonEBP/OREBP (3) and that Rac1 can activate PLC-γ1 (16, 17). We compared the effect of Rac1 in PLC-γ1–null MEFs to its effect in the same cells in which PLC-γ1 is reconstituted (18). These cells express approximately the same amount of TonEBP/OREBP whether PLC-γ1 is reconstituted or not (Fig. 5A). caRac1 does not increase TonEBP/OREBP transcriptional activity in the null cells, but it does when PLC-γ1 is reconstituted (Fig. 5B). Similarly, dominant-negative Rac1 does not inhibit TonEBP/OREBP in PLC-γ1–null cells unless they are reconstituted with PLC-γ1 (Fig. 5C). We conclude that Rac1 activates TonEBP/OREBP via PLC-γ1.
Fig. 5.
Rac1 activates TonEBP/OREBP via PLC-γ1. (A) Expression of TonEBP/OREBP and Rac1 is equivalent in PLC-γ1+/+ and PLC-γ1−/− MEFs. (B and C Upper) Representative Western blots of expressed transfected PLC-γ1, caRac1 or dominant-negative Rac1 (dnRac1). (B) PLC-γ1+/+ and PLC-γ1−/− MEFs were transfected with caRac1 plus ORE reporter for 32 h, then the medium was changed, either maintaining osmolality at 290 mosmol/kg or increasing it to 500 mosmol/kg (NaCl added) for 16 h before measuring TonEBP/OREBP transcriptional activity (*P < 0.05 versus respective control, n = 3). (C) As in B, except cells were transfected with dominant-negative Rac1 (*P < 0.05 versus respective control, n = 3).
Discussion
Rac1–OSM–MEKK3–MKK3–p38–TonEBP/OREBP?
Our original purpose was to directly test the hypothesis (13) that Rac1 contributes to TonEBP/OREBP activity via an OSM–MEKK3–MKK3–p38 pathway (11). We find that knockdown of Rac1 or OSM reduces TonEBP/OREBP activity, and knockdown of both Rac1 and OSM reduces TonEBP/OREBP activity similarly to each alone (Fig. 1), indicating a common pathway. Thus, our data are in accord with a tonicity-dependent physical association between Rac1 and OSM (11). However, activation of TonEBP/OREBP is not via a Rac1–OSM–MEKK3–MKK3–p38 pathway because knockdown of MKK3 does not affect TonEBP/OREBP transcriptional activity (Fig. 3) and knockdown of Rac1 or OSM increases, rather than decreases, p38 activity (phosphorylation) (Fig. 4A). The effects of Rac1 and p38 on TonEBP/OREBP protein abundance differ in that siRNA knockdown of Rac1 reduces TonEBP/OREBP protein at both 300 and 500 mosmol/kg (Fig. 2D), but the p38 inhibitor SB203580 decreases TonEBP/OREBP abundance only at 500 mosmol/kg (Fig. 4B). The increase of p38 phosphorylation when we knocked down Rac1 is somewhat surprising because Rac1 generally activates p38 (19), but there are other exceptions. For example, Rac1 contributes to EGF-induced phosphorylation of p38 in papilloma cells but not in normal laryngeal cells (20). Furthermore, similar to our finding, knockdown of OSM in human endothelial cells increases phosphorylation of p38 (21).
Rac1 is located in the nucleus as well as in the cytoplasm (22). siRNA against Rac1 knocks it down more in the nucleus than in the cytoplasm (Fig. 2C), probably because it both regulates and is regulated by the cell cycle. Inhibition of Rac1 induces G1 cell-cycle arrest (23), and Rac1 is excluded from the nucleus in early G1 (24). This combination accounts for its disproportionate decrease in the nucleus when it is knocked down.
Rac1–PLC-γ1–TonEBP/OREBP.
Having found that regulation of TonEBP/OREBP by Rac1 is not mediated by MKK3 and p38, we tested whether mediation is via PLC-γ1, which contributes to regulation of TonEBP/OREBP (3), interacts directly with Rac1 (16, 25), and can be a downstream target of Rac1 (16, 17). In addition, Rac1 often acts in combination with PLC-γ1 to regulate cellular signaling events (16, 25, 26). In the present study, we find that caRac1 and dominant-negative Rac1 do not affect TonEBP/OREBP transcriptional activity in PLC-γ1–null MEFs unless PLC-γ1 is reconstituted (Fig. 5). When PLC-γ1 is reconstituted, caRac1 increases TonEBP/OREBP transcriptional activity, and dominant-negative Rac1 reduces it (Fig. 5), similar to the result in HEK293 cells (Fig. 4 C and D). We conclude that, because the effect of caRac1 depends on expression of PLC-γ1, Rac1 must be acting upstream of PLC-γ1 and not vice versa. It should be noted, however, that in other cells and circumstances, PLC-γ1 can act upstream of Rac1 (25).
p38 and TonEBP/OREBP.
p38α contributes to hypertonicity-induced activation of TonEBP/OREBP (9), but full understanding of this interaction has been complicated by opposite effects of p38α and p38δ; p38α increases TonEBP/OREBP activity and p38δ decreases it, but the phosphospecific antibodies commonly used to measure p38 activity do not directly distinguish between p38α activity and p38δ activity (6). In addition, some of the ways of inhibiting p38 inhibit both p38α and p38δ, resulting in no net effect on TonEBP/OREBP activity. For example, MKK3 is an upstream activator of p38, but, although dominant-negative MKK3 prevents high NaCl-induced phosphorylation of p38, it does not inhibit the activation of TonEBP/OREBP (15). That result is confirmed in the present experiments by siRNA knockdown of MKK3 (Fig. 3A). Because MKK3 activates both p38α and p38δ (7), inhibition of MKK3 reduces their opposing effects, resulting in no net change of TonEBP/OREBP activity. Another example is MAPK phosphatase 1 (MKP-1), which also inhibits both p38α and p38δ without any effect on TonEBP/OREBP activity (6). SB203580 is an often-used inhibitor of p38. It inhibits p38α and p38β, but not p38δ (27, 28). SB203580 reduces high NaCl-induced TonEBP/OREBP transcriptional activity, which was interpreted as simply attributable to inhibition of a positive effect of p38 (9, 29). However, the situation evidently is more complicated. Inhibition of the hypertonicity-induced positive effect of p38α by SB203580 also unmasks the accompanying hypertonicity-induced negative effect of p38δ. Similarly, reduction of high NaCl-induced TonEBP/OREBP transcriptional activity by dominant-negative p38α (9) also unmasks the inhibitory effect of p38δ.
Hypertonicity-Induced Activation of p38.
Both MKK3 and MKK6 can activate p38 by phosphorylating it. However, although MKK6 is involved in high NaCl-induced increase of TonEBP/OREBP transcriptional activity, MKK3 is not. Dominant-negative MKK6 reduces high NaCl-induced TonEBP/OREBP transcriptional activity in mouse inner medullary collecting duct cells (30) as siRNA does against MKK6 in HEK293 cells (Fig. 3B). Also, reconstitution of MKK6 increases TonEBP/OREBP transcriptional activity in MKK6-null cells (Fig. 4 C and D). In contrast, siRNA against MKK3 does not reduce TonEBP/OREBP transcriptional activity (Fig. 3A). MKK3 and MKK6 have different effects because they activate different p38s. Both of them activate p38α (31), but MKK3 strongly activates p38δ in response to hyperosmolality, whereas MKK6 does not (32). As discussed above, activation of p38α alone increases TonEBP/OREBP activity, but activation of both p38α and p38δ does not because of their opposing effects.
Additional Considerations.
In the present study, we find that a Rac1–PLC-γ1 pathway contributes to regulation of TonEBP/OREBP transactivating activity. We previously found that PLC-γ1 regulates TonEBP/OREBP transactivating activity via its SH3 domain, independent of its lipase activity (3). In addition, PLC-γ1 also regulates TonEBP/OREBP in a way that apparently does not involve Rac1. PLC-γ1, through its C-SH2 domain, regulates nuclear localization of TonEBP/OREBP, dependent on its lipase activity (3). This activation evidently is independent of Rac1 because Rac1 does not contribute to TonEBP/OREBP nuclear localization (Fig. 2C). Phosphatidylinositol 3-kinase (PI3K) may be an upstream activator of the Rac1–PLC-γ1–TonEBP/OREBP pathway. PI3K increases high NaCl-dependent transactivating activity of TonEBP/OREBP (33), and PI3K is known to activate Rac1 in other contexts (34).
MKK6–p38α–TonEBP/OREBP Pathway.
MKK6 (Fig. 3D), like p38α (9), increases TonEBP/OREBP transactivating activity, but neither MKK6 nor p38α increases its nuclear localization (Fig. 3 E and F). MEKK3 may be the upstream activator of the MKK6–p38α–TonEBP/OREBP pathway because MEKK3 is known to regulate TonEBP/OREBP activity (8) and to act upstream of MKK6 (35). However, this role remains to be established. Also, at present there is no direct indication of how p38α increases high NaCl-dependent TonEBP/OREBP transactivating activity.
Rac1 activation by hyperosmotic stress was previously shown in HEK293 (11) and LLC-PK1 cells (36). In both cases, the activation was transient. Activity peaked at 30 s, after which it rapidly fell back to baseline and even below it, which raises the question of whether the osmotically induced increase of Rac1 activity is required for TonEBP/OREBP signaling or only some Rac1 activity—as a background—is needed for optimal TonEBP/OREBP stimulation. The fact that dominant-negative and siRNA-mediated knockdown of Rac1 reduce TonEBP/OREBP activity both at 300 and 500 mosmol/kg is consistent with the possibility that only background activity of Rac1 is necessary. We cannot distinguish between these possibilities but would emphasize that knockdown of Rac1 reduces the high NaCl-induced increase of TonEBP/OREBP activity (Figs. 1B, 4D, and 5C) and that even a transient increase of Rac1 activity could initiate longer-lived downstream effects.
Finally, it is not surprising that Rac1 and p38α contribute separately to high NaCl-induced activation of TonEBP/OREBP because signaling networks often include parallel pathways. Robustness is a property that allows a system to maintain its functions against internal and external perturbations, and redundancy is a critical strategy for robustness (37). Other examples of kinases that independently signal high NaCl-induced activation of TonEBP/OREBP are Fyn and p38 (9).
Materials and Methods
Cells, Chemicals, and Antibodies.
See SI Materials and Methods for details.
Plasmids, siRNAs, Transfections, and Luciferase Activity.
Plasmids pcDNA3.1-myc-caRac1 and pcDNA3.1-myc-dnRac1 were gifts from Andras Kapus (University of Toronto, Toronto, ON, Canada). The conditional expression construct of dominant-negative MKK6, pTRE2-Flag-dnMKK6, was provided by Norman P. Curthoys (Colorado State University, Fort Collins, CO). pcDNA3.0-Flag-MKK6 was purchased from Addgene. The human ORE-X, ORE, and mutated ORE luciferase reporters were described previously (38). The control siRNA was also described previously (33). The two siRNAs against OSM, described previously (11), were combined in equal amounts. siRNA against human Rac1 (5′-GGUAUUUUACAGCACCAAUCUCCUUAG-3′ and 5′-Phos/AAGGAGAUUGGUGCUGUAAAAUACC-3′) was synthesized by Integrated DNA Technologies. MKK3 and MKK6 siRNAs were purchased from Qiagen. We used Effectene, according the manufacturer's protocol (Qiagen), for transient transfection of plasmids into HEK293 cells. Transfection of siRNAs and transfection of DNA plasmids into MEFs were done with Lipofectamine 2000 in the recommended ratio of siRNA or DNA to Lipofectamine 2000 (Invitrogen). All transfections were done by adding cell suspensions to plated DNA or siRNA and transfection reagent. Luciferase activity was measured as described previously (38).
TonEBP/OREBP Transactivating Activity and Nuclear Localization.
TonEBP/OREBP transactivating activity was analyzed in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system, as previously described (39). To measure TonEBP/OREBP nuclear localization, cytoplasmic and nuclear proteins were extracted separately with NE-PER (Pierce). TonEBP/OREBP in each fraction was measured by Western blot analysis, and nuclear to cytoplasmic ratio was calculated from the concentration in cytoplasmic and nuclear extracts and the relative volumes of the extracts (40). The cytoplasmic protein β-tubulin and nuclear protein Brg-1 were monitored in each extract to exclude the possibility that the ratio was affected by inadequate separation of nuclear and cytoplasmic proteins. To eliminate variability in transfection efficiency, transfected cells were pooled and then split into dishes to receive medium change.
Statistics.
Data are expressed as mean ± SEM. Analyses were performed by paired t test with P < 0.05 considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Roger J. Davis for MKK6−/− MEFs, Dr. Graham Carpenter for PLC-γ1+/+ and PLC-γ1−/− MEFs, Dr. Andras Kapus for Rac1 mutant constructs, and Dr. Norman P. Curthoys for pTRE2-Flag-dnMKK6 plasmid. This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services and by Grant CO83WU from the Uniformed Services University of the Health Sciences, Department of Defense.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108107108/-/DCSupplemental.
References
- 1.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 2.Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol. 2001;280:C248–C253. doi: 10.1152/ajpcell.2001.280.2.C248. [DOI] [PubMed] [Google Scholar]
- 3.Irarrazabal CE, et al. Phospholipase C-γ1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA. 2010;107:906–911. doi: 10.1073/pnas.0913415107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Gallazzini M, Burg MB, Ferraris JD. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA. 2010;107:7072–7077. doi: 10.1073/pnas.1002795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallazzini M, Yu MJ, Gunaratne R, Burg MB, Ferraris JD. c-Abl mediates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP. FASEB J. 2010;24:4325–4335. doi: 10.1096/fj.10-157362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Ferraris JD, Dmitrieva NI, Liu Y, Burg MB. MKP-1 inhibits high NaCl-induced activation of p38 but does not inhibit the activation of TonEBP/OREBP: Opposite roles of p38α and p38δ. Proc Natl Acad Sci USA. 2008;105:5620–5625. doi: 10.1073/pnas.0801453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 8.Padda R, et al. MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am J Physiol Renal Physiol. 2006;291:F874–F881. doi: 10.1152/ajprenal.00377.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ko BC, et al. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP) J Biol Chem. 2002;277:46085–46092. doi: 10.1074/jbc.M208138200. [DOI] [PubMed] [Google Scholar]
- 10.Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol. 2010;7:637–647. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- 11.Uhlik MT, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 12.Kino T, et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2:ra5. doi: 10.1126/scisignal.2000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander RT, Grinstein S. Activation of kinases upon volume changes: Role in cellular homeostasis. Contrib Nephrol. 2006;152:105–124. doi: 10.1159/000096319. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh-Hamad D, Gustin MC. MAP kinases and the adaptive response to hypertonicity: Functional preservation from yeast to mammals. Am J Physiol Renal Physiol. 2004;287:F1102–F1110. doi: 10.1152/ajprenal.00225.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kültz D, Garcia-Perez A, Ferraris JD, Burg MB. Distinct regulation of osmoprotective genes in yeast and mammals. Aldose reductase osmotic response element is induced independent of p38 and stress-activated protein kinase/Jun N-terminal kinase in rabbit kidney cells. J Biol Chem. 1997;272:13165–13170. doi: 10.1074/jbc.272.20.13165. [DOI] [PubMed] [Google Scholar]
- 16.Rao JN, et al. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-γ1 after wounding. Am J Physiol Cell Physiol. 2008;295:C1499–C1509. doi: 10.1152/ajpcell.00232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Singleton PA, Diedrich F. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-γ promotes phospholipase Cγ1 activation, Ca2+ signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem. 2004;279:29654–29669. doi: 10.1074/jbc.M403608200. [DOI] [PubMed] [Google Scholar]
- 18.Liao HJ, Ji QS, Carpenter G. Phospholipase C-γ1 is required for the induction of immediate early genes by platelet-derived growth factor. J Biol Chem. 2001;276:8627–8630. doi: 10.1074/jbc.C100030200. [DOI] [PubMed] [Google Scholar]
- 19.Platanias LC. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther. 2003;98:129–142. doi: 10.1016/s0163-7258(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 20.Wu R, Coniglio SJ, Chan A, Symons MH, Steinberg BM. Up-regulation of Rac1 by epidermal growth factor mediates COX-2 expression in recurrent respiratory papillomas. Mol Med. 2007;13:143–150. doi: 10.2119/2007-00005.Wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, et al. Differential angiogenesis function of CCM2 and CCM3 in cerebral cavernous malformations. Neurosurg Focus. 2010;29:E1. doi: 10.3171/2010.5.FOCUS1090. [DOI] [PubMed] [Google Scholar]
- 22.Lanning CC, Daddona JL, Ruiz-Velasco R, Shafer SH, Williams CL. The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J Biol Chem. 2004;279:44197–44210. doi: 10.1074/jbc.M404977200. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, et al. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–1668. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 24.Michaelson D, et al. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol. 2008;181:485–496. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Wang Q, Wang Y, Chen X, Wang Z. PLC-γ1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol Endocrinol. 2009;23:901–913. doi: 10.1210/me.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones NP, Katan M. Role of phospholipase Cγ1 in cell spreading requires association with a β-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol. 2007;27:5790–5805. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38δ. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 28.Avitzour M, et al. Intrinsically active variants of all human p38 isoforms. FEBS J. 2007;274:963–975. doi: 10.1111/j.1742-4658.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 29.Nadkarni V, Gabbay KH, Bohren KM, Sheikh-Hamad D. Osmotic response element enhancer activity. Regulation through p38 kinase and mitogen-activated extracellular signal-regulated kinase kinase. J Biol Chem. 1999;274:20185–20190. doi: 10.1074/jbc.274.29.20185. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, et al. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest. 2009;119:1647–1658. doi: 10.1172/JCI35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brancho D, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remy G, et al. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cell Signal. 2010;22:660–667. doi: 10.1016/j.cellsig.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welch HC, Coadwell WJ, Stephens LR, Hawkins PT. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546:93–97. doi: 10.1016/s0014-5793(03)00454-x. [DOI] [PubMed] [Google Scholar]
- 35.Deacon K, Blank JL. MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J Biol Chem. 1999;274:16604–16610. doi: 10.1074/jbc.274.23.16604. [DOI] [PubMed] [Google Scholar]
- 36.Thirone AC, et al. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: Key role in the osmotically triggered F-actin response. Am J Physiol Cell Physiol. 2009;296:C463–C475. doi: 10.1152/ajpcell.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–837. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol. 2005;289:F377–F385. doi: 10.1152/ajprenal.00463.2004. [DOI] [PubMed] [Google Scholar]
- 39.Ferraris JD, et al. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc Natl Acad Sci USA. 2002;99:739–744. doi: 10.1073/pnas.241637298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraris JD, Burg MB. Tonicity-regulated gene expression. Methods Enzymol. 2007;428:279–296. doi: 10.1016/S0076-6879(07)28016-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.