Abstract
Two distinct defects are thought to be important for the pathophysiology of schizophrenia. One is an increase of D2 receptors (D2Rs) in the striatum and another is a decrease in the GABAergic function in the prefrontal cortex (PFC). Whether these two defects are functionally linked is not known. We previously reported that selective overexpression of D2Rs in the striatum of the mouse causes behavioral abnormality associated with PFC functions. Using patch-clamp recording, we find that overexpression of D2Rs in the striatum affects inhibitory transmission in the PFC and dopamine (DA) sensitivity. The overexpression of D2Rs in the striatum caused an increase in frequency of spontaneous excitatory postsynaptic currents (EPSCs) in layer V pyramidal neurons, whereas their neuronal excitability was unaffected. In contrast, both the frequency and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) were significantly decreased in these mice, indicating a reduced inhibitory transmission. Furthermore, in D2R transgenic mice the dopaminergic modulation of evoked IPSCs was shifted, with reduced sensitivity. The change in dopamine sensitivity in the PFC of D2R transgenic mice appears specific for D2Rs because in D2R transgenic mice the effects of D2 agonist but not D1 agonist, on both evoked IPSCs and EPSCs, were reduced. Together, these results indicate that overexpression of D2Rs in the striatum leads to a functional deficit in the GABAergic system. These results provide a functional link between D2R overexpression and GABAergic inhibition in the PFC and suggest that the postulated deficit in GABAergic function in schizophrenia could be secondary to alterations in the striatal dopamine system.
Keywords: psychiatric disorders, synaptic transmission, animal model, cognitive functions
The original dopamine (DA) hypothesis of schizophrenia proposed that hyperactivity of dopaminergic transmission leads to the symptoms of schizophrenia. This hypothesis was supported by the observation that all antipsychotic drugs block D2 receptors (D2Rs) (1, 2). Imaging studies provided a direct evidence for the DA hypothesis suggesting a hyperfunction of the striatal DA system. These studies found increased F-Dopa uptake, increased amphetamine-induced DA release, and an increase in the occupancy and density of D2Rs in the striatum (3–7). On the other hand, another deficit, a hypofunction of the prefrontal cortex (PFC), has been associated with the cognitive symptoms. The nature of this hypofunction is unclear (8, 9) but both a hypofunction of the DA system and a hypofunction of the GABAergic system have been postulated to account for this cognitive deficit. Whereas the evidence for a dopaminergic hypofunction in the PFC is not substantial (10–13), several studies have found decreased expression of GABAergic markers in the cortex, including the PFC (14–16). These findings raise the important question: Are the cortical GABAergic hypofunction and the subcortical dopaminergic hyperfunction related?
We previously generated a mouse model of dopaminergic hyperfunction by overexpressing D2Rs selectively in the striatum. These mice showed deficits in prefrontal-dependent cognitive tasks (17–19), and a decrease in incentive motivation (19, 20). Our studies further showed that increased density of D2Rs in the striatum leads to alterations in the DA system of the PFC that could be responsible for the cognitive deficits. Those alterations include increased DA tissue levels, a decrease in DA turnover, and an increase in D1 receptor activation in the PFC (17). Chronic changes in cortical DA function may also have profound direct effects on the electrophysiological properties of PFC neurons and thereby alter synaptic transmission in the PFC. To address this question, we asked: Does up-regulation of D2Rs in the striatum alter synaptic transmission in the PFC? Due to the postulated importance of the GABA system in schizophrenia, we specifically focused on whether D2R up-regulation in the striatum could lead to a hypofunction of the GABAergic system in the PFC. We found that overexpression of D2Rs in the striatum decreased inhibitory transmission and increased excitatory transmission in layer V neurons of the prelimbic area of the PFC. Furthermore, we found a decrease in inhibitory postsynaptic current (IPSC) sensitivity in response to DA due to a decrease in D2R function. These results suggest that the D2R overexpression in the striatum could contribute to the hypofunction of the GABAergic system in the PFC.
Results
D2R Overexpression in the Striatum Results in Increased Excitatory Synaptic Transmission in PFC Layer V Pyramidal Neurons.
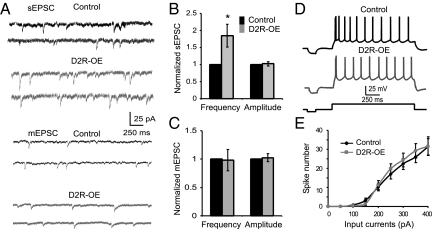
Selective D2R overexpression in the striatum leads to persistent abnormalities in the functioning of the PFC (17). Because of the importance of synaptic transmission for PFC function, we examined whether D2R overexpression in the striatum would change excitatory synaptic transmissions in the PFC. To explore this question, we first recorded spontaneous EPSCs (sEPSCs) in layer V pyramidal neurons in slices of the medial PFC. Compared with control mice, the frequency of sEPSCs recorded from D2R transgenic mice (D2R-OE mice) was significantly increased by 84.7 ± 33.4% (n = 6, P < 0.05; Fig. 1 A and B), whereas the amplitude of the sEPSCs was unaltered (n = 6, P = 0.53; Fig. 1 A and B). The results indicate that excitatory synaptic transmission in the PFC was enhanced in D2R-OE mice. To further explore the mechanisms of the change in sEPSC in D2R-OE mice, we recorded the miniature EPSCs (mEPSCs) and the neuronal excitability of layer V pyramidal neurons. The mEPSCs were recorded in the presence of tetrodotoxin (TTX), which prevents the opening of voltage-gated sodium channels required for the generation of action potentials. In D2R-OE mice, neither frequency nor amplitude of mEPSCs was altered compared with control (n = 6, P = 0.77 for frequency and P = 0.82 for amplitude; Fig. 1C). Further analysis indicated that the input resistance (240.9 ± 10.9 MΩ in D2R-OE vs. 210.3 ± 10.8 MΩ in control, n = 10, P = 0.31) was not significantly altered, whereas the resting membrane potential (−71.2 ± 2.28 mV in D2R-OE vs. −64.1 ± 1.97 mV in control, n = 10, P = 0.07) showed a trend for a more negative potential in D2R-OE mice, indicating a possible change in potassium channels. The spike numbers induced by step current injection in layer V pyramidal neurons were similar, without significant difference (n = 10, P > 0.05 for both; ANOVA F = 0.11, P = 0.99; Fig. 1 D and E). In addition, neither the threshold of action potentials nor the first spike latency in D2R-OE mice exhibited significant changes compared with those from control mice (n = 10, P = 0.25, and P = 0.99, respectively). These results indicate that neuronal excitability of layer V pyramidal neurons was relatively unaffected by the overexpression of D2 in the striatum.
Fig. 1.
D2R overexpression in the striatum results in increased frequency of sEPSC in layer V pyramidal neurons. (A) Sample traces showing the sEPSCs and mEPSCs. (B) The frequency of the sEPSC was significantly increased almost twofold (n = 6, *P < 0.05) whereas the sEPSC amplitude was unaffected in mice that overexpressed D2 (n = 6, P = 0.53). (C) In contrast, both the frequency and amplitude of the mEPSCs were similar in control littermates and D2R-OE mice, without statistical difference (n = 6, P = 0.77 for frequency and P = 0.82 for amplitude). (D) Sample traces of action potentials from layer V pyramidal neurons in control and D2R-OE mice. (E) The relationship between spike numbers and injected currents in both control and D2R-OE mice. D2R overexpression in the striatum did not affect the excitabilities of layer V pyramidal neurons in the PFC (n = 10, P > 0.05).
D2R Overexpression in the Striatum Results in Decreased Inhibitory Synaptic Transmission in PFC Layer V Pyramidal Neurons.
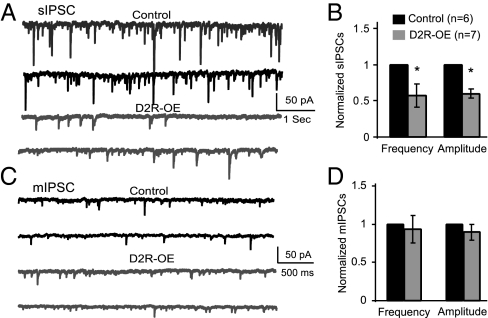
To explore whether inhibitory synaptic transmission in the PFC also was affected by D2R overexpression in the striatum, sIPSCs and mIPSCs were recorded in layer V pyramidal neurons in slices prepared from D2R-OE and control mice. Both the frequency and the amplitude of the sIPSCs recorded from D2R-OE mice were significantly decreased compared with those in control (Fig. 2 A and B, frequency decreased by 42.8 ± 16.2%, P < 0.05; amplitude decreased by 40.3 ± 6.31%, P < 0.05). When we blocked the action potentials in the slice with TTX, both the frequency and amplitude of the mIPSCs were not affected by overexpressing D2Rs in the striatum (P = 0.28 for frequency and P = 0.19 for amplitude; Fig. 2C). These data indicate that D2R overexpression in the striatum attenuated inhibitory synaptic transmission in the PFC.
Fig. 2.
D2R overexpression in the striatum results in decreases in both frequency and amplitude of sIPSCs in layer V pyramidal neurons. (A) Sample traces of the sIPSC recorded from layer V pyramidal neurons in control and D2R-OE mice in the presence of AP5 (50 μM) and NBQX (20 μM). (B) The sIPSC frequency and amplitude recorded from the control and D2R-OE mice (P < 0.05 for both). (C) Sample traces of the mIPSC recorded from layer V pyramidal neurons in control and D2R-OE mice in the presence of TTX. (D) The mIPSCs in both mice genotypes seem to be similar without any statistical difference (n = 5, P = 0.28 for frequency and P = 0.19 for amplitude).
D2R Overexpression in the Striatum Results in Reduced Sensitivity of GABAa Receptor-Mediated IPSCs to DA in PFC Layer V Pyramidal Neurons.
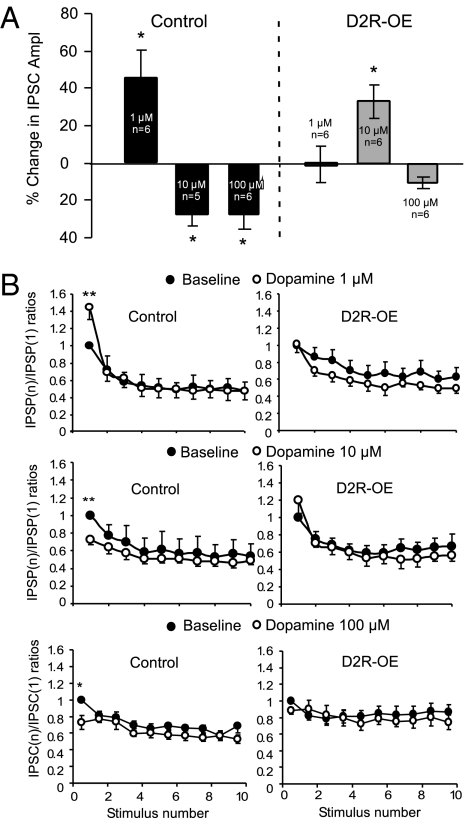
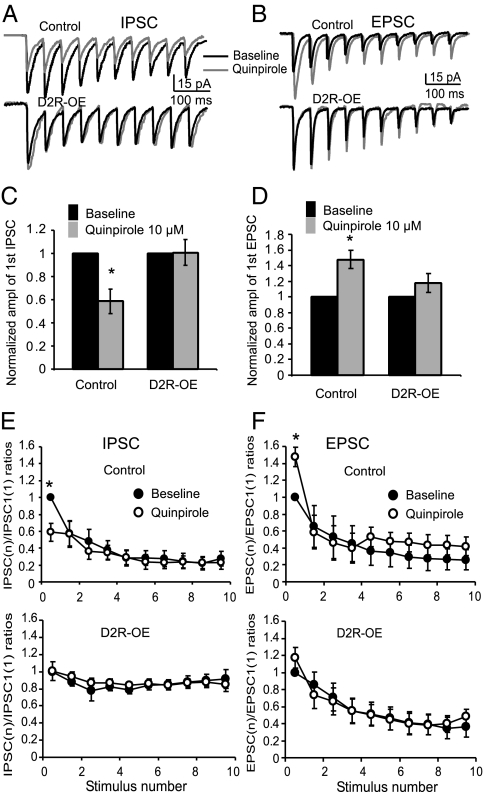
Because of the attenuated inhibitory synaptic transmission in the PFC of striatal D2R-OE mice, we tested the response of GABAa receptor-mediated IPSCs to different concentrations of DA in the layer V pyramidal neurons in the PFC of young adult (3–4 mo) mice. Consistent with results from previous studies (21, 22), we found the effects of DA on inhibitory synaptic transmission to be bidirectional and concentration dependent in the control mice. At a relatively low concentration of 1 μM, DA significantly enhanced the amplitude of evoked IPSCs by 45.1 ± 14.9% (n = 6, P < 0.05; Fig. 3 A and B), whereas at a higher concentration of 10 μM or 100 μM, DA decreased the amplitude of evoked IPSCs by about 27.1 ± 6.23% and 27.2 ± 8.30%, respectively (n = 6, P < 0.05 for both; Fig. 3). Surprisingly, the dopaminergic responses to evoked IPSCs in the D2R-OE mice were significantly shifted compared with the responses in control mice. Application of 1 μM DA had no obvious effect on the amplitude of the IPSCs (increased by 1.15 ± 9.88%, n = 6, P = 0.99). In contrast, 10 μM DA significantly increased the amplitude of the IPSCs by 32.9 ± 8.97% (n = 6, P < 0.05), whereas 100 μM DA caused a small decrease in the amplitude of IPSC (11.8 ± 4.02%) without statistical significance (n = 6, P = 0.06; ANOVA F = 21.1, P < 0.001; Fig. 3A). These data indicate that D2R overexpression decreased the sensitivity of inhibitory synaptic transmission in the PFC to regulation by DA.
Fig. 3.
D2R overexpression in the striatum results in reduction of the sensitivity to DA of GABAa receptor-mediated IPSC in layer V pyramidal neurons. The IPSCs were isolated in the presence of AP5 (50 μM) and NBQX (20 μM). (A) In control mice, DA induced distinctly bidirectional effects on evoked IPSCs, with significant enhancement at 1 μM but suppression at 10 μM and 100 μM (*P < 0.05). In contrast, in the D2R-OE mice, DA at 1 μM had almost no effect on evoked IPSCs (n = 6, P = 0.99) but significantly increased the amplitude by almost 40% at 10 μM (n = 6, *P < 0.05). When the concentration was increased to 100 μM, the IPSC amplitude decreased again but had no significance (n = 6, P = 0.06; ANOVA F = 21.1, P < 0.001). (B) The effects of DA on evoked IPSCs trains in control and mice that overexpressed D2 (n = 6 at each concentration, *P < 0.05). The IPSC currents elicited in the train were normalized to the amplitude of the first IPSC recorded in the baseline condition and were plotted against the stimulus numbers. These data show that DA significantly changed the amplitude of the IPSCs (*P < 0.05) but exhibited no clear effect on the ratios of the IPSCs elicited by repetitive pulses (i.e., IPSC2-10, P > 0.05), suggesting potential postsynaptic effects.
To further explore the action of DA in the pre- or postsynaptic site in the GABAergic synapses, we used a 10-pulse 20-Hz train to elicit IPSCs and test dopaminergic modulation. We calculated the paired-pulse ratio (PPR) using the nth IPSC divided by the first IPSC within a train before and after application of DA. Despite the significant changes in the amplitudes of the IPSCs at some of the DA concentrations described above, we did not see any significant changes in the PPRs from the 2nd to 10th IPSCs before and after administering different doses of DA in either D2R-OE or control mice, indicating that all IPSCs from the 2nd to the 10th were equally modulated by DA (Fig. 3B, ANOVA P > 0.05 for all; SI Text and Fig. S1). These data suggest that the effects of DA on the evoked IPSCs likely involved postsynaptic mechanisms, consistent with results from previous studies (21, 22) but differed from others recorded in layer II/III pyramidal neurons (23).
D2R Overexpression in the Striatum Does Not Alter the Effects of the D1 Agonist on both IPSCs and EPSCs in the PFC.
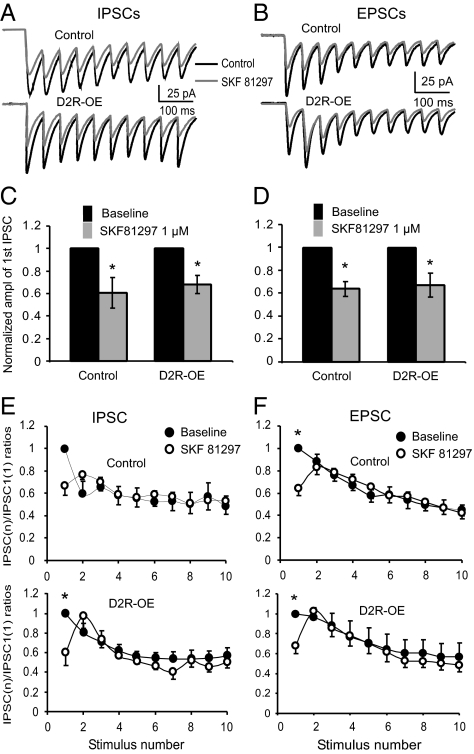
To determine which type of DA receptors are involved in the change in sensitivity of DA to synaptic transmission in layer V pyramidal neurons in striatal D2R-OE mice, we tested the effects of the D1/D5 agonist SKF-81297 on the evoked IPSC and EPSC trains in both transgenic and control mice. SKF-81297 is a highly D1-selective agonist, which has a Ki for the D1 receptor of ∼2 nM but has a Ki for the D2R of ∼1,000 nM (24). We and others recently reported that SKF-81297 at a concentration of 1 μM significantly enhanced NMDA receptor (NMDAR) currents and trafficking in the PFC neurons (25, 26). We found that bath application of SKF-81297 (1 μM) for 10 min caused significant depression in IPSCs trains recorded from both D2R-OE and control mice. Fig. 4 A and C show that the amplitudes of the first IPSCs were significantly decreased by 39.5 ± 13.8% (n = 6, P < 0.05) in control mice and by 36.0 ± 5.95% in D2R-OE mice (n = 6, P < 0.05; Fig. 4 A and C). We noted no difference between the decreases in these two groups (P = 0.58). Furthermore, the PPRs in the IPSC trains were also similar, without significant differences before and after application of SKF-81297 in both D2R-OE and control mice (n = 6, ANOVA, F = 0.19, P = 1.00 in control and F = 0.61, P = 0.77 in D2-OE mice; Fig. 4E). Similarly, SKF-81297 also significantly depressed the evoked EPSCs but had limited effects on the PPRs of the EPSCs in both control and D2R-OE mice. The amplitudes of the first EPSCs were significantly decreased by 39.8 ± 6.73% in control mice and by 32.0 ± 8.04% in D2R-OE mice (n = 6, P < 0.05; Fig. 4 B and D), but no difference between the decreases in these two groups (P = 0.70). The PPRs of the EPSCs in the 10-pulse trains were also unaffected by SKF-81297 applications in both control and D2R-OE mice (n = 6; ANOVA F = 0.28, P = 0.97 in control and F = 0.09, P = 1.00 in D2R-OE mice; Fig. 4F). We also measured the 20–80% rise time of the IPSCs in the neurons recorded from both control and D2R-OE mice and we found no statistical difference between the two groups (1.63 ± 0.13 ms in control vs. 1.67 ± 0.93 ms in D2R-OE, n = 12, P = 0.88). Together, these data indicate that D1/D5 receptors are not involved in the change of DA sensitivity in layer V pyramidal neurons in the PFC induced by overexpressing D2Rs in the striatum.
Fig. 4.
D2R overexpression in the striatum does not affect the effect of the D1 agonist on evoked IPSCs and EPSCs in layer V pyramidal cells. Representative traces showing evoked IPSCs (A) and EPSCs (B) recorded from control and D2R-OE mice before and after application of the D1 agonist SKF-81297 (1 μM). Effects of the D1 agonist SKF-81297 on the first IPSCs (C) or EPSCs (D) in control mice were similar to those in mice that overexpressed D2. D1 receptor stimulation caused significant decrease of either IPSC1 or EPSC1 amplitude (n = 6, *P < 0.05) but had no significant effects on subsequent IPSCs (E) and EPSCs (F) in the trains (n = 6, P > 0.05 for all by ANOVA).
D2R Overexpression in the Striatum Decreases the Effect of the D2 Agonist on both IPSCs and EPSCs in the PFC.
We next examined the effects of D2 agonism on evoked IPSCs and EPSCs in layer V pyramidal neurons in the PFC. Bath application of the selective D2/D3 receptor agonist quinpirole (10 μM) for 10 min caused significant depression of the amplitudes of the IPSCs in the IPSC trains in control mice, but had no clear effects on the IPSC trains in D2R-OE mice. A single time point analysis showed that the first IPSC was decreased by 41.3 ± 10.6% in the control mice (n = 6, P < 0.05) compared with a decrease of 0.82 ± 11.1% in the D2R-OE mice (n = 6, P > 0.05; Fig. 5 A and C). Multiple time point analysis showed that the PPRs in the IPSC trains were not affected by application of quinpirole in both control and D2R-OE mice (n = 6; ANOVA F = 0.01, P = 1.00 in control and F = 0.27, P = 0.97 in D2R-OE mice; Fig. 5E). In contrast, quinpirole (10 μM) significantly enhanced the amplitudes of the EPSCs in the EPSC trains recorded from control mice, but had no effects on the EPSC trains recorded from the D2R-OE mice. The first EPSC was increased by 47.8 ± 11.6% in control mice (n = 6, P < 0.05; Fig. 5 B and D) compared with a slight increase of 17.6 ± 12.1% (n = 6, P = 0.24; Fig. 5 B and D). Similarly, quinpirole had no clear effects on the PPRs as multiple time point analysis exhibited in both control and D2R-OE mice (n = 6; ANOVA F = 0.35, P = 0.94 in control and F = 0.13, P = 0.10 in D2R-OE mice; Fig. 5F). These data indicate that the altered sensitivity to DA in synaptic transmission induced by D2 overexpression in the striatum appears to be mediated by the changes in D2Rs in the PFC.
Fig. 5.
D2R overexpression in the striatum desensitizes the effect of the D2 agonist on both IPSCs and EPSCs. Representative traces showing evoked IPSCs (A) and EPSCs (B) recorded from control and D2R-OE mice before and after application of the D2 agonist quinpirole (10 μM). Quinpirole significantly decreased the amplitude of the first IPSCs (C, n = 6, *P < 0.05) but increased the amplitude of EPSCs (D, n = 6, *P < 0.05) in control mice. In contrast, quinpirole exhibited no effects on the amplitudes of both evoked IPSCs and EPSCs in D2R-OE mice (C and D, n = 6, P = 0.24). We did not observe any significant changes in subsequent IPSCs (E) and EPSCs (F) in the trains before and after application of quinpirole, indicating unaltered paired-pulse ratios in both IPSC and EPSC (n = 6, P > 0.05 for all by ANOVA).
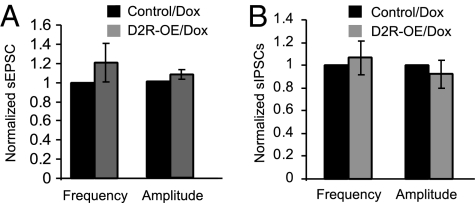
Reversal of D2R Overexpression by Doxycycline Recovers the Changes in both sEPSC and sIPSC.
To further determine whether the alterations in synaptic transmission is coincident with D2R overexpression, we recorded both sEPSC and sIPSC in layer V pyramidal neurons in both control and D2R-OE mice that were fed for 14 d with doxycycline-supplemented food (40 mg/kg) after reaching adulthood (17). We found that after switching off the D2 gene with doxycycline feeding, there were no differences in either frequency or amplitude in both sEPSCs and sIPSCs between control and D2R-OE mice (n = 6, P = 0.46 for frequency and P = 0.34 for amplitude, Fig. 6A; n = 6, P = 0.83 for frequency and P = 0.16 for amplitude, Fig. 6B).
Fig. 6.
Changes in synaptic transmissions were reversed after switching off transgenic D2R expression by feeding the mice doxycycline for two weeks. (A) Frequency and amplitude of sEPSCs in control and D2R-OE mice fed for 2 wk with doxycycline (n = 6, P = 0.46 for frequency and P = 0.34 for amplitude). (B) Frequency and amplitude of sIPSCs between control and D2R-OE mice fed for two weeks with doxycycline (n = 6, P = 0.83 for frequency and P = 0.16 for amplitude).
Discussion
The last two decades have led to an increased awareness of the importance of the negative and cognitive symptoms in patients with schizophrenia and their resistance to D2R antagonism. These insights have led to a reformulation of the classical DA hypothesis. Functional brain imaging studies suggested that the cognitive and negative symptoms might arise from altered PFC functions (27) and preclinical studies documented the importance of prefrontal D1 receptors for optimal PFC performance (13, 28). These observations led to the hypothesis that an alteration in D1 receptor function in the PFC might be implicated in the cognitive and negative symptoms of schizophrenia, whereas the excess subcortical DA transmission may be related only to core or positive symptoms such as hallucinations and delusions (11, 29–31). Therefore, the predominant hypothesis for schizophrenia was based on the idea that there is an imbalance in DA resulting in (i) hypoactive mesocortical DA projections to the PFC (resulting in hypostimulation of D1 receptors, negative symptoms, and cognitive impairment) and (ii) hyperactive subcortical projections (resulting in hyperstimulation of D2Rs and positive symptoms).
The hypothesis that hyperstimulation of D2Rs in the striatum is relevant only for the positive symptoms was challenged by the observation that D2R-OE mice which showed deficits in prefrontal dependent cognitive tasks that resemble the cognitive deficits of schizophrenia (7, 17–19). The cognitive defects observed in the D2R-OE mice persisted, even after the transgene had been switched off, suggesting a developmental origin (32). In contrast, however, the deficit in incentive motivation (which is a component of the negative symptoms of schizophrenia) was rescued when the transgene was switched off, suggesting that concurrent up-regulation of D2Rs in the adult animal is necessary for the motivational deficit.
Here we explored the nature of the prefrontal deficit at the level of individual neurons. We found that the frequency of sEPSCs recorded from the PFC layer V pyramidal neuron was significantly increased, whereas the frequency and amplitude of sIPSCs were significantly decreased, indicating an increased excitatory and a decreased inhibitory transmission in the PFC of D2R-OE mice compared with control littermates. The changes in excitatory synaptic transmission could be caused by alterations in presynaptic glutamate release or postsynaptic glutamate receptors. Traditionally the frequency of sEPSCs has been interpreted as being presynaptic in origin. However, recent studies indicate that the change in sEPSC frequency could also be attributable to alterations in number of postsynaptic receptors (25, 33). The change in inhibitory synaptic transmission could also be derived from changes in either presynaptic release or postsynaptic GABAa receptors because not only the frequency but also the amplitude of the sIPSCs was decreased. Independent of the mechanisms involved, the imbalance in the excitatory and inhibitory activities in the PFC networks that we observed could contribute to the behavioral deficits of D2R-OE mice. For example, the altered synaptic transmission in the PFC could lead to the deficits in working memory and conditional associative learning (CAL) that we have observed in D2R-OE mice (17–19). However, switching off the transgene and thereby normalizing D2R expression by feeding the mice for two weeks with doxycycline reverses the alterations in inhibitory and excitatory transmission but not the deficits in the working memory and the CAL tasks. Therefore, the changes in synaptic transmission are not sufficient to explain the prefrontal dependent cognitive deficits in D2R-OE mice, although they could partially contribute. Because the PFC is also involved in motivation (34) and D2R-OE mice show motivational deficits that are reversed after doxycycline treatment (19, 20), it is possible that the altered synaptic transmission in the PFC of D2R-OE mice underlies, or at least contributes to their motivational deficits.
How could hyperactive D2Rs in the striatum affect the functioning of the PFC? The striatum and PFC are anatomically and functionally linked by several parallel frontostriatal loops (7). The striatum receives direct glutamatergic inputs from the PFC and sends feedback to the PFC via the basal ganglia output nuclei and the thalamus (35–37). Thus, a D2R overexpression in the ventral striatum could potentially alter the ventral pallidum-mediodorsal thalamic-medial prefrontal loop (37). Alternatively, increased signaling of striatal D2Rs could also regulate the activity of the dopaminergic neurons, including those projecting to the cortex, thereby affecting DA release in the PFC (7, 38, 39). Our data show that an increase in D2Rs in the striatum led to reduced sensitivity of inhibitory synaptic transmission to DA in PFC layer V pyramidal neurons and that these effects were likely mediated by D2Rs in the PFC. The sensitivity change is unlikely attributable to the developmental change of the dopamine system (32) because all of these data were obtained from the young adult mice (3–4 mo). One possibility would be that increased DA release in the PFC of D2R-OE mice leads to a desensitization of D2Rs and therefore we measured a decreased modulation of IPSCs and EPSCs to the D2 agonist quinpirole. Although we still do not know the exact mechanism of reduced D2R modulation, it may involve desensitization of the receptor due to reduced protein levels, increased internalization, or altered phosphorylation. In contrast, we found that the D1 agonist SKF-81297 similarly depressed the amplitudes of evoked IPSCs and EPSCs in both control and D2R-OE mice, indicating that D1 receptors might not be involved in the change of DA sensitivity in D2R-OE mice. This seems to be contradictory with our previous study (17) in which we found that D1 receptor activation by systemic application of Cl-APB induced significant increase of c-fos activity in the PFC. The reason for this discrepancy is unclear, but as we discussed previously (17), the increased activation of D1 receptors is probably not due to increased absolute amounts of D1 receptors in the mPFC because neither in vitro ligand binding nor real-time PCR revealed evidence for increased D1 receptor expression levels in the PFC. This conclusion is consistent with our findings in the current study. The D1 receptor activation by systemic application of Cl-APB may not be altered in the PFC but in the striatum. Altered activation of the striatum by Cl-APB may indirectly affect prefrontal cortical c-fos activation via the striato-thalamocortical loops. Alternatively, our recording in this study is focused in layer V pyramidal neurons only, whereas the c-fos activation was measured in all cortical layers.
Decreased GABAergic function in the PFC has become an extensive focus in schizophrenia research (16). Several studies have found decreased expression of interneuron markers including parvalbumin, GAD67 and NPY, and these findings have been replicated in independent sample populations (14–16). Although previous studies have proposed that dysfunction of the dopamine system might be derived from the NMDA hypofunction in GABAergic interneurons (9), it is still unknown how the dopamine, glutamate, and GABA systems are affected during the pathological process. Whether the dysfunctions of these systems occur simultaneously or sequentially is unknown. Our results of reduced synaptic transmission of PFC interneurons as a consequence of striatal D2R up-regulation raise the interesting possibility that decreased GABAergic function in schizophrenia could also be secondary to alterations in the striatal DA system, at least in the D2R-OE model. Such a relationship might suggest that alterations in D2 expression in the striatum produce both developmental (nonreversible) and transient (reversible) change in prefrontal cortical function that negatively affect behaviors related to both cognitive and negative symptoms of schizophrenia.
Materials and Methods
Detailed procedures can be found in the SI Materials and Methods. Briefly, mice expressing the human D2R under the tetO minimal promoter were generated on a C57BL/6-CBA(J) F2 background (17). Most of the data were obtained from young adult mice (3–4 mo) except those with doxycycline-supplemented chow (2 mo, Fig. 6). Offspring were genotyped by PCR for tTA and tet-O transgenes (18). Whole-cell patch clamp recordings were conducted in the medial PFC. The layer V pyramidal neurons were recorded in the voltage-clamp mode at −70 mV in the presence of picrotoxin (50 μM) for sEPSCs, or with both picrotoxin and TTX (0.5 μM) for mEPSCs. The evoked EPSCs were elicited by stimulating layer II/III with a 10-pulse 20-Hz train through a bipolar electrode. The sIPSC and mIPSCs at the layer V pyramidal neurons were recorded at −70 mV in the presence of d-AP5 (50 μM) and NBQX (10 μM) without or with TTX (0.5 μM), respectively. The data were analyzed with the Student's t test and were presented as mean ± SE.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grant R01MH232395 (to W.-J.G.), Silvio O. Conte Center for Schizophrenia Research Grant MH 66171 (to E.H.S., C.K., and E.R.K.), a National Alliance for Research on Schizophrenia and Depression Young Investigator award (to C.K.), and a generous gift from Harold and Shari Levy for schizophrenia research (to E.R.K. and E.H.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109718108/-/DCSupplemental.
References
- 1.Seeman P, Lee T. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 2.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Dargham A, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot PS, Laruelle M. The role of in vivo molecular imaging with PET and SPECT in the elucidation of psychiatric drug action and new drug development. Eur Neuropsychopharmacol. 2002;12:503–511. doi: 10.1016/s0924-977x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 5.Wong DF, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 6.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10:40–68, 5. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 10.Akil M, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 11.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 12.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 13.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: Insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 14.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 15.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 16.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 17.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Bach M-E, et al. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci USA. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward RD, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci. 2009;123:720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drew MR, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- 24.Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- 25.Li YC, Liu G, Hu JL, Gao WJ, Huang YQ. Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. J Neurochem. 2010;114:62–73. doi: 10.1111/j.1471-4159.2010.06720.x. [DOI] [PubMed] [Google Scholar]
- 26.Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem. 2008;106:2489–2501. doi: 10.1111/j.1471-4159.2008.05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- 28.Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 30.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 31.Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- 32.Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 35.Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.